Abstract
Fasting triggers a constellation of physiological and behavioral changes, including increases in peripherally produced ghrelin and centrally produced hypothalamic neuropeptide Y (NPY). Refeeding stimulates food intake in most species; however, hamsters primarily increase foraging and food hoarding with smaller increases in food intake. Fasting-induced increases in foraging and food hoarding in Siberian hamsters are mimicked by peripheral ghrelin, central NPY, and NPY Y1 receptor agonist injections. Because fasting stimulates ghrelin and subsequently NPY synthesis/release, it may be that fasting-induced increased hoarding is mediated by NPY Y1 receptor activation. Therefore, we asked: Can an Y1 receptor antagonist block fasting- or ghrelin-induced increases in foraging, food hoarding, and food intake? This was accomplished by injecting the NPY Y1 receptor antagonist 1229U91 intracerebroventricularly in hamsters fasted, fed, or given peripheral ghrelin injections and housed in a running wheel-based food delivery foraging system coupled with simulated-burrow housing. Three foraging conditions were used: 1) no running wheel access, free food, 2) running wheel access, free food, or 3) foraging requirement (10 revolutions/pellet) for food. Fasting was a more potent stimulator of foraging and food hoarding than ghrelin. Concurrent injections of 1229U91 completely blocked fasting- and ghrelin-induced increased foraging and food intake and attenuated, but did not always completely block, fasting- and ghrelin-induced increases in food hoarding. Collectively, these data suggest that the NPY Y1 receptor is important for the effects of ghrelin- and fasting-induced increases in foraging and food intake, but other NPY receptors and/or other neurochemical systems are involved in increases in food hoarding.
Keywords: food deprivation, intracerebroventricular, antagonist, 1229U91, hamster, Siberian hamster
determining the physiological factors that regulate ingestive behavior is critical to understanding the etiology of obesity. Ingestive behavior occurs in two phases 1) the actual eating of the food or the “consummatory” phase and 2) the acquisition and storage of food or the “appetitive” phase (11). The consummatory aspects of ingestive behavior have received almost exclusive attention in the quest to understand the mechanisms underlying food intake. As for the appetitive phase of ingestive behavior, however, there is comparatively little known about the mechanisms underlying the search for food or “foraging,” which is surprising, given its pervasive nature across animal taxa (for a review, see Ref. 24). Perhaps, the lack of attention to this initial phase of ingestive behavior is due to the difficulty in conducting field studies of foraging or the problem of creating a laboratory-based analog of this behavior. In addition, “food hoarding”—the storage of food for later ingestion, is another appetitive ingestive behavior with widespread expression among animal species (for a review, see Ref. 57), but studies of the mechanisms underlying this appetitive ingestive behavior also have received little attention compared with consummatory ingestive behavior (food intake; for a review see Ref. 5).
Siberian hamsters (Phodopus sungorus) and other hamster species (for a review, see Ref. 6) primarily increase foraging (5, 14) and food hoarding (3, 4, 62) in response to energetic challenges rather than food intake. Siberian hamsters and other animals that have the capacity to transport significant amounts of food (for a review, see Ref. 57) use food hoarding as a crucial part of their ingestive behavioral repertoire in response to many naturally occurring energetic challenges [e.g., pregnancy, lactation (3, 5); for a review, see Ref. 5].
Another naturally occurring energetic challenge is fasting, a condition that triggers a plethora of alterations in peripheral metabolism, peripheral signaling peptides, and central neurotransmitters (for reviews, see Refs. 37 and 46). Once fasting is terminated with food availability, marked increases in appetitive (foraging and food hoarding) ingestive behaviors occur in Siberian hamsters with little change in food intake (3, 4, 14, 62). The exact mechanisms underlying these fasting-induced increases in appetitive ingestive behaviors is unknown, but there are increases in peripheral and central peptide synthesis/release implicated in the stimulation of food intake that are associated with fasting. For example, fasting triggers increases in circulating concentrations of the largely stomach-derived peptide ghrelin in Siberian hamsters (32), as it does in laboratory rats (53, 56). In addition, peripherally administered ghrelin that creates 24–48 h fasting-like plasma active ghrelin concentrations in Siberian hamsters markedly stimulates foraging, food hoarding, and, to a lesser degree, food intake (32). Fasting also increases arcuate nucleus (ARC) gene expression of the orexigenic peptides neuropeptide Y (NPY) and agouti-related peptide (AgRP) in Siberian hamsters (40, 41), as it does in other species such as laboratory rats and mice (9, 33, 42, 43). When AgRP (13), NPY (16), or a NPY Y1 receptor agonist, [Pro34]NPY (16), are administered to Siberian hamsters centrally, food hoarding strikingly increases, whereas food intake increases minimally.
Several lines of research suggest that ghrelin, which is increased with fasting, may be stimulating NPY synthesis and release, resulting in effects on food intake. For example, the mRNA for the ghrelin receptor (growth hormone secretagogue receptor) is expressed in 94% of the NPY neurons in the ARC as seen by double-labeling in situ hybridization histochemistry (22, 38, 45, 59). In addition, 90% of ghrelin-induced Fos-positive (activated) ARC neurons express NPY mRNA (59), suggesting that ghrelin stimulates NPY synthesizing neurons in this nucleus. Moreover, ghrelin increases the expression of ARC neuron NPY mRNA (2, 45, 50), and intracerebroventricular administration of a NPY Y1 antagonist inhibits ghrelin-stimulated food intake (38, 45). Collectively, these findings suggest that the NPY neurons in the ARC could be important effectors for the orexigenic action of ghrelin; indeed, ghrelin also stimulates the release of NPY from hypothalamic explants in vitro. (63). Thus, because fasting increases circulating ghrelin concentrations, which, in turn, stimulate NPY-producing ARC neurons (18, 19, 35, 36, 49) and presumably NPY synthesis/release (63), which finally may stimulate NPY Y1 receptors in the hypothalamic paraventricular nucleus, the activation of these downstream Y1 receptors might partially underlie the fasting- and ghrelin-induced increases in appetitive ingestive behaviors. Therefore, we asked: Can an Y1 receptor antagonist block fasting- or ghrelin-induced increases in foraging and food hoarding? This was accomplished by testing the ability of a third ventricularly injected NPY Y1 receptor antagonist (1229U91; 29) to block fasting- and ghrelin-induced increases in foraging, food hoarding, and food intake in hamsters housed in a simulated burrow/running wheel-based foraging system.
METHODS
Animals
All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with Public Health Service and United States Department of Agriculture guidelines. Adult male Siberian hamsters, ∼3.5 mo old and weighing 35–43 g were obtained from our breeding colony. The lineage of this colony has been described recently (8). Hamsters were group-housed and reared in a long-day photoperiod (16:8-h light-dark cycle, with lights on at 0200) from birth. Room temperature was maintained at 21 ± 2.0°C.
Hamsters were acclimated for 2 wk in specially designed hoarding apparatuses, as previously described (15, 16) that would serve as their housing for the duration of the experiment. More specifically, two cages were connected with a convoluted polyvinylchloride tubing system (38.1 mm ID and ∼1.52 m long) with corners and straightways for horizontal and vertical climbs. The diet (75-mg pellets, Purified Rodent Diet; Research Diets, New Brunswick, NJ) and tap water were available ad libitum. A running wheel (524-mm circumference) and pellet dispenser were attached to the food (top) cage. Wheel revolutions were counted using a magnetic detection system and monitored by a computer-based hardware-software program (Med Associates, Lancaster, NH). Hamsters were trained in these apparatuses (16, 32) and received a third ventricular cannula (13, 16), as previously described and described briefly in Cannula Implantation.
Foraging Training Regimen
We used a wheel-running training regimen that eases the hamsters into their foraging efforts without large changes in body mass or food intake (15). Specifically, hamsters were given free access to the food pellets for 2 days while they adapted to the running wheel. In addition to the free food, a 75-mg food pellet was dispensed upon completion of every 10 wheel revolutions. On the third day, the free-food condition was replaced by a response-contingent condition where only every 10 wheel revolutions triggered the delivery of a pellet. This condition was in effect for 5 days during which time body mass, food intake, food hoarding, wheel revolutions, and pellets earned were measured daily. At the end of this acclimation period (7 days total), all animals were removed from the foraging apparatuses and temporarily housed in shoebox cages where the same food pellets were available ad libitum with no foraging requirements. Guide cannulas were then surgically implanted in these hamsters (see Cannula Implantation for details). After a 1-wk postsurgical recovery period in shoebox cages, all hamsters were returned to the hoarding/foraging apparatus and retrained to the following schedule: 2 days for adaptation with free access to food pellets and 5 days at 10 revolutions/pellet.
Cannula Implantation
Cannulas were stereotaxically implanted into the third ventricle, as described previously (13). Briefly, the animals were anesthetized with isoflurane and the fur at the top of the head was removed to expose the area to be incised. A hole was trephined at the intersection of bregma and the midsaggital sinus and the guide cannula (26-gauge stainless steel; Plastics One, Roanoke, VA) was lowered using the following stereotaxic coordinates (level skull, anterior-posterior from bregma 0, medial-lateral from midsaggital sinus 0, and dorsal-ventral from the top of the skull −5.0 mm) targeted for placement just above the third ventricle. The guide cannula was secured to the skull using cyanoacrylate ester gel, 3/16 mm jeweler's screws, and dental acrylic. A removable obturator sealed the opening in the guide cannula throughout the experiment, except when it was removed for the injections. Hamsters received 0.2 mg/kg buprenorphine at 12 and 24 h postsurgery to minimize discomfort and subsequently were allowed 1 wk to recover fully in the shoebox cage housing before being returned to their simulated burrow housing.
Cannula Verification
Following the last test, an injection of 0.4 μl bromophenol blue dye was given to confirm placement of the cannula in the third ventricle. The animals were killed with an overdose of pentobarbital sodium (75 mg/kg); their brains were removed and then postfixed in 10% paraformaldehyde for a minimum of 2 days. Each brain was sliced manually for verification of the cannulas. Cannulas were considered to be located in the third ventricle if the dye were visible in any part of this ventricle. Only the data from animals with confirmed third-ventricle cannula placements were included in the analyses.
Intracerebroventricular Injection Protocol
The inner cannula (33-gauge stainless steel; Plastics One) extended 5.5 mm below the top of the skull, and all hamsters were injected with a 0.4 μl volume. All injections were given at the beginning of the dark phase of the photoperiod. Animals were lightly restrained by hand during the 30-s injection, and the injection needle remained in place ∼30 s before withdrawal, as we have done previously (13, 16).
Experimental Design
At the end of the 7-day retraining period, the hamsters were separated into three groups matched for their current body mass and average hoard size across these last 3 days of training at 10 revolutions/pellet (n = 8/group). The three groups consisted of 10 revolutions/pellet foraging requirement (10 revs, foraging group, contingent food delivery), no foraging requirement with an active running wheel (free wheel; exercise control group, noncontingent food), or no foraging requirement with a blocked wheel (blocked wheel; sedentary control group, noncontingent food). Selection of the 10 revolutions/pellet foraging effort and inclusion of the free and blocked wheel groups was based on our previous study in Siberian hamsters using this foraging/hoarding system (15). For experiment 1, each group received all drug combinations: intraperitoneal ghrelin + intracerebroventricular saline, intraperitoneal ghrelin + intracerebroventricular 1229U91, intraperitoneal saline + intracerebroventricular saline, and intraperitoneal saline + intracerebroventricular 1229U91, and they were given in a counterbalanced schedule to control for possible order effects of peptide administration. For experiment 2, each group was observed during a baseline ad libitum, saline-treated period that was followed by 48 h of fasting, at which time either intracerebroventricular saline or 1229U91 injections were given and the ingestive behaviors compared with each animal's own baseline behaviors.
Measurement of Foraging, Food Hoarding and Food Intake
Foraging (pellets earned) was defined as the number of pellets delivered upon completion of the requisite wheel revolutions. Food hoarding (pellets hoarded) was defined as the number of pellets found in the bottom “burrow” cage in addition to those removed from the cheek pouches. For the 10-rev group, food intake (pellets eaten) was defined as pellets earned − surplus pellets − hoarded pellets = food intake. For the free and blocked wheel groups, food intake (pellets eaten) was defined as pellets given − pellets left in the top cage − hoarded pellets = food intake. The electronic balance used to weigh the food pellets was set to “parts” measurement rather then obtaining fractions of a pellet in milligrams; thus the smallest unit measured was one 75-mg food pellet and equal to 1.
Experiment 1: Does 1229U91 Inhibit Ghrelin-Induced Stimulation of Appetitive and Consummatory Ingestive Behaviors in Siberian Hamsters?
A repeated-measures design was chosen to minimize variability; therefore, all animals received all of the following possible injection combinations: intraperitoneal ghrelin (30 μg/kg; Bachem Biosciences, King of Prussia, PA) + 1229U91 (17 nmol; Neosystems, Strasbourg, France), intraperitoneal saline + 1229U91 (17 nmol), intraperitoneal ghrelin (30 μg/kg) + intracerebroventricular saline, intraperitoneal saline + intracerebroventricular saline. This dose of ghrelin was chosen because it results in plasma active ghrelin concentrations within the physiological range of fasting for 12–48 h in this species (32). Because there were no carryover effects of ghrelin at this dose for any behavior beyond 7 days (32), a washout period of this length occurred between the counterbalanced injections.
Experiment 2: Does the NPY Y1 Receptor Antagonist, 1229U91, Inhibit Fasting-Induced Stimulation of Appetitive and Consummatory Ingestive Behaviors in Siberian Hamsters?
Two weeks after the last injection from experiment 1, food intake, foraging and food hoarding were monitored during baseline ad libitum fed, saline injection conditions, and then animals were divided into two groups, rebalanced for body weight and hoarding and were food deprived for 48 h. Half of the animals received intracerebroventricular 1229U91 (17 nmol), and the other half received intracerebroventricular saline. In our previous studies of food hoarding, we have used fasts ranging from 12 to 56 h (Institutional Animal Care and Use Committee approved), with the latter length appearing somewhat severe and/or “nonphysiological” at first blush. In the utopian conditions of the laboratory, however, Siberian hamsters are almost 50% body fat compared with as low as ∼25% in nature (60); therefore, short fasts in the laboratory of 12–24 h are minimally energetically challenging in these animals, and thus stimulation of food hoarding is minimal (Clein MR, and Bartness TJ, unpublished results). Therefore, we selected 48-h food deprivation to trigger the behavior nearly maximally. It also seems reasonable to envision these fast lengths as on a physiological continuum with the intermeal intervals occurring naturally of much shorter lengths in hamsters (∼4 h) (7).
Statistical Analyses
For all measures of food intake, foraging and food hoarding in experiments 1 and 2, if under the saline treatment an animal had not eaten, foraged (earned pellets), or hoarded for a given time interval (i.e., a value of 0), the minimum value (1 pellet) was assigned to avoid a zero in the denominator. For experiment 1, each animal was given saline + saline, ghrelin + saline, 1129U91 + saline, and ghrelin + 1229U91. The percent saline value was calculated by dividing each animal's ingestive behavior response to each of the drug conditions by that of the saline + saline control condition multiplied by 100; thus, this yields data for each animal expressed as a % of saline value. All the individual % saline values were then averaged to yield means ± SE across animals for each foraging condition. For experiment 2, food intake, foraging, and food hoarding were monitored during an ad libitum-fed, saline-injection treatment baseline period, and then each animal was food deprived followed by treatment with either saline or 1229U91. Similar to experiment 1, the percent saline value was calculated by dividing each animal's ingestive behavior response to either food deprivation + saline or to food deprivation + 1229U91 by each animal's ad libitum saline baseline value multiplied by 100; thus, this yields data for each animal expressed as a percentage of saline value. Once again, all of the individual %saline values were then averaged to yield means ± SE across animals for each foraging condition. Therefore, the data are graphed as the mean percent of saline ± SE for food intake, food hoarding, foraging (10 revolutions/pellet group), and wheel running (free wheel group). The data from both experiments were analyzed for each time interval to identify time points postinjection at which the effects were occurring; therefore, the data are presented noncumulatively. No statistical comparisons were made across time (i.e., all statistical comparisons were made within each time interval) because the intervals were of unequal duration [0–1 h and 1–2 h (1-h duration), 2–4 h (2-h duration), 4–24 h (20-h duration), 24–48 (24-h duration)]. Data were analyzed using a repeated-measures, two-way ANOVA (experiment 1: foraging group × drug combination: 3 × 4; experiment 2: foraging group × drug treatment; 3 × 2), and Bonferroni's post hoc tests were used for individual pairwise comparisons. Differences between means were considered statistically significant if P < 0.05. Exact probabilities and test values were omitted for simplicity and clarity of the presentation of the results.
RESULTS
Experiment 1: Does 1229U91 Inhibit Ghrelin-Induced Stimulation of Appetitive and Consummatory Ingestive Behaviors in Siberian Hamsters?
Wheel running.
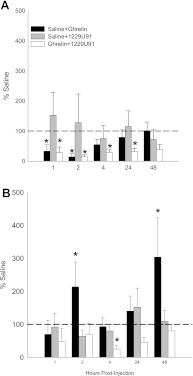
As seen previously (32), ghrelin did not stimulate wheel running by the free wheel hamsters (Fig. 1) suggesting there were not nonspecific increases in locomotor activity triggered by the peptide. Instead, wheel running was significantly decreased, but only at 0–1 and 1–2 h (P < 0.05; Fig. 1). 1229U91 did not affect wheel running at any time interval. There were a few time points at which ghrelin + saline or ghrelin +1229U91 inhibited wheel-running activity, but this effect was not consistent over the observation period and probably represents behavioral competition with feeding (Fig. 1).
Fig. 1.
Values are means ± SE of foraging as a percentage of the intracerebroventricularly and intraperitoneally saline-injected controls for the effects of intraperitoneal ghrelin treatment with intracerebroventricular saline (black bars), intraperitoneal saline treatment with intracerebroventricular 1229U91 (gray bars) and intraperitoneal ghrelin treatment with intracerebroventricular 1229U91 (white bars) on hamsters without a foraging requirement and with a freely moving wheel (free wheel) (A) and with a foraging requirement (10 revolutions/pellet) (B). *P < 0.05 compared with the saline control condition.
Foraging.
As seen previously (32), ghrelin significantly stimulated foraging by the 10-rev group (range: ∼200–300%) at 1–2 and 24–48 h compared with saline (P < 0.05; Fig. 1) and appears to be bona fide increased foraging because it did not occur in the free wheel group, as described above. 1229U91 completely blocked the ghrelin-induced increased foraging at 1–2 and 24–48 h. Although ghrelin did not significantly increase foraging at 2–4 h postinjection, 1229U91 decreased foraging in these hamsters to a level significantly below the saline-treated controls (P < 0.05; Fig. 1).
Food intake.
Ghrelin significantly stimulated food intake for all groups (∼200–350%) at 0–1 h compared with saline (P < 0.05; Fig. 2) and additionally at the 1–2, 4–24 and 24–48 h time intervals for the foraging hamsters (10-rev group, P < 0.05; Fig. 2C). 1229U91 completely blocked all of the ghrelin-induced increases in food intake for all groups (Fig. 2). 1229U91 treatment alone produced an unexpected small, but significant increase in food intake in the foraging hamsters (10-rev), but only at 4–24 h (P < 0.05; Fig. 2).
Fig. 2.
Values are means ± SE of food intake as a percentage of the intracerebroventricularly and intraperitoneally saline-injected controls for the effects of intraperitoneal ghrelin treatment with intraventricular saline (black bars), intraperitoneal saline treatment with intracerebroventricular 1229U91 (gray bars), and intraperitoneal ghrelin treatment with intracerebroventricular 1229U91 (white bars) on hamsters without a foraging requirement and a stationary wheel (blocked wheel; A), hamsters with no foraging requirement and a freely moving wheel (free wheel; B) and hamsters with a foraging requirement (10 revolutions/pellet; C). *P < 0.05 compared with the saline control condition.
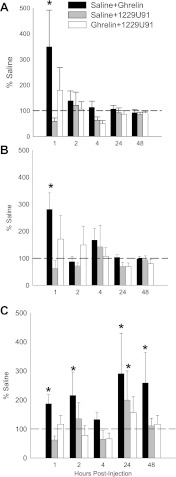
Food hoarding.
Ghrelin alone significantly increased food hoarding (range: ∼200–600%) for the foraging hamsters (10-rev group) at all time intervals compared with saline controls (P < 0.05; Fig. 3). In the free wheel and blocked wheel groups, ghrelin significantly increased food hoarding at all but the 1 to 2- and 24 to 48-h intervals (range: ∼400–800%; P < 0.05; Fig. 3). 1229U91 blocked the ghrelin-induced increased food hoarding only at 0–1 h in the blocked wheel group and only at 2–4 h in the free wheel group compared with saline (P < 0.05; Fig. 3, A and B). 1229U91 blocked the ghrelin-induced increased food hoarding at the 0–1, 2–4, and 4–24 h in the foraging hamsters (10-rev group) compared with saline injections (P < 0.05; Fig. 3C).
Fig. 3.
Values are means ± SE of food hoarding as a percentage of the intracerebroventricularly and intraperitoneally saline-injected controls for the effects of intraperitoneal ghrelin treatment with intraventricular saline (black bars), intraperitoneal saline treatment with intracerebroventricular 1229U91 (gray bars), and intraperitoneal ghrelin treatment with intracerebroventricular 1229U91 (white bars) on hamsters without a foraging requirement and a stationary wheel (blocked wheel; A), hamsters with no foraging requirement and a freely moving wheel (free wheel; B) and hamsters with a foraging requirement (10 revolutions/pellet; C). *P < 0.05 compared with the saline control condition.
Experiment 2: Does the NPY Y1 Receptor Antagonist, 1229U91, Inhibit Fasting-Induced Stimulation of Appetitive and Consummatory Ingestive Behaviors in Siberian Hamsters?
Wheel running.
Wheel running was not affected by food deprivation, 1229U91 treatment, or their combination in the free wheel group (data not shown), suggesting there was not nonspecific stimulation or inhibition of locomotor activity.
Foraging.
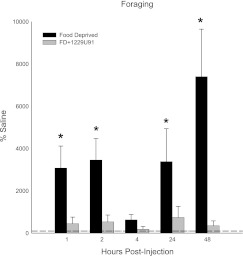
Fasting for 48 h markedly stimulated foraging [∼2,500–7,500 at all but 1–2 h after refeeding compared with saline-injected ad libitum-fed controls (P < 0.05; Fig. 4) ]. 1229U91 blocked all fasting-induced increases in foraging (Fig. 4).
Fig. 4.
Values are means ± SE of foraging as a percentage of the saline-injected ad libitum-fed control condition for the effects of food deprivation with intracerebroventricular saline injection (black bars) and food deprivation with intracerebroventricular 1229U91 injection (gray bars) on hamsters with a foraging requirement (10 revolutions/pellet) *P < 0.05 compared with saline-injected ad libitum control condition.
Food intake.
Food deprivation significantly increased food intake 0–1 h post-refeeding for all groups and also at 1–2 h for the free wheel group, as well as at 2–4 and 24–48 h for the 10-rev group compared with the vehicle-treated ad libitum-fed controls (range: ∼50–300%; P < 0.05; Fig. 5). 1229U91 blocked all of the fasting-induced increases in food intake by all groups compared with vehicle-treated ad libitum-fed controls (P < 0.05; Fig. 5). Occasionally, 1229U91 decreased food intake below that of the ad libitum-fed, saline-treated condition in the 10-rev group at 4–24 h, the free wheel group at 1–2 h and blocked wheel group at 2–4 h post-refeeding (P < 0.05; Fig. 5).
Fig. 5.
Values are means ± SE of food intake as a percentage of the saline-injected ad libitum fed control condition for the effects of food deprivation with intracerebroventricular saline injection (black bars) and food deprivation with intracerebroventricular 1229U91 injection (gray bars) on hamsters without a foraging requirement and a stationary wheel (blocked wheel; A), hamsters with no foraging requirement and a freely moving wheel (free wheel; B), and hamsters with a foraging requirement (10 revolutions/pellet; C) *P < 0.05 compared with saline injected ad libitum control condition.
Food hoarding.
Fasting-stimulated food hoarding in all groups compared with ad libitum-fed hamsters (range: ∼500–7,000%; Fig. 6). Specifically, the least significant increase in food hoarding was by blocked wheel hamsters with a ∼500% increase 0–1 h postfast and greater increases at 2–4 h and 4–24 h compared with the ad libitum-fed saline-treated controls (2,000% and 4,000% increases, respectively: P < 0.05; Fig. 6). The fasting-induced increased food hoarding was significant at all time intervals by the free wheel hamsters with the greatest increase occurring later postfast (4–24 h and 24–48 h of ∼5,000%; P < 0.05; Fig. 6). The fasting-induced increased food hoarding was most striking by the foraging hamsters (10-rev group), with at least an ∼3,000% increases during the 0–1 h, 1–2 h, and 4–24 h postfast with a maximal increase (∼7,000%) occurring late (24–48 h; P < 0.05; Fig. 6).
Fig. 6.
Values are means ± SE food hoarding as a percentage of the saline-injected ad libitum fed control condition for the effects of food deprivation with intracerebroventricular saline injection (black bars) and food deprivation with intracerebroventricular 1229U91 injection (gray bars) on hamsters without a foraging requirement and a stationary wheel (blocked wheel; A), hamsters with no foraging requirement and a freely moving wheel (free wheel; B), and hamsters with a foraging requirement (10 revolutions/pellet; C) *P < 0.05 compared with saline injected ad libitum fed control condition.
1229U91 inhibited fasting-induced increased food hoarding during at least one time interval for all groups, but its effectiveness in doing so differed across the foraging groups (Fig. 6). Specifically, this Y1 receptor antagonist only inhibited the fasting-induced increased food hoarding by the free wheel group 24–48 h (P < 0.05; Fig. 6B) and the blocked wheel group at 2–4 h and 4–24 h postfast (P < 0.05; Fig. 6A), whereas it completely inhibited postfast increases in food hoarding at all intervals for the foraging hamsters (10-rev group; P < 0.05; Fig. 6C).
DISCUSSION
This study was predicated on the notion that the fasting-induced increases in appetitive (foraging and food hoarding) and ingestive behaviors were due to fasting-induced increases in circulating ghrelin concentrations that, in turn, stimulated NPY release and activated NPY Y1 receptors. This idea is partially, but not wholly, supported by the results of the present study using peripheral ghrelin injections and fasting to stimulate the ingestive behaviors and third ventricular injections of the Y1 receptor antagonist 1229U91 to block these responses. In laboratory rats, ghrelin-induced increases in food intake appear to be mediated through the NPY Y1 receptor subtype (17, 38, 50), and in the present study, ghrelin-induced increased food intake was consistently blocked by third ventricular injections of 1229U91. The ghrelin-induced increased food hoarding, however, was not consistently blocked by this NPY Y1 receptor antagonist with the effects of 1229U91 often being time and foraging effort-dependent. In addition, the fasting-induced increases in food intake of refed laboratory rats appear to at least partially act through the NPY Y1 receptor subtype (e.g., 29, 31, 47, 61), and in the present study, when food intake was elevated postfast, 1229U91 blocked these increases for groups at all time points during refeeding. 1229U91 also effectively blocked the fasting-induced increases in food hoarding by foraging hamsters, with lesser effects in the other groups. Finally, Y1 receptor antagonism blocked ghrelin- or fasting-induced increased forging without consistently and nonspecifically decreasing locomotor activity indicating a bona fide effect on this appetitive ingestive behavior.
We previously demonstrated that Y1 receptor stimulation, produced by third ventricular injection of [Pro34]NPY, stimulated foraging and food hoarding with a significantly smaller stimulation of food intake (16). Additional evidence implicating Y1 receptors in these ingestive behaviors in Siberian hamsters is that third-ventricular injection of the Y1 receptor antagonist 1229U91 inhibits NPY-triggered increases in food hoarding and food intake (Day DE and Bartness TJ, unpublished observations). As G. P. Smith (51) so insightfully noted, to make a compelling case for the physiological functions of a peptide or in this case one of its receptors, it is essential that a receptor “antagonist” produces the opposite effect of exogenous administration of the peptide or blocks the physiological response thought to involve the peptide. Thus, it might be expected that Y1 receptor antagonism would block or inhibit these appetitive ingestive behaviors triggered by fasting, a physiological stimulus thought to be under at least some control by NPY (5, 16, 16, 44), or peripheral ghrelin administration that produces fasting-like circulating concentrations of the peptide (32). Therefore, the partial or complete blockade of these appetitive ingestive behaviors by the Y1 receptor antagonist lends further credence to the involvement of this receptor in foraging and food hoarding in addition to its well-established role in food intake (consummatory ingestive behavior; e.g., Ref. 52).
We previously reported that peripherally administered ghrelin (32) or fasting (14) stimulated foraging by Siberian hamsters, effects that cannot be accounted for simply by nonspecific increases in locomotor activity (wheel running). These effects were duplicated here. The Y1 receptor completely blocked both ghrelin- and fasting-induced increased foraging in these experiments and, because the Y1 receptor antagonist did not inhibit wheel running when given by itself, it is difficult to argue that this inhibition of foraging was due to a nonspecific malaise. This suggests that the NPY Y1 receptor is involved in this appetitive behavior. This notion is supported by our previous work showing more consistent stimulation of foraging by a Y1 receptor agonist, [Pro34]NPY, than a Y5 receptor agonist, [d-Trp34]NPY, although the latter did markedly stimulate foraging late postinjection (4–24 h; Ref. 16). NPY itself stimulates foraging in this species using this foraging/hoarding model (16). In addition, it was anecdotally noted during tests of the effects of central NPY injections on cardiac function that laboratory rats increased locomotor behaviors reminiscent of foraging (21, 58). Collectively, these data suggest an involvement of NPY in foraging. To our knowledge this is the first indication of a specific receptor involved in foraging behavior. Because foraging is such an important appetitive ingestive behavior, it seems unlikely that NPY and the Y1 receptor are the only neurochemicals mediating it.
The effects of ghrelin on food intake were relatively short-lived in the present experiment, only being significantly elevated during the initial 0- to 4-h postinjection interval, except for the hamsters foraging for their food (10-rev group), where food intake also was significantly increased 4–24 h and 24–48 h postinjection. These data are consistent with our initial study of ghrelin's effects on ingestive behaviors in this species (32) and the relatively early postinjection stimulation of food intake by ghrelin in laboratory rats (27, 56, 64) and mice (2, 56). The protracted (4–24 h postinjection) small elevation of food intake by the 10-rev group suggests a postreceptor mechanism, given that we found an absence of circulating active ghrelin at this time using the same ghrelin dose, foraging/hoarding system and species (32). Because ghrelin activates arcuate AgRP neurons (38) and increases its gene and protein expression (27, 28) and because we have previously found that third ventricularly administered AgRP stimulates food intake 2–5 days postinjection in Siberian hamsters (13), as it does in laboratory rats (20, 39), AgRP may be the mediator of the persisting effects of ghrelin on food intake seen here [and food hoarding (13) and see below].
1229U91 was effective in blocking ghrelin- and fasting-induced increases in food intake by groups at all time points when it was elevated. These data support the findings in laboratory rats and mice where Y1 receptor antagonists block fasting- (29, 31, 47, 61) and ghrelin- (38, 50) induced increases in food intake. These data might appear somewhat at odds with our recent finding that third ventricularly injected Y1 receptor agonist [Pro34]NPY stimulates food hoarding to a greater degree than food intake (16); the stimulation of food intake by this Y1 receptor agonist, although substantial (∼250% increase vs. vehicle), was dwarfed by its striking ability to increase food hoarding (∼450–1,100%; Ref. 16), however. Note though that a third ventricularly administered Y5 agonist, [d-Trp34]NPY, stimulated food intake (∼225–800%) more than food hoarding, especially for foraging hamsters (16) and is consistent with data suggesting the involvement of this NPY receptor subtype in this consummatory ingestive behavior in other laboratory rodents (e.g., 10, 12, 23, 30, 54, 65).
The Y1 receptor antagonist was partially effective in blocking fasting- or ghrelin-induced increased food hoarding. This ability was highly dependent on the stimulus that triggered the hoarding (fasting vs. ghrelin), as well as being dependent on the foraging effort in the fasted-refed, but not the ghrelin-treated hamsters. That is, the fasting-induced increased food hoarding was abolished for the blocked-wheel group at two of three intervals when it was increased (2–4 h and 4–24 h, but not at 0–1 h), at only one of the five intervals when it was increased by free wheel hamsters (24–48 h) but at all of the five intervals of increased hoarding by the foraging (10-rev group) hamsters. By contrast, 1229U91 was relatively ineffective in blocking ghrelin-induced increased food hoarding, regardless of the foraging effort group. These interesting, albeit complicated findings, suggest several points. First, the foraging effort-dependent effects of the Y1 receptor blockade for the fasting-induced increased food hoarding is a frequent finding in our studies, including those where third ventricular injections of NPY or a Y1 or Y5 agonist (16) or AgRP (13) or systemic ghrelin injections (32) increase hoarding. We are not the first to notice that that the effects of a peptide on ingestive behaviors are dependent on the presence of an appetitive behavioral component. For example, intracerebroventricular NPY stimulates consumption of sucrose when an animal has to move to a sipper tube to drink (eat), but does not do so when the sucrose is delivered via an intraoral catheter (no appetitive response) (48). Food intake after fasting or ghrelin injections in the present study, as well as after administration of intracerebroventricular NPY (16) or AgRP (13) in our previous studies using this model, is maximal when animals are foraging for their food (10 revolutions/pellet). In addition, if the appetitive effort (bar pressing requirement) is too energetically demanding, the ability of intracerebroventricular NPY to stimulate food intake by laboratory rats is less than when food is freely available (26). These types of effects add a cautionary note to the interpretation of the roles of peptides and likely other neurochemicals in ingestive behavior, suggesting their effects are highly modifiable by the testing environment in the laboratory and therefore also likely highly modifiable by the environment in nature. Second, although a Y1 receptor agonist, [Pro34]NPY, given to Siberian hamsters in the same foraging effort groups here increased food hoarding more markedly than did a Y5 receptor agonist, [d-Trp34]NPY, the relative inability of this Y1 receptor to inhibit food hoarding illustrates possible differences in the control of food hoarding that may depend on the stimulus triggering this appetitive behavior. Finally, the neural sites where stimulation of the various NPY receptor subtypes triggers this foraging or hoarding are unknown and only grossly indicated by the third ventricular injections as being in the hypothalamus, midbrain, and/or brainstem. In a pilot experiment, however, we found that parenchymal microinjections of an Y1 receptor agonist, [Pro34]NPY, into the hypothalamic paraventricular nucleus markedly stimulated foraging and food hoarding (Day DE and Bartness TJ, unpublished observations), suggesting the involvement of at least this or neighboring brain areas.
Collectively, the results of this study combined with known relations among fasting, ghrelin, NPY/AgRP arcuate nucleus neurons and food hoarding reviewed above suggest the following scenario. First, fasting induces increases in plasma active ghrelin (1, 32, 55), which, in turn, stimulates arcuate nucleus NPY/AgRP synthesizing neurons (28, 28, 34, 49, 59, 63). Release of NPY and its action at the Y1 receptor in the paraventricular nucleus of the hypothalamus (Day DE and Bartness TJ, unpublished results), and perhaps other areas, stimulates circuits underlying the postfast increases in food hoarding, foraging, and food intake in this species (3, 4, 14, 62). The persisting stimulation of food hoarding may be due to AgRP-activated circuits, given that NPY administration does not produce long-lasting (>24 h) increases in food hoarding (16). As noted above, the neurochemical/neuroanatomical underlying mechanisms of these appetitive and consummatory behaviors are not this simple, but instead involve a suite of neurochemicals and a network of circuits in addition to peripheral factors, with the present data adding to this complex picture.
Supplementary Material
Acknowledgments
The authors thank Raven Jackson, Constance Foster, and Teal Pelish for help with data collection.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86: 4753–4758, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 120: 337–345, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Bartness TJ Food hoarding is increased by pregnancy, lactation, and food deprivation in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 272: R118–R125, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Bartness TJ, Clein MR. Effects of food deprivation and restriction, and metabolic blockers on food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 266: R1111–R1117, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Bartness TJ, Day DE. Food hoarding: a quintessential anticipatory appetitive behavior. Prog Psychobiol Physiol Psychol 18: 69–100, 2003. [Google Scholar]
- 6.Bartness TJ, Demas GE. Comparative studies of food intake: lessons from non-traditionally studied species. In: Food and Fluid Intake, edited by Stricker EM and Woods SC. New York: Plenum, 2004, p. 423–467.
- 7.Bartness TJ, Morley JE, Levine AS. Photoperiod-peptide interactions in the energy intake of Siberian hamsters. Peptides 7: 1079–1085, 1986. [DOI] [PubMed] [Google Scholar]
- 8.Bowers RR, Festuccia WTL, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol Regul Integr Comp Physiol 286: R1167–R1175, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology 52: 441–447, 1990. [DOI] [PubMed] [Google Scholar]
- 10.Cabrele C, Langer M, Bader R, Wieland HA, Doods HN, Zerbe O, and Beck-Sickinger AG. The first selective agonist for the neuropeptide YY5 receptor increases food intake in rats. J Biol Chem 275: 36043–36048, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Craig W Appetites and aversions as constituents of instincts. Biol Bull 34: 91–107, 1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Criscione L, Rigollier P, Batzl-Hartmann C, Rueger H, Stricker-Krongrad A, Wyss P, Brunner L, Whitebread S, Yamaguchi Y, Gerald C, Heurich RO, Walker MW, Chiesi M, Schilling W, Hofbauer KG, Levens N. Food intake in free-feeding and energy-deprived lean rats is mediated by the neuropeptide Y5 receptor. J Clin Invest 102: 2136–2145, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Day DE, Bartness TJ. Agouti-related protein increases food hoarding, but not food intake by Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 286: R38–R45, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Day DE, Bartness TJ. Fasting-induced increases in hoarding are dependent on the foraging effort level. Physiol Behav 78: 655–668, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Day DE, Bartness TJ. Effects of foraging effort on body fat and food hoarding by Siberian hamsters. J Exp Zool 289: 162–171, 2001. [PubMed] [Google Scholar]
- 16.Day DE, Keen-Rhinehart E, Bartness TJ. Role of NPY and its receptor subtypes in foraging, food hoarding and food intake by Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 289: R29–R36, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Faulconbridge LF, Grill HJ, Kaplan JM. Distinct forebrain and caudal brainstem contributions to the neuropeptide Y mediation of ghrelin hyperphagia. Diabetes 54: 1985–1993, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Guan JL, Wang QP, Kageyama H, Takenoya F, Kita T, Matsuoka T, Funahashi H, Shioda S. Synaptic interactions between ghrelin- and neuropeptide Y-containing neurons in the rat arcuate nucleus. Peptides 24: 1921–1928, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van Der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 48: 23–29, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Hagan MM, Rushing PA, Pritchard LM, Schwartz MW, Strack AM, Van Der Ploeg LH, Woods SC, Seeley RJ. Long-term orexigenic effects of AgRP-(83—132) involve mechanisms other than melanocortin receptor blockade. Am J Physiol Regul Integr Comp Physiol 279: R47–R52, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Harland D, Bennett T, Gardiner SM. Cardiovascular actions of neuropeptide Y in the hypothalamic paraventricular nucleus of conscious Long Evans and Brattleboro rats. Neurosci Lett 85: 121–123, 1988. [DOI] [PubMed] [Google Scholar]
- 22.Hewson AK, Dickson SL. Systemic administration of ghrelin induces Fos and Egr-1 proteins in the hypothalamic arcuate nucleus of fasted and fed rats. J Neuroendocrinol 12: 1047–1049, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Hwa JJ, Witten MB, Williams P, Ghibaudi L, Gao J, Salisbury BG, Mullins D, Hamud F, Strader CD, Parker EM. Activation of the NPY Y5 receptor regulates both feeding and energy expenditure. Am J Physiol Regul Integr Comp Physiol 277: R1428–R1434, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Illius AW, Tolkamp BJ, Yearsley J. The evolution of the control of food intake. Proc Nutr Soc 61: 465–472, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Iyengar S, Li DL, Simmons RM. Characterization of neuropeptide Y-induced feeding in mice: do Y1-Y6 receptor subtypes mediate feeding? J Pharmacol Exp Ther 289: 1031–1040, 1999. [PubMed] [Google Scholar]
- 26.Jewett DC, Cleary J, Levine AS, Schaal DW, Thompson T. Effects of neuropeptide Y on food-reinforced behavior in satiated rats. Pharmacol Biochem Behav 42: 207–212, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology 141: 4797–4800, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Kamegai J, Tamura H, Shimizu T, Ishii S, Sugihara H, Wakabayashi I. Chronic central infusion of ghrelin increases hypothalamic neuropeptide Y and agouti-related protein mRNA levels and body weight in rats. Diabetes 50: 2438–2443, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Kanatani A, Ishihara A, Asahi S, Tanaka T, Ozaki S, Ihara M. Potent neuropeptide YY1 receptor antagonist, 1229U91: blockade of neuropeptide Y-induced and physiological food intake. Endocrinology 137: 3177–3182, 1996. [DOI] [PubMed] [Google Scholar]
- 30.Kanatani A, Ishihara A, Iwaasa H, Nakamura K, Okamoto O, Hidaka M, Ito J, Fukuroda T, MacNeil DJ, Van Der Ploeg LH, Ishii Y, Okabe T, Fukami T, Ihara M. l-152,804: orally active and selective neuropeptide Y Y5 receptor antagonist. Biochem Biophys Res Commun 272: 169–173, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Kask A, Rago L, Harro J. Evidence for involvement of neuropeptide Y receptors in the regulation of food intake: studies with Y1-selective antagonist BIBP3226. Br J Pharmacol 124: 1507–1515, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 288: R716–R722, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Kim EM, Welch CC, Grace MK, Billington CJ, Levine AS. Effects of palatability-induced hyperphagia and food restriction on mRNA levels of neuropeptide-Y in the arcuate nucleus. Brain Res 806: 117–121, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Kim MS, Namkoong C, Kim HS, Jang PG, Kim Pak YM, Katakami H, Park JY, Lee KU. Chronic central administration of ghrelin reverses the effects of leptin. Int J Obes Relat Metab Disord 28: 1264–1271, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Kim MS, Yoon CY, Park KH, Shin CS, Park KS, Kim SY, Cho BY, Lee HK. Changes in ghrelin and ghrelin receptor expression according to feeding status. Neuroreport 14: 1317–1320, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Kohno D, Gao HZ, Muroya S, Kikuyama S, Yada T. Ghrelin directly interacts with neuropeptide-Y-containing neurons in the rat arcuate nucleus: Ca2+ signaling via protein kinase A and N-type channel-dependent mechanisms and cross-talk with leptin and orexin. Diabetes 52: 948–956, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Konturek SJ, Konturek JW, Pawlik T, Brzozowski T. Brain-gut axis and its role in the control of food intake. J Physiol Pharmacol 55: 137–154, 2004. [PubMed] [Google Scholar]
- 38.Lawrence CB, Snape AC, Baudoin FM, Luckman SM. Acute central ghrelin and GH secretagogues induce feeding and activate brain appetite centers. Endocrinology 143: 155–162, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Lu XY, Nicholson JR, Akil H, Watson SJ. Time course of short-term and long-term orexigenic effects of Agouti- related protein (86–132). Neuroreport 12: 1281–1284, 2001. [DOI] [PubMed] [Google Scholar]
- 40.Mercer JG, Lawrence B, Beck B, Burlet A, Atkinson T, Barrett P. Hypothalamic NPY and preproNPY mRNA in Djungarian hamsters: effects of food deprivation and photoperiod. Am J Physiol Regul Integr Comp Physiol 269: R1099–R1106, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Mercer JG, Moar KM, Ross AW, Morgan PJ. Regulation of leptin receptor, POMC and AGRP gene expression by photoperiod and food deprivation in the hypothalamic arcuate nucleus of the male Siberian hamster (Phodopus sungorus). Appetite 34: 109–111, 2000. [DOI] [PubMed] [Google Scholar]
- 42.Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology 140: 4551–4557, 1999. [DOI] [PubMed] [Google Scholar]
- 43.Mizuno TM, Mobbs CV. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology 140: 814–817, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Morley JE, Levine AS, Gosnell BA, Kneip J, Grace M. Effect of neuropeptide Y on ingestive behaviors in the rat. Am J Physiol Regul Integr Comp Physiol 252: R599–R609, 1987. [DOI] [PubMed] [Google Scholar]
- 45.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature 409: 194–198, 2001. [DOI] [PubMed] [Google Scholar]
- 46.Newsholme EA, Leech AR. Biochemistry for the Medical Sciences. Chichester, NY: Wiley, 1983.
- 47.Polidori C, Ciccocioppo R, Regoli D, Massi M. Neuropeptide Y receptor(s) mediating feeding in the rat: characterization with antagonists. Peptides 21: 29–35, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Seeley R, Payne CJ, Woods SC. Neuropeptide Y fails to increase intraoral intake in rats. Am J Physiol Regul Integr Comp Physiol 268: R423–R427, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Seoane LM, Lopez M, Tovar S, Casanueva FF, Senaris R, Dieguez C. Agouti-related peptide, neuropeptide Y, and somatostatin-producing neurons are targets for ghrelin actions in the rat hypothalamus. Endocrinology 144: 544–551, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Shintani M, Ogawa Y, Ebihara K, izawa-Abe M, Miyanaga F, Takaya K, Hayashi T, Inoue G, Hosoda K, Kojima M, Kangawa K, Nakao K. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes 50: 227–232, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Smith GP Introduction to the reviews on peptides and the control of food intake and body weight. Neuropeptides 33: 323–328, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Stanley BG, Magdalin W, Seirafi A, Nguyen MM, Leibowitz SF. Evidence for neuropeptide Y mediation of eating produced by food deprivation and for a variant of the Y1 receptor mediating this peptide's effect. Peptides 13: 581–587, 1992. [DOI] [PubMed] [Google Scholar]
- 53.Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol 23: 7973–7981, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tang-Christensen M, Kristensen P, Stidsen CE, Brand CL, Larsen PJ. Central administration of Y5 receptor antisense decreases spontaneous food intake and attenuates feeding in response to exogenous neuropeptide Y. J Endocrinol 159: 307–312, 1998. [DOI] [PubMed] [Google Scholar]
- 55.Toshinai K, Mondal MS, Nakazato M, Date Y, Murakami N, Kojima M, Kangawa K, Matsukura S. Upregulation of ghrelin expression in the stomach upon fasting, insulin- induced hypoglycemia, and leptin administration. Biochem Biophys Res Commun 281: 1220–1225, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 407: 908–913, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Vander Wall SB Food Hoarding in Animals. Chicago, IL: Univ. Chicago Press, 1990.
- 58.VanNess JM, DeMaria JE, Overton JM. Increased NPY activity in the PVN contributes to food-restriction induced reductions in blood pressure in aortic coarctation hypertensive rats. Brain Res 821: 263–269, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Wang L, Saint-Pierre DH, Tache Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y-synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci Lett 325: 47–51, 2002. [DOI] [PubMed] [Google Scholar]
- 60.Weiner J Limits to energy budget and tactics in energy investments during reproduction in the Djungarian hamster (Phodopus sungorus sungorus Pallas 1770). Symp Zool Soc Lond 57: 167–187, 1987. [Google Scholar]
- 61.Wieland HA, Engel W, Eberlein W, Rudolf K, Doods HN. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO 3304 and its effect on feeding in rodents. Br J Pharmacol 125: 549–555, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wood AD, Bartness TJ. Food deprivation-induced increases in hoarding by Siberian hamsters are not photoperiod-dependent. Physiol Behav 60: 1137–1145, 1996. [DOI] [PubMed] [Google Scholar]
- 63.Wren AM, Small CJ, Fribbens CV, Neary NM, Ward HL, Seal LJ, Ghatei MA, Bloom SR. The hypothalamic mechanisms of the hypophysiotropic action of ghrelin. Neuroendocrinology 76: 316–324, 2002. [DOI] [PubMed] [Google Scholar]
- 64.Wren AM, Small CJ, Ward HL, Murphy KG, Dakin CL, Taheri S, Kennedy AR, Roberts GH, Morgan DG, Ghatei MA, Bloom SR. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 141: 4325–4328, 2000. [DOI] [PubMed] [Google Scholar]
- 65.Wyss P, Stricker-Krongrad A, Brunner L, Miller J, Crossthwaite A, Whitebread S, Criscione L. The pharmacology of neuropeptide Y (NPY) receptor-mediated feeding in rats characterizes better Y5 than Y1, but not Y2 or Y4 subtypes. Regul Pept 75–76: 363–371, 1998. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.