Abstract
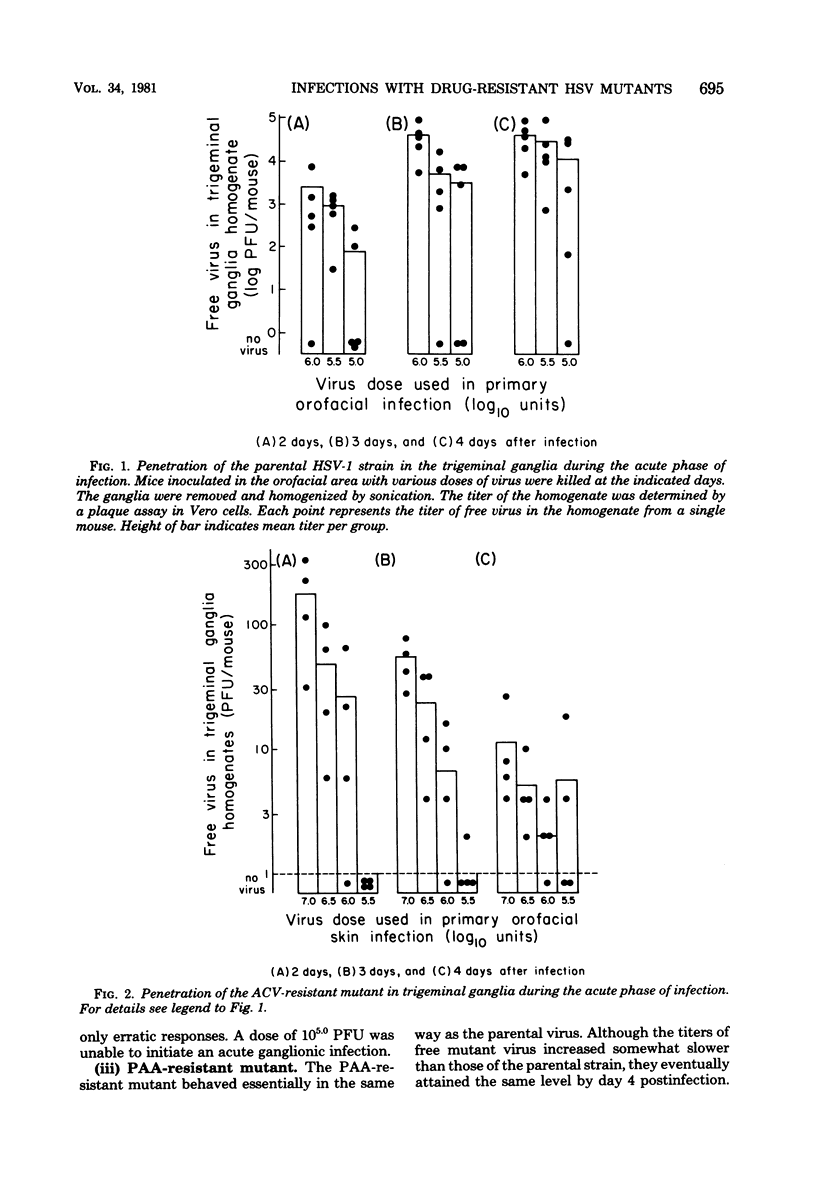
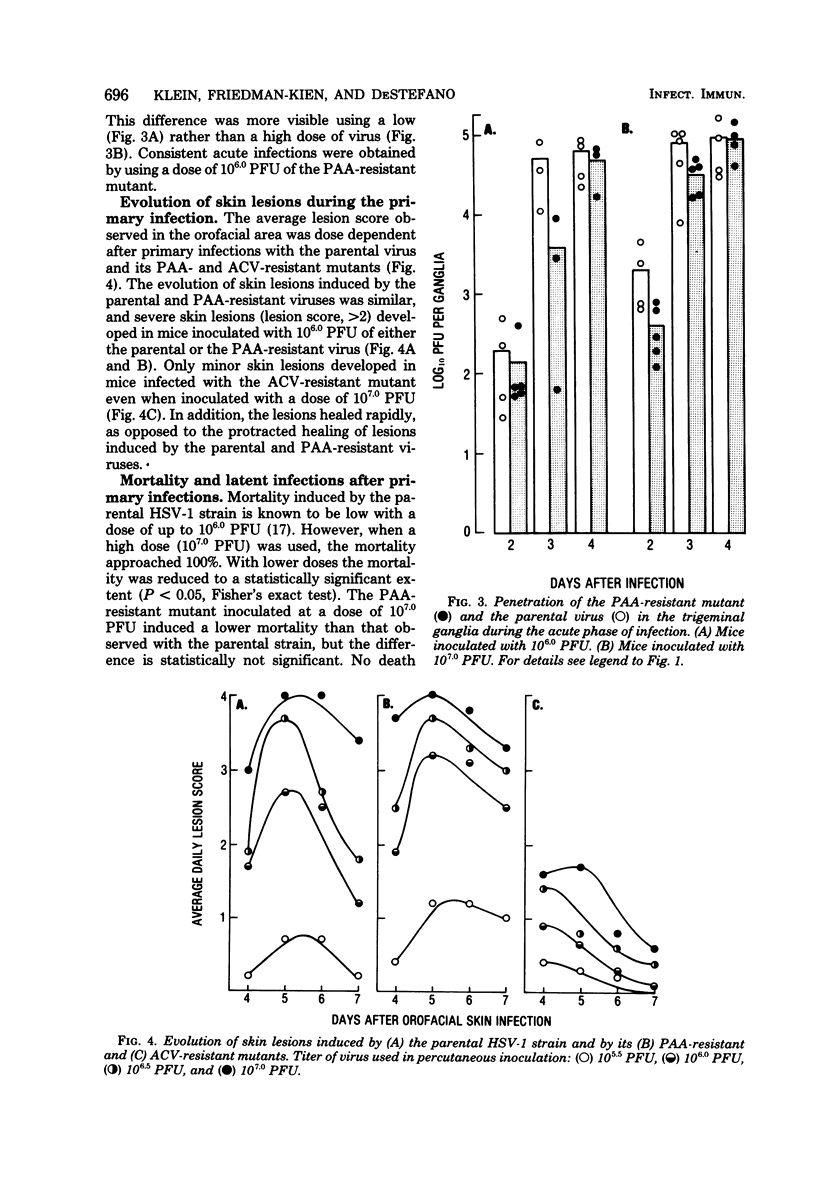
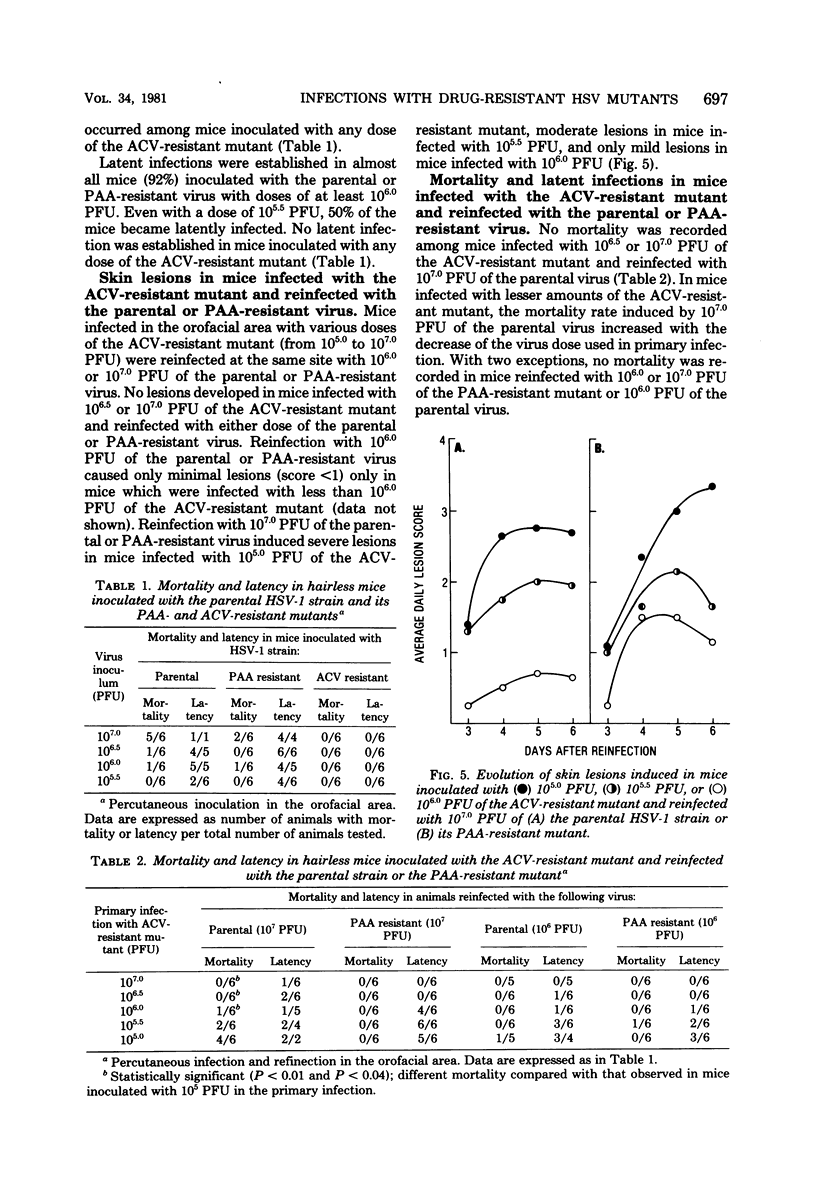
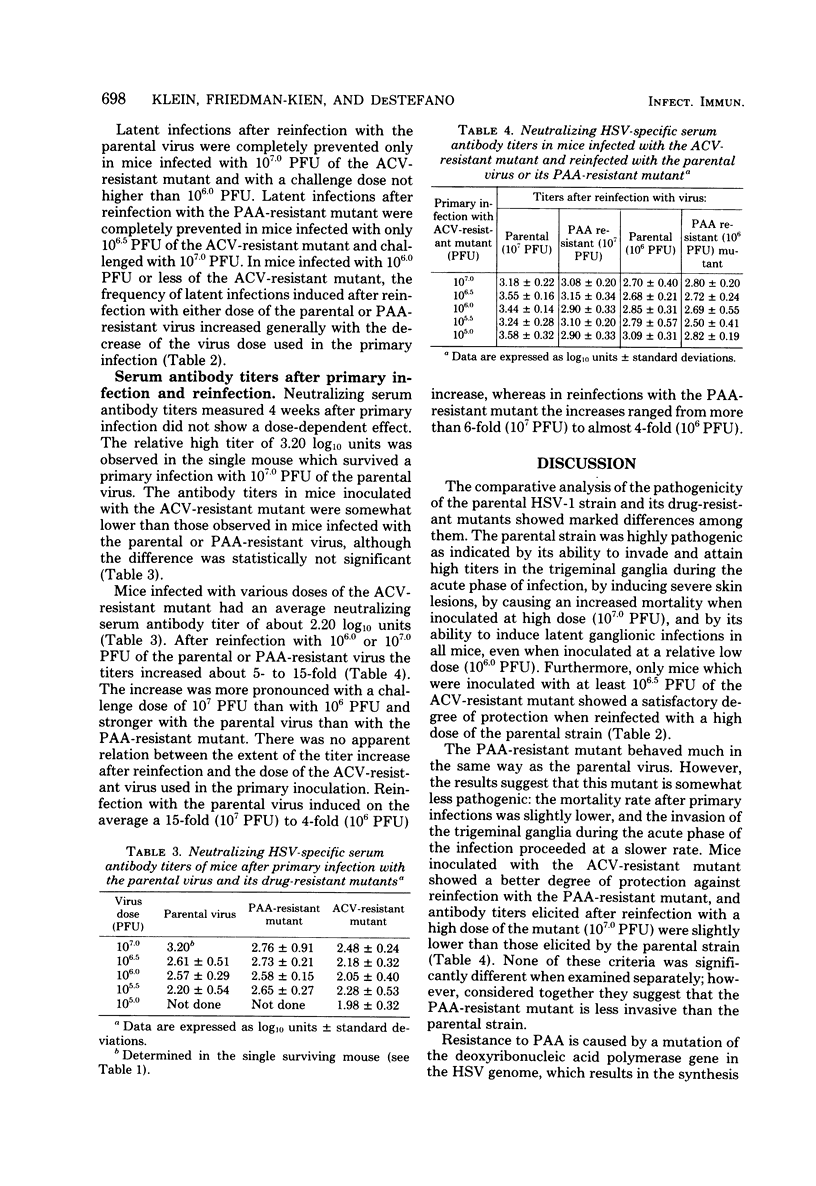
The comparative analysis of the pathogenicity of a parental herpes simplex virus type 1 strain and its phosphonoacetic acid (PAA)-resistant and acyclovir (ACV)-resistant mutants showed marked differences among them. After orofacial skin inoculation of hairless mice the parental and PAA-resistant viruses were detected during the first 4 days after infection at high and increasing titers in the trigeminal ganglia; the ACV-resistant mutant was present at low and decreasing titers in the ganglia. Severe and slow-healing skin lesions were produced by the parental and PAA-resistant viruses; mild and rapidly healing lesions were produced by the ACV-resistant mutant. Virus titers in ganglia and the intensity of skin lesions were related to the virus dose used in the primary infection. Latent infections became established in trigeminal ganglia of mice inoculated with 106.0 plaque-forming units of the parental or PAA-resistant virus; no latent infections were detected in ganglia of mice inoculated with 107.0 plaque-forming units of the ACV-resistant mutant. Serum antibody titers attained similar values 4 weeks after primary infection with both mutants and the parental virus. Mice infected with the ACV-resistant mutant were reinfected with the parental and PAA-resistant viruses; the degree of protection against development of skin lesions, mortality, and latency was related to the dose of ACV-resistant virus used in the primary infection. Mortality was prevented by a dose of 106.0 plaque-forming units, skin lesions were prevented by a dose of 106.5 plaque-forming units, and latency was prevented by a dose of 107.0 plaque-forming units of the ACV-resistant mutant. Protection against reinfection with the PAA-resistant mutant was achieved with lower doses than protection against the parental virus. Serum antibody titers showed a 4- to 15-fold increase after reinfection. The results suggest that the ACV-resistant, latency-negative mutant has many attributes of a live attenuated herpes simplex virus vaccine.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswell J. F., Allen G. P., Jamieson A. T., Campbell D. E., Gentry G. A. Antiviral activity of arabinosylthymine in herpesviral replication: mechanism of action in vivo and in vitro. Antimicrob Agents Chemother. 1977 Aug;12(2):243–254. doi: 10.1128/aac.12.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. M., Subak-Sharpe J. H., Warren K. G., Wroblewska Z., Koprowski H. Detection by complementation of defective or uninducible (herpes simplex type 1) virus genomes latent in human ganglia. Proc Natl Acad Sci U S A. 1979 May;76(5):2364–2368. doi: 10.1073/pnas.76.5.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Darby G. Pathogenicity in mice of strains of herpes simplex virus which are resistant to acyclovir in vitro and in vivo. Antimicrob Agents Chemother. 1980 Feb;17(2):209–216. doi: 10.1128/aac.17.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Darby G., Wildy P. Isolation and characterization of acyclovir-resistant mutants of herpes simplex virus. J Gen Virol. 1980 Jul;49(1):115–124. doi: 10.1099/0022-1317-49-1-115. [DOI] [PubMed] [Google Scholar]
- Field H. J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 1978 Oct;81(2):267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Hay J., Subak-Sharpe J. H. Mutants of herpes simplex virus types 1 and 2 that are resistant to phosphonoacetic acid induce altered DNA polymerase activities in infected cells. J Gen Virol. 1976 Apr;31(1):145–148. doi: 10.1099/0022-1317-31-1-145. [DOI] [PubMed] [Google Scholar]
- Hirano A., Yumura K., Kurimura T., Katsumoto T., Moriyama H., Manabe R. Analysis of herpes simplex virus isolated from patients with recurrent herpes keratitis exhibiting "treatment-resistance" to 5-iodo-2'-deoxyuridine. Acta Virol. 1979 May;23(3):226–230. [PubMed] [Google Scholar]
- JOHNSON R. T. THE PATHOGENESIS OF HERPES VIRUS ENCEPHALITIS. I. VIRUS PATHWAYS TO THE NERVOUS SYSTEM OF SUCKLING MICE DEMONSTRATED BY FLUORESCENT ANTIBODY STAINING. J Exp Med. 1964 Feb 1;119:343–356. doi: 10.1084/jem.119.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. J., DeStefano E., Brady E., Friedman-Kien A. E. Experimental skin infection with an acyclovir resistant herpes simplex virus mutant: response to antiviral treatment and protection against reinfection. Arch Virol. 1980;65(3-4):237–246. doi: 10.1007/BF01314540. [DOI] [PubMed] [Google Scholar]
- Klein R. J., Friedman-Kien A. E., Brady E. Latent herpes simplex virus in ganglia of mice after primary infection and reinoculation at a distant site. Arch Virol. 1978;57(2):161–166. doi: 10.1007/BF01315677. [DOI] [PubMed] [Google Scholar]
- Klein R. J., Friedman-Kien A. E. Phosphonoacetic acid-resistant herpes simplex virus infection in hairless mice. Antimicrob Agents Chemother. 1975 Mar;7(3):289–293. doi: 10.1128/aac.7.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. J., Friedman-Kien A. E., Yellin P. B. Orofacial herpes simplex virus infection in hairless mice: latent virus in trigeminal ganglia after topical antiviral treatment. Infect Immun. 1978 Apr;20(1):130–135. doi: 10.1128/iai.20.1.130-135.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. J. Isolation of herpes simplex virus clones and drug resistant mutants in microcultures. Arch Virol. 1975;49(1):73–80. doi: 10.1007/BF02175598. [DOI] [PubMed] [Google Scholar]
- Koyama H., Kasahara S. Induction of herpes simplex virus immunity in newborn mice. Infect Immun. 1975 Dec;12(6):1472–1474. doi: 10.1128/iai.12.6.1472-1474.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M., Schafer T. W., Came P. E. Chemotherapy of cutaneous herpesvirus infection of hairless mice. J Invest Dermatol. 1973 Apr;60(4):203–206. doi: 10.1111/1523-1747.ep12724476. [DOI] [PubMed] [Google Scholar]
- Lopez C. Genetics of natural resistance to herpesvirus infections in mice. Nature. 1975 Nov 13;258(5531):152–153. doi: 10.1038/258152a0. [DOI] [PubMed] [Google Scholar]
- Marcialis M. A., La Colla P., Schivo M. L., Flore O., Firinu A., Loddo B. Low virulence and immunogenicity in mice and in rabbits of variants of Herpes simplex virus resistant to 5-iodo-2-deoxyuridine. Experientia. 1975 Apr 15;31(4):502–503. doi: 10.1007/BF02026404. [DOI] [PubMed] [Google Scholar]
- Nesburn A. B., Robinson C., Dickinson R. Adenine arabinoside effect on experimental idoxuridine-resistant herpes simplex infection. Invest Ophthalmol. 1974 Apr;13(4):302–304. [PubMed] [Google Scholar]
- Price R. W., Walz M. A., Wohlenberg C., Notkins A. L. Latent infection of sensory ganglia with herpes simplex virus: efficacy of immunization. Science. 1975 May 30;188(4191):938–940. doi: 10.1126/science.166432. [DOI] [PubMed] [Google Scholar]
- Schnipper L. E., Crumpacker C. S. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2270–2273. doi: 10.1073/pnas.77.4.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröeder C. H., Engler H., Kirchner H. Protection of mice by an apathogenic strain HSV-1 against lethal infection by a pathogenic strain of HSV-1. J Gen Virol. 1981 Jan;52(Pt 1):159–161. doi: 10.1099/0022-1317-52-1-159. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Dunstan M. E. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology. 1979 Dec;99(2):417–422. doi: 10.1016/0042-6822(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Miller R. L., Rapp F. Trigeminal ganglion infection by thymidine kinase-negative mutants of herpes simplex virus. Science. 1979 Aug 31;205(4409):915–917. doi: 10.1126/science.224454. [DOI] [PubMed] [Google Scholar]
- Trousdale M. D., Nesburn A. B., Miller C. A. Assessment of acyclovir on acute ocular infection induced by drug-resistant strains of HSV-1. Invest Ophthalmol Vis Sci. 1981 Feb;20(2):230–235. [PubMed] [Google Scholar]
- Underwood G. E., Elliott G. A., Buthala D. A. Herpes keratitis in rabbits: pathogenesis and effect of antiviral nucleosides. Ann N Y Acad Sci. 1965 Jul 30;130(1):151–167. doi: 10.1111/j.1749-6632.1965.tb12549.x. [DOI] [PubMed] [Google Scholar]
- White D. O., Shew M. A., Howsam K. G., Robertson I. F. Herpes simplex virus infection of the cornea. Med J Aust. 1968 Jul 13;2(2):59–63. doi: 10.5694/j.1326-5377.1968.tb29290.x. [DOI] [PubMed] [Google Scholar]


