Abstract
Food is acquired (obtained by foraging) and frequently stored (hoarded) across animal taxa, including humans, but the physiological mechanisms underlying these behaviors are virtually unknown. We found that peptides that stimulate food intake in rats stimulate food foraging and/or hoarding more than intake in Siberian hamsters. Neuropeptide Y (NPY) is a potent orexigenic peptide that increases food foraging and hoarding (appetitive behavior) and food intake (consummatory behavior). Given that NPY injections into the hypothalamic paraventricular nucleus (PVH) or perifornical area (PFA) increase food intake by rats, it is possible that these injections may stimulate food foraging or hoarding by Siberian hamsters. We also tested whether antagonism of the NPY Y1 receptor (Y1-R), the agonism of which stimulates hoarding, would inhibit post-food-deprivation increases in foraging and hoarding. We injected one of three doses of NPY or vehicle into the PVH or PFA of animals housed in a simulated foraging-hoarding housing system and measured these behaviors at 1, 2, 4, and 24 h. A subset of animals was subsequently food deprived and then given PVH or PFA Y1-R antagonist microinjections before they were refed. NPY PVH microinjections decreased foraging but increased hoarding and food intake, whereas NPY PFA microinjections increased all three behaviors, but the greatest increase was in hoarding. Y1-R antagonist inhibited post-food-deprivation increases in hoarding when injected into the PVH and PFA and inhibited foraging when injected into the PFA. These results support the view that NPY is involved in appetitive and consummatory ingestive behaviors, but each may be controlled by different brain areas and/or NPY receptor subtypes.
Keywords: food intake, Siberian hamster, neuropeptide Y1 receptor, antagonist
obesity is increasing worldwide and is the result of energy intake chronically exceeding energy expenditure. Despite our expanded understanding of the factors that control food intake, obesity is literally and figuratively expanding, with ∼65% of Americans overweight and ∼30% obese (37). With the considerable focus placed on the underlying mechanisms controlling food intake, other factors that could play significant roles in controlling energy input are often ignored. For example, the mechanisms involved in food acquisition (foraging) and food storage (hoarding) (for review see Ref. 3) have received little attention, even though it is almost always necessary, even for humans, to forage for food, and food is often stored for some period of time before it is consumed (hoarded). Therefore, we have been studying the mechanisms underlying food foraging and food hoarding (appetitive ingestive behaviors), as well as food intake (consummatory ingestive behavior) (13), in Siberian hamsters (Phodopus sungorus) housed in a simulated burrow system with a wheel-running-based foraging component (16). Siberian hamsters, unlike laboratory rats (10, 51) or mice (42, 44), provide a unique model of food hoarding, because they have cheek pouches that allow for the transport of large amounts of foraged food and naturally hoard food in the laboratory and in the wild (for review see Ref. 3). In addition, Siberian hamsters, unlike rats and mice (3), exhibit changes in food hoarding independently of changes in food intake (17). This differential expression of appetitive and consummatory ingestive behaviors suggests at least a partially independent physiology that subserves each ingestive behavior. Thus Siberian hamsters are an ideal species for study of the effects of environmental and physiological challenges on foraging, food hoarding, and food intake, including the role of neuropeptides related to energy balance, on these behaviors.
Of the many possible central factors that could be involved in food foraging and hoarding, we initially chose neuropeptide Y (NPY), because it is the most potent orexigenic agent known (11, 47). In addition, we previously found that NPY injection into the third ventricle in Siberian hamsters markedly increases foraging and food hoarding, with food hoarding occurring to a greater extent than food intake (19). Moreover, we also found that injections of a Y1 receptor (Y1-R) agonist into the third ventricle stimulated foraging and, especially, food hoarding to a greater degree than food intake and, conversely, that a Y5 receptor agonist stimulated food intake to a greater extent than foraging or hoarding (19). The sites of action for these effects of NPY are not known, inasmuch as injections of any peptide into the third ventricle affect many brain areas in the vicinity of the third and fourth ventricles. Site-specific injections of NPY into the paraventricular nucleus of the hypothalamus (PVH) (48) or the perifornical area (PFA) (50) elicit a robust increase in food intake in laboratory rats, suggesting that these two sites are significant loci for the effects of NPY on consummatory ingestive behavior. This effect of parenchymal microinjections of NPY is on firm neuroanatomic ground, because the PVH and PFA possess NPY Y receptors (38, 39) and contain numerous NPY-immunoreactive fibers (20). Microinjections of a Y1-R agonist into the PVH or PFA trigger a clear dose-dependent increase in food intake in laboratory rats (49), and, conversely, prior or coinjection of an NPY Y1-R antagonist into the PVH blocks the ability of PVH NPY injections to increase food intake (54, 57). Because Siberian hamsters often respond to factors that stimulate food intake in laboratory rats and mice by particularly and impressively increasing foraging and/or food hoarding [e.g., food deprivation (2, 17), systemic ghrelin injections (28), 3rd ventricular injections of agouti-related protein (18), and 3rd ventricular injections of NPY (19)], it may be that microinjections of NPY or an NPY Y1-R agonist into the PVH and/or PFA will primarily enhance appetitive ingestive behaviors as well.
Therefore, on the basis of the robust orexigenic effect of NPY in the PFA and PVH (48, 50), apparent NPY-induced increases in appetitive responses in laboratory rats (1, 5, 26, 43), and the significant role of the Y1-R in foraging and food hoarding in Siberian hamsters (19), we hypothesized that 1) PVH and PFA NPY should increase foraging and food hoarding to a greater extent than food intake and 2) the post-food-deprivation increases in food foraging and hoarding normally seen in these hamsters (17) will be blocked/attenuated by an NPY Y1-R antagonist injected into the PVH or PFA. We housed male Siberian hamsters in a running wheel-based food delivery-foraging system coupled with simulated burrow housing (16). Three foraging conditions were used: 1) a foraging requirement to earn food (10 wheel revolutions per pellet), 2) running wheel access and noncontingently available food (free food), or 3) blocked running wheel and free food (see below). Animals in all foraging conditions were microinjected with saline or three doses of NPY into the PVH or PFA, and food foraging, hoarding, and intake were measured 1, 2, 4, and 24 h after injection. Animals were then food deprived or remained ad libitum fed and injected with a vehicle or Y1-R antagonist before refeeding, and the same behavioral measures were obtained 1, 2, 4, and 24 h after injection.
METHODS
Animals and Housing
Adult male Siberian hamsters (Phodopus sungorus sungorus; ∼3 mo old, 35–46 g body wt) were obtained from our breeding colony, which was established in 1988 with several outbreedings (for detailed genealogy see Ref. 7). Hamsters were group housed and reared from birth in a 16:8-h light-dark cycle (lights on at 2030). Room temperature was maintained at 21 ± 2°C and relative humidity at 50 ± 10%. All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and were in accordance with the US Public Health Service and US Department of Agriculture guidelines.
Two sets of 64 animals were acclimated for 1 wk in our hoarding-foraging apparatus as previously shown and described (16). Briefly, two cages are connected with a convoluted polyvinyl chloride tubing system (38.1 mm ID, ∼1.52 m long), with corner and straightways for horizontal and vertical climbs. The top or “food cage” (456 mm long × 234 mm wide × 200 mm high) was equipped with a water bottle and running wheel. The bottom or “burrow cage” (290 mm long × 180 mm wide × 130 mm high) was covered to simulate the darkness of a natural burrow. The burrow cage contained Alpha-Dri (Specialty Papers, Kalamazoo, MI) bedding and cotton nesting material. Tap water and 75-mg food pellets (Purified Rodent Diet, Research Diets, New Brunswick, NJ) were available ad libitum during this period.
At the end of this acclimation period, all animals were removed from the foraging apparatus and housed in a single-shoebox cage with 75-mg food pellets and water available ad libitum. Guide cannulas were then surgically implanted in all hamsters (see Cannula Implantation). After a 1-wk postsurgical recovery period, all hamsters were returned to the foraging-hoarding apparatus, and baseline measures were taken.
Training and Baseline Measures
Hamsters were trained to forage for their food on the basis of our previously published procedures (16). Briefly, hamsters were given free access to food for 2 days while they adapted to the running wheel. In addition to the free food, a 75-mg food pellet was dispensed on completion of every 10 wheel revolutions. Wheel revolutions were counted using a magnetic detection system and monitored by a computer-based hardware-software system (Med Associated, Lancaster, NH). On the 3rd day, the free food condition was replaced by a response-contingent condition, in which only every 10 wheel revolutions triggered the delivery of a pellet. This condition was in effect for the remaining 5 days of the 1-wk training period. The hamsters were then separated into three foraging groups that were matched for percent change in body mass and average hoard size. The three foraging groups were as follows: 10 revolutions per pellet (10 Rev; a 75-mg food pellet was delivered on completion of the 10 wheel revolutions); free wheel-free food [FW; food was available noncontingently (not earned), but the running wheel was active (locomotor activity control group)], or blocked wheel-free food [BW; food was available noncontingently (not earned), but the running wheel was blocked (sedentary control group)].
Measurement of Foraging, Food Hoarding, and Food Intake
Foraging (pellets earned) was defined as the number of pellets delivered on completion of the requisite 10 wheel revolutions. Food hoarding (pellets hoarded) was defined as the number of pellets found in the bottom (burrow) cage in addition to those removed from the cheek pouches. For the 10 Rev group, food intake (pellets eaten) was defined as pellets earned − pellets left in the top cage − pellets hoarded. For the FW and BW groups, food intake (pellets eaten) was defined as pellets given (400 pellets) − pellets left in the top cage − pellets hoarded. An electronic balance used to weigh the food pellets was set to measure “parts,” rather than fractions of a pellet in milligrams; thus one 75-mg food pellet = 1, and fractions of a pellet were computed by the scale.
Cannula Implantation
The animals were anesthetized with isoflurane, and the fur at the top of the head was removed to expose the area to be incised. A guide cannula (26-gauge stainless steel; Plastics One, Roanoke, VA) was unilaterally implanted, stereotaxically targeted for the PVH (−0.03 cm anteroposterior, −0.03 cm mediolateral, −0.55 cm dorsoventral) or the PFA (−0.04 cm anteroposterior, −0.045 cm mediolateral, −0.6 cm dorsoventral). Specifically, the skull was trephined at the specified coordinates, and the cannula was stereotaxically lowered into place. The guide cannula was secured to the skull using a 3/16-mm jeweler's screw and dental acrylic. A removable obturator was placed into the guide cannula throughout the experiment, except when it was removed for the injections.
Injection Protocol
Injections consisted of vehicle (sterile 0.15 M NaCl) or one of three doses (0.176, 0.352, or 0.704 nmol) of NPY (American Peptide, Sunnyvale, CA) via an internal cannula (33-gauge stainless steel; Plastics One) that penetrated below the top of the skull 0.6 cm into the PVH and 0.65 cm into the PFA. The lowest dose was 10 times less than that used for third ventricular injections of NPY in our animals (19) and fell within the range of effective doses used previously for site-specific microinjections of NPY (48). The highest dose used was ineffective in stimulating foraging, hoarding, or food intake if injected into the third ventricle (unpublished results). The inner cannula was connected to a microsyringe via polyethylene tubing, and the injection volume for the vehicle or NPY was 100 nl. Each animal in the three foraging groups received all doses of NPY or its vehicle in a counterbalanced schedule to control for possible order effects of peptide administration. They were injected twice a week for 2 wk, with 3 days between injections to serve as a washout period on the basis of our previous experience with third ventricular injections of NPY (19).
At 2 h before the onset of the dark cycle, food was removed from the pouches of the hamsters, they were placed in clean burrow cages, and access to the tubes was blocked. Animals were restrained by hand during the ∼30-s injection period, and the injection needle remained in place for ∼30 s. Hamsters were returned to their respective cages, and access to the tube was reinstated. Foraging, food hoarding, and food intake were measured 1, 2, 4, and 24 h after injection.
Food Deprivation Protocol
After completion of the NPY or vehicle injection cycles and the last 3-day washout period, half of the animals were food deprived for 56 h. In our previous studies of food hoarding, we used food deprivation periods of 12–56 h (with approval of the Institutional Animal Care and Use Committee), with the 56-h duration appearing somewhat severe and/or nonphysiological. In the laboratory, however, Siberian hamsters are ∼50% body fat compared with ∼25% body fat in nature (53). Short food deprivation periods of 12–24 h are minimally energetically challenging in these animals, and stimulation of food hoarding is low (2; M. R. Clein and T. J. Bartness, unpublished results). Therefore, on the basis of this published report (2) and the unpublished results, we selected a 56-h period of food deprivation to trigger the behavior nearly maximally.
Before refeeding, a Y1-R antagonist (BIBO 3304, 17.6 nmol; a gift from Boehringer Ingelheim) or vehicle control (0.15 M saline, 10% dimethyl sulfoxide, and 2.5% glacial acetic acid; 100 nl volume) injected unilaterally into the PVH or PFA of half of the food-deprived and non-food-deprived animals (see Injection Protocol). This dose was chosen because it fell within the range of lower effective doses that decreased food intake in previous studies (40, 45, 54) and was 10 times the NPY dose used for the present study. Hamsters were returned to their respective cages, and foraging, food hoarding, and food intake were measured 1, 2, 4, and 24 h after injection.
Cannula Verification
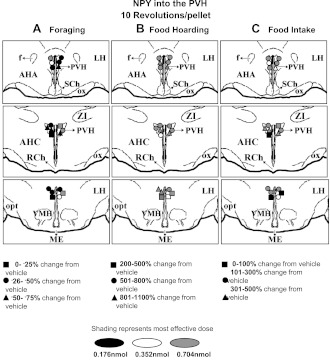
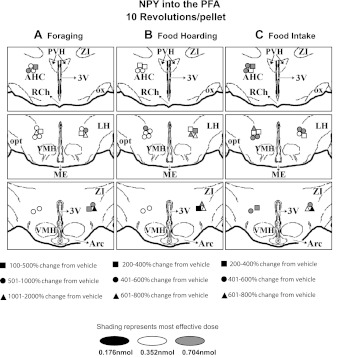
After the end of all injection cycles, methylene blue dye (100 nl) was injected to confirm placement of the cannula in the PVH or PFA. The animals were transcardially perfused with 0.9% saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were placed in fixative overnight and then transferred to a 30% sucrose solution for 48 h. Coronal brain sections were sliced (80 μm) using a microtome. Sections were then mounted on glass slides and stained with cresyl violet. Injection sites were determined on the basis of dye deposition and cannula tip placement and plotted onto Siberian hamster atlas plates (unpublished). The greatest percent change in foraging, hoarding, or food intake stimulated by NPY (Fig. 1) or blocked by the Y1-R antagonist (Fig. 2) was then matched to each injection site for the 10 Rev group, the only group that had to forage for their food and, therefore, the only group that displayed all three behaviors (i.e., foraging, hoarding, and intake). Animals whose cannula placement was not within the PVH or PFA were considered misses, and their data were not included in the analyses.
Fig. 1.
Mapping of neuropeptide Y (NPY) hypothalamic paraventricular nucleus (PVH) injections for the 10 revolutions per pellet (10 Rev) group, with corresponding greatest increases/decreases in foraging (A), food hoarding (B), and food intake (C). AHC, anterior hypothalamus, central; LH, lateral hypothalamus; AHA, anterior hypothalamic area; RCh, retrochiasmatic area; SCh, suprachiasmatic area; VMH, ventromedial hypothalamus; ME, median eminence; f, fornix; opt, optic tract; ox, optic chiasm.
Fig. 2.
Mapping of NPY PFA injections for the 10 Rev group, with corresponding greatest increases/decreases in foraging (A), food hoarding (B), and food intake (C). Arc, arcuate nucleus.
Statistical Analysis
Behavioral measures were analyzed using a three-way mixed-model ANOVA for repeated measures (foraging effort group × drug × time: 3 × 4 × 4 for experiment 1 and 3 × 2 × 4 for experiment 2) using Number Crunching Statistical Software version 2000 (Kaysville, UT). For analysis of cumulative differences in behavioral measures, data were analyzed using a two-way ANOVA with repeated measures for experiment 1 (foraging effort group × drug: 3 × 4) and a two-way ANOVA for experiment 2 (foraging effort group × drug: 3 × 2) using the same statistical software. For analysis of differences between behavioral measures within a group (10 Rev, FW, or BW) at a given time point for NPY PVH or PFA injections, percent change data were analyzed using a two-way repeated-measures ANOVA (behavior × dose: 3 × 3). For analysis of differences between NPY PVH and PFA injections for a given behavior (foraging, food hoarding, or food intake), percent change data were analyzed using a three-way mixed-model ANOVA (foraging effort group × injection site × dose: 3 × 2 × 3). Duncan's new multiple range tests were used for post hoc tests when appropriate. Differences among groups were considered statistically significant if P < 0.05. Exact probabilities and test values were omitted for simplicity and clarity of the presentation. Data are presented as percent change from vehicle injections for experiment 1 and percent change from nonfasted hamsters for experiment 2 to simplify the graphs. To allow for calculation of the percent change from control values when the control value was “0,” we added “1” to all values.
RESULTS
Experiment 1: Does Microinjection of NPY Into the PVH or PFA Increase Foraging, Food Hoarding, or Food Intake in the Siberian Hamster?
Wheel revolutions.
PVH.
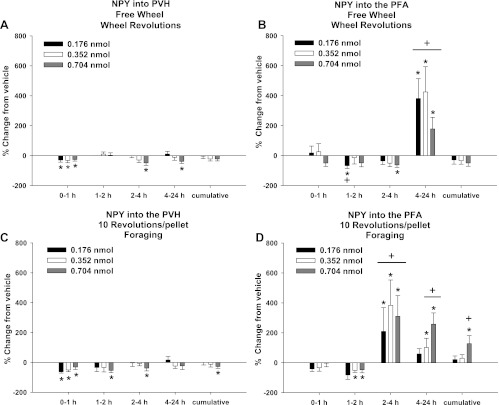
Wheel running in the FW group, a test for general locomotor activity effects of NPY, was significantly decreased by all three doses of NPY into the PVH at 0–1 h and additionally decreased by the highest dose at 2–4 and 4–24 h compared with vehicle injections (P < 0.05 for each; Fig. 3A).
Fig. 3.
Percent change from vehicle for each NPY dose in wheel revolutions for the free-wheel (FW) group and foraging for the 10 Rev group after injections of NPY into the PVH (A and C) and perifornical area (PFA; B and D). A: percent change in wheel revolutions after vehicle injections into the PVH for the FW group [absolute values: 294.8 ± 166.5 (0–1 h), 96.2 ± 49.3 (1–2 h), 473.3 ± 264.7 (2–4 h), 201.3 ± 105.7 (4–24 h), and 985.2 ± 544.8 (cumulative)]. B: percent change in wheel revolutions after vehicle injections into the PFA for the FW group [absolute values: 181.0 ± 65.4 (0–1 h), 134.1 ± 55.9 (1–2 h), 282.1 ± 99.7 (2–4 h), 730.4 ± 202.5 (0–24 h), and 1,326.9 ± 309.6 (cumulative)]. C: percent change in foraging after vehicle injections into the PVH for the 10 Rev group [absolute values: 480.4 ± 104.9 (0–1 h), 252.8 ± 79.4 (1–2 h), 631.8 ± 188.0 (2–4 h), 518.1 ± 125.9 (4–24 h), and 1,880.1 ± 382.3 (cumulative)]. D: percent change in foraging after vehicle injections into the PFA for the 10 Rev group [absolute values: 194.3 ± 75.4 (0–1 h), 119.33 ± 52.16 (1–2 h), 154.2 ± 109.5 (2–4 h), 173.7 ± 42.7 (0–24 h), and 640.4 ± 162.1 (cumulative)]. Values are means ± SE. *P < 0.05 vs. vehicle control. +P < 0.05 vs. PVH.
PFA.
Wheel running in the FW group was significantly decreased after PFA NPY injections at 1–2 h for the low dose of NPY and 2–4 h for the high dose followed by increased wheel running for all NPY doses at 4–24 h compared with vehicle (P < 0.05 for each; Fig. 3B).
PVH VS. PFA.
Wheel running was significantly increased across all NPY doses 4–24 h after PFA NPY injections compared with PVH NPY injections (P < 0.05; Fig. 3, A and B). Wheel running also was significantly decreased at 1–2 h after the low NPY dose was injected into the PFA compared with PVH NPY injections (P < 0.05 each; Fig. 3, A and B).
Foraging.
PVH.
Foraging in the 10 Rev group was significantly decreased after PVH NPY injections at 0–1 h for all NPY doses, with additional decreased foraging at the highest dose at 1–2 and 2–4 h and cumulatively across the 0- to 24-h postinjection period compared with vehicle injections (P < 0.05 each; Fig. 3C).
PFA.
Foraging in the 10 Rev group was significantly decreased after PFA NPY injections at 1–2 h for all doses of NPY but was significantly increased at 2–4 h for all doses and at 4–24 h for the intermediate and high NPY doses compared with vehicle injections (P < 0.05 each; Fig. 3D). Finally, foraging was significantly increased cumulatively across the 0- to 24-h postinjection period for the high NPY dose compared with vehicle injections (P < 0.05; Fig. 3D).
PVH VS. PFA.
Foraging was significantly increased for all NPY doses at 2–4 h after PFA injection, for the intermediate and high doses at 4–24 h, and for the high dose cumulatively across the 0- to 24-h postinjection period compared with PVH NPY injections (P < 0.05 each; Fig. 3, C and D).
Food hoarding.
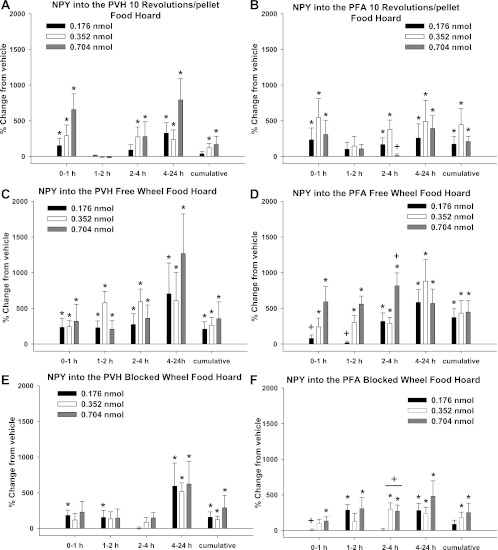
PVH.
In the 10 Rev group, food hoarding was significantly increased after PVH NPY injections for all three NPY doses at 0–1, 2–4, and 4–24 h and cumulatively across the 0- to 24-h postinjection period for the intermediate and high NPY doses compared with vehicle injections (P < 0.05 each; Fig. 4A). In the FW group, food hoarding was significantly increased after PVH NPY injections for all three doses across all times compared with vehicle injections (P < 0.05 each; Fig. 4C), whereas in the BW group, it was significantly increased for all three doses at 4–24 h and cumulatively (P < 0.05 each; Fig. 4E). The lowest NPY dose for the latter group significantly increased hoarding at 0–1 and 1–2 h compared with vehicle injections (P < 0.05 each; Fig. 4E).
Fig. 4.
Percent change from vehicle for each NPY dose in food hoarding for the 10 Rev group, FW group, and blocked-wheel (BW) group after injections of NPY into the PVH (A, C, and E) and PFA (B, D, and F). A: percent change in food hoarding after vehicle injections into the PVH for the 10 Rev group [absolute values: 1.7 ± 0.7 (0–1 h), 1.5 ± 0.5 (1–2 h), 3.8 ± 2.1 (2–4 h), 7.2 ± 3.7 (4–24 h), and 15.1 ± 3.9 (cumulative)]. B: percent change in food hoarding after vehicle injections into the PFA for the 10 Rev group [absolute values: 1 ± 0 (0–1 h), 1 ± 0 (1–2 h), 1.7 ± 0.7 (2–4 h), 1.1 ± 0.1 (4–24 h), and 4.8 ± 0.7 (cumulative)]. C: percent change in food hoarding after vehicle injections into the PVH for the FW group [absolute values: 1.7 ± 0.7 (0–1 h), 1.6 ± 0.5 (1–2 h), 1.9 ± 0.9 (2–4 h), 9.4 ± 3.5 (4–24 h), and 15.5 ± 3.5 (cumulative)]. D: percent change in food hoarding after vehicle injections into the PFA for the FW group [absolute values: 1 ± 0 (0–1 h), 1.1 ± 0.1 (2 h), 2.4 ± 1.0 (4 h), 6.7 ± 3.1 (24 h), and 11.3 ± 3.1 (cumulative)]. E: percent change in food hoarding after vehicle injections into the PVH for the BW group [absolute values: 1.5 ± 0.3 (0–1 h), 1 ± 0 (1–2 h), 2.2 ± 0.5 (2–4 h), 8.2 ± 3.5 (4–24 h), and 13.8 ± 3.8 (cumulative)]. F: percent change in food hoarding after vehicle injections into the PFA for the BW group [absolute values: 1.5 ± 0.5 (0–1 h), 1 ± 0 (1–2 h), 2.9 ± 0.9 (2–4 h), 9.9 ± 3.1 (4–24 h), and 15.2 ± 3.1 (cumulative)]. Values are means ± SE. *P < 0.05 vs. vehicle control. +P < 0.05 vs. PVH.
PFA.
Similar to the PVH NPY injections, food hoarding in the 10 Rev group was significantly increased after PFA NPY injections for all three NPY doses, but with slight differences in timing, such that increases occurred only at 0–1 and 4–24 h and cumulatively across the 0- to 24-h postinjection period (but not at 2–4 h), with additional significantly increased hoarding for the lowest and intermediate doses at 2–4 h, compared with vehicle injections (P < 0.05 each; Fig. 4B). In the FW group, food hoarding was significantly increased for all three NPY doses at 2–4 and 4–24 h and cumulatively, with the intermediate and high NPY doses triggering increased hoarding at 0–1 and 1–2 h compared with vehicle injections (P < 0.05 each; Fig. 4D). In the BW group, food hoarding was significantly increased for all three NPY doses at 4–24 h in addition to several other postinjection times compared with vehicle injections, but in a varied fashion (P < 0.05 each; Fig. 4F): for the lowest dose at 1–2 h, for the intermediate dose at 2–4 h, and for the high dose at 0–1, 1–2, and 2–4 h compared with vehicle injections (P <0.05 each; Fig. 4F). Food hoarding was significantly increased cumulatively across the 0- to 24-h postinjection period for the intermediate and high NPY doses compared with vehicle injections as well (P < 0.05 each; Fig. 4F).
PVH VS. PFA.
At the high dose, PFA NPY injection significantly increased food hoarding 2–4 h after injection by the FW group compared with PVH injections (P < 0.05; Fig. 4, C and D). At this same postinjection time, intermediate and high NPY doses injected into the PFA significantly increased food hoarding by the BW group compared with comparable PVH injections (P < 0.05 each; Fig. 4, E and F).
Food intake.
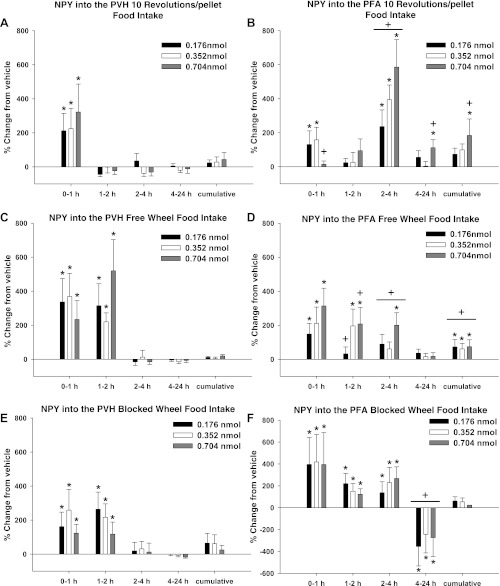
PVH.
In the 10 Rev group, food intake was significantly increased after PVH NPY injections for all three NPY doses at 0–1 h compared with vehicle (P < 0.05 each; Fig. 5A). In the FW and BW groups, food intake was significantly increased for all three NPY doses at 0–1 and 1–2 h compared with vehicle injections (P < 0.05 each; Fig. 5, C and E).
Fig. 5.
Percent change from vehicle for each NPY dose in food intake for 10 Rev, FW, and BW groups after injections of NPY into the PVH (A, C, and E) and PFA (B, D, and F). A: percent change in food intake after vehicle injections into the PVH for the 10 Rev group [absolute values: 7.3 ± 2.9 (0–1 h), 6.2 ± 3.4 (1–2 h), 14.3 ± 6.1 (2–4 h), 34.4 ± 5.7 (4–24 h), and 63.2 ± 16.5 (cumulative)]. B: percent change in food intake after vehicle injections into the PFA for the 10 Rev group [absolute values: 2.3 ± 0.9 (0–1 h), 1.8 ± 0.8 (1–2 h), 1.6 ± 0.6 (2–4 h), 14.89 ± 3.3 (4–24 h), and 640.4 ± 162.1 (cumulative)]. C: percent change in food intake after vehicle injections into the PVH for the FW group [absolute values: 8.6 ± 0.8 (0–1 h), 1.3 ± 0.2 (1–2 h), 5.8 ± 0.9 (2–4 h), 36.2 ± 1.9 (4–24 h), and 47.9 ± 2.0 (cumulative)]. D: percent change in food intake after vehicle injections into the PFA for the FW group [absolute values: 1.4 ± 0.3 (0–1 h), 2 ± 0.5 (1–2 h), 3.8 ± 1.5 (2–4 h), 22.9 ± 3.6 (4–24 h), and 30 ± 3.03 (cumulative)]. E: percent change in food intake after vehicle injections into the PVH for the BW group [absolute values: 3.7 ± 0.7 (0–1 h), 1.8 ± 0.5 (1–2 h), 6.73 ± 1.3 (2–4 h), 33.18 ± 3.6 (4–24 h), and 46.3 ± 4.6 (cumulative)]. F: percent change in food intake after vehicle injections into the PFA for the BW group [absolute values are as follows: 1.5 ± 0.5 (0–1 h), 1 ± 0 (2–4 h), 4.5 ± 1.3 (2–4 h), 28.6 ± 3.9 (4–24 h), and 35.7 ± 4.1 (cumulative)]. Values are means ± SE. *P < 0.05 vs. vehicle control. +P < 0.05 vs. PVH.
PFA.
Somewhat similarly, food intake in the 10 Rev group was significantly increased after PFA NPY injections at all three doses at 2–4 h, at 0–1 h for the low and intermediate doses, and at 4–24 h and cumulatively for the high dose compared with vehicle injections (P < 0.05 each; Fig. 5B). In the FW group, food intake was significantly increased with all three NPY doses at 0–1, at 1–2 h for the intermediate dose, and at 1–2 and 2–4 h for the high dose compared with vehicle injections (P < 0.05 each; Fig. 5D). In the 10 Rev group, cumulative food intake from 0 to 24 h after injection was significantly increased for all three NPY doses injected into the PFA compared with vehicle injections (P < 0.05 each; Fig. 5D). In the BW group, food intake was significantly increased for all three NPY doses at 0–1, 1–2, and 2–4 h after injection but was significantly decreased at 4–24 h compared with vehicle injections (P < 0.05 each; Fig. 5F).
PVH VS. PFA.
Food intake was significantly increased after NPY PFA injections at later time points in the 10 Rev and FW groups compared with PVH NPY injections in the same groups (P < 0.05 each; Fig. 5, A–D). Specifically, in the 10 Rev group, all three NPY doses injected into the PFA significantly increased food intake at 2–4 h after injection, and the highest dose increased food intake at 4–24 h and cumulatively compared with PVH NPY injections (P < 0.05 each; Fig. 5, A and B). Somewhat similarly, in the FW group, all three NPY doses injected into the PFA significantly increased food intake 2–4 h and cumulatively after injection compared with PVH NPY injections (P < 0.05 each; Fig. 5, C and D). By contrast, food intake was significantly decreased in the BW group by all three NPY doses injected into the PFA at 4–24 h after injection compared with PVH NPY injections (P < 0.05 each; Fig. 5, E and F).
Comparison between behaviors for PVH NPY and PFA NPY injections.
NPY injected into the PVH or PFA increased food hoarding to a greater degree (∼100–1,200%) than food intake (75–600%). Specifically, PVH NPY injections increased food hoarding ∼200–1,200% and PFA NPY injections increased food hoarding ∼100–800% across time. By contrast, PVH NPY injections increased food intake ∼200–500% only at the earliest time point, whereas PFA NPY injections increased food intake ∼75–600% for up to 4 h after injection. Moreover, food hoarding was significantly increased compared with food intake after PVH NPY injections at 2–4 and 4–24 h and cumulatively (P < 0.05 each; Figs. 4 and 5). For PFA NPY injections, food hoarding was significantly greater than food intake at 2–4 and 4–24 h and cumulatively (P < 0.05 each; Figs. 4 and 5).
There was a differential effect of NPY injected into the PVH vs. the PFA on wheel running and foraging. Although PVH NPY and PFA NPY injections decreased both behaviors for up to 2 h after injection, wheel running and foraging remained decreased after NPY injections into the PVH, whereas PFA NPY injections resulted in later (2–24 h after injection) increased foraging and wheel running (P < 0.05 each; Fig. 3). Specifically, PFA NPY injections increased wheel running at 4–24 h and foraging at 2–4 and 4–24 h and cumulatively compared with PVH NPY injections (P < 0.05 each; Fig. 3).
Experiment 2: Does Antagonism of NPY Y1 Receptors in PVH or PFA Block Post-Food-Deprivation Increases in Food Hoarding in Siberian Hamsters?
Wheel revolutions.
PVH.
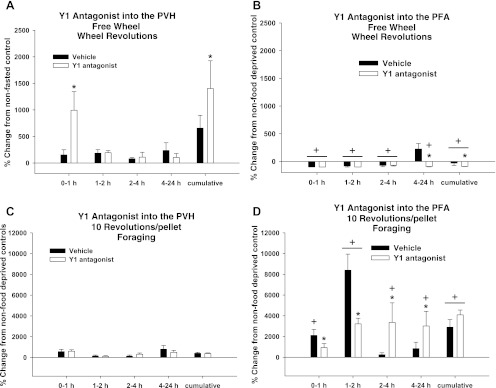
Food deprivation triggered the expected significantly increased wheel revolutions (19) across all time points compared with ad libitum-fed hamsters (P < 0.05 each; Fig. 6A). The Y1-R antagonist significantly exaggerated this food deprivation-induced increase in wheel running at 0–1 h and cumulatively 0–24 h after injection compared with their vehicle-injected counterparts (P < 0.05 each; Fig. 6A).
Fig. 6.
Percent change from non-food-deprived hamsters in wheel revolutions for the FW group and foraging for the 10 Rev group after injections of vehicle or Y1 antagonist into the PVH (A and C) and PFA (B and D). A: percent change in wheel revolutions of non-food-deprived hamsters of the FW group injected with vehicle [absolute values: 64 ± 21.7 (0–1 h), 0 ± 0 (1–2 h), 140 ± 35.2 (2–4 h), 237 ± 0.7 (4–24 h), and 441 ± 5 (cumulative)] and Y1 antagonist [absolute values: 74.8 ± 14.9 (0–1 h), 4 ± 3.5 (1–2 h), 162 ± 62.4 (2–4 h), 104.2 ± 74.4 (4–24 h), and 345 ± 144.8 (cumulative)] into the PVH. B: percent change in wheel revolutions of non-food-deprived hamsters of the FW group injected with vehicle [absolute values: 276.5 ± 21.5 (0–1 h), 105 ± 12 (1–2 h), 359 ± 32.7 (2–4 h), 77 ± 6.2 (4–24 h), and 817.5 ± 129.5 (cumulative)] and Y1 antagonist [absolute values: 267.7 ± 59.2 (0–1 h), 152.3 ± 45.7 (1–2 h), 291.7 ± 29.2 (2–4 h), 261.3 ± 60.8 (4–24 h), and 973 ± 96.4 (cumulative)] into the PFA. C: percent change in foraging of non-food-deprived hamsters of the 10 Rev group injected with vehicle [absolute values: 63 ± 12.6 (0–1 h), 1 ± 0 (1–2 h), 140 ± 72.9 (2–4 h), 153.3 ± 58.0 (4–24 h), and 357.0 ± 77.8 (cumulative)] and Y1 antagonist [absolute values: 50 ± 9.2 (0–1 h), 1 ± 0 (1–2 h), 70 ± 13.8 (2–4 h), 172 ± 59.6 (4–24 h), and 293.9 ± 59.1 (cumulative)] into the PVH. D: percent change in foraging of non-food-deprived hamsters of the 10 Rev group injected with vehicle [absolute values: 10.5 ± 9.5 (0–1 h), 1 ± 0 (1–2 h), 27 ± 13.5 (2–4 h), 129.5 ± 64.5 (4–24 h), and 267 ± 8.7 (cumulative)] and Y1 antagonist [absolute values: 30 ± 8.5 (0–1 h), 1 ± 0 (1–2 h), 15 ± 1 (2–4 h), 104.3 ± 23.9 (4–24 h) and 150.7 ± 17.3 (cumulative)] into the PFA. Values are means ± SE. *P < 0.05 vs. non-food-deprived hamsters. +P < 0.05 vs. PVH.
PFA.
Food deprivation also significantly increased wheel revolutions, but only at 4–24 h after injection compared with ad libitum-fed hamsters (P < 0.05; Fig. 6B). The Y1-R antagonist significantly decreased wheel running at 4–24 h and cumulatively compared with vehicle-injected hamsters (P < 0.05 each; Fig. 6B).
PVH VS. PFA.
The Y1-R antagonist injected into the PFA significantly decreased wheel running compared with PVH injections at 4–24 h and cumulatively (P < 0.05 each; Fig. 6, A and B).
Foraging.
PVH.
Food deprivation triggered the expected significantly increased foraging (19) at 0–1 and 4–24 h and cumulatively across the 0- to 24-h postinjection period compared with ad libitum-fed hamsters (P < 0.05 each; Fig. 6C). The Y1-R antagonist had no effect on this post-food-deprivation increase in foraging (Fig. 6C).
PFA.
Similarly, food deprivation significantly increased foraging at 0–1, 1–2, and 4–24 h, as well cumulatively across the 0- to 24-h postinjection period, compared with ad libitum-fed hamsters (P < 0.05 each; Fig. 6D). The Y1-R antagonist significantly inhibited this post-food-deprivation increase in foraging at 0–1 and 1–2 h compared with vehicle-injected hamsters (P < 0.05 each; Fig. 6D). Thereafter, the Y1-R antagonist significantly exaggerated the post-food-deprivation increase in foraging at 2–4 and 4–24 h after injection compared with vehicle-injected animals (P < 0.05 each; Fig. 6D).
PVH VS. PFA.
Foraging was significantly increased after Y1-R antagonist PFA injections at 1–2, 2–4, and 4–24 h and cumulatively 0–24 h after injection compared with PVH injections (P < 0.05 each; Fig. 6, C and D).
Food hoarding.
PVH.
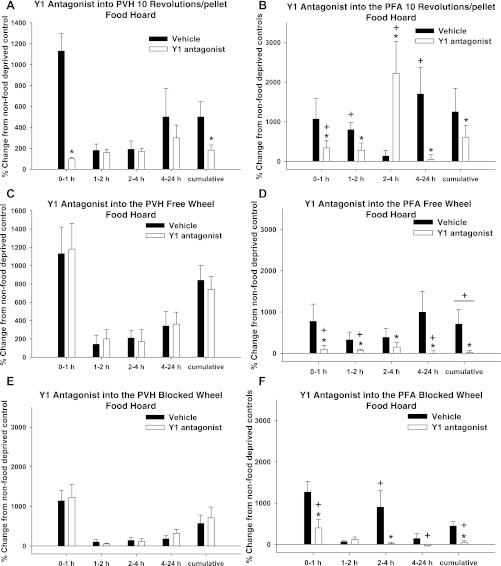
The normal food-deprivation-induced significant increase in food hoarding (19) occurred across all time points for the 10 Rev group and at various times for the FW and BW groups (P < 0.05 each; Fig. 7). The Y1-R antagonist significantly inhibited the post-food-deprivation increase in food hoarding at 0–1 h and cumulatively 0–24 h after injection compared with their vehicle-injected counterparts in the 10 Rev group only (P < 0.05 each; Fig. 7A). There was no effect of the Y1 antagonist on food hoarding in the FW or BW group (Fig. 7, C and E).
Fig. 7.
Percent change from non-food-deprived hamsters in food hoarding for 10 Rev, FW, and BW groups after injection of vehicle or Y1 antagonist into the PVH (A, C, and E) and PFA (B, D, and F). A: percent change in food hoarding of non-food-deprived hamsters of the 10 Rev group injected with vehicle [absolute values: 4.0 ± 1.4 (0–1 h), 0 ± 0 (2 h), 2 ± 1.5 (2–4 h), 0 ± 0 (4–24 h), and 6 ± 3.1 (cumulative)] and Y1 antagonist [absolute values: 1.4 ± 0.4 (0–1 h), 0 ± 0 (1–2 h), 0 ± 0 (2–4 h), 2 ± 0.5 (4–24 h), and 3.4 ± 1.4 (cumulative)] into the PVH. B: percent change in food hoarding of non-food-deprived hamsters of the 10 Rev group injected with vehicle [absolute values: 0 ± 0 (0–1 h), 0 ± 0 (1–2 h), 3 ± 1.3 (2–4 h), 0 ± 0 (4–24 h), and 3 ± 1.3 (cumulative)] and Y1 antagonist [absolute values: 0 ± 0 (0–1 h), 0 ± 0 (1–2 h), 0 ± 0 (2–4 h), 0 ± 0 (4–24 h), and 0 ± 0 (cumulative)] into the PFA. C: percent change in food hoarding of non-food-deprived hamsters of the FW group injected with vehicle [absolute values: 2 ± 1.3 (0–1 h), 0 ± 0 (1–2 h), 3 ± 2.3 (2–4 h), 2 ± 1.7 (4–24 h), and 7 ± 3.9 (cumulative)] and Y1 antagonist [absolute values: 1 ± 0.3 (0–1 h), 0 ± 0 (1–2 h), 4 ± 2.4 (2–4 h), 0 ± 0 (4–24 h), and 5 ± 3.2 (cumulative)] into the PVH. D: percent change in food hoarding of non-food-deprived hamsters of the FW group injected with vehicle [absolute values: 1 ± 0 (0–1 h), 1 ± 0.3 (1–2 h), 4 ± 3.2 (2–4 h), 5.5 ± 1.5 (4–24 h), and 11.5 ± 1.5 (cumulative)] and Y1 antagonist [absolute values: 1.7 ± 0.7 (0–1 h), 1 ± 0.3 (1–2 h), 1 ± 0 (2–4 h), 14.3 ± 3.3 (4–24 h), and 18 ± 3.1 (cumulative)] into the PFA. E: percent change in food hoarding of non-food-deprived hamsters of the BW group injected with vehicle [absolute values: 0 ± 0 (0–1 h), 1 ± 0.7 (1–2 h), 3 ± 2.3 (2–4 h), 1 ± 0.7 (4–24 h), and 5 ± 3.2 (cumulative)] and Y1 antagonist [absolute values: 0 ± 0.7 (0–1 h), 2 ± 1.3 (1–2 h), 2 ± 0.7 (2–4 h), 1 ± 0 (4–24 h), and 5 ± 1.7 (cumulative)] into the PVH. F: percent change in food hoarding of non-food-deprived hamsters of the BW group injected with vehicle [absolute values: 1 ± 0 (0–1 h), 1 ± 0.3 (1–2 h), 1 ± 0.7 (2–4 h), 3 ± 2.3 (4–24 h), and 6 ± 3.9 (cumulative)] and Y1 antagonist [absolute values: 1 ± 0.7 (0–1 h), 1 ± 0.7 (1–2 h), 3 ± 2.3 (2–4 h), 4 ± 1.5 (4–24 h), and 9 ± 2.9 (cumulative)] into the PFA. Values are means ± SE. *P < 0.05 vs. non-food-deprived hamsters. +P < 0.05 vs. PVH.
PFA.
Similarly, food hoarding was significantly increased in food-deprived hamsters at 0–1, 1–2, and 4–24 h for the 10 Rev group, at all time points for the FW group, and at 0–1 and 2–4 h for the BW group (P < 0.05 each; Fig. 7). The Y1-R antagonist significantly inhibited the post-food-deprivation increase in food hoarding in all groups. Specifically, in the 10 Rev group, the Y1-R antagonist significantly inhibited hoarding at 0–1, 1–2, and 4–24 h compared with their vehicle-injected counterparts (P < 0.05 each; Fig. 7B), but exaggerated food hoarding at 2–4 h. In the FW group, food-deprivation-induced hoarding was inhibited across all time points by PFA Y1-R antagonist injection (P < 0.05 each; Fig. 7D). In the BW group, hoarding was inhibited 0–1 and 2–4 h after Y1-R antagonist injection, as well as cumulatively (P < 0.05 each; Fig. 7F).
PVH VS. PFA.
Food hoarding was significantly decreased after Y1-R antagonist injections into the PFA compared with PVH injections at 0–1 h in the 10 Rev group, 0–1, 1–2, and 4–24 h and cumulatively in the FW group, and 0–1 and 4–24 h and cumulatively in the BW group (P < 0.05 each; Fig. 7). In the 10 Rev group, food hoarding was significantly increased 2–4 h after Y1-R antagonist PFA injection compared with comparable injections into the PVH (P < 0.05 each; Fig. 7).
Food intake.
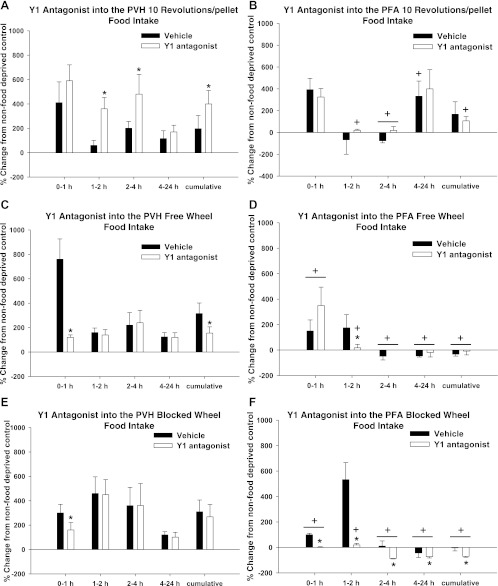
PVH.
Food deprivation triggered significantly increased food intake by all groups at various times. Specifically, food intake increased in the food-deprived 10 Rev group at 0–1, 2–4, and 4–24 h, as well as cumulatively, compared with their ad libitum-fed counterparts (P < 0.05 each; Fig. 8A). Food intake significantly increased in food-deprived FW and BW animals at all times compared with their ad libitum-fed counterparts (P < 0.05 each; Fig. 8, C and E). In the 10 Rev group, the Y1-R antagonist significantly exaggerated this increased food intake at 1–2 and 2–4 h and cumulatively (P < 0.05 each; Fig. 8A). By contrast, food intake was significantly suppressed by the Y1-R antagonist in the FW and BW groups at 0–1 h after injection and significantly suppressed cumulatively across the 0- to 24-h postinjection period in the FW group compared with vehicle-injected animals (P < 0.05 each; Fig. 8, C and E).
Fig. 8.
Percent change from non-food-deprived hamsters in food intake for 10 Rev, FW, and BW groups after injection of vehicle or Y1 antagonist into the PVH (A, C, and E) and PFA (B, D, and F). A: percent change in food intake of non-food-deprived hamsters of the 10 Rev group injected with vehicle [absolute values: 2 ± 1.7 (0–1 h), 0 ± 0 (1–2 h), 9 ± 3.2 (2–4 h), 15 ± 6.2 (4–24 h), and 26 ± 6.7 (cumulative)] and Y1 antagonist [absolute values: 4 ± 2.3 (0–1 h), 0 ± 0 (1–2 h), 6 ± 1.7 (2–4 h), 15 ± 4.9 (4–24 h), and 25 ± 3.7 (cumulative)] into the PVH. B: percent change in food intake of non-food-deprived hamsters of the 10 Rev group injected with vehicle [absolute values: 1 ± 1 (0–1 h), 1 ± 0 (1–2 h), 9.5 ± 1.5 (2–4 h), 12.5 ± 6.5 (4–24 h), and 23 ± 6 (cumulative)] and Y1 antagonist [absolute values: 1 ± 0.3 (0–1 h), 0 ± 0 (1–2 h), 1 ± 0 (2–4 h), 15 ± 3.2 (4–24 h), and 17.3 ± 2.8 (cumulative)] into the PFA. C: percent change in food intake of non-food-deprived hamsters of the FW group injected with vehicle [absolute values: 3 ± 1.3 (0–1 h), 0 ± 0 (1–2 h), 8 ± 2.3 (2–4 h), 18 ± 3.7 (4–24 h), and 29 ± 3.9 (cumulative)] and Y1 antagonist [absolute values: 2 ± 0.3 (0–1 h), 0 ± 0 (1–2 h), 12 ± 5.1 (2–4 h), 10 ± 3.7 (4–24 h), and 24 ± 2.3 (cumulative)] into the PVH. D: percent change in food intake of non-food-deprived hamsters of the FW group injected with vehicle [absolute values: 1 ± 0 (0–1 h), 1 ± 0.7 (1–2 h), 4.5 ± 0.5 (2–4 h), 32.0 ± 4.0 (4–24 h), and 38.5 ± 4.5 (cumulative)] and Y1 antagonist [absolute values: 1 ± 0.7 (0–1 h), 1 ± 0.7 (1–2 h), 2.3 ± 0.9 (2–4 h), 12.33 ± 6.7 (4–24 h), and 16.7 ± 7.1 (cumulative)] into the PFA. E: percent change in food intake of non-food-deprived hamsters of the BW group injected with vehicle [absolute values: 3 ± 0.7 (0–1 h), 1.0 ± 0.3 (1–2 h), 3.6 ± 1.5 (2–4 h), 18.0 ± 2.6 (4–24 h), and 25.6 ± 3.2 (cumulative)] and Y1 antagonist [absolute values: 0.2 ± 0.2 (0–1 h), 1.0 ± 1.0 (1–2 h), 3.0 ± 1.8 (2–4 h), 17.9 ± 3.9 (4–24 h) and 22.0 ± 2.6 (cumulative)] into the PVH. F: percent change in food intake of non-food-deprived hamsters of the BW group injected with vehicle [absolute values: 1 ± 0 (0–1 h), 1 ± 0.3 (1–2 h), 3 ± 2.2 (2–4 h), 17.1 ± 1.3 (4–24 h), and 22 ± 3.0 (cumulative)] and Y1 antagonist [absolute values: 1 ± 0.3 (0–1 h), 1 ± 0.3 (1–2 h), 6 ± 3.2 (2–4 h), 20 ± 6.4 (4–24 h), and 28 ± 8.5 (cumulative)] into the PFA. Values are means ± SE. *P < 0.05 vs. non-food-deprived hamsters. +P < 0.05 vs. PVH.
PFA.
Food deprivation significantly increased food intake at early time points after injection for all groups and also significantly increased food intake by the 10 Rev group later, at 4–24 h (P < 0.05 each; Fig. 8). The Y1-R antagonist significantly inhibited these increased food intakes at 1–2 h after injection in the FW group and at 0–1 and 1–2 h in the BW group (P < 0.05 each; Fig. 8, D and F), whereas food intake also was decreased in the BW group at 2–4 and 4–24 h and cumulatively after Y1-R antagonist injection compared with vehicle-injected controls (P < 0.05 each; Fig. 8F).
PVH VS. PFA.
Food intake was significantly decreased after Y1-R antagonist injections into the PFA compared with PVH injections. Specifically, food intake was decreased at 1–2 and 2–4 h and cumulatively in the 10 Rev group; at 1–2, 2–4, and 4–24 h and cumulatively in the FW group; and across all time points in the BW group (P < 0.05 each; Fig. 8). By contrast, there was a significant increase in food intake 0–1 h after Y1-R antagonist injections into the PFA in the FW group compared with injections into the PVH in the same group (P < 0.05 each; Fig. 8).
Comparison between behaviors for NPY Y-1 antagonist PVH and PFA injections.
The Y1-R antagonist injected into the PVH significantly inhibited food hoarding (∼1,000%) and increased food intake (∼350%) in the 10 Rev group after food deprivation; in the FW and BW groups, however, the Y1-R antagonist had no effect on fasting-induced food hoarding but inhibited food intake in the FW group (∼700%). Specifically, in the 10 Rev group, food hoarding was significantly decreased compared with food intake at 0–1, 1–2, and 2–4 h and cumulatively 0–24 h after Y1-R antagonist injection (P < 0.05 each; Figs. 7A and 8A). The Y1-R antagonist-induced decrease in food intake at 0–1 h and cumulatively in the FW group and at 0–1 h in the BW group was significant compared with food hoarding at the same time points in each group (P < 0.05 each; Fig. 7 and Fig. 8, C and E).
PFA.
In the 10 Rev group, foraging was significantly increased compared with food hoarding or food intake, whether the animals were injected with vehicle or the Y1-R antagonist (P < 0.05 each; Figs. 6D, 7B, and 8B). Moreover, food hoarding in the 10 Rev group was significantly increased compared with food intake at 0–1, 1–2, and 2–4 h and cumulatively after Y1-R antagonist injections (P < 0.05 each; Figs. 7B and 8B). In the FW group, food hoarding was significantly increased compared with wheel revolutions at all time points and food intake at 1–2, 2–4, and 4–24 h and cumulatively (P < 0.05 each; Figs. 6B, 7D, and 8D). The Y1-R-induced changes in food hoarding were significantly increased compared with food intake at 0–1 and 2–4 h and cumulatively for the BW group (P < 0.05 each; Figs. 7F and 8F).
DISCUSSION
NPY is a powerful orexigenic neuropeptide that stimulates appetitive and consummatory ingestive behaviors under a variety of conditions (for review see Ref. 46). The results of the present study support the view that the PVH and PFA are two nuclei that mediate NPY-related effects on the consummatory ingestive behavior of food intake (48, 50) and extend previous findings to show that NPY microinjections into the PVH and PFA also can alter the appetitive ingestive behaviors of foraging and food hoarding. Specifically, NPY injected into the PVH or PFA increased food hoarding to a greater extent than foraging or food intake. Moreover, the NPY Y1-R appears to play a role in controlling food hoarding through the PVH and PFA on the basis of the ability of a Y1-R antagonist to inhibit post-food-deprivation increases in food hoarding. Finally, it appears that the PFA Y1-R played an additional role in controlling foraging, because PFA Y1-R antagonism inhibited post-food-deprivation increases in foraging. In general, the results from the present study support the view that NPY does not just increase food intake per se, but it also may be important in increasing appetitive ingestive behaviors. Which ingestive behavior, to what extent, and when it is displayed may depend on which NPY receptors and brain sites are activated, as well as the physiological state of the animal and changes in its environment.
NPY injected into the PVH or PFA stimulated food hoarding across all time points and to a greater extent than food intake. This result supports the notion that NPY may play a greater role in the appetitive than the consummatory phase of ingestive behavior (1, 19), especially in this species. Despite the many studies demonstrating impressive increases in food intake after ventricular or site-specific injections of NPY (6, 11, 12, 34, 47, 48), the effects of NPY on appetitive vs. consummatory ingestive behaviors were not separable in these studies. The intraoral feeding model, however, only tests consummatory ingestive behavior whereby food is infused into the mouth via a cheek fistula, and rejection responses indicate satiation. With use of this model, central NPY stimulates (5), fails to change (43), or inhibits (1) food intake but does not trigger the marked and robust increase in food intake seen in freely feeding animals (6, 11, 12, 34, 47, 48). It is likely that the increase in food intake after central injections of NPY in other studies occurs consistently only when appetitive ingestive behaviors are displayed to gain access to the food (even if appetitive behaviors are not directly measured). For example, central NPY injections stimulate consumption of pellets from a feeder (26) or sucrose from a bottle (1, 5, 43), both of which require some degree of appetitive ingestive behavior. In the present study using a simulated foraging-hoarding housing system, where we measured changes in appetitive and consummatory ingestive responses, NPY injected into the PVH or PFA significantly increased food hoarding (appetitive) to a greater degree (∼100–1,200%) than food intake (consummatory ingestive behavior; 75–600%), suggesting that the effects of NPY on these two categories of ingestive behaviors are at least partially separable.
NPY injections into the PVH or PFA also increased food intake, albeit not to the same extent as food hoarding. The percent increase in food intake was greater across all groups after PFA NPY injections (175–600% across time for the 10 Rev group, 75–375% across time for the FW group, and 100–400% for the BW group) than PVH NPY injections (200–300% only at 1 h for the 10 Rev group, 200–500% only up to 2 h after injection for the FW group, and 100–250% up to 2 h after injection for the BW group), which supports the data showing that NPY is a more potent stimulator of food intake in the PFA than PVH (50). Although it has been suggested that the effect of NPY on food intake is greater if the antecedent behavior to eating (an appetitive response) is required to gain access to the food (43), we found that to be true for NPY PFA, but not NPY PVH, injections in the present experiment. Specifically, the addition of the foraging requirement (10 wheel revolutions per pellet) resulted in greater increases in NPY-induced food intake than nonforaging conditions after PFA, but not PVH, injections. It is possible that this site-specific difference in the ability of a substantial appetitive response (e.g., foraging) to increase food intake is a function of the number of pellets earned. That is, because foraging was inhibited consistently (∼25–50% across time) by PVH NPY injections but only transiently (decreased ∼75% up to 4 h after injection with later increases of 100–400% up to 24 h after injection) by PFA NPY injections, animals receiving the former injection earned fewer pellets and, thus, there was less food to be eaten or hoarded. These food intake data, as well as those for hoarding and foraging in the present experiment, suggest that there are site-specific differences in the effects of NPY on appetitive and consummatory ingestive behaviors.
The role of the NPY Y1-R in mediating changes in appetitive and consummatory ingestive behaviors appears to be different between the PVH and PFA and depends on whether the animals have to earn their food or food is freely given. For example, the NPY Y1-R antagonist injected into the PVH significantly inhibited food hoarding (∼1,000%) and increased food intake (∼350%) in the 10 Rev group after food deprivation, whereas in the FW and BW groups, the Y1-R antagonist had no effect on food-deprivation-induced food hoarding but inhibited food intake in the FW group (∼700%). This inhibition of food intake in animals that were not required to earn their food is consistent with previous studies in laboratory rats where prior injection or coinjection of a Y1-R antagonist into the PVH blocked the ability of subsequent NPY PVH injections to increase food intake (54, 57). By contrast with the effects of Y1-R antagonist injections into the PVH, Y1-R antagonist injections into the PFA significantly inhibited all three food-deprivation-triggered ingestive behaviors in all groups except food intake in the 10 Rev group. Thus the effects of Y1-R antagonism are site specific, and, moreover, within a site, the effect of Y1-R antagonism can be modulated by the effort required to obtain food. By contrast to the present data, laboratory rats required to earn food by pressing a lever show a reduction in food earned/eaten in response to a third ventricular NPY injection compared with animals that had free access to food (26). This effect may be due to the high level of lever pressing required for the food pellet, and a similar result might have been seen in the present experiment if we increased the foraging effort above the 10 revolutions per pellet. The underlying mechanisms for the site-specific and foraging effort-specific effect of the Y1-R antagonist are unknown, but when laboratory mice are tested during various physiological states, they exhibit site-specific differences in Y1-R expression [e.g., food deprivation (58), pregnancy (36), leptin (58), diet-induced obesity (22)]. This suggests that Y1-R expression between the PVH and PFA could vary under altered metabolic states such as foraging and food deprivation and is site specific. Another possible explanation for this site-specific effect of the Y1-R antagonist may be the different neuronal phenotypes and their efferent projections for these two brain sites. In the PVH, one of the neuronal phenotypes of neurons expressing the Y1-R are thyrotropin-releasing hormone cells (8), and Y1-positive nerve terminals are found in close proximity to corticotrophin-releasing hormone neurons (31). Although the phenotype of the Y1-R-positive neurons in the PFA, to our knowledge, has not been directly studied, the PFA contains orexin and melanin-concentrating hormone neurons, which have been implicated in mediating changes in ingestive behaviors and could be potential targets for NPY and express Y1-Rs (27). Therefore, the Y1-R expression level and neuronal phenotype of Y1-R-containing neurons, as well as the efferent projections of these cells in both nuclei, could potentially affect the action of NPY on appetitive and consummatory ingestive behaviors.
There was a differential effect of NPY on wheel running and foraging when it was injected into the PVH vs. the PFA. Although PVH NPY and PFA NPY injections decreased wheel running and foraging for up to 2 h after injection, both behaviors remained decreased after NPY injections into the PVH, whereas foraging and wheel running significantly increased later (2–24 h after injection) after NPY injections into the PFA. Decreased wheel running by the FW group can signal nonspecific suppression of locomotor activity and/or malaise; however, NPY-injected hamsters in both brain site groups displayed increases in other behaviors, especially a marked increase in food hoarding and, to a lesser extent, food intake often exceeding 200%, compared with their vehicle-injected counterparts. It is difficult to envision a nonspecific locomotor/malaise effect that would not also inhibit, or at the very least maintain, hoarding and food intake at control levels, yet in this case, both behaviors were markedly increased during the same time period of the foraging/wheel running suppression. One interpretation of the early inhibition of foraging/wheel running by NPY in both brain sites is behavioral competition, where the animals are not foraging/wheel running because they are hoarding and eating. This does not, however, account for the continued inhibition of foraging in the NPY PVH hamsters. Nevertheless, these NPY-induced decreases (PVH and PFA injections) or later increases (PFA injections only) in wheel running or foraging contrast with our previous findings and those of others in studies of NPY injected into the third ventricle. Using the same foraging-hoarding apparatus and species, we previously found that NPY injections into the third ventricle increase wheel running and foraging at these early time points with no changes 2 h after injection (19). Increases in wheel running have been reported after NPY injections into the third ventricle in laboratory rats with free access to food (35). One obvious difference between the present study and those experiments is that we gave parenchymal microinjections of NPY into the PVH or PFA, whereas periventricular hypothalamic, midbrain, and brain stem sites were potentially stimulated by the NPY injections into the third ventricle (19, 35). Although the PVH NPY-induced decreases in wheel running and foraging are in contrast to the increases seen after injections into the third ventricle, these data support the notion that PVH NPY increases anabolic responses and decreases catabolic responses seen by others (6, 29). That is, PVH NPY injections increase feeding and white adipose tissue (WAT) lipoprotein lipase mRNA (an enzyme that stimulates lipid uptake into WAT), which are anabolic responses, and simultaneously, decrease brown adipose tissue thermogenesis, as indicated by expression of uncoupling protein-1 gene, a catabolic response (6, 29). Thus, PVH NPY increased energy input (feeding) and storage into WAT and, simultaneously, decreased energy output, a combined effect that would direct the animal into a positive energy balance. Somewhat analogously, PVH NPY microinjections here increased food intake and hoarding (anabolic responses) and decreased foraging/wheel running (a catabolic response). Others have also documented differential effects of NPY injections into the PVH vs. the PFA. For example, NPY injections into the PVH increase the respiratory quotient, indicative of lipid storage and carbohydrate fuel oxidation, increase food intake (15, 33), and decrease body temperature (14), whereas PFA NPY injections increase feeding without affecting the respiratory quotient (15) or body temperature (14). Collectively, it is not surprising that there appears to be a differential effect of NPY on foraging/wheel running, whether it is injected into the PVH or PFA. In addition, PVH NPY injections promote anabolic responses (food intake and hoarding) and decrease catabolic responses (foraging/wheel running), whereas PFA NPY injections only increase anabolic responses (food intake and hoarding).
The increase in wheel running and foraging after PFA, but not PVH, NPY injections could be meditated by orexin neurons, which are largely restricted to the PFA and lateral hypothalamus (24). Central injections of orexin-A and orexin-B mimic central NPY responses, in that orexins stimulate locomotor activity (25, 30), sympathetic nervous system activity (56), and food intake (for review see Ref. 55). Finally, NPY receptors are found in the PFA (23; M. J. Dailey and T. J. Bartness, unpublished observations) where orexins are expressed (27), suggesting a possible link between the NPY and orexin pathways. Although NPY and orexins appear to interact in laboratory rats and mice, such a relation does not appear to occur in Siberian hamsters. That is, at least in terms of the effects of third ventricular injections of orexin-A or orexin-B or orexin receptor antagonism, there is no clear effect on wheel running or foraging or on hoarding or food intake (E. Keen-Rhinehart and T. J. Bartness, unpublished observations).
Together, the present data and findings of others discussed above suggest the need for a more integrative view of the role of NPY in controlling ingestive behaviors. It appears that we can no longer think of NPY as only a potent orexigenic agent that acts to increase food intake in all circumstances. The results from the present study support a broader view that NPY plays a role in affecting appetitive ingestive behaviors in addition to food intake and that increases in appetitive ingestive behaviors do not necessitate increases in food intake (consummatory ingestive behavior), at least in Siberian hamsters. These results also further dissociate the effects of NPY injected into the PVH and PFA on appetitive and consummatory ingestive behaviors and demonstrate that Y1-Rs appear to mediate changes in food hoarding more so than foraging or food intake, as we first reported by the effects of NPY Y1-R agonist injections into the third ventricle using this same model and species (19). Finally, the present study shows the usefulness of models that can measure appetitive and consummatory ingestive behaviors independently to elucidate more completely the functions of neuropeptides involved in feeding.
Perspectives and Significance
Current approaches to the obesity problem primarily focus on the mechanisms controlling the consummatory ingestive behavior of food intake and have been largely unsuccessful. Food, however, almost always has to be acquired (foraged for) and frequently is stored for subsequent consumption (hoarded) (52). Even though most humans in modern industrialized areas do not have to expend much energy to acquire food, as do nonhuman animals, humans still have to expend some effort and time to obtain food, and human food acquisition can be altered by changes in energy balance. For example, if you go to the grocery store hungry, you will bring home more food than if you go when you are full (4, 21, 32). Moreover, obese people bring home more high-fat foods and more calories per person than lean people (41). Once food is acquired and stored in our refrigerators/freezers and pantries, we are more likely to eat this stored food than to go out and eat food away from our domiciles; indeed, 85% of all food purchased is consumed at home (41). Therefore, understanding the NPY-mediated control of human foraging and food storing (hoarding) could provide an additional target for pharmaceutical or behavioral manipulations in the treatment or prevention of obesity.
GRANTS
This research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-078358 to T. J. Bartness.
Acknowledgments
We thank Maisie Adiviani and Omuwa Braimah for help collecting the data and processing the tissue and Dr. B. Glenn Stanley for advice on microinjections.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ammar AA, Sederholm F, Saito TR, Scheurink AJ, Johnson AE, Sodersten P. NPY-leptin: opposing effects on appetitive and consummatory ingestive behavior and sexual behavior. Am J Physiol Regul Integr Comp Physiol 278: R1627–R1633, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Bartness TJ, Clein MR. Effects of food deprivation and restriction and metabolic blockers on food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 266: R1111–R1117, 1994. [DOI] [PubMed] [Google Scholar]
- 3.Bartness TJ, Day DE. Food hoarding: a quintessential anticipatory appetitive behavior. Prog Psychobiol Physiol Psychol 18: 69–100, 2003. [Google Scholar]
- 4.Beneke WM, Davis CH. Relationship of hunger, use of a shopping list and obesity to food purchases. Int J Obes 9: 391–399, 1985. [PubMed] [Google Scholar]
- 5.Benoit SC, Clegg DJ, Woods SC, Seeley RJ. The role of previous exposure in the appetitive and consummatory effects of orexigenic neuropeptides. Peptides 26: 751–757, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Billington CJ, Briggs JE, Harker S, Grace M, Levine AS. Neuropeptide Y in hypothalamic paraventricular nucleus: a center coordinating energy metabolism. Am J Physiol Regul Integr Comp Physiol 266: R1765–R1770, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Bowers RR, Festuccia WTL, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol Regul Integr Comp Physiol 286: R1167–R1175, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Broberger C, Visser TJ, Kuhar MJ, Hokfelt T. Neuropeptide Y innervation and neuropeptide-Y-Y1-receptor-expressing neurons in the paraventricular hypothalamic nucleus of the mouse. Neuroendocrinology 70: 295–305, 1999. [DOI] [PubMed] [Google Scholar]
- 9.Brown CM, Coscina DV, Fletcher PJ. The rewarding properties of neuropeptide Y in perifornical hypothalamus vs. nucleus accumbens. Peptides 21: 1279–1287, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Calhoun JB The Ecology and Sociology of the Norway Rat. Washington, DC: US Government Printing Office, 1962.
- 11.Clark JT, Kalra PS, Crowley WR, Kalra SP. Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115: 427–429, 1984. [DOI] [PubMed] [Google Scholar]
- 12.Corp ES, Melville LD, Greenberg D, Gibbs J, Smith GP. Effect of fourth ventricular neuropeptide Y and peptide YY on ingestive and other behaviors. Am J Physiol Regul Integr Comp Physiol 259: R317–R323, 1990. [DOI] [PubMed] [Google Scholar]
- 13.Craig W Appetites and aversions as constituents of instincts. Biol Bull 34: 91–107, 1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currie PJ, Coscina DV. Dissociated feeding and hypothermic effects of neuropeptide Y in the paraventricular and perifornical hypothalamus. Peptides 16: 599–604, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Currie PJ, Coscina DV. Regional hypothalamic differences in neuropeptide Y-induced feeding and energy substrate utilization. Brain Res 737: 238–242, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Day DE, Bartness TJ. Effects of foraging effort on body fat and food hoarding by Siberian hamsters. J Exp Zool 289: 162–171, 2001. [PubMed] [Google Scholar]
- 17.Day DE, Bartness TJ. Fasting-induced increases in hoarding are dependent on the foraging effort level. Physiol Behav 78: 655–668, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Day DE, Bartness TJ. Agouti-related protein increases food hoarding, but not food intake by Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 286: R38–R45, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Day DE, Keen-Rhinehart E, Bartness TJ. Role of NPY and its receptor subtypes in foraging, food hoarding, and food intake by Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 289: R29–R36, 2005. [DOI] [PubMed] [Google Scholar]
- 20.de Quidt ME, Emson PC. Distribution of neuropeptide Y-like immunoreactivity in the rat central nervous system. II. Immunohistochemical analysis. Neuroscience 18: 545–618, 1986. [DOI] [PubMed] [Google Scholar]
- 21.Dodd DK, Stalling RB, Bedell J. Grocery purchases as a function of obesity and assumed food deprivation. Int J Obes 1: 43–47, 1977. [PubMed] [Google Scholar]
- 22.Eva C, Serra M, Mele P, Panzica G, Oberto A. Physiology and gene regulation of the brain NPY Y1 receptor. Front Neuroendocrinol 27: 308–339, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Fetissov SO, Byrne LC, Hassani H, Emfors P, Hokfelt T. Characterization of neuropeptide Y Y2 and Y5 receptor expression in the mouse hypothalamus. J Comp Neurol 470: 256–265, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Hakansson M, De LL, Sutcliffe JG, Yanagisawa M, Meister B. Leptin receptor- and STAT3-immunoreactivities in hypocretin/orexin neurones of the lateral hypothalamus. J Neuroendocrinol 11: 653–663, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Ida T, Nakahara K, Katayama T, Murakami N, Nakazato M. Effect of lateral cerebroventricular injection of the appetite-stimulating neuropeptide orexin and neuropeptide Y on the various behavioral activities of rats. Brain Res 821: 526–529, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Jewett DC, Cleary J, Levine AS, Schaal DW, Thompson T. Effects of neuropeptide Y on food-reinforced behavior in satiated rats. Pharmacol Biochem Behav 42: 207–212, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Kalra SP, Dube MG, Shuye P, Bin X, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev 20: 68–100, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 288: R716–R722, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Kotz CM, Briggs JE, Grace MK, Levine AS, Billington CJ. Divergence of the feeding and thermogenic pathways influenced by NPY in the hypothalamic PVN of the rat. Am J Physiol Regul Integr Comp Physiol 275: R471–R477, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul Pept 104: 27–32, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Li C, Chen P, Smith MS. Corticotropin releasing hormone neurons in the paraventricular nucleus are direct targets for neuropeptide Y neurons in the arcuate nucleus: an anterograde tracing study. Brain Res 854: 122–129, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Mela DJ, Aaron JI, Gatenby SJ. Relationships of consumer characteristics and food deprivation to food purchasing behavior. Physiol Behav 60: 1331–1335, 1996. [DOI] [PubMed] [Google Scholar]
- 33.Menendez JA, McGregor IS, Healey PA, Atrens DM, Leibowitz SF. Metabolic effects of neuropeptide Y injections into the paraventricular nucleus of the hypothalamus. Brain Res 516: 8–14, 1990. [DOI] [PubMed] [Google Scholar]
- 34.Morley JE, Levine AS, Gosnell BA, Kneip J, Grace M. Effect of neuropeptide Y on ingestive behaviors in the rat. Am J Physiol Regul Integr Comp Physiol 252: R599–R609, 1987. [DOI] [PubMed] [Google Scholar]
- 35.Nergardh R, Ammar A, Brodin U, Bergstrom J, Scheurink A, Sodersten P. Neuropeptide Y facilitates activity-based anorexia. Psychoneuroendocrinology 32: 493–502, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Oberto A, Mele P, Zammaretti F, Panzica G, Eva C. Evidence of altered neuropeptide Y content and neuropeptide Y1 receptor gene expression in the hypothalamus of pregnant transgenic mice. Endocrinology 144: 4826–4830, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 295: 1549–1555, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Parker R, Herzog H. Localization of Y-receptor subtype mRNAs in rat brain by digoxigenin labeled in situ hybridization. Methods Mol Biol 153: 165–183, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci 11: 1431–1448, 1999. [DOI] [PubMed] [Google Scholar]
- 40.Polidori C, Ciccocioppo R, Regoli D, Massi M. Neuropeptide Y receptor(s) mediating feeding in the rat: characterization with antagonists. Peptides 21: 29–35, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Ransley JK, Donnelly JK, Botham H, Khara TN, Greenwood DC, Cade JE. Use of supermarket receipts to estimate energy and fat content of food purchased by lean and overweight families. Appetite 41: 141–148, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Ross I, Smith WI. The hoarding behavior of the mouse. II. The role of deprivation, satiation and stress. J Gen Psychol 82: 279–297, 1953. [DOI] [PubMed] [Google Scholar]
- 43.Seeley R, Payne CJ, Woods SC. Neuropeptide Y fails to increase intraoral intake in rats. Am J Physiol Regul Integr Comp Physiol 268: R423–R427, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Smith WI, Ross S. The hoarding behavior in the mouse. I. The role of previous feeding experience. J Gen Psychol 8: 299, 1953. [DOI] [PubMed] [Google Scholar]
- 45.Souza SC, Muliro KV, Liscum L, Lien P, Yamamoto MT, Schaffer JE, Dallal GE, Wang X, Kraemer FB, Obin M, Greenberg AS. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J Biol Chem 277: 8267–8272, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Stanley BG Neuropeptide Y in multiple hypothalamic sites controls eating behavior, endocrine, and autonomic systems for body energy balance. In: The Biology of Neuropeptide Y and Related Peptides, edited by Colmers WF and Whalestedt C. Totowa, NJ: Humana, 1993, p. 457–509.
- 47.Stanley BG, Leibowitz SF. Neuropeptide Y: stimulation of feeding and drinking by injection into the paraventricular nucleus. Life Sci 35: 2635–2642, 1984. [DOI] [PubMed] [Google Scholar]
- 48.Stanley BG, Leibowitz SF. Neuropeptide Y injected in the paraventricular hypothalamus: a powerful stimulant of feeding behavior. Proc Natl Acad Sci USA 82: 3940–3943, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stanley BG, Magdalin W, Seirafi A, Nguyen MM, Leibowitz SF. Evidence for neuropeptide Y mediation of eating produced by food deprivation and for a variant of the Y1 receptor mediating this peptide's effect. Peptides 13: 581–587, 1992. [DOI] [PubMed] [Google Scholar]
- 50.Stanley BG, Magdalin W, Seirafi A, Thomas WJ, Leibowitz SF. The perifornical area: the major focus of (a) patchily distributed hypothalamic neuropeptide Y-sensitive feeding system(s). Brain Res 604: 304–317, 1993. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi LK, Lore RK. Foraging and food hoarding of wild Rattus norvegicus in an urban environment. Behav Neural Biol 29: 527–531, 1980. [DOI] [PubMed] [Google Scholar]
- 52.Vander Wall SB Food Hoarding in Animals. Chicago: University of Chicago Press, 1990.
- 53.Weiner J Limits to energy budget and tactics in energy investments during reproduction in the Djungarian hamster (Phodopus sungorus sungorus Pallas 1770). Symp Zool Soc Lond 57: 167–187, 1987. [Google Scholar]
- 54.Wieland HA, Engel W, Eberlein W, Rudolf K, Doods HN. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br J Pharmacol 125: 549–555, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci 24: 429–458, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Yasuda T, Masaki T, Kakuma T, Hara M, Nawata T, Katsuragi I, Yoshimatsu H. Dual regulatory effects of orexins on sympathetic nerve activity innervating brown adipose tissue in rats. Endocrinology 146: 2744–2748, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Yokosuka M, Kalra PS, Kalra SP. Inhibition of neuropeptide Y (NPY)-induced feeding and c-Fos response in magnocellular paraventricular nucleus by a NPY receptor antagonist: a site of NPY action. Endocrinology 140: 4494–4500, 1999. [DOI] [PubMed] [Google Scholar]
- 58.Zammaretti F, Panzica G, Eva C. Fasting, leptin treatment, and glucose administration differentially regulate Y1 receptor gene expression in the hypothalamus of transgenic mice. Endocrinology 142: 3774–3782, 2001. [DOI] [PubMed] [Google Scholar]