Abstract
Food deprivation stimulates foraging and hoarding and to a much lesser extent, food intake in Siberian hamsters. Leptin, the anorexigenic hormone secreted primarily from adipocytes, may act in the periphery, the brain, or both to inhibit these ingestive behaviors. Therefore, we tested whether leptin given either intracerebroventricularly or intraperitoneally, would block food deprivation-induced increases in food hoarding, foraging, and intake in animals with differing foraging requirements. Hamsters were trained in a running wheel-based food delivery foraging system coupled with simulated burrow housing. We determined the effects of food deprivation and several peripheral doses of leptin on plasma leptin concentrations. Hamsters were then food deprived for 48 h and given leptin (0, 10, 40, or 80 μg ip), and additional hamsters were food deprived for 48 h and given leptin (0, 1.25, 2.5, or 5.0 μg icv). Foraging, food intake, and hoarding were measured postinjection. Food deprivation stimulated food hoarding to a greater degree and duration than food intake. In animals with a foraging requirement, intracerebroventricular leptin almost completely blocked food deprivation-induced increased food hoarding and intake, but increased foraging. Peripheral leptin treatment was most effective in a sedentary control group, completely inhibiting food deprivation-induced increased food hoarding and intake at the two highest doses, and did not affect foraging at any dose. Thus, the ability of leptin to inhibit food deprivation-induced increases in ingestive behaviors differs based on foraging effort (energy expenditure) and the route of administration of leptin administration.
Keywords: foraging, wheel running, feeding, hypothalamus, Siberian hamsters
obesity is a disease of, literally and figuratively, enormous proportions. As of yet, there are currently no effective treatments for obesity, and this disease continues to run rampant throughout developed and underdeveloped countries. Therefore, innovative and alternative lines of basic research are needed to forge the beginnings of pathways to new potential obesity treatments. One critical area of basic research involves determining the neuroendocrine factors that regulate ingestive behavior. Often ingestive behavior is thought of in terms of food intake only, but it is important to consider the entire sequence of events associated with food, and this includes two phases: 1) the acquisition and storage of food or the appetitive phase and 2) the actual eating of the food or the consummatory phase (20). The consummatory aspects of ingestive behavior have received the most attention in the quest to understand the energy intake portion of the obesity phenomenon. As for the appetitive phase of ingestive behavior, consisting of foraging and food hoarding, there is comparatively little known about the mechanisms underlying these widely expressed behaviors across animal species (for review, see Refs. 32 and 60). Therefore, understanding how both the appetitive and consummatory phases of ingestive behavior are controlled may provide key insights into the etiology of obesity that could lead to new avenues for its treatment.
Siberian hamsters (Phodopus sungorus) and other hamster species (for a review, see Ref. 11) primarily increase foraging (10, 22) and food hoarding (8, 9, 62), that is, appetitive behaviors, in response to energetic challenges. Specifically, Siberian hamsters (and other animals that have the capacity to transport significant amounts of food; for a review see Ref. 60) use food hoarding as a crucial part of their ingestive behavioral repertoire in response to many naturally-occurring energy demands [e.g., food shortages, pregnancy, lactation (8, 10); for a review see Ref. 10]. In this manner, Siberian hamsters are not unlike humans that transport food back to their domiciles in their vehicles and store it in their refrigerators/pantries for later consumption, as evidenced by the finding that 85% of all purchased food is eaten at home (50). Therefore, Siberian hamsters are an ideal model for studying the neuroendocrine factors that regulate both appetitive and consummatory ingestive behaviors compared with other rodents, where appetitive behaviors are a smaller part of their naturally occurring ingestive behavior repertoire (41) or where both appetitive and consummatory ingestive behaviors increase or decrease together, such as in laboratory rats and mice (for a review, see Ref 10).
Food deprivation is a naturally-occurring energetic challenge encountered by Siberian hamsters and triggers a plethora of alterations in peripheral metabolism and signaling peptides as well as in central neurochemicals (for a review, see Ref. 49). When food is available again, Siberian hamsters markedly increase their appetitive ingestive behaviors (foraging and especially hoarding), with either no increase in food intake or relatively minor increases compared with other species tested (for a review, see Ref 10). The exact mechanisms underlying these food deprivation-induced increases in appetitive ingestive behaviors in Siberian hamsters are just beginning to be uncovered. For example, we now know that food deprivation triggers increases in circulating concentrations of the largely stomach-derived peptide ghrelin in Siberian hamsters (34), as it does in laboratory rats (57) and humans (2), and that peripheral ghrelin treatment stimulates foraging and food hoarding and, to a lesser extent, food intake in Siberian hamsters (34). We also now know that the ability of food deprivation or ghrelin to stimulate appetitive and consummatory ingestive behaviors is impaired by central treatment with anorexigenic agents such as the neuropeptide Y (NPY), a Y1- receptor antagonist 1229U91, and the melanocortin 3/4 receptor agonist melanotan II (35, 36).
A physiological factor that may participate in the termination of appetitive ingestive behaviors is leptin, the product of the obesity gene (Ob) that is synthesized and primarily secreted by white adipocytes (63). Circulating leptin concentrations are decreased by food deprivation and increased by feeding (1). In rodents, central leptin reduces voluntary food intake (25, 54). Leptin treatment following food deprivation abolishes the normal increases in food intake seen in laboratory rats (42) and mice (51). Not surprising, given that food deprivation stimulates ghrelin secretion (see above), is that leptin blocks the ability of ghrelin to stimulate food intake (3). The possible acute inhibitory/satiety effects of leptin on appetitive consummatory ingestive behaviors have been studied in Siberian hamsters, but chronic exogenous administration of the cytokine results in decreases in food intake (4, 38) or, in one case, increases in food intake (27). Acute effects of leptin have not been studied nor have there been any tests of leptin on appetitive ingestive behaviors in this species. Leptin does, however, decrease food hoarding by Syrian hamsters [Mesocricetus auratus; (53)], but foraging was not assessed, central application of leptin was not done, and food deprivation-induced changes in serum leptin concentrations, as well as serum leptin concentrations after its administration were not measured. Therefore, we asked: can either peripheral or central leptin treatment block food deprivation-induced increases in foraging, food hoarding, and food intake in Siberian hamsters? This was accomplished by attempting to block 48-h food deprivation-induced increases in foraging and food hoarding by injecting murine leptin either intraperitoneally or intracerebroventricularly into the third ventricle of food-deprived, long day-housed, male Siberian hamsters living in a running wheel-based, food delivery-foraging system that is coupled with simulated burrow-housing (21). Murine leptin was used because of the unavailability of purified hamster leptin and because there is ∼95% amino acid sequence homology between hamster and mouse leptin (44).
METHODS
Animals
All procedures were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with Public Health Service and United States Department of Agriculture guidelines. Adult male Siberian hamsters, n = 104, ∼3.5 mo old, and weighing 35–50 g were obtained from our breeding colony. The lineage of this colony has been described recently (15). In brief, the breeding stock for this colony was donated by Dr. Bruce Goldman (University of Connecticut in 1988), based on founder stock from Dr. Klaus Hoffman (Germany), and interbred with second generation wild-trapped hamsters donated by Dr. Katherine Wynne-Edwards (Queens College) in 1990. In 1995, the colony hamsters were interbred with animals donated by Dr. G. Robert Lynch (University of Colorado) that also had originated from the original Hoffman stock but had been kept isolated for ∼20 yr. Later in 1999, F2 generation wild-trapped hamsters were interbred with the colony through a generous gift from Dr. Stephan Steinlechner (School of Veterinary Medicine, Hannover, Germany). Hamsters were group-housed and raised in a long-day photoperiod (16:8-h light-dark cycle; lights on at 0200) from birth. Room temperature was maintained at 21 ± 2.0°C.
Experiment 1: Does Food Deprivation Decrease Plasma Leptin in Siberian Hamsters, and How Is It Affected by Different Doses of Peripheral Leptin Treatment?
Twenty male Siberian hamsters with body masses ranging from 35–50 g kept in standard shoebox cage housing were used in this experiment. Intraorbital blood samples were drawn on each hamster at the onset of the experiment and then again after 48 h of food deprivation. The animals were then given one of the following treatments: saline; 10, 40, or 80 μg leptin ip (recombinant murine leptin, Peprotech, Rocky Hill, NJ); refed; and blood samples taken at 4 and 24 h after animals postrefeeding/injection. Blood samples were spun in a centrifuge at 3,000 rpm for 40 min, and the serum was collected. Serum samples were then analyzed in a leptin ELISA as described below.
Leptin ELISA.
The serum leptin concentrations in plasma obtained from animals in experiment 1 were determined using a mouse ELISA kit (Linco Research, St. Charles, MO) according to the manufacturer's instructions, as reported previously (16, 17, 40). All samples were run in duplicate in the same assay, and the limits of the assay were 0.05 to 30 ng/ml.
General food foraging, hoarding, and food intake protocols for experiments 2 and 3, hoarding apparatuses.
Hamsters used in experiments 2 and 3 were acclimated for 2 wk in specially designed hoarding apparatuses as previously described (21) that would serve as their housing for the duration of the experiment. More specifically, two cages were connected with a convoluted polyvinylchloride tubing system (38.1 mm ID and ∼1.52-m long) with corners and straightaways for horizontal and vertical climbs. The diet (75 mg pellets, Purified Rodent Diet; Research Diets, New Brunswick, NJ) and tap water were available ad libitum. A running wheel (524 mm circumference) and magazine-type pellet dispenser, which holds ∼1,750 pellets and always delivers one whole pellet at a time, were attached to the food (top) cage. Wheel revolutions were counted using a magnetic-detection response system that produced a switch closure every 360 degrees of wheel rotation that was monitored by a computer-based hardware/software program (Med Associates, Lancaster, NH). For experiment 3 only, hamsters were first trained in these apparatuses (24, 37) and then received a third ventricular cannula (23, 24), as previously described and described in brief below.
Measurement of foraging, food hoarding, and food intake.
Foraging (pellets earned) was defined as the number of pellets delivered upon completion of the requisite wheel revolutions. Food hoarding (pellets hoarded) was defined as the number of pellets found in the bottom cage (burrow) in addition to those removed from the cheek pouches. For the 10 Revolutions/Pellet groups, food intake (pellets eaten) was defined as: pellets earned − surplus pellets − hoarded pellets = food intake. For the Free and Blocked Wheel groups, food intake (pellets eaten) was defined as: pellets given − pellets left in the top cage − hoarded pellets = food intake. An electronic balance used to weigh the food pellets was set to parts measurement rather than obtaining fractions of a pellet in milligrams; thus, one 75-mg food pellet = 1, and fractions of a pellet were computed by the scale.
Foraging training regimen.
We used a wheel-running training regimen that eases the hamsters into their foraging efforts without changes in body mass or food intake (21). Specifically, hamsters were given free access to food pellets for 2 days while they adapted to the running wheel. In addition to the free food, a 75-mg food pellet was dispensed upon completion of every 10 wheel revolutions. On day 3, the free food condition was replaced by a response-contingent condition, where only every 10 wheel revolutions triggered the delivery of a pellet. This condition was in effect for 5 days during which time body mass, food intake, food hoarding, wheel revolutions, and pellets earned were measured daily. During this time there was little or no evidence of changes in food intake or body weight. Because these animals are outbred from wild-caught populations, we did observe the expected inherent individual variability in food intake, food hoarding, and foraging in this population of animals, which has evolved polymorphic and plastic responses due to the potential for fluctuating food availability in their natural environment. At the end of this acclimation period (7 days total), animals in the intracerebroventricular leptin experiment were removed from the foraging apparatuses and temporarily housed in shoebox cages where the same food pellets were available ad libitum with no foraging requirements. Guide cannulas were then surgically implanted in these hamsters (see below for details). Following a 1-wk postsurgical recovery period, all hamsters were returned to the hoarding/foraging apparatus and retrained in the following schedule: 2 days for adaptation with free access to food pellets and 5 days at 10 revolutions/pellet.
Experimental design for experiments 2 and 3.
At the end of training, the hamsters for use in experiments 2 and 3 were separated into three groups matched for their current body mass and average hoard size across these last 3 days of training at 10 Revolutions/pellet (n = 14/group). The three groups consisted of 10 revolutions/pellet foraging requirement, no foraging requirement with an active running wheel (Free Wheel; exercise control group), or no foraging requirement with a blocked wheel (Blocked Wheel; sedentary control group); each of the last two had food available noncontingently. Selection of the 10 revolutions/pellet foraging effort was based on a previous study in Siberian hamsters using this foraging/hoarding system to maximize hoarding levels (21).
Experiment 2: Does Peripheral Leptin Treatment Block the Effects of Food Deprivation on Ingestive Behaviors in Siberian Hamsters?
An additional 42 male hamsters with a starting average body mass of 39.68 + 0.71 g were trained in the foraging/hoarding apparatuses for experiment 2. These animals were separated into three groups: 10 Revolutions/Pellet (foraging) group (n = 14), Free Wheel (exercise) group (n = 14), and Blocked Wheel (sedentary) group (n = 14) as described above; were food deprived at 39.88 + 0.89 g average body mass for 48 h; and were then injected intraperitoneally with one of four solutions at the onset of the dark phase of the light cycle: sterile saline vehicle or 10, 40, or 80 μg of leptin (recombinant murine leptin; Peprotech, Rocky Hill, NJ). Following these intraperitoneal injections, food intake, wheel running, and food hoarding were monitored at 1, 2, 4, 24, and 48 h postinjection. After a 2-wk recovery/washout period, animals were reassigned to one of the four treatments listed above in a counterbalanced fashion, and the same behavioral measurements were performed a second time. In our previous studies of food hoarding, we have used food-deprivation periods ranging from 12 to 56 h (Institutional Animal Care and Use Committee approved) with the latter length appearing somewhat lengthy or nonphysiological at first blush. In the utopian conditions of the laboratory, however, Siberian hamsters are almost 50% body fat compared with as low as ∼25% in nature (61); therefore, short periods of food deprivation in the laboratory of 12–24 h are minimally energetically challenging in these animals, and thus stimulation of food hoarding is minimal (Clein MR and Bartness TJ, unpublished results). Therefore, we selected 48-h food deprivation to trigger the behavior nearly maximally. It also seems reasonable to envision these food-deprivation lengths as on a physiological continuum with the intermeal intervals occurring naturally of much shorter lengths in hamsters [∼4 h (12)].
Experiment 3: Does Intracerebroventricular Leptin Treatment Block the Effects of Food Deprivation on Ingestive Behaviors in Siberian Hamsters?
An additional 42 male hamsters with a starting average body mass of 39.73 + 0.81 g were implanted with third ventricular cannulas for experiment 3, as described below. These animals were separated into three groups: 10 Revolutions/Pellet (foraging) group (n = 14), Free Wheel (exercise) group (n = 14), and Blocked Wheel (sedentary) group (n = 14) as described above, and after recovery at an average body weight of 39.07 + 0.84 g, they were food deprived for 48 h and then injected intracerebroventricularly with one of four solutions at the onset of the dark phase of the light cycle, i.e., sterile saline vehicle or 1.25 μg, 2.5 μg or 5.0 μg of leptin (recombinant murine leptin; Peprotech, Rocky Hill, NJ). Following these intracerebroventricular injections, food intake, wheel running, and food hoarding were monitored at 1, 2, 4, 24, and 48 h postinjection. After a 2-wk washout period, animals were reassigned to one of the four treatments listed above in a counterbalanced fashion, and the same behavioral measurements were performed a second time.
Cannula implantation.
Cannulas were stereotaxically implanted into the third ventricle of hamsters used in experiment 3, as described previously (23). In brief, the animals were anesthetized with isoflurane and the fur at the top of the head was removed to expose the area to be incised. A hole was trephined at the intersection of bregma and the midsaggital sinus, and the guide cannula (26-gauge stainless steel; Plastics One, Roanoke, VA) was lowered using the following stereotaxic coordinates (level skull, anterior-posterior from bregma 0, medial-lateral from midsaggital sinus 0, and dorsal-ventral from the top of the skull −5.0 mm) targeted for placement just above the third ventricle. The guide cannula was secured to the skull using cyanoacrylate ester gel, 3/16-mm jeweler's screws, and dental acrylic. A removable obturator sealed the opening in the guide cannula throughout the experiment, except when it was removed for the injections. Hamsters received 0.2 mg/kg buprenorphine at 12 and 24 h postsurgery to minimize discomfort, and subsequently were allowed 1 wk to recover fully in the shoebox cage housing before being returned to their simulated burrow housing.
Intracerebroventricular injection protocol.
The inner cannula (33-gauge stainless steel, Plastics One, Roanoke, VA) extended 5.5 mm below the top of the skull and all hamsters were injected with a 0.4-μl volume. All injections were given at the beginning of the dark phase of the photoperiod. Animals were lightly restrained by hand during the 30-s injection, and the injection needle remained in place ∼30 s before withdrawal as we have done previously (23, 24).
Cannulas verification.
Following the last test in experiment 3, an injection of 0.4 μl bromophenol blue dye was given to confirm placement of the cannula in the third ventricle. The animals were anesthetized with an overdose of pentobarbital sodium (100 mg/kg), and their brains removed and postfixed in 10% paraformaldehyde for a minimum of two days. Each brain was sliced manually for cannula verification. Cannulas were considered to be located in the third ventricle if the dye was visible in any part of this ventricle. Only the data from animals with confirmed third-ventricle cannula placements were included in the analyses (n = 42), and there was no incidence of cannula loss during the study.
Statistical Analyses
In experiment 1, leptin concentrations in response to food deprivation were analyzed by a Student's t-test, the plasma leptin concentrations in response to intraperitoneal leptin injection were analyzed by one-way ANOVA, and Tukey's multiple-comparison tests were used for individual pairwise comparison. For all measures of food intake, foraging, and food hoarding in experiments 2 and 3, the data were analyzed for each time interval to identify intervals where the effects were occurring; thus, no statistical comparisons were made across time points (i.e., there was no effect of time analysis). Therefore, the data from one time interval only are compared with data within that time interval. Data were analyzed using a two-way ANOVA (Foraging Effort group × leptin treatment: 3 × 4) and Bonferroni's post hoc tests were used for individual pairwise comparison. All statistical analyses were done using GraphPad Prism Software (San Diego, CA). Differences between means were considered statistically significant if P < 0.05. Exact probabilities and test values were omitted for simplicity and clarity of the presentation of the results.
RESULTS
Experiment 1: Does Food Deprivation Decrease Plasma Leptin in Siberian Hamsters, and How Is It Affected by Different Doses of Peripheral Leptin Treatment?
Plasma leptin.
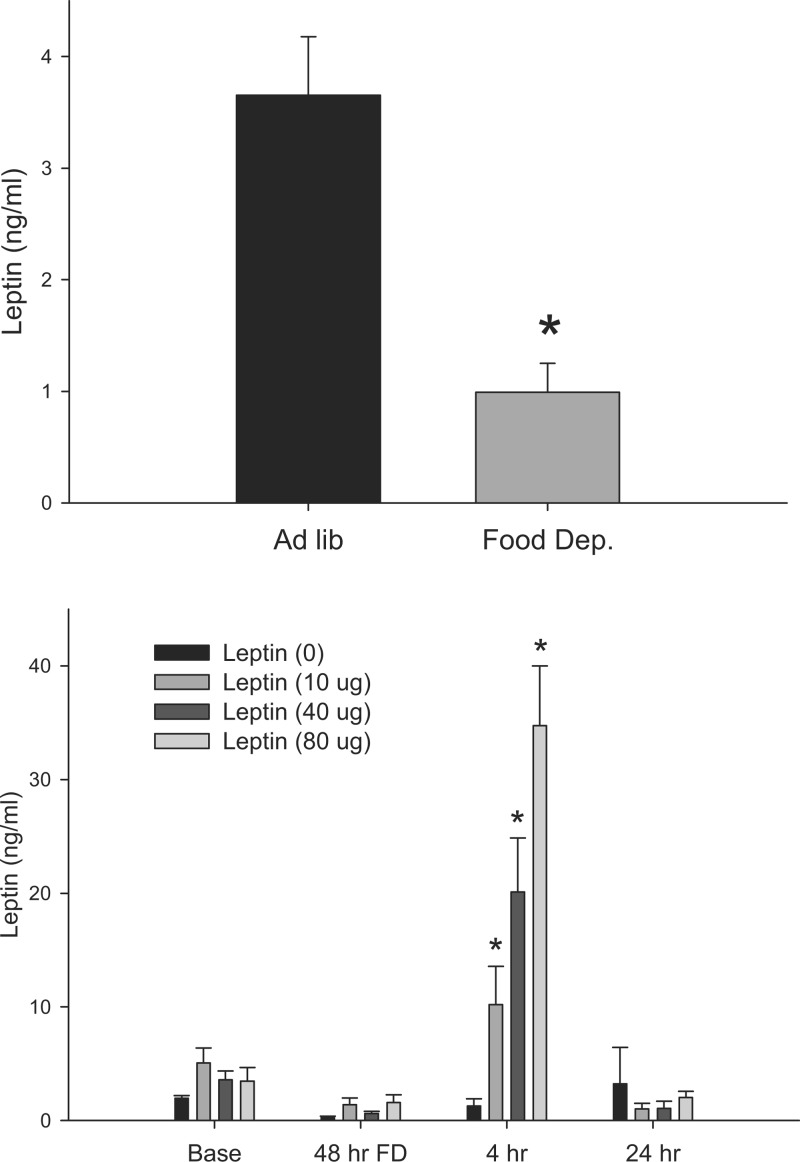
Forty-eight hours of food deprivation significantly decreased serum leptin concentration by ∼4-fold (Fig. 1; P < 0.05). Peripheral leptin treatment dose-dependently increased serum leptin concentrations up to 4 h postinjection, such that the 10, 40, and 80 μg of intraperitoneal leptin resulted in approximately two-, four- and sevenfold increases compared with baseline values (Fig. 1; P < 0.05). By 24 h, postinjection serum leptin concentrations had returned to baseline (Fig. 1).
Fig. 1.
Top: mean ± SE plasma leptin expressed in nanograms per milliliter at baseline (black bar, n = 10) and after a 48-h food deprivation (grey bar, n = 10). *P < 0.05 compared with adlibitum-fed controls. Bottom: means ± SE plasma leptin expressed in nanograms per milliliter at baseline, after 48 h food deprivation, and after refeeding with 0 μg (n = 5), 10 μg (n = 5), 40 μg (n = 5), and 80 μg (n = 5) peripheral leptin treatment. *P < 0.05 compared with 0 μg leptin baseline condition.
Experiment 2: Does Peripheral Leptin Treatment Block the Effects of Food Deprivation on Ingestive Behaviors in Siberian Hamsters?
Wheel running.
Leptin did not affect cumulative wheel running after food deprivation, a general measure of locomotor activity, by the Free Wheel group (data not shown).
Foraging.
Peripheral leptin treatment did not significantly alter foraging (pellets earned) after food deprivation by the 10 Revolutions/Pellet groups (data not shown).
Food intake.
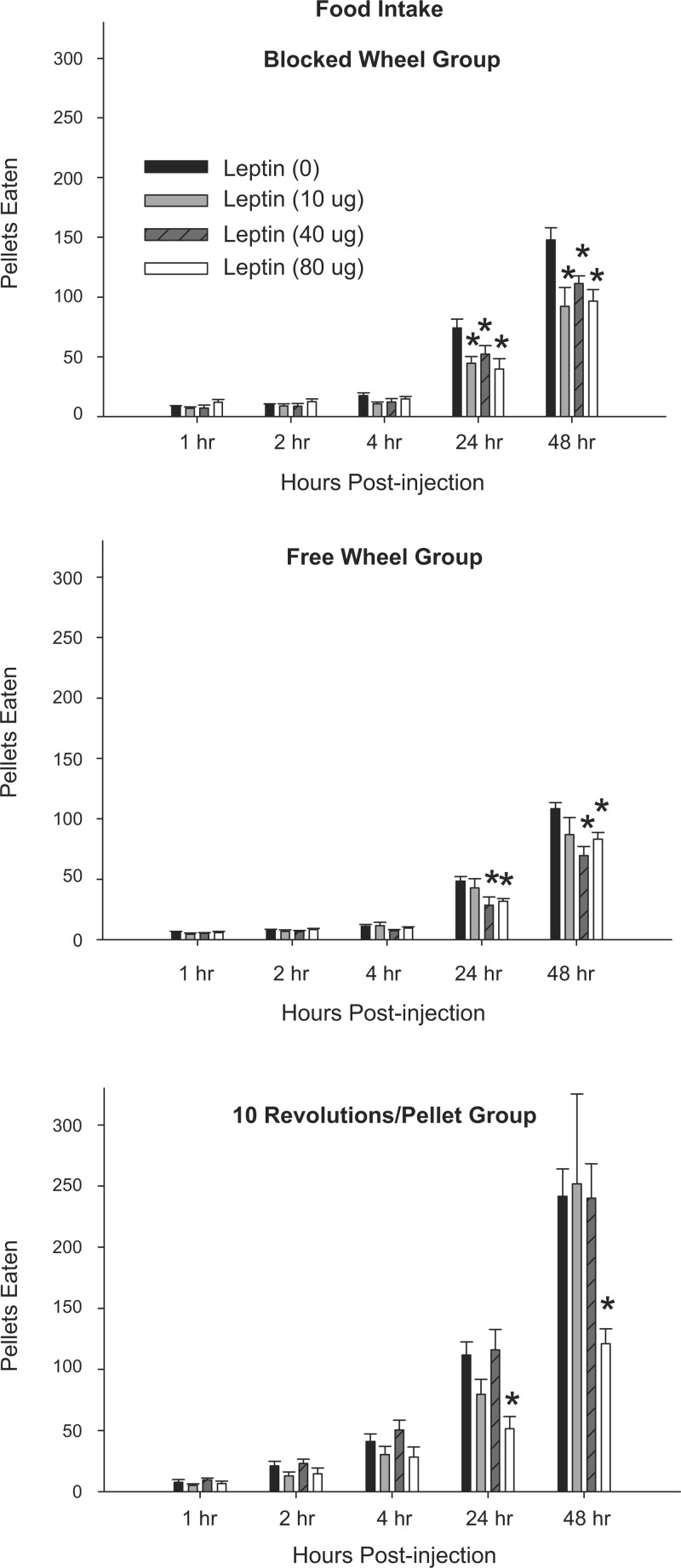
The hamsters with an explicit foraging requirement (10 Revolutions/Pellet) ate approximately twice as much as the Free Wheel and Blocked Wheel groups (P < 0.05; Fig. 2). Peripheral leptin treatment most effectively inhibited food deprivation-induced increased food intake by the Blocked Wheel hamsters, decreasing the cumulative number of pellets eaten at 24 and 48 h postinjection at all doses compared with saline injections (Fig. 2, P < 0.05). Leptin also significantly decreased the food deprivation-induced increased cumulative food intake by the Free Wheel group at the two higher doses (40 and 80 μg) at 24 and 48 h postinjection compared with saline (Fig. 2, P < 0.05). Peripheral leptin treatment was the least effective at inhibiting food deprivation-induced increased food intake by the 10 Revolutions/Pellet group, only significantly decreasing the cumulative number of consumed pellets at the 80-μg dose at 24 and 48 h postinjection compared with saline (Fig. 2, P < 0.05).
Fig. 2.
Mean ± SE cumulative food intake expressed as the number of pellets eaten after 48-h food deprivation with 0 μg (n = 7), 10-μg (n = 7), 40-μg (n = 7), and 80-μg doses (n = 7) of peripheral leptin for hamsters without a foraging requirement and a stationary wheel (Blocked Wheel group; top), hamsters with no foraging requirement and a freely moving wheel (Free Wheel group; middle), and hamsters with a foraging requirement (10 Revolutions/Pellet group; bottom) *P < 0.05 compared with the food-deprived group with 0 μg leptin control condition.
Food hoarding.
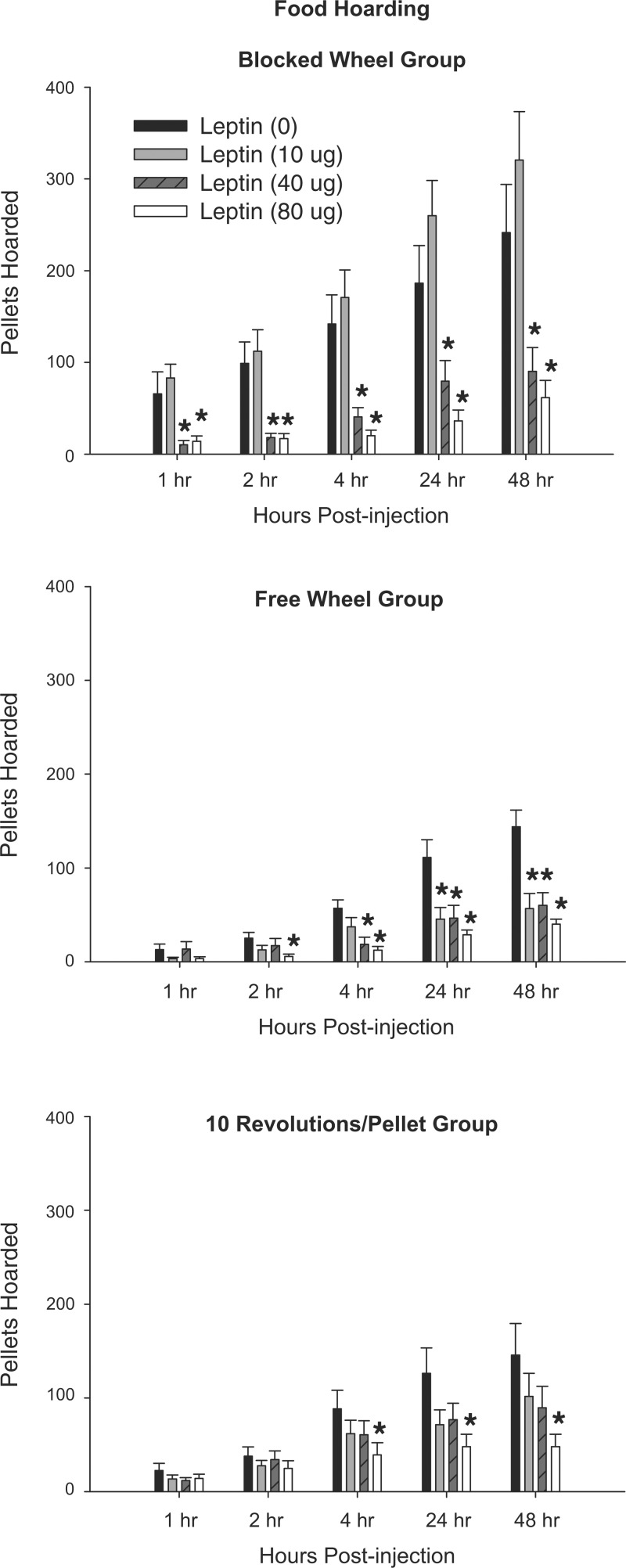
Peripheral leptin treatment significantly blocked the effects of food deprivation on food hoarding in a time-dependent, dose-dependent, and foraging effort-dependent manner. Specifically, in the most sedentary groups of hamsters (Blocked Wheel groups), peripheral leptin treatment at the two highest doses (40 and 80 μg) decreased the cumulative number of pellets hoarded at all times postinjection compared with saline injections (Fig. 3, P < 0.05). In the Free Wheel group, leptin was moderately effective at counteracting the effects of food deprivation on food hoarding. In this group, the lowest dose of leptin significantly inhibited food deprivation-induced food hoarding, but only at the 24 and 48 h postinjection, whereas the 40-μg dose was effective in significantly inhibiting hoarding at 4, 24, and 48 h postinjection compared with the saline vehicle (Fig. 3, P < 0.05). The highest leptin dose (80 μg) was most effective, inhibiting food deprivation-induced increased food hoarding at all times except for the first hour postinjection compared with saline injections in this Free Wheel group (Fig. 3, P < 0.05). In the 10 Revolutions/Pellet groups, leptin was the least effective at blocking the food deprivation-induced increased hoarding vs. the other two groups. Thus, only the highest leptin dose (80 μg) significantly decreased the cumulative number of pellets hoarded at 4, 24, and 48 h postinjection compared with saline injections (Fig. 3, P < 0.05).
Fig. 3.
Mean ± SE of cumulative food hoarding expressed as the number of pellets hoarded after 48-h food deprivation with 0-μg (n = 7), 10-μg (n = 7), 40-μg (n = 7), and 80-μg (n = 7) doses of peripheral leptin for hamsters without a foraging requirement and a stationary wheel (Blocked Wheel group, top), hamsters with no foraging requirement and a freely moving wheel (Free Wheel group, middle), and hamsters with a foraging requirement (10 Revolutions/Pellet group, bottom). *P < 0.05 compared with the food-deprived group with 0 μg leptin control condition.
Experiment 3: Does Intracerebroventricular Leptin Treatment Block the Effects of Food Deprivation on Ingestive Behaviors in Siberian Hamsters?
Wheel running.
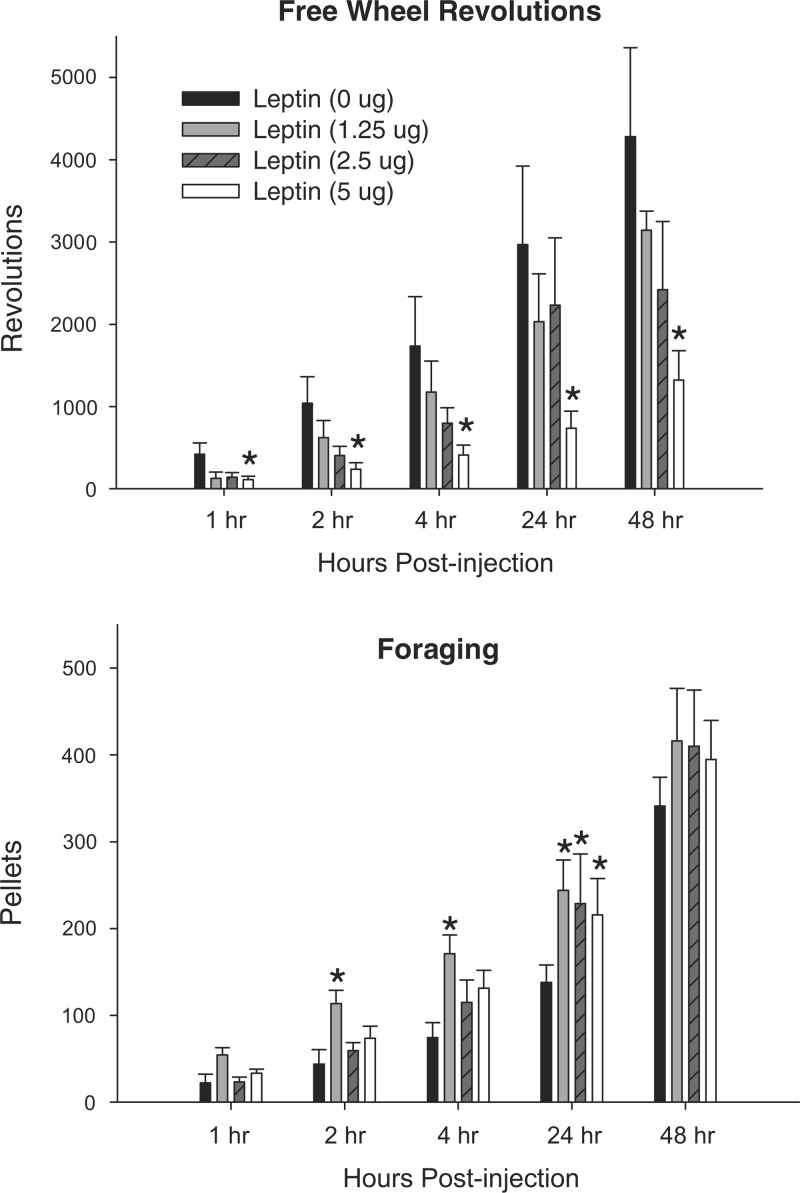
Central leptin treatment at the highest dose (5 μg) significantly decreased cumulative wheel running that was not associated with food delivery (Free Wheel group) at all time points postinjection compared with saline injections (Fig. 4, P < 0.05), an apparent nonspecific effect not seen with the other two lower central leptin doses (1.25 and 2.5 μg).
Fig. 4.
Mean ± SE cumulative wheel revolutions run after 48-h food deprivation with 0-μg (n = 7), 1.25-μg (n = 7), 2.5-μg (n = 7), and 5-μg doses (n = 7) of central leptin for hamsters with no foraging requirement and a freely moving wheel (free wheel revolutions, top) and hamsters with a foraging requirement (foraging, bottom). *P < 0.05 compared with the food-deprived with 0 μg leptin control condition.
Foraging.
Central leptin treatment significantly increased foraging by the 10 Revolutions/Pellet hamsters at 2, 4, and 24 h postinjection at the lowest dose (1.25 μg), and at the two higher doses (2.5 and 5 μg) at 24 h postinjection compared with saline (Fig. 4, P < 0.05).
Food intake.
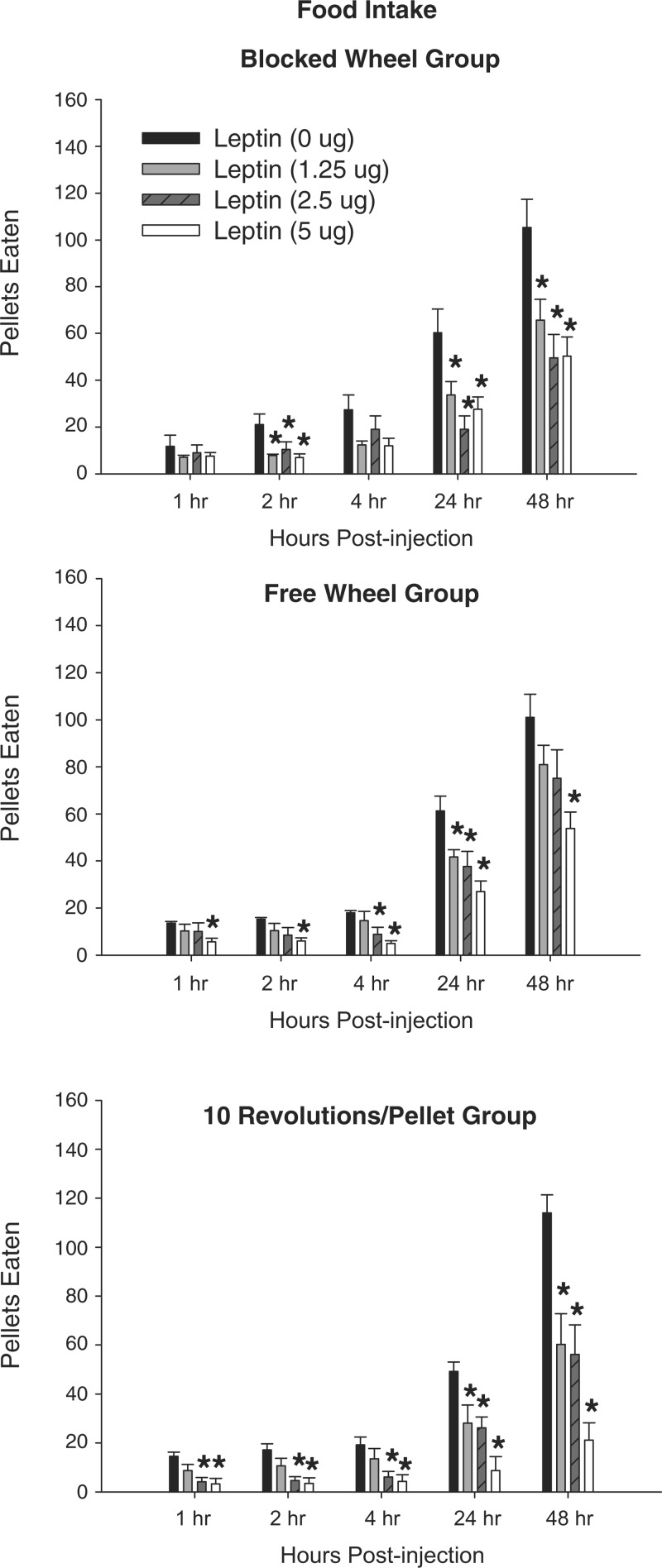
Central leptin treatment at all doses significantly inhibited food deprivation-induced increased food intake in the Blocked Wheel group, decreasing the cumulative number of pellets eaten at 2, 24, and 48 h postinjection (Fig. 5, P < 0.05). For the Free Wheel hamsters, the highest dose of central leptin (5.0 μg) decreased food intake at all times (Fig. 5, P < 0.05), In addition, 2.5 μg leptin treatment only decreased food intake at 4 and 24 h postinjection, whereas the 1.25 μg leptin dose only decreased food intake 24 h postinjection (Fig. 5, P < 0.05). All doses of leptin inhibited food deprivation-induced food intake in the 10 Revolutions/Pellet group at 24 and 48 h postinjection, and the two highest doses (2.5 and 5 μg) also inhibited food deprivation-induced food intake at 1, 2, and 4 h postinjection compared with saline treatment (Fig. 5, P < 0.05).
Fig. 5.
Mean ± SE of cumulative food intake expressed as the number of pellets eaten after 48-h food deprivation with 0-μg (n = 7), 1.25-μg (n = 7), 2.5-μg (n = 7), and 5-μg (n = 7) doses of central leptin for hamsters without a foraging requirement and a stationary wheel (Blocked Wheel group, top), hamsters with no foraging requirement and a freely moving wheel (Free Wheel group, middle), and hamsters with a foraging requirement (10 Revolutions/Pellet group, bottom). *P < 0.05 compared with the food-deprived with 0 μg leptin control condition.
Food hoarding.
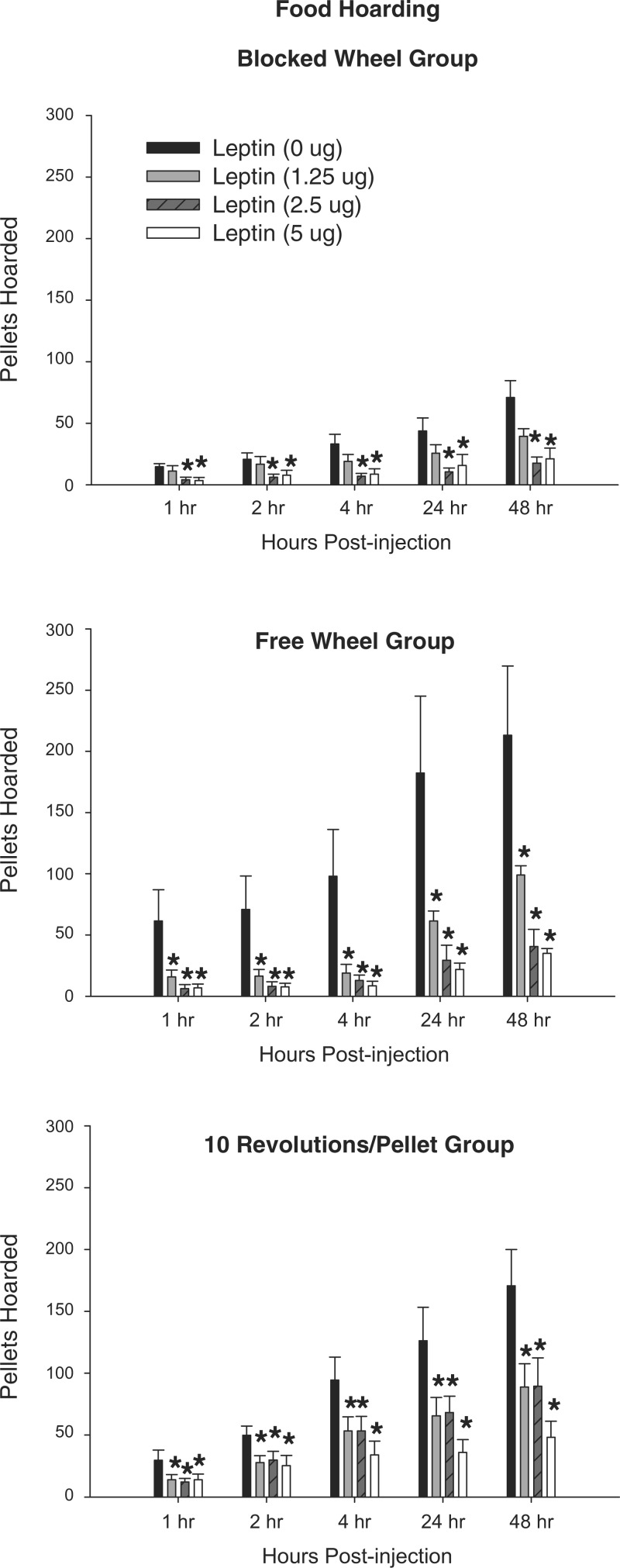
Leptin decreased the cumulative food hoarded at all times for the two higher doses (2.5 and 5.0 μg) in the Blocked Wheel group compared with vehicle-injected controls (Fig. 6, P < 0.05). For both the Free Wheel and 10 Revolutions/Pellet groups, all doses of leptin significantly decreased food deprivation-induced increased food hoarding at all times, postinjection compared with their vehicle-injected counterparts (Fig. 6, P < 0.05).
Fig. 6.
Mean ± SE food hoarding expressed as the number of pellets hoarded after 48-h food deprivation with 0-μg (n = 7), 1.25-μg (n = 7), 2.5-μg (n = 7), and 5.0-μg (n = 7) doses of central leptin for hamsters without a foraging requirement and a stationary wheel (Blocked Wheel group, top), hamsters with no foraging requirement and a freely moving wheel (Free Wheel group, middle), and hamsters with a foraging requirement (10 Revolutions/Pellet group, bottom) *P < 0.05 compared with the food-deprived with 0 μg leptin control condition.
DISCUSSION
The results of this study show that leptin not only inhibits food deprivation-induced increases in consummatory ingestive behavior, but it also effectively inhibits food deprivation-induced increases in food hoarding when given either intracerebroventricularly or peripherally. These data also indicate that the ability of leptin to inhibit food deprivation-induced increases in appetitive and consummatory ingestive behaviors can differ based on foraging effort (energy expenditure) and the route of leptin administration.
Food deprivation decreases plasma leptin concentrations in humans (14), laboratory rats, or mice (28), pigs (7), horses (18), and Siberian hamsters (40, 58). In one report, 24-h food-deprived female Siberian hamsters did not exhibit significant decreases in serum leptin concentrations (29), although this may have been due to the relatively short length of the food deprivation. Others find that food-deprived female Siberian hamsters have decreased serum leptin concentrations when food is withheld for 48 (58) or 56 h (40), and here, we found significantly decreased serum leptin concentrations in male Siberian hamsters food deprived for 48 h.
Peripheral leptin injection did not inhibit food deprivation-induced foraging, but it did inhibit food hoarding and intake as discussed below. Central leptin injections at the middle (2.5 μg) and high (5.0 μg) doses, however, significantly increased foraging, whereas it inhibited food hoarding and intake (also discussed below). This appears to be a bona fide specific central leptin-induced increase in foraging because there was not a nonspecific increase in wheel running in the Free Wheel group where food was not contingent on this locomotor activity. By contrast, wheel running was decreased instead in these hamsters, at least at the highest central leptin dose (5 μg). Interestingly, we recently found that parenchymal microinjections of NPY into the hypothalamic paraventricular nucleus (PVH) dose relatedly decreased foraging (Daily ME and Bartness TJ, unpublished observation). Given the presence of leptin receptors on Arc NPY/agouti-related protein (AgRP) neurons (45) and that leptin inhibits their activity (55), the ability of central leptin to increase foraging fits with the capability of PVN NPY to inhibit foraging. Why peripheral leptin also does not do so is unclear, although this may reflect the general finding of central leptin being more effective in altering ingestive behaviors than peripheral leptin (discussed below).
It is accepted that leptin acts in the central nervous system to alter most ingestive behaviors (19), with central treatments often being more effective than peripheral. Therefore, as noted above for foraging, central leptin treatment appears considerably more effective in inhibiting food deprivation-induced increased food intake, and indeed, significantly decreased food intake within 1–2 h for most doses in most groups; whereas peripheral leptin had unusually long delays in decreasing food intake, becoming significantly much later (24 and 48 h) and often only at the higher doses (Free Wheel and 10 Revolutions/Pellet groups). Similarly, central leptin injections were more effective in significantly decreasing food hoarding, although the rapidity of the central vs. peripheral effects was not as disparate as for the leptin-induced decreases in food intake. That is, peripheral leptin decreased food hoarding at 1, 2, and 4 h postinjection for the Blocked Wheel, Free Wheel, and 10 Revolutions/Pellet groups, respectively, whereas central leptin began decreasing food hoarding for all groups 1 h after injection. The more rapid effect of central compared with peripheral leptin likely reflects the more direct access to central leptin target sites, such as the periventricular hypothalamic region [e.g., (52)] and periventricular brain stem (30) sites of action, than peripheral leptin where transport across the blood brain-barrier might not only require longer time to access such targets, but also might deliver less leptin because of transporter saturation properties (6). Indeed, the nonphysiological level of peripheral leptin resulting from our exogenous administration which could, as with high concentrations of endogenous leptin (5), saturate the uptake system. For example, both obese humans and Siberian hamsters housed in long-day photoperiod have abundant adipose tissue that elevates peripheral leptin concentrations, thereby likely saturating the leptin transporter system and potentially diminishing access of leptin into the brain. Obviously, centrally administered leptin does not have to work through this obstruction to its brain sites of action, and this may be at least part of the observed greater effectiveness of central vs. peripheral leptin on these ingestive behaviors. We also realize, however, that it is impossible to compare the results of central to peripheral injections of any substance in terms of equivalency of doses (47). Thus, an alternative hypothesis is that any differences between centrally vs. peripherally injected leptin could be that the central dose is higher and this could be independent of penetration to the leptin sites of action.
We previously have used this Siberian hamster foraging/hoarding model to help determine the mechanisms underlying food deprivation-induced increases in the appetitive ingestive behaviors of food foraging and hoarding. The present study continues this work to further understand the ability of food deprivation to stimulate foraging and hoarding with refeeding. Thus, to date, it appears that food deprivation stimulates the release of ghrelin from the stomach, as evidenced by a positive relation between the length of food deprivation and circulating ghrelin concentrations (34). Presumably, ghrelin then stimulates its growth hormone secretagogue receptors in the brain [i.e., GHS-R1a; (31, 59)], especially arcuate nucleus NPY/AgRP neurons that have GHS-R1a [e.g.,(46)] to increase the expression and release of these peptides into the PVH and other projection sites (e.g., perifornical area) to increase these appetitive ingestive behaviors. In support of this notion is the ability of central NPY (24) or AgRP (23) to trigger impressive food-deprivation-like increases in foraging and food hoarding. With the present data, and the evidence that leptin may be a physiological antagonist of ghrelin, decreasing NPY and AgRP expression in the hypothalamus and inhibiting food intake [e.g., (48)], there is continued support for this functional conceptualization of how food deprivation stimulates appetitive ingestive behaviors in this and, likely, other species.
Perspectives and Significance
How might circulating leptin affect foraging/hoarding and food intake naturally in Siberian hamsters? With food deprivation, circulating leptin levels drop, as seen in the present experiment, likely due to increases in the sympathetic nervous system drive to white adipose tissue (16) that is known to inhibit leptin secretion (56). In addition, food deprivation triggers increases in ghrelin release that, in turn, stimulates its GHS-R1a receptors, some of which are located on Arc NPY/AgRP neurons. Stimulation of these neurons can cause the release of these peptides in several sites, primarily the PVN [e.g., (33)]. Microinjection of NPY or the NPY Y-1 receptor agonist BIBO 3304 increases foraging/food hoarding similarly to food deprivation in ad libitum-fed Siberian hamsters (Daily ME and Bartness TJ, unpublished observation). With refeeding, however, the physiological milieu is reversed, such that sympathetic drive to white adipose tissue is decreased, and, consequently, leptin secretion is increased [e.g., (39)]. Foraging and hoarding increase, and concomitant with these initial large increases in these appetitive ingestive behaviors comes smaller increases in food intake [e.g., (21); and present study].
Understanding the underlying basis for the fundamental behaviors of foraging (for a review, see Ref. 32) and hoarding (for reviews, see Refs. 10 and 60) that are so pervasive across animal taxa, including humans [e.g., (26)], has great importance for understanding the development of obesity. For example, as your mother said, do not go to the grocery store hungry because you will bring home more food than if you go after you have eaten; and indeed, hungry people bring home more food than sated people [e.g.,(13, 26, 43)]. Obese people bring home more high-fat foods and more calories per person than do lean people (50). Therefore, understanding the underlying mechanisms involved in human foraging and food storing (hoarding) behaviors could greatly impact the obesity epidemic, especially because 85% of purchased food is eaten at home (50). Thus, foraging and hoarding of food may provide another point of attack for pharmacological and/or behavioral intervention.
GRANT
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01-DK-078358 (to T. J. Bartness).
Acknowledgments
The authors thank Raven Jackson, Constance Foster, and Teal Pelish for their help with data collection.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahima RS, Prabakaran D, Mantzoros CS, Qu D, Lowell BB, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 382: 250–252, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86: 4753–4758, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 120: 337–345, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Atcha Z, Cagampang FR, Stirland JA, Morris ID, Brooks AN, Ebling FJ, Klingenspor M, Loudon AS. Leptin acts on metabolism in a photoperiod-dependent manner, but has no effect on reproductive function in the seasonally breeding Siberian hamster (Phodopus sungorus). Endocrinology 141: 4128–4135, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J Physiol Endocrinol Metab 278: E1158–E1165, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Banks WA, Kastin AJ, Huang WT, Jaspan JB, Maness LM. Leptin enters the brain by a saturable system independent of insulin. Peptides 17: 305–311, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Barb CR, Barrett JB, Kraeling RR, Rampacek GB. Serum leptin concentrations, luteinizing hormone and growth hormone secretion during feed and metabolic fuel restriction in the prepuberal gilt. Domest Anim Endocrinol 20: 47–63, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Bartness TJ Food hoarding is increased by pregnancy, lactation and food deprivation in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 272: R118–R125, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Bartness TJ, Clein MR. Effects of food deprivation and restriction, and metabolic blockers on food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 266: R1111–R1117, 1994. [DOI] [PubMed] [Google Scholar]
- 10.Bartness TJ, Day DE. Food hoarding: a quintessential anticipatory appetitive behavior. Prog Psychobiol Physiol Psychol 18: 69–100, 2003. [Google Scholar]
- 11.Bartness TJ, Demas GE. Comparative studies of food intake: lessons from non-traditionally studied species. In: Food and Fluid Intake, edited by Stricker EM and Woods SC. New York: Plenum, 2004, p. 423–467.
- 12.Bartness TJ, Morley JE, Levine AS. Photoperiod-peptide interactions in the energy intake of Siberian hamsters. Peptides 7: 1079–1085, 1986. [DOI] [PubMed] [Google Scholar]
- 13.Beneke WM, Davis CH. Relationship of hunger, use of a shopping list and obesity to food purchases. Int J Obes 9: 391–399, 1985. [PubMed] [Google Scholar]
- 14.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. J Clin Endocrinol Metab 81: 3419–3423, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Bowers RR, Festuccia WTL, Song CK, Shi H, Migliorini RH, Bartness TJ. Sympathetic innervation of white adipose tissue and its regulation of fat cell number. Am J Physiol Regul Integr Comp Physiol 286: R1167–R1175, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Brito MN, Brito NA, Baro DJ, Song CK, Bartness TJ. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 148: 5339–53347, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Brito NA, Brito MN, Bartness TJ. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am J Physiol Regul Integr Comp Physiol 294: R1445–R1452, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Buff PR, Morrison CD, Ganjam VK, Keisler DH. Effects of short-term feed deprivation and melatonin implants on circadian patterns of leptin in the horse. J Anim Sci 83: 1023–1032, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Campfield LA, Smith FJ, Burn P. The OB protein (leptin) pathway–a link between adipose tissue mass and central neural networks. Horm Metab Res 28: 619–632, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Craig W Appetites and aversions as constituents of instincts. Biol Bull 34: 91–107, 1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day DE, Bartness TJ. Effects of foraging effort on body fat and food hoarding by Siberian hamsters. J Exp Zool 289: 162–171, 2001. [PubMed] [Google Scholar]
- 22.Day DE, Bartness TJ. Fasting-induced increases in hoarding are dependent on the foraging effort level. Physiol Behav 78: 655–668, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Day DE, Bartness TJ. Agouti-related protein increases food hoarding, but not food intake by Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 286: R38–R45, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Day DE, Keen-Rhinehart E, Bartness TJ. Role of NPY and its receptor subtypes in foraging, food hoarding, and food intake by Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 289: R29–R36, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Dhillon H, Kalra SP, Kalra PS. Dose-dependent effects of central leptin gene therapy on genes that regulate body weight and appetite in the hypothalamus. Mol Ther 4: 139–145, 2001. [DOI] [PubMed] [Google Scholar]
- 26.Dodd DK, Stalling RB, Bedell J. Grocery purchases as a function of obesity and assumed food deprivation. Int J Obes 1: 43–47, 1977. [PubMed] [Google Scholar]
- 27.Drazen DL, Demas GE, Nelson RJ. Leptin effects on immune function and energy balance are photoperiod dependent in Siberian hamsters (Phodopus sungorus). Endocrinology 142: 2768–2775, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Frederich RC, Lollmann B, Hamann A, Napolitano-Rosen A, Kahn BB, Lowell BB, Flier JS. Expression of ob mRNA and its encoded protein in rodents. Impact of nutrition and obesity. J Clin Invest 96: 1658–1663, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman DA, Lewis DA, Kauffman AS, Blum RM, Dark J. Reduced leptin concentrations are permissive for display of torpor in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 287: R97–R103, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Grill HJ, Schwartz MW, Kaplan JM, Foxhall JS, Breininger J, Baskin DG. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143: 239–246, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, Smith RG, Van Der Ploeg LH, Howard AD. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res 48: 23–29, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Illius AW, Tolkamp BJ, Yearsley J. The evolution of the control of food intake. Proc Nutr Soc 61: 465–472, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Kalra SP, Dube MG, Sahu A, Phelps CP, Kalra PS. Neuropeptide Y secretion increases in the paraventricular nucleus in association with increased appetite for food. Proc Natl Acad Sci USA 88: 10931–10935, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keen-Rhinehart E, Bartness TJ. Peripheral ghrelin injections stimulate food intake, foraging and food hoarding in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol 288: R716–R722, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Keen-Rhinehart E, Bartness TJ. MTII attenuates ghrelin- and food deprivation-induced increases in food hoarding and food intake. Horm Behav 52: 612–620, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keen-Rhinehart E, Bartness TJ. NPY Y1 receptor is involved in ghrelin- and fasting-induced increases in foraging, food hoarding, and food intake. Am J Physiol Regul Integr Comp Physiol 292: R1728–R1737, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keen-Rhinehart E, Kalra SP, Kalra PS. AAV-mediated leptin receptor installation improves energy balance and the reproductive status of obese female Koletsky rats. Peptides 26: 2567–2578, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Klingenspor M, Niggemann H, Heldmaier G. Modulation of leptin sensitivity by short photoperiod acclimation in the Djungarian hamster, Phodopus sungorus. J Comp Physiol [B] 170: 37–43, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Kmiec Z, Pokrywka L, Kotlarz G, Kubasik J, Szutowicz A, Mysliwski A. Effects of fasting and refeeding on serum leptin, adiponectin and free fatty acid concentrations in young and old male rats. Gerontology 51: 357–362, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Leitner C, Bartness TJ. Food deprivation-induced changes in body fat mobilization after neonatal monosodium glutamate treatment. Am J Physiol Regul Integr Comp Physiol 294: R775–R783, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Lore RK, Flannelly DJ. Comparative studies of wild and domestic rats: some difficulties in isolating the effects of geneotype and environment. Aggress Behav 7: 253–257, 1978. [Google Scholar]
- 42.McMinn JE, Sindelar DK, Havel PJ, Schwartz MW. Leptin deficiency induced by fasting impairs the satiety response to cholecystokinin. Endocrinology 141: 4442–4448, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Mela DJ, Aaron JI, Gatenby SJ. Relationships of consumer characteristics and food deprivation to food purchasing behavior. Physiol Behav 60: 1331–1335, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Mercer JG, Beck B, Burlet A, Moar KM, Hoggard N, Atkinson T, Barrett P. Leptin (ob) mRNA and hypothalamic NPY in food-deprived/refed Syrian hamsters. Physiol Behav 64: 191–195, 1998. [DOI] [PubMed] [Google Scholar]
- 45.Mercer JG, Hoggard N, Williams LM, Lawrence CB, Hannah LT, Morgan PJ, Trayhurn P. Coexpression of leptin receptor and preproneuropeptide Y mRNA in arcuate nucleus of mouse hypothalamus. J Neuroendocrinol 8: 733–735, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Mondal MS, Date Y, Yamaguchi H, Toshinai K, Tsuruta T, Kangawa K, Nakazato M. Identification of ghrelin and its receptor in neurons of the rat arcuate nucleus. Regul Pept 126: 55–59, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Myers RD Handbook of Drug and Chemical Stimulation of the Brain. Behavioral: Pharmacological and Physiological Aspects. New York: Van Nostrand Reinhold, 1974.
- 48.Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature 409: 194–198, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Newsholme EA, Leech AR. Biochemistry for the Medical Sciences. Chichester, UK: Wiley, 1983.
- 50.Ransley JK, Donnelly JK, Botham H, Khara TN, Greenwood DC, Cade JE. Use of supermarket receipts to estimate energy and fat content of food purchased by lean and overweight families. Appetite 41: 141–148, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Rentsch J, Levens N, Chiesi M. Recombinant ob-gene product reduces food intake in fasted mice. Biochem Biophys Res Commun 214: 131–136, 1995. [DOI] [PubMed] [Google Scholar]
- 52.Satoh N, Ogawa Y, Katsuura G, Hayase M, Tsuji T, Imagawa K, Yoshimasa Y, Nishi S, Hosoda K, Nakao K. The arcuate nucleus as a primary site of satiety effect of leptin in rats. Neurosci Lett 224: 149–152, 1997. [DOI] [PubMed] [Google Scholar]
- 53.Schneider JE, Buckley CA. Food hoarding is increased by food deprivation and decreased by leptin treatment in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 285: R1021–R1029, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Shiraishi T, Oomura Y, Sasaki K, Wayner MJ. Effects of leptin and orexin-A on food intake and feeding related hypothalamic neurons. Physiol Behav 71: 251–261, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 146: 1043–1047, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Trayhurn P, Duncan JS, Rayner DV, Hardie LJ. Rapid inhibition of ob gene expression and circulating leptin levels in lean mice by the beta 3-adrenoceptor agonists BRL 35135A and ZD2079. Biochem Biophys Res Commun 228: 605–610, 1996. [DOI] [PubMed] [Google Scholar]
- 57.Tschop M, Smiley DL, Heiman ML. Ghrelin induces adiposity in rodents. Nature 407: 908–913, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Tups A, Ellis C, Moar KM, Logie TJ, Adam CL, Mercer JG, Klingenspor M. Photoperiodic regulation of leptin sensitivity in the Siberian hamster, Phodopus sungorus, is reflected in arcuate nucleus SOCS-3 (suppressor of cytokine signaling) gene expression. Endocrinology 145: 1185–1193, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Tups A, Helwig M, Khorooshi RM, Archer ZA, Klingenspor M, Mercer JG. Circulating ghrelin levels and central ghrelin receptor expression are elevated in response to food deprivation in a seasonal mammal (Phodopus sungorus). J Neuroendocrinol 16: 922–928, 2004. [DOI] [PubMed] [Google Scholar]
- 60.Vander Wall SB Food Hoarding in Animals. Chicago, IL: University of Chicago Press, 1990.
- 61.Weiner J Limits to energy budget and tactics in energy investments during reproduction in the Djungarian hamster (Phodopus sungorus sungorus Pallas 1770). Symp Zool Soc Lond 57: 167–187, 1987. [Google Scholar]
- 62.Wood AD, Bartness TJ. Food deprivation-induced increases in hoarding by Siberian hamsters are not photoperiod-dependent. Physiol Behav 60: 1137–1145, 1996. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432, 1994. [DOI] [PubMed] [Google Scholar]