Abstract
Unlike most species, after food deprivation, Siberian hamsters increase foraging and food hoarding, two appetitive ingestive behaviors, but not food intake, a consummatory ingestive behavior. We previously demonstrated (Wood AD, Bartness TJ, Am J Physiol Regul Integr Comp Physiol 272: R783−R792, 1997) that increases in food hoarding are triggered by directly decreasing body fat levels through partial surgical lipectomy; however, we did not test if lipectomy affected foraging, nor if the magnitude of the lipid deficit affected food hoard size. Therefore, we tested whether varying the size of the lipectomy-induced lipid deficit and/or foraging effort affected foraging, food hoarding, or food intake. This was accomplished by housing adult male Siberian hamsters in a foraging/hoarding system and removing (x) both epididymal white adipose tissue (EWATx) pads, both inguinal white adipose tissue (IWATx) pads, or both EWAT and IWAT pads (EWATx + IWATx) and measuring foraging, food hoarding, and food intake for 12 wk. The lipectomy-induced lipid deficit triggered different patterns of white adipose tissue mass compensation that varied with foraging effort. Foraging for food (10 wheel revolutions to earn a food pellet) abolished the EWATx-induced compensation in IWAT pad mass. The magnitude of the lipid deficit did not engender a proportional change in any of the appetitive or consummatory ingestive behaviors. EWATx caused the greatest increase in food hoarding compared with IWATx or EWATx + IWATx, when animals were required to forage for their food. Collectively, it appears that the magnitude of a lipid deficit does not affect appetitive or consummatory behaviors; rather, when energy (foraging) demands are increased, loss of specific (gonadal) fat pads can preferentially stimulate increases in food hoarding.
Keywords: appetitive behavior, carcass composition, Siberian hamster, white adipose tissue
goal-oriented behaviors, such as ingestive behaviors, of nonhuman and human animals consists of the dichotomy of appetitive and consummatory phases (6). The mechanisms underlying the consumption of food (consummatory ingestive behavior) have received considerable attention compared with those underlying the approach, transport, and storage of food (appetitive ingestive behaviors). Both phases contribute to the overall energy strategy and balance of animals. For example, after food deprivation, changes in appetitive and consummatory ingestive behaviors occur, but vary among species (for review, see Refs. 3, 4). Specifically, food-deprived laboratory rats increase their foraging for food and, when they find it, eat it (e.g., Ref. 22). By contrast, hamster species do not overeat following resumption of feeding after food deprivation (for review, see Ref. 3). Instead, food-deprived Siberian and Syrian hamsters increase foraging (7, 15) and food hoarding (1, 2, 7, 15, 26). This separation of appetitive from consummatory ingestive behaviors in Siberian hamsters, as seen after food deprivation but also under other energy-demanding conditions [e.g., metabolic blockers, pregnancy, lactation (1, 2)], makes them an ideal model to study the differential expression of each of these phases of ingestive behavior.
The physiological triggers that elicit post-food deprivation-induced increases in foraging and food hoarding in Siberian hamsters are not known. One of the myriad of physiological effects of food deprivation is an increase in lipid mobilization from white adipose tissue (WAT) depots (e.g., Refs. 11, 14). Food deprivation-induced reductions in body fat are due to the secondary consequences of decreased energy intake and are not the only outcome of negative energy balance [e.g., decreased thermogenesis (11, 17), decreased reproductive system activity (27)]. Therefore, to test the notion that decreases in body fat per se trigger increases in these appetitive ingestive behaviors by Siberian hamsters, we chose a direct method that, at least initially, only decreases body fat: surgical removal of WAT (partial lipectomy, referred heretofore as lipectomy). Our laboratory previously demonstrated that lipectomized Siberian hamsters increase food hoarding, but not food intake (31). Moreover, the enhanced food hoarding returned to normal at a time when there is a complete recovery of lipectomy-induced lipid deficits, a response occurring exclusively in the nonexcised WAT pads (Ref. 31; for review see Ref. 21). In that experiment, however, the housing system did not require the animals to explicitly forage for their food (i.e., the animals only had to traverse a convoluted tube of ∼1.5 m long to get to the food source, which could be accomplished rather easily and quickly), and the size of the surgically induced lipid deficit was not varied [both inguinal (IWAT) and epididymal WAT (EWAT) pads were removed]; thus no relation between the magnitude of the lipid deficit and the size of the food hoards could be established. Therefore, the purpose of the present experiment was to test the effect of varied lipectomy-induced lipid deficits on appetitive (foraging and food hoarding) and consummatory (food intake) ingestive behaviors, with and without a required foraging effort. We have combined the foraging model of Perrigo and Bronson (24) to our laboratory's previously developed hoarding system (2), where animals are required not only to traverse the tube, but also to meet a prescribed number of revolutions in a wheel to obtain a pellet of food (8). This allowed us to answer the following questions. 1) Does foraging effort (and thus to some degree energy expenditure) affect the compensation of fat pad masses after lipectomy? 2) Does a greater lipid deficit trigger a greater increase in foraging, food hoarding, or food intake? This was accomplished by housing male Siberian hamsters in our foraging/hoarding system and removing (x) the pair of EWAT (EWATx) or IWAT (IWATx) pads, or both pairs of EWAT and IWAT pads (EWATx + IWATx), or their respective sham surgeries and measuring foraging, food hoarding, and food intake for 12 wk, a time by which body fat levels return to control values for lipectomized Siberian hamsters (for review, see Ref. 21).
METHODS
Animals and Housing
Adult male Siberian hamsters, ∼3.5 mo old and weighing 36–46 g, were obtained from our breeding colony. The colony was established in 1988, and its genealogy was described recently (5). Hamsters were group housed and reared from birth in a 16:8-h light-dark cycle (lights on at 2030). Room temperature was maintained at 21 ± 2°C, and relative humidity was 50 ± 10%. All procedures were approved by the Georgia State University Institutional Animals Care and Use Committee and were in accordance with the Public Health Service and United States Department of Agriculture guidelines.
Animals were tested in two identical replicates of 68 animals due to limited foraging/hoarding apparatuses. Each hamster was acclimated for 1 wk in our hoarding/foraging apparatus, as previously shown and described (8). Briefly, two cages were connected with a convoluted polyvinyl-chloride tubing system (38.1 mm inner diameter and ∼1.52 m long), with corner and straightways for both horizontal and vertical climbs. The top or “food cage” was 456 × 234 × 200 mm (length × width × height) and equipped with a water bottle and running wheel. The bottom or “burrow cage” was 290 × 180 × 130 mm and was covered to simulate the darkness of a natural burrow. The burrow cage contained Alpha-Dri (Specialty Papers, Kalamazoo, MI) bedding and cotton nesting material. The test diet (75 mg pellets: Purified Rodent Diet; Research Diets, New Brunswick, NJ) and tap water were available ad libitum during this period.
At the end of the adaptation period, hamsters were then trained to forage for their food based on our laboratory's previously published procedure (8). In brief, hamsters were given free access to food for 2 days while they adapted to the running wheel. In addition to the free food, a 75-mg food pellet was dispensed upon completion of every 10 wheel revolutions. Wheel revolutions were counted using a magnetic detection system and monitored by a computer-based hardware/software system (Med Associated, Lancaster, NH). On the 3rd day, the free food condition was replaced by a response-contingent condition in which only every 10 wheel revolutions triggered the delivery of a pellet. This condition was in effect for the remaining 5 days of the 1-wk-long training period. The hamsters were then separated into 12 groups (3 foraging groups × 4 lipectomy conditions) that were matched for percent change in body mass and average hoard size. The three foraging groups were 10 Revolutions/pellet group, free wheel/free food [Free Wheel group; food was available noncontingently (not earned), but the running wheel was active (locomotor activity control group)], or blocked wheel/free food [Blocked Wheel group; food was available noncontingently (not earned), but the running wheel was blocked (sedentary control group)].
Surgery
Animals within each of the foraging groups underwent one of four lipectomy conditions: 1) bilateral EWATx and sham IWATx (EWATx), 2) bilateral IWATx and sham EWATx (IWATx), 3) bilateral EWATx and IWATx (EWATx + IWATx), or 4) sham EWATx and sham IWATx (sham EWATx + sham IWATx). Hamsters were anesthetized with isoflurane. The dorsal and ventral hindquarters of each hamster were shaved and disinfected with ethanol (10% vol/vol). An incision was made lateral to the ventral midline through the dermis and peritoneum. Each EWAT pad was externalized and carefully excised without disrupting the neural and vascular provisions of the testes. The peritoneal incision was sutured shut with sterile silk, and the dermal incision was closed with sterile wound clips. Each IWAT pad was exposed and excised through an incision to the dorsal midline immediately adjacent to each hind leg. The IWAT pads were blunt dissected, and the incision was closed with sterile wound clips. Sham lipectomy included making an incision, visualization of the fat pads, and closure of the incision. Nitrofurozone powder was applied to all incisions to minimize infection. Hamsters had a 1-wk postsurgery recovery period where they were housed singly in polystyrene shoebox cages (290 × 189 × 130 mm) and given food (75 mg pellets) and water ad libitum. Fresh apple slices were given to facilitate fluid and caloric intake for the first 2–3 days postsurgery. The animals also received subcutaneous buprenorphine (0.05 mg/kg sc) injections for 2 days after surgery. After recovery, hamsters were returned to their respective hoarding/foraging cages, maintaining the same group membership. They subsequently were trained for an additional 1-wk training period, as previously described (8), to recover baseline measures of foraging, food intake, and food hoarding.
Measurements of Food Hoarding, Foraging, and Food Intake
Foraging (pellets earned) was defined as the number of pellets delivered upon completion of the requisite wheel revolutions. Food hoarding (pellets hoarded) was defined as the number of pellets found in the bottom “burrow” cage in addition to those removed from the cheek pouches. Surplus pellets were defined as the number of pellets removed from the top food cage that were earned, but neither eaten nor hoarded. For the 10 Revolutions/pellet group, food intake (pellets eaten) was defined as pellets earned − surplus pellets − hoarded pellets = food intake. For the Free Wheel and Blocked Wheel groups, food intake (pellets eaten) was defined as pellets given (400 pellets) − pellets left in the top cage − hoarded pellets = food intake.
Food hoard size and food intake were measured to the nearest whole pellet by setting the electronic balance to “parts” measurement rather then obtaining fractions of a pellet in milligrams; thus the smallest unit measured was one 75-mg food pellet and is equal to 1. These measures were made daily before the onset of the dark cycle (between 0830 and lights off at 1230) for the 1-wk training period and the 12 wk of the experiment that followed the surgical recovery period. Foraging was monitored by the computer system continuously during the experiment. Body mass was measured weekly to the nearest 0.01 g.
Blood Collection and Hormone Assays
Blood samples were drawn once a week after the surgical recovery period. Animals were lightly anesthetized with isoflurane, blood samples (∼300 μl) were drawn from the retroorbital sinus, and animals were returned to their respective housing conditions. The samples were centrifuged at 4°C for 30 min at 2,500 rpm. Serum was recovered and stored at −20°C until subsequent measurement of testosterone (testosterone EIA kit, ALPCO Diagnostics, Salem, NH) and leptin (mouse leptin ELISA kit, Millipore, St. Charles, MO), using commercially available ELISA kits, according to the manufacturers' instructions.
Tissue Harvesting and Carcass Composition
Twelve weeks after surgery, the hamsters were killed by an overdose of pentobarbital sodium (100 mg/kg), and their WAT pads [bilateral EWAT, IWAT, retroperitoneal WAT (RWAT), dorsal subcutaneous WAT (DWAT)] were removed, blotted dry, and weighed to the nearest 0.01 g.
Carcass composition was determined according to a modification of the method of Leshner et al. (16). Briefly, the carcass was shaved, and the gastrointestinal tract was removed and discarded. The carcass was weighed to establish the carcass wet weights. The carcass was then dehydrated in an oven at 70°C and weighed every 1–2 days until dry to determine total carcass water content. The carcass was blended, and an ∼1-g sample of the homogenous material was processed for lipid extraction using petroleum ether to determine lipid content gravimetrically. The remaining dried delipidated carcass sample was termed fat-free dry mass (FFDM). Total carcass lipid was then back-calculated based on the analysis of these samples and their respective carcass wet weights. The lipid, water, and FFDM of the dissected WAT pads was estimated according to the estimations of Newby et al. (23) and added to each carcass component to determine total values.
Statistical Analyses
Body mass, foraging, food hoarding, and food intake were analyzed using a three-way ANOVA for repeated measures (NCSS version 2000, Kaysville, UT) with a surgery × foraging effort × time (4 × 3 × 12) design for each behavioral measure. A separate, non-repeated-measures two-way ANOVA design (surgery × foraging effort; 4 × 3) was done on cumulative foraging, food hoarding, food intake, and final body mass measures. Terminal measures, including individual WAT pad mass and carcass composition, were analyzed by a two-way ANOVA design (surgery × foraging effort; 4 × 3). Measures of serum hormone concentrations were compared across surgical groups using a one-way ANOVA design. Post hoc analyses for significant differences between group means were determined using Duncan's new multiple-range tests when appropriate. Differences among groups were considered statistically significant if P < 0.05. Exact probabilities and test values were omitted for simplicity and clarity of the presentation of the results.
RESULTS
Hormone Assays
Leptin.
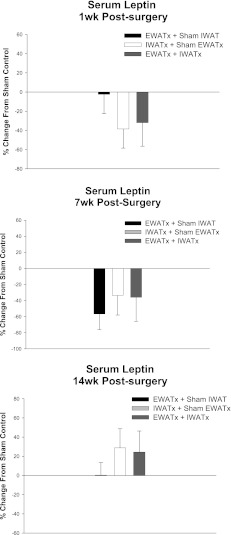
There were no differences in the percent change in serum leptin of the lipectomized groups relative to their respective sham controls at any of the three time points tested (Fig. 1). One week after surgery, however, the absolute serum leptin concentrations were significantly decreased in the IWATx condition (mean ± SE = 1.17 ± 0.29 ng/ml) compared with sham control animals (mean ± SE = 1.90 ± 0.41 ng/ml; P < 0.05; Fig. 1), likely reflecting the surgery-induced lipid deficit. Seven weeks after surgery (the approximate midpoint of the experiment), animals in the EWATx condition still had significantly decreased absolute serum leptin concentrations (mean ± SE = 0.47 ± 0.36 ng/ml) compared with the sham controls (mean ± SE = 1.09 ± 0.27 ng/ml; P < 0.05; Fig. 1). No differences in the absolute serum leptin concentrations were seen by 14 wk after surgery (the end point of the study) among the lipectomy conditions.
Fig. 1.
Percent change in serum leptin concentrations from sham epidiymal white adipose tissue (EWAT) lipectomy and sham inguinal white adipose tissue (IWAT) lipectomy (sham EWATx + sham IWATx) for each lipectomy condition [bilateral EWAT lipectomy and sham IWAT lipectomy (EWATx), bilateral IWAT lipectomy and sham EWAT lipectomy (IWATx), bilateral EWAT lipectomy and IWAT lipectomy (EWATx + IWATx)] 1 wk, 7 wk, or 14 wk after surgical lipectomy. Serum leptin concentrations of sham EWATx + sham IWATx at 1 wk (1.90 ± 0.41 ng/ml), 7 wk (1.08 ± 0.27 ng/ml), and 14 wk (2.48 ± 0.06 ng/ml) are given. Values are means ± SE.
Testosterone.
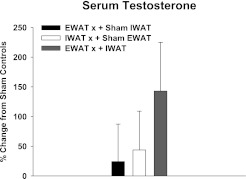
There were no differences in the percent change in serum testosterone concentrations of the lipectomized groups relative to the respective sham controls at the end of the experiment (Fig. 2), nor were there differences in the absolute serum testosterone concentrations.
Fig. 2.
Percent change in serum testosterone concentrations from sham EWATx + sham IWATx for each lipectomy condition (see Fig. 1 legend). Serum testosterone concentrations of sham EWATx + sham IWATx at 1 wk (1.88 ± 0.77 ng/ml) are given. Values are means ± SE.
Body mass.
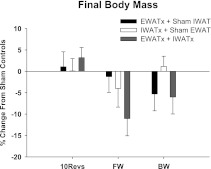
There were no differences in the percent change in the final body mass from that of the respective sham controls among the lipectomy conditions (Fig. 3). Absolute body mass was affected by the foraging effort; however, the final body mass was significantly lower in the 10 Revolutions/pellet group (mean ± SE = 39.5 ± 0.66 g) compared with Free Wheel (mean ± SE = 45.2 ± 0.92 g) and Blocked Wheel (mean ± SE = 43.8 ± 0.85 g) animals (P < 0.05). Whereas the animals in the Free Wheel and Blocked Wheel groups increased their body mass progressively after surgery (∼14 and 10%, respectively), animals in the 10 Revolutions/pellet group did not begin to increase their body mass until the 9th wk after surgery (albeit never reaching a statistically significant difference from their postsurgical body mass; data not shown).
Fig. 3.
Percent change in body mass from sham EWATx + sham IWATx for each lipectomy condition (see Fig. 1 legend) in groups 10 Revolutions/pellet (10Revs), Free Wheel (FW), and Blocked Wheel (BW). Body mass of sham EWATx + sham IWATx in 10Revs (39.52 ± 1.95 g), FW (47.58 ± 1.94 g), and BW (44.81 ± 2.36 g) groups are given. Values are means ± SE.
Carcass composition.
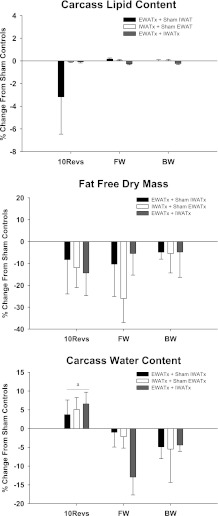
There were no differences in carcass lipid, water content, or FFDM among the lipectomy conditions, despite removal of various WAT pads (Fig. 4). Animals in the 10 Revolutions/pellet group had significantly increased total water content compared with Free Wheel and Blocked Wheel groups (P < 0.05; Fig. 4).
Fig. 4.
Percent change in carcass lipid mass, fat free dry mass, and carcass water content from sham EWATx + sham IWATx for each lipectomy condition (see Fig. 1 legend) for each group (10Revs, FW, and BW). aP < 0.05 compared with FW and BW. Carcass lipid mass for sham EWATx + sham IWATx in 10Revs (9.71 ± 1.46 g), FW (10.35 ± 1.61 g), and BW (10.80 ± 2.36 g) groups are given. Fat free dry mass for sham EWATx + sham IWATx in 10Revs (0.04 ± 0.01 g), FW (0.01 ± 0.01 g), and BW (0.03 ± 0.01 g) groups are given. Carcass water content for sham EWATx + sham IWATx in 10Revs (17.62 ± 0.68 g), FW (19.75 ± 1.04 g), and BW (18.89 ± 1.22 g) groups are given. Values are means ± SE.
Fat Pad Mass
EWAT.
There was no significant compensatory increase in EWAT mass after EWATx (Fig. 5), as expected from our laboratory's previous studies (e.g., Refs. 18, 28, 31).
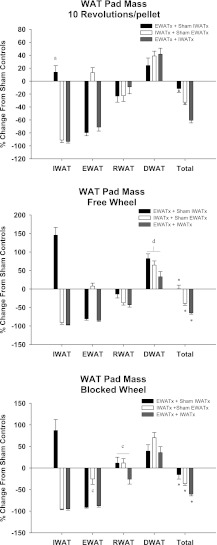
Fig. 5.
Percent change in fat pad mass from sham EWATx + sham IWATx for each lipectomy condition (see Fig. 1 legend) in groups 10Revs, FW, and BW. *P < 0.05 compared with other lipectomy conditions. aP < 0.05 compared with FW and BW. cP < 0.05 compared with 10Revs and FW. dP < 0.05 compared with 10Revs. Fat pad mass of sham EWATx + sham IWATx in 10Revs group for IWAT (1.45 ± 0.31 g), EWAT (0.77 ± 0.13 g), retroperitoneal white adipose tissue (RWAT; 0.18 ± 0.06 g), dorsal subcutaneous white adipose tissue (DWAT; 1.23 ± 0.25 g), and total (3.68 ± 0.57 g) are given. Fat pad mass of sham EWATx + sham IWATx in FW group for IWAT (2.08 ± 0.41 g), EWAT (0.89 ± 0.12 g), RWAT (0.21 ± 0.07 g), DWAT (1.59 ± 0.15 g), and total (4.77 ± 0.64 g) are given. Fat pad mass of sham EWATx + sham IWATx in the BW group for IWAT (1.56 ± 0.29 g), EWAT (1.01 ± 0.19 g), RWAT (0.14 ± 0.04 g), DWAT (1.48 ± 0.22 g), and total (4.19 ± 0.66 g) are given. Values are means ± SE.
IWAT.
Similar to EWATx, there was no significant compensatory increase in IWAT mass after IWATx, as expected (18, 20, 31). IWAT mass was significantly increased by EWATx in both the Free Wheel and Blocked Wheel groups compared with animals that were in the 10 Revolutions/pellet group (P < 0.05; Fig. 5).
RWAT.
There were no significant compensatory increases in RWAT mass after either EWATx and/or IWATx in any of the foraging groups. Both 10 Revolutions/pellet and Free Wheel groups had significantly decreased RWAT mass after EWATx and/or IWATx compared with animals in the Blocked Wheel group (P < 0.05; Fig. 5).
DWAT.
The only statistically significant change in DWAT mass after lipectomy was a significant increase in mass for the Free Wheel group compared with the 10 Revolutions/pellet group for all lipectomies (P < 0.05; Fig. 5).
Total WAT.
By adding EWAT, IWAT, RWAT, and DWAT to obtain total dissectible WAT, the original graded lipid deficit that was produced by EWATx and/or IWATx was still evident 12 wk after surgery (P < 0.05; Fig. 5). That is, EWATx animals show the smallest or no total lipid deficit, IWATx animals show a significantly larger lipid deficit, and the EWATx + IWATx animals show the largest lipid deficit (P < 0.05; Fig. 5).
Behavioral Measures
Wheel running.
There was no effect of lipectomy on wheel running (total revolutions, data not shown), suggesting no debilitating effect of the surgery or nonspecific stimulation of locomotor activity.
Foraging.
Consistent with our laboratory's previous findings (7, 8), animals in the 10 Revolutions/pellet group ran significantly more wheel revolutions than the Free Wheel animals (P < 0.05; mean ± SE = 3,625 ± 101 and 2,555 ± 92 revolutions, respectively). See Fig. 6.
Fig. 6.
Percent change in cumulative foraging from sham EWATx + sham IWATx for each lipectomy condition (see Fig. 1 legend) in 10Revs group. Cumulative foraging of sham EWATx + sham IWATx in 10Revs group (3,788 ± 110) is given. Values are means ± SE.
Food hoarding.
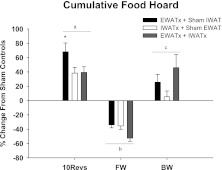
Animals in the 10 Revolutions/pellet group hoarded significantly more food than animals in the Free Wheel or Blocked Wheel groups (P < 0.05; Fig. 7), consistent with our laboratory's previous findings (8). Among the 10 Revolutions/pellet groups, EWATx hamsters significantly hoarded more food compared with IWATx or EWATx + IWATx animals (P < 0.05; Fig. 7).
Fig. 7.
Percent change in cumulative food hoard from sham EWATx + sham IWATx for each lipectomy condition (see Fig. 1 legend) in groups 10Revs, FW, and BW. *P < 0.05 compared with IWATx and EWATx + IWATx. aP < 0.05 compared with FW and BW. bP < 0.05 compared with 10Revs and BW. cP < 0.05 compared with 10Revs and FW. Cumulative food hoard of sham EWATx + sham IWATx in 10Revs (96 ± 6), FW (23 ± 1), and BW (11 ± 1) groups is given. Values are means ± SE.
Food intake.
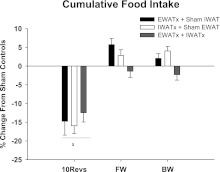
Animals in the 10 Revolutions/pellet group ate significantly less than animals in the Free Wheel or Blocked Wheel groups (P < 0.05; Fig. 8). There was no effect of lipectomy on food intake (Fig. 4), a result we also have seen previously (e.g., Refs. 18, 31).
Fig. 8.
Percent change in cumulative food intake from sham EWATx + sham IWATx for each lipectomy condition (see Fig. 1 legend) in groups 10Revs, FW, and BW. aP < 0.05 compared with FW and BW. Cumulative food intake of sham EWATx + sham IWATx in 10Revs (72 ± 2), FW (46 ± 0.6), and BW (41 ± 0.6) groups is given. Values are means ± SE.
DISCUSSION
The major results of the present study were that 1) compensatory increases in nonexcised WAT masses occurred for all lipectomies, regardless of the foraging effort; 2) foraging for food (10 Revolutions/pellet group) abolished the EWATx-induced increase in IWAT mass; 3) the magnitude of the lipid deficit did not elicit a proportional change in appetitive or consummatory ingestive behaviors; and 4) although EWATx produced the smallest lipid deficit (∼50% of IWATx, ∼33% of EWATx + IWATx), it triggered the largest increase in food hoarding when animals experienced the greatest foraging effort (10 Revolutions/pellet group).
The EWATx-specific effects seen in the present experiment, and discussed in detail below, are not likely due to compromised testicular function (i.e., decreased testosterone synthesis and release). Although EWATx can decrease paired testes wet weight to some extent and did so here, no differences between the lipectomy conditions or circulating testosterone concentrations were found here or previously (19).
The results of the present experiment support previous studies showing that total body fat can be tightly regulated, as evidenced by the lipectomy-induced compensatory increases in nonexcised WAT mass, a response that occurs not only in Siberian hamsters (e.g., Refs. 18, 28, 31), but in laboratory rats and mice, lambs, ground squirrels, and Syrian hamsters (for review, see Ref. 21), including suggestive evidence in humans (25, 33). Thus there was no difference in carcass lipid content of EWATx and/or IWATx hamsters compared with their respective sham lipectomized controls 12 wk after lipectomy. Foraging effort differentially affected the pattern and degree of lipectomy-induced compensatory WAT mass increases. Specifically, the Blocked Wheel hamsters increased IWAT mass after EWATx to approximately the same extent as seen in our laboratory's previous studies (∼50% compared with sham controls; e.g., Refs. 18, 28, 31), as might be expected, given essentially the same housing conditions. By contrast, the Free Wheel EWATx hamsters more strikingly increased their IWAT mass (∼145% compared with sham controls). Thus the increased energy expenditure that likely occurred by wheel running, whether voluntary (Free Wheel) or imposed (10 Revolutions/pellet), triggered a fat pad-specific augmentation of the compensatory increase in IWAT mass compared with standard shoebox cage housing, as in our laboratory's previous lipectomy studies (e.g., Refs. 18, 28, 31). If the exercise-induced increase in energy expenditure was too great, however, as likely occurred with the 10 Revolutions/pellet group that ran significantly more (∼42% more) than the Free Wheel group, this response was abolished. Thus it would appear that, in the case for the greater wheel running by the 10 Revolutions/pellet hamsters, the presumed additional increase in energy expenditure apparently curtailed any supplemental storage of energy in IWAT. Instead, the animals may have utilized this energy to offset the increased energy demands of the required foraging effort.
In the present study, the total lipectomy-induced body fat losses were not associated with proportional increases in the appetitive or consummatory ingestive behaviors measured here. As noted above, the smallest lipid deficit (EWATx) produced the largest increase in food hoarding. Instead of increases in ingestive behaviors being stimulated by the size of the lipid deficit, loss of lipid stores from specific fat pads appears to be more important in triggering some of the changes in these ingestive behaviors. That is, there was no significant correlation between the amount of WAT removed and the degree to which any of these ingestive behaviors were expressed (data not shown). Conversely, hamsters required to forage for their food (10 Revolutions/pellet) hoarded significantly more when only EWAT (EWATx) was removed then when either IWAT (IWATx) alone was removed or when both EWAT and IWAT were removed (EWATx + IWATx). It is unclear why the EWATx-induced increase in food hoarding did not occur when EWAT was removed in the EWATx + IWATx hamsters, although it may be because this maximal lipid deficit overcame any EWATx-specific effects. That is, we previously have seen the elimination of fat pad-specific effects triggered by lipectomy, if the lipid deficit became too large (20), as may be occurring with the EWATx + IWATx procedure, implying that, at some point, the size of the decrease in overall lipid reserves may affect behavior and physiology. This idea that the site of lipid loss may be more important than the size of the total lipid loss (at least until a more extreme level of fat loss occurs) is not unique to the present experiment. For example, in a recent study (Y. Chu, G. Huddleston, R. Bowers, A. Clancy, R. Harris, and T. Bartness, unpublished observations), EWATx of Syrian hamsters completely inhibits spermatogenesis, as it does in laboratory rats (30); however, IWATx of Syrian hamsters, which produces twice the lipid deficit of EWATx, does not affect spermatogenesis. Therefore, it may be that lipid loss from fat pads that are most critical to the animal, such as those related to reproduction (EWAT in males, parametrial WAT in females), affects physiology (spermatogenesis) and behavior (hoarding, in 10 Revolutions/pellet group in the present study) more than does the size of the total lipid deficit or lipid deficits in nonreproductive associated WAT pads. If this is the case, then circulating factors, such as the largely adipocyte-derived cytokine leptin, thought by some to convey body fat levels to the brain (e.g., Ref. 32), cannot be involved in such fat pad-specific responses, because leptin released from any WAT pad, or other tissue, would not carry with it specific signaling of its origin. Moreover, our laboratory has shown previously that a functioning leptin signaling system is not necessary for lipectomy-induced compensatory increases in the mass of nonexcised WAT pads, because ob/ob and db/db mice show normal lipectomy-triggered compensation in total body fat for their surgical lipid deficit (12). In the present study, there were no differences in the relative serum leptin concentrations among any of the lipectomy groups, regardless of the graded lipid deficit or the specific fat pad removed; however, there was a significant absolute decrease in leptin concentration by IWAT compared with controls 1 wk after lipectomy. Alternatively, the sensory innervation of WAT (9, 10, 28) has the potential to convey such fat pad-specific information. Indeed, selective destruction of sensory nerves in EWAT via locally injected capsaicin, a sensory nerve-specific neurotoxin (13), triggers an increase in WAT pad mass by the noninjected WAT pads that is indistinguishable from the compensatory increases in WAT pad mass triggered by actual EWATx (28).
The lipectomy-induced compensatory increase in RWAT mass seen in our laboratory's previous studies, in which hamsters were housed in standard shoebox cages (18, 20, 29), also appears to be affected by increases in foraging effort. That is, RWAT was not increased by any of the lipectomy conditions in the present study, regardless of the foraging group. This may be because all animals had to minimally climb to the top cage to obtain food (Blocked Wheel), and some animals had the additional energy drains of noncontingently running in the wheel (Free Wheel) or contingently running in the wheel to obtain food (10 Revolutions/pellet) in the present study. If this notion is correct, then it would be predicted that the Blocked Wheel group would expend the least amount of energy (no wheel running) and, therefore, might marshal a greater RWAT compensatory response than the two groups with running wheels (Free Wheel and 10 Revolutions/pellet). Although RWAT mass was not significantly increased by lipectomy in any group, RWAT mass was not decreased in two of the three lipectomy groups in the Blocked Wheel condition, as occurred in all of the other groups compared with their respective sham controls. Thus differences in the pattern of fat pad mass compensation after lipectomy between our laboratory's present and former studies seems to be most readily explained by the requirement to explicitly forage for food (10 Revolutions/pellet group), or the opportunity to wheel run (Free Wheel group) and necessity to traverse the convoluted tubing connecting the burrow cage to the upper cage for all groups, including the Blocked Wheel group.
Collectively, the present data suggest that 1) appetitive ingestive behaviors are not altered by the total amount of fat mass loss, but instead are altered by lipid deficits in gonadal WAT; and 2) apparent increases in energy expenditure due to increases in foraging effort can differentially affect the ability of the nonexcised WAT pads to compensate for lipectomy-induced lipid deficits. Understanding the complex interactions among environmental factors affecting food availability and consumption, internal energy reserves (body fat), and external energy reserves (food hoards) is in its infancy, but, fully explored, should yield important information about the control of appetitive behaviors that are understudied relative to their consummatory counterparts.
GRANTS
This research was supported by National Science Foundation Research Grant IBN 9876495 and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK078358 to T. J Bartness.
Acknowledgments
The authors thank Kelly Johnson for assistance with data collection.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bartness TJ Food hoarding is increased by pregnancy, lactation and food deprivation in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 272: R118–R125, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Bartness TJ, Clein MR. Effects of food deprivation and restriction, and metabolic blockers on food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 266: R1111–R1117, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Bartness TJ, Day DE. Food hoarding: a quintessential anticipatory appetitive behavior. Prog Psychobiol Physiol Psychol 18: 69–100, 2003. [Google Scholar]
- 4.Bartness TJ, Demas GE. Comparative studies of food intake: lessons from non-traditionally studied species. In: Food and Fluid Intake, edited by Stricker EM and Woods SC. New York: Plenum, 2004, p. 423–467.
- 5.Bowers RR, Festuccia WTL, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol Regul Integr Comp Physiol 286: R1167–R1175, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Craig W Appetites and aversions as constituents of instincts. Biol Bull 34: 91–107, 1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day DE, Bartness TJ. Fasting-induced increases in hoarding are dependent on the foraging effort level. Physiol Behav 78: 655–668, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Day DE, Bartness TJ. Effects of foraging effort on body fat and food hoarding by Siberian hamsters. J Exp Zool 289: 162–171, 2001. [PubMed] [Google Scholar]
- 9.Fishman RB, Dark J. Sensory innervation of white adipose tissue. Am J Physiol Regul Integr Comp Physiol 253: R942–R944, 1987. [DOI] [PubMed] [Google Scholar]
- 10.Giordano A, Morroni M, Carle F, Gesuita R, Marchesi GF, Cinti S. Sensory nerves affect the recruitment and differentiation of rat periovarian brown adipocytes during cold acclimation. J Cell Sci 111: 2587–2594, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Harris RB, Kelso EW, Flatt WP, Bartness TJ, Grill HJ. Energy expenditure and body composition of chronically maintained decerebrate rats in the fed and fasted condition. Endocrinology 147: 1365–1376, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Harris RBS, Hausman DB, Bartness TJ. Compensation for partial lipectomy in mice with genetic alterations of leptin and its receptor subtypes. Am J Physiol Regul Integr Comp Physiol 283: R1094–R1103, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Jancso G, Kiraly E, Jancso-Gabor A. Direct evidence for an axonal site of action of capsaicin. Naunyn Schmiedebergs Arch Pharmacol 313: 91–94, 1980. [DOI] [PubMed] [Google Scholar]
- 14.Krotkiewski M, Bjorntorp P. The effects of dexamethasone and starvation on body composition and regional adipose tissue cellularity in the rat. Acta Endocrinol (Copenh) 80: 667–675, 1975. [DOI] [PubMed] [Google Scholar]
- 15.Lea SEG, Tarpy RM. Hamsters' demand for food to eat and hoard as a function of deprivation and cost. Anim Behav 34: 1759–1768, 1986. [Google Scholar]
- 16.Leshner AI, Litwin VA, Squibb RL. A simple method for carcass analysis. Physiol Behav 9: 281–282, 1972. [DOI] [PubMed] [Google Scholar]
- 17.Ma SW, Foster DO. Starvation-induced changes in metabolic rate, blood flow and regional energy expenditure in rats. Can J Physiol Pharmacol 64: 1252–1258, 1986. [DOI] [PubMed] [Google Scholar]
- 18.Mauer MM, Bartness TJ. Body fat regulation following partial lipectomy in Siberian hamsters is photoperiod-dependent and fat pad-specific. Am J Physiol Regul Integr Comp Physiol 266: R870–R878, 1994. [DOI] [PubMed] [Google Scholar]
- 19.Mauer MM, Bartness TJ. A role for testosterone in the maintenance of seasonally-appropriate body mass, but not in lipectomy-induced body fat compensation in Siberian hamsters. Obes Res 3: 31–41, 1995. [DOI] [PubMed] [Google Scholar]
- 20.Mauer MM, Bartness TJ. Fat pad-specific compensatory mass increases after varying degrees of partial lipectomy in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 273: R2117–R2123, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Mauer MM, Harris RBS, Bartness TJ. The regulation of total body fat: lessons learned from lipectomy studies. Neurosci Biobehav Rev 25: 15–28, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Morgan CT, Stellar E, Johnson O. Food-deprivation and hoarding in rats. J Comp Physiol 35: 275–295, 1943. [Google Scholar]
- 23.Newby DF, DiGirolamo M, Cotsonis GA, Kutner MH. Model of spontaneous obesity in aging male Wistar rats. Am J Physiol Regul Integr Comp Physiol 259: R1117–R1125, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Perrigo G, Bronson FH. Behavioral and physiological responses of female house mice to foraging variation. Physiol Behav 34: 437–440, 1985. [DOI] [PubMed] [Google Scholar]
- 25.Scarborough DA, Bisaccia E. The occurrence of breast enlargement in females following liposuction. Am J Cosmetic Surg 8: 97–99, 1991. [Google Scholar]
- 26.Schneider JE, Buckley CA. Food hoarding is increased by food deprivation and decreased by leptin treatment in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 285: R1021–R1029, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Schneider JE, Wade GN. Availability of metabolic fuels controls estrous cyclicity of Syrian hamsters. Science 244: 1326–1328, 1989. [DOI] [PubMed] [Google Scholar]
- 28.Shi H, Bartness TJ. White adipose tissue sensory nerve denervation mimics lipectomy-induced compensatory increases in adiposity. Am J Physiol Regul Integr Comp Physiol 289: R514–R520, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Shi H, Bowers RR, Bartness TJ. Norepinephrine turnover in brown and white adipose tissue after partial lipectomy. Physiol Behav 81: 535–543, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan V, Thombre DP, Lakshmanan S, Chakrabarty AS. Effect of removal of epididymal fat on spermatogenesis in albino rats. Indian J Exp Biol 24: 487–488, 1986. [PubMed] [Google Scholar]
- 31.Wood AD, Bartness TJ. Partial lipectomy, but not PVN lesions, increases food hoarding by Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 272: R783–R792, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Woods SC, Schwartz MW, Baskin DG, Seeley RJ. Food intake and the regulation of body weight. Annu Rev Psychol 51: 255–277, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Yost TJ, Rodgers CM, Eckel RH. Suction lipectomy: Outcome relates to region-specific lipoprotein lipase activity and internal weight change. Plast Reconstr Surg 92: 1101–1109, 1993. [PubMed] [Google Scholar]