Abstract
Background and Aims
While marine omega-3 fatty acids have been associated with a lower mortality in heart failure patients, data on omega-3 and incident heart failure are inconsistent. We systematically reviewed the evidence on the association of omega-3 fatty acids and fish intake with the incidence of heart failure in this meta-analysis.
Methods
We identified relevant studies by searching MEDLINE and EMBASE databases up to August 31, 2011 without restrictions and by reviewing reference lists from retrieved articles.
Results
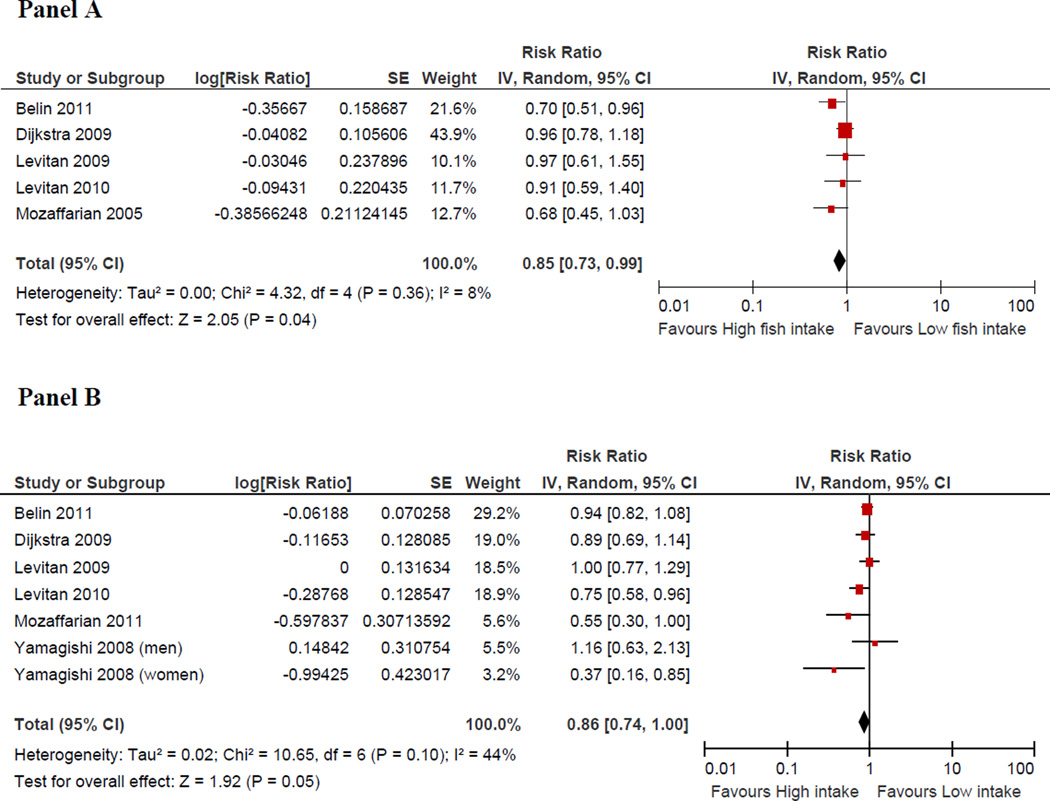
A total of 176,441 subjects and 5,480 incident cases of heart failure from 7 prospective studies were included in this analysis. Using random effect model, the pooled relative risk for heart failure comparing the highest to lowest category of fish intake was 0.85 (95% CI; 0.73–0.99), p=0.04; corresponding value for marine omega-3 fatty acids was 0.86 (0.74–1.00), p=0.05. There was no evidence for heterogeneity across studies of fish consumption (I2=8%). In contrast, there was modest heterogeneity for omega-3 fatty acid analysis (I2= 44%). Lastly, there was no evidence for publication bias.
Conclusions
This meta-analysis is consistent with a lower risk of heart failure with intake of marine omega-3 fatty acids. These observational findings should be confirmed in a large randomized trial.
Keywords: Heart failure, epidemiology, diet, nutrition, omega-3 fatty acids, risk factors
Heart failure (HF) remains a major public health burden1–4. Despite medical progress, mortality after onset of HF remains high, ranging from 20% to 50%5–8. With a rising prevalence of obesity9 and diabetes10, and improved treatment of and survival from myocardial infarction and hypertension, the prevalence of HF is expected to increase in the coming years. Coronary heart disease (CHD) and hypertension are major contributors to HF incidence11–15, suggesting that lowering the risk of CHD and hypertension might reduce the incidence of HF. The DASH trial16 demonstrated beneficial effects of healthy diet on the risk of hypertension. Accumulating evidence suggest that marine omega-3 fatty acids may reduce the risk of CHD deaths17. However, their association with non-fatal CHD18–21 or blood pressure22–24 has been inconsistent. In animal experimental and short-term human clinical trials, marine omega-3 fatty acids improved hemodynamics25, left ventricular structure and function26–28, and inflammation29–31, and thereby play an important role in the development of HF. However, few studies have examined the association between omega-3 fatty acids and HF risk. While some studies have reported a lower risk of HF with consumption of baked or broiled fish32–34 as well as higher plasma of dietary EPA/DHA35, such findings have not been consistent across studies. The observational arm of the Women’s Health Initiative36 reported a lower risk of HF with fish consumption but no association between dietary EPA/DHA and incident HF. Given the inconsistency in the literature on the role of omega-3 fatty acids and HF risk, it is important to clarify whether marine omega-3 fatty acids confer a lower risk of HF. Hence, we conducted a meta-analysis to review current evidence on the association of fish consumption and marine omega-3 (EPA and DHA) with the incidence of HF.
Materials and Methods
We followed the guidelines published by the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group37 to complete the meta-analysis.
Study selection
All relevant cohort studies published in English-language journals from 1966 to August 2011, that evaluated the association between fish consumption or omega-3 fatty acids and HF, were identified by searching EMBASE, MEDLINE, Web of Science and CABI abstracts. We used the terms “fish,” “seafood,” “n-3 fatty acids, “animal products,” “omega-3 fatty acids,” in combination with “congestive heart failure,” and “heart failure.” In addition, we also manually reviewed the references of all retrieved articles and recent reviews to identify relevant studies. Two of our investigators (LD and AOA) independently conducted the search, reviewed all relevant articles and identified eligible studies. We resolved any discrepancy by group discussion. Overall, we included any paper that provided multivariable adjusted relative risks (RRs) and their corresponding 95% confidence intervals for HF, comparing categories of fish consumption, dietary intake or blood concentrations of EPA and DHA. If a study reported RR and 95% CI for men and women separately, and the effect of fish or EPA/DHA intake on the risk of HF was modified by sex, we treated the results by sex as 2 separate studies in the meta-analysis. Finally, where more than one study was published from the same cohort, we only included data from the report with biomarker assessment of marine omega-3 fatty acids or study with more incident heart failure.
Data extraction
Two investigators (LD and AOA) independently abstracted data and entered them in a customized data collection form. The data collection’s form included the first author’s last name, year of publication, country where the study was conducted, duration of follow-up, age range for study participants at baseline, sample size, proportion of men, number of HF events, methods used to assess marine omega-3 fatty acids (measured in plasma, red blood cells, or diet) and fish intake, variables included in the multivariable model. We also recorded median level of exposure, person-years of follow-up, number of cases and the multivariable-adjusted risk estimates and corresponding 95% CI in each exposure category. Dr. Yamagishi38 kindly provided exposure category-specific median levels of circulating EPA and DHA, which were not previously published.
Study quality evaluation
The quality of each study was assessed using the Newcastle-Ottawa Scale39. This scale ranges from 1 to 9 stars and judges each study on three broad categories: selection of the study groups; the comparability of the groups; and the ascertainment of the outcome of interest. Any disagreement was resolved through discussion with two authors (LD and AOA).
Statistical Analysis and data Synthesis
We used RevMan 5.1.4 software (The Cochrane Collaboration, Oxford, England) and STATA (StataCorp, College Station, TX) for the meta-analysis. We transformed hazard ratios by taking their natural logarithms and calculating standard errors from the corresponding 95% CI as follows: Ln[upper limit of CI] – Ln[lower limit of CI])/3.92. To estimate a pooled effect and corresponding 95% CI for the highest versus lowest levels of consumption, we weighted the logarithm of the hazard ratios by the inverse of their variance. The Q test and I2 were used to assess heterogeneity among studies40. In the presence of relevant heterogeneity (I2 >50%), the used the DerSimonian and Laird random effect model41 to obtain a pooled estimate of effect. Publication bias was evaluated by visually inspecting funnel plots for asymmetry42 and by using the Egger’s test43. In a sensitivity analysis, we use the “leave one out” method44 to evaluate studies with substantial impact on between-study heterogeneity. Lastly, we assessed potential heterogeneity in study results by geographic location (US vs. Europe).
Description of method for dose-response
The generalized least-squares method for trend estimation of summarized dose-response data was used to calculate relative risks per unit of exposure based on the Greenland and Longnecker method45. These analyses were carried out for fish and marine omega-3 fatty acid intake and HF risk only, as there was insufficient data for EPA/DHA biomarkers. To check for significant non-linear associations (p<0.05), spline knots were created, using the command MKSPLINE. Piecewise and restricted cubic spline regression models were constructed to assess non-linear associations and the optimal model selected based on the Akaike Information Criterium. The xblc command was used to create the dose-response plot of the linear and non-linear relationships (Stata Journal, 2011, 11:1). Dose-response analyses were completed using Stata 10.0 (StataCorp, College Station, TX).
Results
Search results
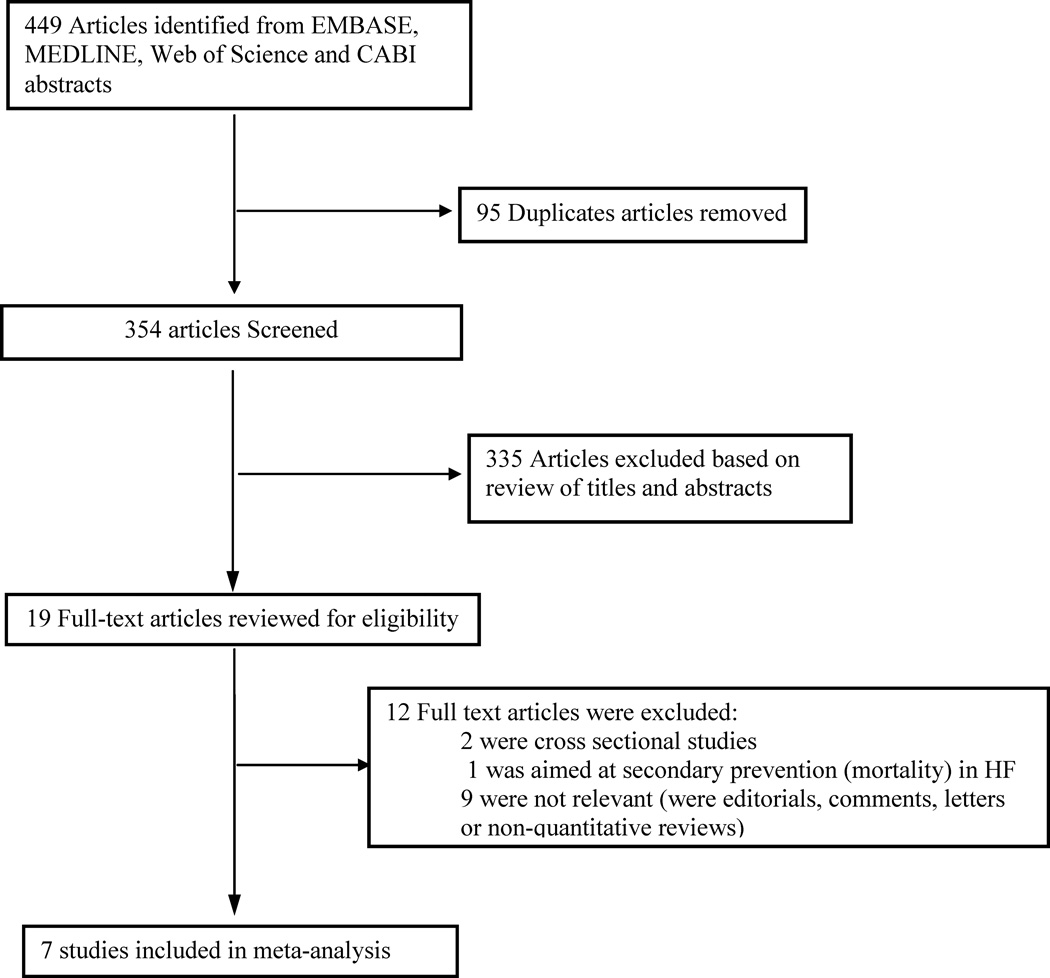
The literature search yielded 449 papers of which seven articles were included in the current analyses after various exclusions (Figure 1). We retained seven prospective studies conducted in the US (n=4) or Europe (n=3) with 176,441 participants in whom 5,480 incident HF occurred. The sample sizes varied across studies from 2,735 in the Cardiovascular Health study35 to 84,493 in the Women’s Health Initiative36. The average duration of follow-up was 13.33 years (range 7 to 16 years). Data on fish intake were obtained via food frequency questionnaires and estimates of dietary EPA/DHA intake was derived from nutrients (n=4 studies) 36,46,33,34or plasma phospholipid omega-3 measurements (n=2 studies)35,47. All reported relative measures of effect for HF in each study were adjusted for multiple covariates. Details on these seven cohorts are provided in Table 1.
Figure 1.
Search and selection of studies included in the meta-analysis
Table 1.
Characteristics of prospective studies on fish or marine omega-3 fatty acids and heart failure*
| Study | Location/Duration (average follow-up) |
% female |
Age, (Y) |
No. of HF cases |
Criteria for incident HF ascertainment |
No. of participants |
Measure of exposure intake |
Study Quality* |
Adjusted Covariates |
|---|---|---|---|---|---|---|---|---|---|
| Belin et al. 2011 | United States/1992–2008 (10.0 Y) | 100 | 50–79 | 1 858 | Adjudication of hospitalized HF events defined by Symptoms and signs consistent with congestive HF, plus: pulmonary edema by chest X-ray; or dilated ventricle or poor ventricular function by imaging studies; or physician diagnosis of HF and receiving medical treatment. | 84 493 | Dietary fish: <1 serving/mth(C1), ≥5 serving/wk (C5). DHA+ EPA intake: <0.020 (Qt1), ≥0.071 (Qt4). |
7 | Age, ethnicity, education, physical activity, smoking, alcohol, DM, HTN, AF, MI/CABG/PTCA, BMI, time-dependent MI, fiber, fruit/vegetable servings, fried fish servings, saturated fat intake (%), DHA+EPA (%), ALA (%), linoleic acid (%), fried food servings, and sodium intake (mg) |
| Dijkstra et al.2009 | Netherlands/ 1990– 2008(11.4 Y) | 59 | ≥55 | 669 | Definite HF defined in accordance with the HF criteria of the European Society of Cardiology (a combination of HF diagnosed by a medical specialist and the presence of typical symptoms, such as breathlessness at rest or during exertion, ankle oedema, and pulmonary crepitations, confirmed by evidence of cardiac dysfunction e.g. chest X-ray, echocardiography) or Probable HF (defined as at least two typical symptoms suggestive of HF were present, and at least 1 of the following: history of cardiovascular disease (e.g., myocardial infarction, hypertension), response to treatment for HF, or objective evidence of cardiac dysfunction, while symptoms could not be attributed to another underlying disease, such as chronic obstructive pulmonary disease) | 5 299 | Dietary fish: none(C1), ≥20g/day(C3). DHA+ EPA intake: <28mg/day(Q1), ≥212mg/day (Q5). |
7 | Age, sex, total energy intake, smoking, BMI, education, and intake of alcohol, fat, saturated fat, trans-fat and meat. |
| Levitan et al. 2009 | Sweden/1998–2004 (7.0M Y) |
0 | 45–79 | 597 | Hospitalization for or death from HF was identified by codes 428 (International Classification of Disease-9), I50, or I11.0 (International Classification of Disease-10) as the primary diagnosis | 39 367 | Dietary fish: never (C1), ≥3 serving/wk (C5). Marine Omega-3 intake: <0.15g/day(Q1), ≥0.71g/day (Q5). |
7 | Age, BMI, physical activity, energy, alcohol, fibre, sodium, and red or processed meat consumption, education, family history of MI at <60 years, cigarette smoking, marital status, self-reported history of HTN, and high cholesterol. |
| Levitan et al. 2010 | Sweden/1998–2006 (NA) |
100 | 48–83 | 651 | Hospitalization for or death from HF was identified by codes 428 (International Classification of Disease-9), I50, or I11.0 (International Classification of Disease-10) as the primary diagnosis | 36 234 | Dietary fish: <1 serving/wk(C1), ≥3 serving/wk (C4). Marine Omega-3 intake: <0.14g/day(Q1), ≥0.57g/day(Q5). |
7 | Age, education, BMI, physical activity, cigarette smoking, living alone, postmenopausal hormone use, total energy intake, alcohol intake, fiber intake, sodium intake, intake of red or processed meat, family history of MI before 60 years, self-reported history of HTN, self-reported history of high cholesterol. |
| Mozaffarian et al. 2005 | United States/1990–2002 (NA) |
58 | ≥65 | 955 | Confirmation of CHF required each of the following: 1) a diagnosis of CHF by a treating physician; 2) either CHF symptoms (shortness of breath, fatigue, orthopnea, or paroxysmal nocturnal dyspnea) plus signs (edema, rales, tachycardia, gallop rhythm, or displaced apical impulse) or supportive clinical findings on echocardiography, contrast ventriculography, or chest radiography; and 3) medical therapy for CHF, defined as diuretics plus either digitalis or a (diuretics and either digitalis or a vasodilator [nitroglycerin, hydralazine, angiotensin- converting enzyme inhibitor]). | 4 738 | Dietary fish: <1 serving/mth(C1), ≥5 serving/wk (C5). |
8 | Age, gender, race, enrollment site, education, DM, BMI, prevalent CHD, prevalent stroke/transient ischemic attack, smoking, leisure-time physical activity, total caloric intake, intake of fried fish, intakes of saturated fat, fruits, and alcohol intake. |
| Mozaffarian et al. 2011 | United States/1992–2006 (9.7 Y) |
58 | ≥65 | 555 | Confirmation of CHF required each of the following: 1) a diagnosis of CHF by a treating physician; 2) either CHF symptoms (shortness of breath, fatigue, orthopnea, or paroxysmal nocturnal dyspnea) plus signs (edema, rales, tachycardia, gallop rhythm, or displaced apical impulse) or supportive clinical findings on echocardiography, contrast ventriculography, or chest radiography; and 3) medical therapy for CHF, defined as diuretics plus either digitalis or a (diuretics and either digitalis or a vasodilator [nitroglycerin, hydralazine, angiotensin- converting enzyme inhibitor]). | 2 735 | Total Omega-3 intake: (Qt1), (Qt4). |
8 | Age, sex, race, education, enrollment site, smoking status, DM, AF, physical activity, BMI, WC, alcohol use and omega-3 fatty acid concentration over time. |
| Yamagishi et al.2008 | United States/1997–2003 (14.5 Y) |
53 | 45–64 | 195 | Incident HF was defined by the first HF hospitalization (International Classification of Diseases, Ninth Revision [ICD-9] code 428 in any position) or any deaths where the death certificate included a HF code (code 428, ICD-9 or I50, ICD-10, in any position). | 3 575 | Long-chain omega-3 intake: (Q1), (Q5). |
7 | Age, sex, BMI, systolic blood pressure, antihypertensive medication use, plasma total and HDL cholesterol, DM, smoking status, cigarette-years, ethanol and energy intakes, education level and sports index. |
AF, atrial fibrillation; ALA, alpha-linolenic acid; BMI, Body mass index; C, category; CABG, coronary artery by-pass graft; CHD, coronary heart disease; DM, diabetes mellitus; DPA, docosahexaenoic acid;
EPA, eicosapentaenoic acid; F, female; g, grams; HDL, high density lipoprotein; HF, heart failure; HTN, hypertension; MI, myocardial infarction; mth, month; mg, milligrams; NA, not available; No., number;
PTCA, percutaneous transluminal coronary angioplasty; Q, quintile; Qt, quartile; WC, waist circumference; wk, week; y, year.
Study quality assessed using the Newcastle-Ottawa Scale (range, 1–9 stars); MMedian.
Study quality
The overall quality of studies included in these analyses was good with two studies scoring 8 stars on the Newcastle-Ottawa scale and the remaining 5 scoring 7 stars (Table 1). There was consistency in scoring between the two reviewers (kappa=100%).
Fish intake and risk of HF
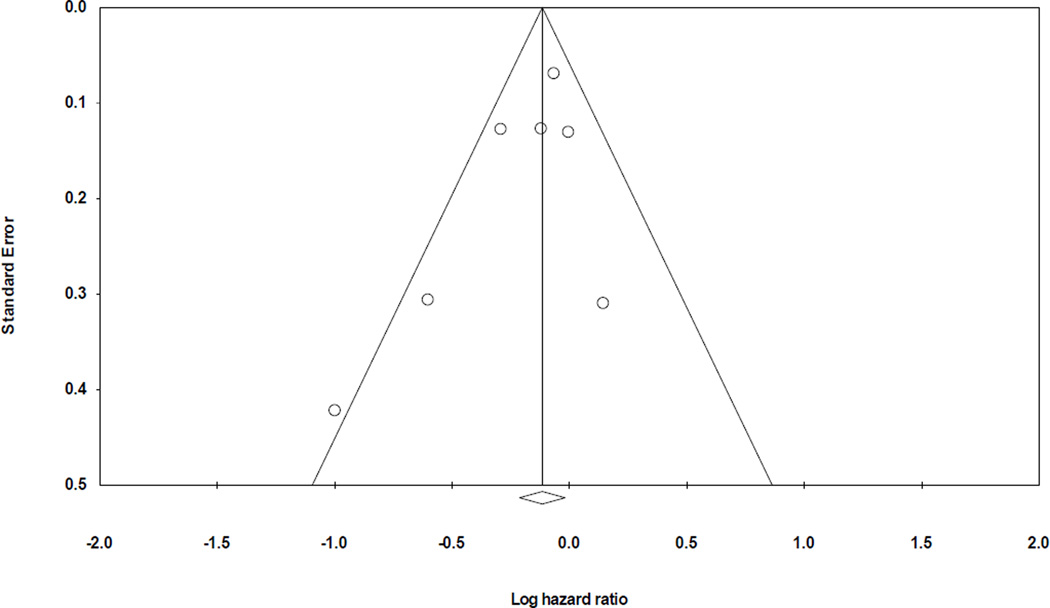
Five prospective studies32–34,36,46 evaluated the association between fish intake and incident HF. In the pooled analysis, a higher intake of fish was associated with a 15% (95% CI: 1% to 27%) lower risk of HF compared with the lowest category of fish intake, Figure 2. There was no evidence for heterogeneity among studies (I2=8%) or publication bias visual inspection of the funnel plot (Figure 3) and by Egger’s test (p = 0.18)
Figure 2.
Adjusted relative risks of heart failure according to the highest vs. lowest category of fish intake (Panel A) and EPA and DHA (Panel B).
CI denotes confidence interval; HF: heart failure; the size of each square is proportional to the study’s weight (inverse of variance –IV).
Figure 3.
Funnel plot assessing publication bias
EPA/DHA and risk of HF
A total of six studies33–36,38,46 examined the association between dietary or plasma levels of EPA/DHA and incident HF. Compared to the lowest category of EPA/DHA, there was a 14% lower risk of HF in the highest category of EPA/DHA (95% CI: 0% to 26%, p=0.05) in the pooled analysis (figure 2). There was no statistically significant evidence for heterogeneity among studies (I2=44%, p=0.10) and no evidence for publication bias (Egger’s test (p = 0.53).
Sensitivity analysis
Amongst studies reporting fish intake and risk of HF, sensitivity analysis omitting one study at a time and calculating the pooled relative risk for the remainder of the studies showed that the study by Dijkstra et al.46 substantially influenced the pooled relative risk. The Pooled RR after excluding Dijkstra et al. 46 was 0.78 (95% CI: 0.64–0.95). Similar exploration done for studies reporting EPA/DHA and the risk of HF revealed that Levitan et al.34 substantially influenced the pooled RR; the pooled RR after exclusion of this study was 0.82 (95% CI: 0.68–0.99). Result for sensitivity analysis based on geographical location showed that studies conducted in the US had a pooled RR for fish intake and the risk of HF of 0.69 (95% CI: 0.54 – 0.89) and that for EPA/DHA intake and the risk of HF was 0.76 (95% CI: 0.50–1.14). Similar but weaker results obtained for studies performed in Europe, was 0.95 (95% CI: 0.80 – 1.13) and 0.87 (95% CI: 0. 74 – 1.03) respectively.
Exposure modeled as a continuous variable
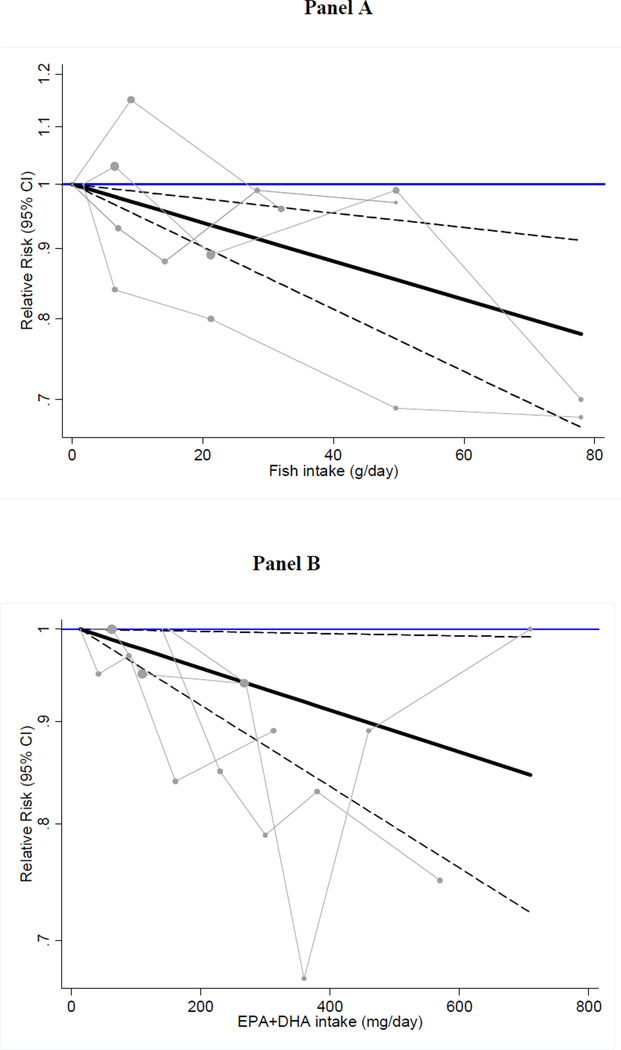
We observed an inverse and linear relation between fish consumption as well as marine omega-3 fatty acids and the risk of HF (Figure 4). For fish consumption, each 15 g/d higher intake of fish (equivalent to one additional 3.5 oz serving of fish per week) was associated with a 5% lower risk of HF [RR: 0.95 (95% CI: 0.93–0.98)]. Spline analyses were suggestive of non-linearity with no further reductions in risk at doses >300 mg/d (p for spline at 300 mg/d = 0.06) (Supplemental Figure 1). Each 125 mg/d increase in marine omega-3 fatty acids (equivalent of about one serving of 3.5 oz of fatty fish per week) was associated with a 3% lower risk of HF [RR: 0.97 (95% CI: 0.94–0.99)] when all studies were pooled.
Figure 4.
Dose-response association of fish intake (Panel A) and dietary EPA+DHA with the risk of heart failure using random effects GLST analysis.
Pooled relative risks (solid black lines) and 95% confidence intervals (dashed lines) at each quantity of intake are reported. Gray lines connect study-specific relative risk according to fish or EPA/DHA levels. Vertical axis is on a log scale represents relative risk. The median intake in the lowest category of fish (0 g/d) and EPA+DHA (14 mg/d) were used as reference groups to estimate the pooled relative risks of the higher levels.
Discussion
In this meta-analysis of prospective cohorts, we found that a higher consumption of fish and a higher dietary or plasma concentration of EPA/DHA were each associated with about 15% lower risk of HF compared with the respective lower exposure category. We also found evidence in support of a linear and inverse association between fish consumption and the risk of HF. There was minimal evidence for heterogeneity across studies or geographic location. These findings extend the observed reduction in mortality rate with EPA/DHA among HF patients in the GISSI-heart failure trial48, where an intervention with 1g of EPA/DHA was associated with a 9% reduction in total mortality compared with placebo after a median follow up of 3.5 years [HR: 0·91 (95% CI: 0·833–0·998}], to include potential decrease in the incidence of HF. What biological mechanisms could support beneficial effects of EPA and DHA on the risk of HF?
Major risk factors of HF include coronary heart disease, hypertension, and diabetes14,49. Of these three major risk factors for HF, the literature supports a beneficial effects of EPA/DHA fatal19,50,51 but not on non-fatal19,51,52 coronary heart disease. Data on the effects of EPA/DHA on hypertension23,24,53 or diabetes54–57 have been inconsistent. Other investigations suggest beneficial effects of EPA/DHA on hemodynamics24, left ventricular indices25,27,28, and inflammation29,58. Fish oil also inhibits natriuretic peptide production59 and alters the diacylglycerol composition in the heart and prevents activation of protein kinase C60,61; chronic activation of protein kinase C has been related to left ventricular hypertrophy and HF62.
Despite the absence of consistent results on the relation between fish and EPA/DHA on non-fatal CHD, hypertension, and diabetes, reported studies have been consistent on the role of EPA/DHA on lipids. In particular, fish oil or EPA/DHA has been consistently associated with a lower concentration of triglycerides and these findings have been confirmed with interventional studies. A favorable effect of EPA/DHA on dyslipidemia could favorably influence the risk of HF via reduction of coronary artery disease events. In addition, EPA/DHA have been shown to favorably influence left ventricular function26–28, heart rate63, and inflammation29–31; suggesting alternative pathways by which EPA/DHA could lower the risk of HF.
Our findings have some limitations that merit additional comments. First, our inference is based on observational studies. Hence, we cannot exclude chance, residual or unmeasured confounding as alternative explanation for our results. This makes it imperative to confirm our findings in a large randomized trial. Second, the limited number of studies available for current analyses precluded detailed analyses stratified by potential modifiers [i.e., diabetics vs. non-diabetics, type of omega-3 assessed (phospholipid, cholesteryl ester, red blood cell membrane, etc)]. Furthermore, we did not have sex-specific estimates of relative risk in studies that consisted of men and women to permit stratified analyses by sex. Third, we did not have data on individual studies to assess HF etiology, types (preserved vs. depressed left ventricular systolic function). At this point, it remains unclear whether omega-3 fatty acids exhibit differential effects on systolic vs. diastolic HF. Fourth, we did not have adequate data from individual studies to assess biologic mechanisms underlying the observed effects. Fifth, each cohort used slightly different criteria for HF ascertainment (Table 1). Hence, it might have been difficult to capture mild cases of HF in some studies or HF treated in ambulatory settings. Such incomplete ascertainment of HF might have biased our results. Nonetheless, our study has numerous strengths including the lack of meaningful heterogeneity, the consistency between relative risk obtained from fish intake and estimated dietary or blood EPA/DHA, the relatively high quality of studies included (quality score ranging from 7 to 8), a large pooled sample size, and robustness of the results in a sensitivity analysis.
If confirmed in a large double blind, placebo controlled randomized clinical trial, EPA/DHA could be added to the list of lifestyle factors and pharmacological agents that can be used for the primary prevention of HF.
In conclusion, our data are consistent with a lower risk of HF among people consuming higher amounts of EPA/DHA or fish.
Supplementary Material
Acknowledgement
Dr. Djousse has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding: Dr Djousse is partially supported by 1R01 HL092946 and R01HL09246S1 from the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contribution:
Study conception: LD
Drafting the manuscript: LD
Literature search and data abstraction: LD and AA
Statistical analysis: AA, JW, ED
Critical review of the manuscript for content: LD, AA, JW, ED, JMG.
Study supervision: LD, JMG.
Conflict of interest
Luc Djousse: Travel reimbursement from the International Nut and Dried Fruit Foundation
Akintunde O. Akinkuolie: None
Jason H.Y. Wu: None
Eric L. Ding: None
J Michael Gaziano: None
References
- 1.Massie BM, Shah NB. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J. 1997;133:703–712. doi: 10.1016/s0002-8703(97)70173-x. [DOI] [PubMed] [Google Scholar]
- 2.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 3.Bhopal RS, Bansal N, Fischbacher CM, Brown H, Capewell S. Ethnic variations in heart failure: Scottish Health and Ethnicity Linkage Study (SHELS) Heart. 2012;98:468–473. doi: 10.1136/heartjnl-2011-301191. [DOI] [PubMed] [Google Scholar]
- 4.Robertson J, McElduff P, Pearson SA, Henry DA, Inder KJ, Attia JR. The health services burden of heart failure: an analysis using linked population health data-sets. BMC Health Serv Res. 2012;12:103. doi: 10.1186/1472-6963-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg RJ, Spencer FA, Farmer C, Meyer TE, Pezzella S. Incidence and hospital death rates associated with heart failure: a community-wide perspective. Am J Med. 2005;118:728–734. doi: 10.1016/j.amjmed.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg RJ, Glatfelter K, Burbank-Schmidt E, Farmer C, Spencer FA, Meyer T. Trends in mortality attributed to heart failure in Worcester, Massachusetts, 1992 to 2001. Am J Cardiol. 2005;95:1324–1328. doi: 10.1016/j.amjcard.2005.01.076. [DOI] [PubMed] [Google Scholar]
- 7.Shahar E, Lee S, Kim J, Duval S, Barber C, Luepker RV. Hospitalized heart failure: rates and long-term mortality. J Card Fail. 2004;10:374–379. doi: 10.1016/j.cardfail.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, Rouleau JL, Tendera M, Castaigne A, Roecker EB, Schultz MK, DeMets DL. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 9.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 10.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 11.Ho KKL, Pinsky JL, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22(4) Suppl A:6a–13a. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 12.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 13.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 14.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 15.Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML, Noble RJ, Packer M, Silver MA, Stevenson LW, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Jacobs AK, Hiratzka LF, Russell RO, Smith SC., Jr ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to revise the 1995 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2001;38:2101–2113. doi: 10.1016/s0735-1097(01)01683-7. [DOI] [PubMed] [Google Scholar]
- 16.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 17.Mozaffarian D, Wu JHY. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. doi: 10.1016/j.jacc.2011.06.063. [DOI] [PubMed] [Google Scholar]
- 18.Hooper L, Thompson RL, Harrison RA, Summerbell CD, Ness AR, Moore HJ, Worthington HV, Durrington PN, Higgins JP, Capps NE, Riemersma RA, Ebrahim SB, Davey SG. Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ. 2006;332:752–760. doi: 10.1136/bmj.38755.366331.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yzebe D, Lievre M. Fish oils in the care of coronary heart disease patients: a meta- analysis of randomized controlled trials. Fundam Clin Pharmacol. 2004;18:581–592. doi: 10.1111/j.1472-8206.2004.00268.x. [DOI] [PubMed] [Google Scholar]
- 20.Ascherio A, Rimm EB, Stampfer MJ, Giovannucci EL, Willett WC. Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. N Engl J Med. 1995;332:977–982. doi: 10.1056/NEJM199504133321501. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 22.Gillum RF, Mussolino ME, Madans JH. Fish consumption and hypertension incidence in African Americans and whites: the NHANES I Epidemiologic Follow-up Study. J Natl Med Assoc. 2001;93:124–128. [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng ZJ, Folsom AR, Ma J, Arnett DK, McGovern PG, Eckfeldt JH. Plasma fatty acid composition and 6-year incidence of hypertension in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol. 1999;150:492–500. doi: 10.1093/oxfordjournals.aje.a010038. [DOI] [PubMed] [Google Scholar]
- 24.Geleijnse JM, Giltay EJ, Grobbee DE, Donders AR, Kok FJ. Blood pressure response to fish oil supplementation: metaregression analysis of randomized trials. J Hypertens. 2002;20:1493–1499. doi: 10.1097/00004872-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Grimsgaard S, Bonaa KH, Hansen JB, Myhre ES. Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemodynamics in humans. Am J Clin Nutr. 1998;68:52–59. doi: 10.1093/ajcn/68.1.52. [DOI] [PubMed] [Google Scholar]
- 26.McLennan PL, Barnden LR, Bridle TM, Abeywardena MY, Charnock JS. Dietary fat modulation of left ventricular ejection fraction in the marmoset due to enhanced filling. Cardiovasc Res. 1992;26:871–877. doi: 10.1093/cvr/26.9.871. [DOI] [PubMed] [Google Scholar]
- 27.Mozaffarian D, Gottdiener JS, Siscovick DS. Intake of tuna or other broiled or baked fish versus fried fish and cardiac structure, function, and hemodynamics. Am J Cardiol. 2006;97:216–222. doi: 10.1016/j.amjcard.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation. 2002;105:2303–2308. doi: 10.1161/01.cir.0000015604.88808.74. [DOI] [PubMed] [Google Scholar]
- 29.Mehra MR, Lavie CJ, Ventura HO, Milani RV. Fish oils produce anti-inflammatory effects and improve body weight in severe heart failure. J Heart Lung Transplant. 2006;25:834–838. doi: 10.1016/j.healun.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Mori TA, Woodman RJ. The independent effects of eicosapentaenoic acid and docosahexaenoic acid on cardiovascular risk factors in humans. Curr Opin Clin Nutr Metab Care. 2006;9:95–104. doi: 10.1097/01.mco.0000214566.67439.58. [DOI] [PubMed] [Google Scholar]
- 31.Bloomer RJ, Larson DE, Fisher-Wellman KH, Galpin AJ, Schilling BK. Effect of eicosapentaenoic and docosahexaenoic acid on resting and exercise-induced inflammatory and oxidative stress biomarkers: a randomized, placebo controlled, cross-over study. Lipids Health Dis. 2009;8:36. doi: 10.1186/1476-511X-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005;45:2015–2021. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 33.Levitan EB, Wolk A, Mittleman MA. Fatty fish, marine omega-3 fatty acids and incidence of heart failure. Eur J Clin Nutr. 2010;64:587–594. doi: 10.1038/ejcn.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levitan EB, Wolk A, Mittleman MA. Fish consumption, marine omega-3 fatty acids, and incidence of heart failure: a population-based prospective study of middle-aged and elderly men. Eur Heart J. 2009;30:1495–1500. doi: 10.1093/eurheartj/ehp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mozaffarian D, Lemaitre RN, King IB, Song X, Spiegelman D, Sacks FM, Rimm EB, Siscovick DS. Circulating long-chain omega-3 fatty acids and incidence of congestive heart failure in older adults: the cardiovascular health study: a cohort study. Ann Intern Med. 2011;155:160–170. doi: 10.1059/0003-4819-155-3-201108020-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belin RJ, Greenland P, Martin L, Oberman A, Tinker L, Robinson J, Larson J, Van Horn L, Lloyd-Jones D. Fish intake and the risk of incident heart failure: the Women's Health Initiative. Circ Heart Fail. 2011;4:404–413. doi: 10.1161/CIRCHEARTFAILURE.110.960450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 38.Yamagishi K, Nettleton JA, Folsom AR. Plasma fatty acid composition and incident heart failure in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J. 2008;156:965–974. doi: 10.1016/j.ahj.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [assessed 2012 May 1]; Available from: URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 40.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 41.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 42.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 43.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37:1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 46.Dijkstra SC, Brouwer IA, van Rooij FJ, Hofman A, Witteman JC, Geleijnse JM. Intake of very long chain n-3 fatty acids from fish and the incidence of heart failure: the Rotterdam Study. Eur J Heart Fail. 2009;11:922–928. doi: 10.1093/eurjhf/hfp126. [DOI] [PubMed] [Google Scholar]
- 47.Yamagishi K, Iso H, Date C, Fukui M, Wakai K, Kikuchi S, Inaba Y, Tanabe N, Tamakoshi A. Fish, omega-3 polyunsaturated fatty acids, and mortality from cardiovascular diseases in a nationwide community-based cohort of Japanese men and women the JACC (Japan Collaborative Cohort Study for Evaluation of Cancer Risk) Study. J Am Coll Cardiol. 2008;52:988–996. doi: 10.1016/j.jacc.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 48.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 49.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 50.Mozaffarian D. Fish and n-3 fatty acids for the prevention of fatal coronary heart disease and sudden cardiac death. Am J Clin Nutr. 2008;87:1991S–1996S. doi: 10.1093/ajcn/87.6.1991S. [DOI] [PubMed] [Google Scholar]
- 51.Bucher HC, Hengstler P, Schindler C, Meier G. N-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med. 2002;112:298–304. doi: 10.1016/s0002-9343(01)01114-7. [DOI] [PubMed] [Google Scholar]
- 52.Kromhout D, Giltay EJ, Geleijnse JM. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 53.Bjorklund K, Lind L, Vessby B, Andren B, Lithell H. Different metabolic predictors of white-coat and sustained hypertension over a 20-year follow-up period: a population- based study of elderly men. Circulation. 2002;106:63–68. doi: 10.1161/01.cir.0000019737.87850.5a. [DOI] [PubMed] [Google Scholar]
- 54.Djousse L, Gaziano JM, Buring JE, Lee IM. Dietary omega-3 fatty acids and fish consumption and risk of type 2 diabetes. Am J Clin Nutr. 2011;93:143–150. doi: 10.3945/ajcn.110.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Djousse L, Biggs ML, Lemaitre RN, King IB, Song X, Ix JH, Mukamal KJ, Siscovick DS, Mozaffarian D. Plasma omega-3 fatty acids and incident diabetes in older adults. Am J Clin Nutr. 2011;94:527–533. doi: 10.3945/ajcn.111.013334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaushik M, Mozaffarian D, Spiegelman D, Manson JE, Willett WC, Hu FB. Long-chain omega-3 fatty acids, fish intake, and the risk of type 2 diabetes mellitus. Am J Clin Nutr. 2009;90:613–620. doi: 10.3945/ajcn.2008.27424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel PS, Sharp SJ, Luben RN, Khaw KT, Bingham SA, Wareham NJ, Forouhi NG. Association between type of dietary fish and seafood intake and the risk of incident type 2 diabetes: the European prospective investigation of cancer (EPIC)-Norfolk cohort study. Diabetes Care. 2009;32:1857–1863. doi: 10.2337/dc09-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mozaffarian D, Lemaitre RN, Kuller LH, Burke GL, Tracy RP, Siscovick DS. Cardiac benefits of fish consumption may depend on the type of fish meal consumed: the Cardiovascular Health Study. Circulation. 2003;107:1372–1377. doi: 10.1161/01.cir.0000055315.79177.16. [DOI] [PubMed] [Google Scholar]
- 59.Shimojo N, Jesmin S, Zaedi S, Maeda S, Soma M, Aonuma K, Yamaguchi I, Miyauchi T. Eicosapentaenoic acid prevents endothelin-1-induced cardiomyocyte hypertrophy in vitro through the suppression of TGF-beta 1 and phosphorylated JNK. Am J Physiol Heart Circ Physiol. 2006;291:H835–H845. doi: 10.1152/ajpheart.01365.2005. [DOI] [PubMed] [Google Scholar]
- 60.Duda MK, O'Shea KM, Lei B, Barrows BR, Azimzadeh AM, McElfresh TE, Hiot BD, Kop WJ, Stanley WC. Dietary supplementation with n-3 PUFA increases adiponectin and attenuates ventricular remodeling and dysfunction with pressure overload. Cardiovasc.Res. 2007;76:303–310. doi: 10.1016/j.cardiores.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takahashi R, Okumura K, Asai T, Hirai T, Murakami H, Murakami R, Numaguchi Y, Matsui H, Ito M, Murohara T. Dietary fish oil attenuates cardiac hypertrophy in lipotoxic cardiomyopathy due to systemic carnitine deficiency. Cardiovasc Res. 2005;68:213–223. doi: 10.1016/j.cardiores.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 62.Bayer AL, Heidkamp MC, Patel N, Porter M, Engman S, Samarel AM. Alterations in protein kinase C isoenzyme expression and autophosphorylation during the progression of pressure overload-induced left ventricular hypertrophy. Mol Cell Biochem. 2003;242:145–152. [PubMed] [Google Scholar]
- 63.Mozaffarian D, Geelen A, Brouwer IA, Geleijnse JM, Zock PL, Katan MB. Effect of fish oil on heart rate in humans: a meta-analysis of randomized controlled trials. Circulation. 2005;112:1945–1952. doi: 10.1161/CIRCULATIONAHA.105.556886. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.