To the Editor
We have previously reported that the GSTM1 null genotype is associated with increased inflammatory response to inhaled ozone (O3) 1 and that O3 modifies markers of innate immunity and inflammation in healthy individuals. 2,3,4 Although it is unclear what inflammatory risk factors account for increased susceptibility to O3, the neutrophil (PMN) response to O3 is a hallmark inflammatory marker that varies in magnitude across individuals – hence it has been used to identify O3 responsive and non-responsive individuals in terms of inflammatory responsiveness. 5,6,3 We hypothesized that PMN responsive individuals would have modified cell responses and airway cytokine levels, and that genetic factors would modulate risk of PMN response to O3.
In a recently published study by our group, 24 healthy adults (age 20-33 years) were examined 18 hours after a 6.6 hour controlled exposure to 0.06 ppm O3 or clean air (CA). 7 That study revealed a significant O3-indcued decrement in FEV1 and an increase in neutrophilic inflammation in the airways. In this report we provide new measurements from that study including genotyping for genes reported to impact risk for response to O3 (GSTM1, NQO1, TNF), sputum cell assessment of markers of innate immune activation and function and inflammatory cells and cytokines. Moreover, in this analysis we stratified the 24 subjects according to their PMN response to O3 defined as, %PMN O3- %PMN CA, such that PMN responsive individuals, or responders, were those who had %PMN O3 - %PMN CA ≥ 10ten (N=13) versus those who had %PMN O3 - %PMN CA < 10 (non-responders, N=11). Specifically, we examined by flow cytometry (BD LSR-II flow cytometer with BD FACSDiva 6.1 software, BD Biosciences Immunocytometry Systems, San Jose, CA) phenotype responses known to be modified by O3 such as cell surface marker expression (mean fluorescence intensity/MFI) of innate immune receptors (CD11b, CD14, CD16, CD86, HLA-DR, CD54), phagocytosis (% of cells undergoing phagocytosis of IgG opsonized zymosan A bioparticles), and inflammatory cytokines (IL-8, IL1β, IL-6, HA, TNFα) as measured by Meso Scale Discovery/MSD (Gaithersburg, MD) 2,4. All flow cytometric endpoints and markers of inflammation were analyzed using non-parametric paired T-Tests comparing post- O3 to post CA exposure time points. Regression analysis was used to determine the association between genotype status and PMN responsiveness. The following regression model was used: logit(P(Responder)) = α0 + α1Age + α2I(Female) + α3BSA + α4BMI + α5I(GSTM1 −) where BSA = body surface area and BMI = body mass index. Statistical significance was determined at p<0.05 level for all analyses.
We observe that the mean (± SEM) PMN response to O3 was significantly elevated in responders versus non-responders (27 ± 3 vs 3 ± 2, p<0.05) and that the mean (±SEM) FEV1 response (% change from CA) was not significantly different between subject cohorts (-1.23% ± 0.8 vs -2.41% ± 2.1). Logistic regression analysis showed that responder status was strongly associated with GSTM1 status. The GSTM1 null genotype significantly (p<0.05) increased the probability of being a responder such that the calculated odds ratio of being a responder for the GSTM1 null genotype was estimated to be 13 (95%CI: 1.071 to 157.8). NQO1 and TNFa genotypes did not have a significant association with PMN response in this cohort. Post CA, responders had significantly (p<0.05) elevated levels of pro-inflammatory cytokines IL-8, IL-6 and TNFα compared to non-responders, and along with Hyaluronic Acid (HA), which has been implicated in inflammatory response to O3, 8,9 these cytokines remained significantly elevated post O3 when compared to post O3 levels in non-responders (Table 1). Only IL-8 was significantly elevated post O3 versus post CA in both responders and non-responders (Table 1). Sputum macrophage phagocytosis was also significantly elevated post CA in responders versus non-responders (51% ± 2 vs 45% ± 3, p<0.05), that together with the inflammatory cytokine data, suggests that PMN responders may have primed immuno-inflammatory features under non O3 exposed conditions. We found that O3 significantly enhanced the expression of immune cell surface receptors on macrophages (CD11b, CD14, CD16) and monocytes (HLA-DR, CD86, CD54) in responders but not in non-responders (Figure 1).
Table 1.
Mean (SEM) Cytokine Levels Following Clean Air and Ozone Exposure
| Responders | Non-Responders | |||
|---|---|---|---|---|
| Cytokine (pg/ml) | Clean Air | Ozone | Clean Air | Ozone |
| IL-8 | 6465 (1618)* # | 9794 (2241) + | 2772 (572) * | 4441 (831) |
| IL-6 | 137 (53) # | 134 (32) + | 39 (10) | 57 (11) |
| IL-1β | 190 (68) | 171 (37) | 67 (13) | 101 (28) |
| TNFα | 15 (5) # | 16 (4) | 4 (1) | 9 (3) |
| HA | 11,737 (1428) | 18,019 (5615) + | 9064 (1474) | 6976 (1522) |
p<0.05 Clean Air vs Ozone;
p<0.05 Clean Air vs Clean Air;
p<0.05 Ozone vs Ozone;
HA hyaluronic Acid; pg/ml picograms per milliliter
Figure 1.
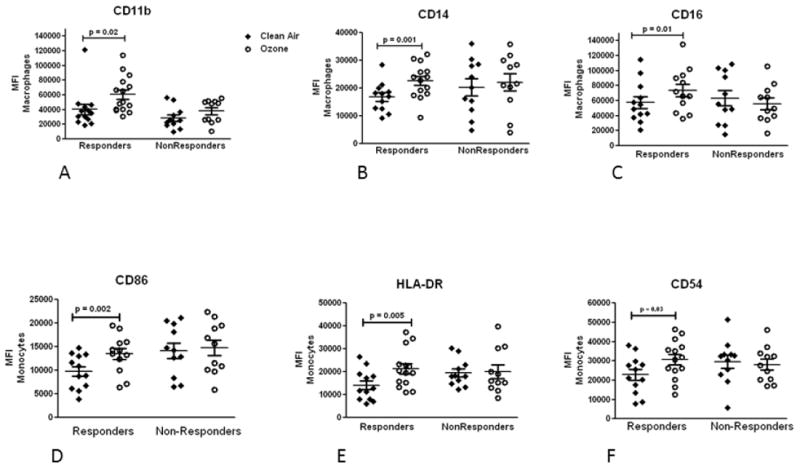
Mean (±SEM) cell surface phenotype expression (MFI) in Responders and NonResponders following clean air (solid diamond) and ozone (open circle) exposure on sputum macrophages (A-C) and monocytes (D-F). Responders, but not NonResponders, had significantly (p<0.05) elevated expression for all markers after O3 versus clean air exposure. MFI: mean fluorescence intensity.
In summary, we report that individuals with an elevated PMN response to low level O3 are 13 times more likely of having the GSTM1null genotype than non-responders. Furthermore, responders have increased immuno-inflammatory responses to O3 compared to non-responders, and have elevated markers of inflammation following CA, suggesting the presence of a primed inflammatory airway in non-O3 exposed conditions. PMN responsiveness was also confirmed to be independent of the spirometric (FEV1) response to low level O3 in healthy people. Since GSTM1 is a risk factor for asthma exacerbation and ozone, these data support the hypothesis that genetic modifiers of oxidative stress modulate the health effects of O3 in individuals with allergic airways disease.
Acknowledgments
Funding Support: U.S. Environmental Protection Agency Internal Fund, U.S. Environmental Protection Agency cooperative agreement CR83346301, and National Institutes of Health grants RC1ES018417, R01ES012706 and U19AI077437 (D.B.P.). Although the research described herein has been funded in part by the United States Environmental Protection Agency, it has not been subjected to the EPA’s required peer and policy review. The findings contained in this report do not necessarily reflect the views of the Environmental Protection Agency or the National Institutes of Health, and no official endorsement should be inferred
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexis NE, Zhou H, Lay JC, Harris B, Hernandez ML, Lu TS, Bromberg PA, Diaz-Sanchez D, Devlin RB, Kleeberger SR, Peden DB. The glutathione-S-transferase Mu 1 null genotype modulates ozone-induced airway inflammation in human subjects. The Journal of allergy and clinical immunology. 2009;124:1222–1228. e1225. doi: 10.1016/j.jaci.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexis NE, Lay JC, Hazucha M, Harris B, Hernandez ML, Bromberg PA, Kehrl H, Diaz-Sanchez D, Kim C, Devlin RB, Peden DB. Low-level ozone exposure induces airways inflammation and modifies cell surface phenotypes in healthy humans. Inhalation toxicology. 2010;22:593–600. doi: 10.3109/08958371003596587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexis NE, Lay JC, Haczku A, Gong H, Linn W, Hazucha MJ, Harris B, Tal-Singer R, Peden DB. Fluticasone propionate protects against ozone-induced airway inflammation and modified immune cell activation markers in healthy volunteers. Environmental health perspectives. 2008;116:799–805. doi: 10.1289/ehp.10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lay JC, Alexis NE, Kleeberger SR, Roubey RA, Harris BD, Bromberg PA, Hazucha MJ, Devlin RB, Peden DB. Ozone enhances markers of innate immunity and antigen presentation on airway monocytes in healthy individuals. The Journal of allergy and clinical immunology. 2007;120:719–722. doi: 10.1016/j.jaci.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Lazaar AL, Sweeney LE, MacDonald AJ, Alexis NE, Chen C, Tal-Singer R. SB-656933, a novel CXCR2 selective antagonist, inhibits ex vivo neutrophil activation and ozone-induced airway inflammation in humans. Br J Clin Pharmacol. 2011 Aug;72(2):282–93. doi: 10.1111/j.1365-2125.2011.03968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holz O, Khalilieh S, Ludwig-Sengpiel A, Watz H, Stryszak P, Soni P, Tsai M, Sadeh J, Magnussen H. SCH527123, a novel CXCR2 antagonist, inhibits ozone-induced neutrophilia in healthy subjects. Eur Resp J. 2010;35:564–70. doi: 10.1183/09031936.00048509. [DOI] [PubMed] [Google Scholar]
- 7.Kim CS, Alexis NE, Rappold AG, Kehrl H, Hazucha MJ, Lay JC, Schmitt MT, Case M, Devlin RB, Peden DB, Diaz-Sanchez D. Lung function and inflammatory responses in healthy young adults exposed to 0.06 ppm ozone for 6.6 hours. Am J Respir Crit Care Med. 2011 May 1;183(9):1215–21. doi: 10.1164/rccm.201011-1813OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez ML, Lay JC, Harris B, Esther CR, Jr, Brickey WJ, Bromberg PA, Diaz-Sanchez D, Devlin RB, Kleeberger SR, Alexis NE, Peden DB. Atopic asthmatic subjects but not atopic subjects without asthma have enhanced inflammatory response to ozone. J Allergy Clin Immunol. 2010 Sep;126(3):537–44. doi: 10.1016/j.jaci.2010.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garantziotis S, Li Z, Potts EN, Kimata K, Zhuo L, Morgan DL, et al. Hyaluronan mediates ozone-induced airway hyperresponsiveness in mice. J Biol Chem. 2009;284:11309–11317. doi: 10.1074/jbc.M802400200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]


