Abstract
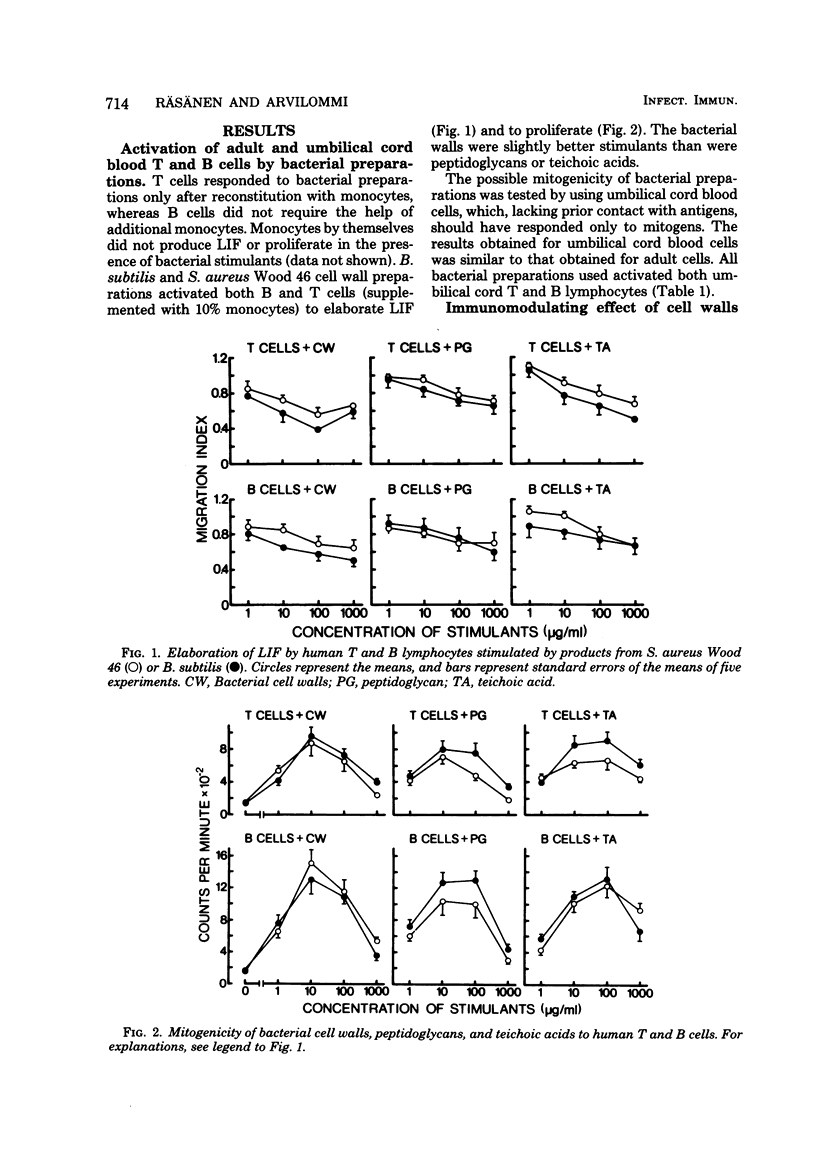
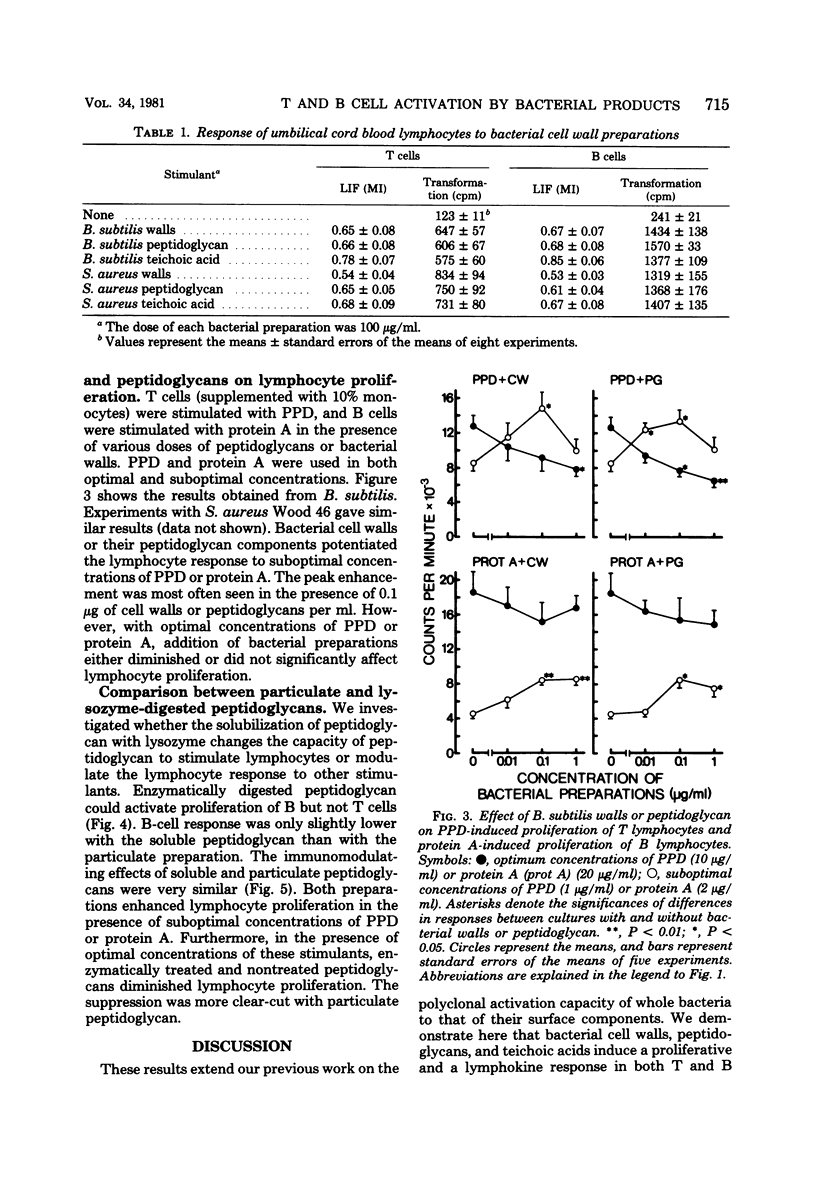
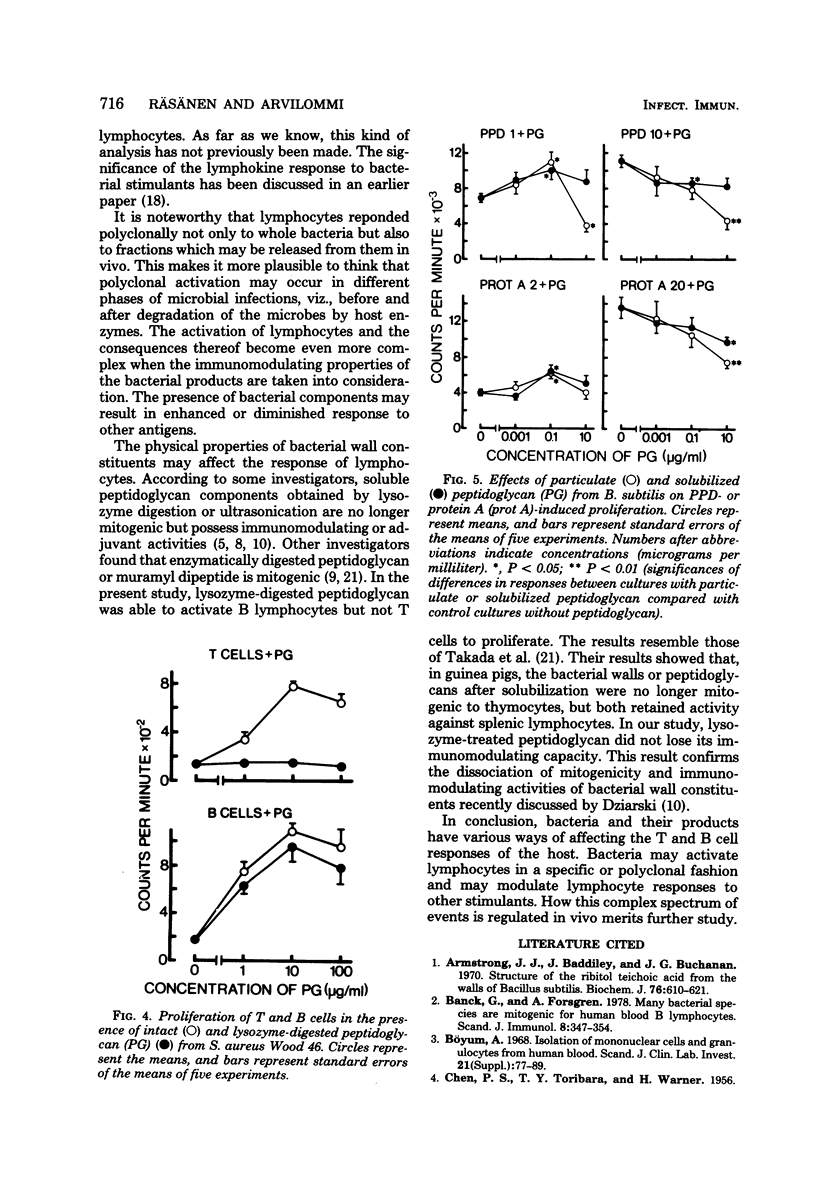
In this study, the mitogenic and immunomodulating effects of bacterial cell wall preparations were investigated. Cell walls, peptidoglycans, and teichoic acids from Bacillus subtilis and Staphylococcus aureus Wood 46 activated both human T cells (supplemented with 10% monocytes) and B cells to proliferative and to produce leukocyte migration inhibitory factor. Similar results were obtained with adult and umbilical cord blood cells, suggesting that these bacterial preparations acted as mitogens. Cell walls and peptidoglycans had a modulating effect on purified protein derivative-induced and protein A-induced proliferation. In the presence of suboptimal concentrations of these stimulants, bacterial components enhanced the proliferative response. However, at optimal concentrations of purified protein derivative or protein A, bacterial components suppressed lymphocyte proliferation. Peptidoglycans solubilized by lysozyme activated B lymphocytes but not T cells. Solubilization had no effect on immunomodulating capacity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG J. J., BADDILEY J., BUCHANAN J. G. Structure of the ribitol teichoic acid from the walls of Bacillus subtilis. Biochem J. 1960 Sep;76:610–621. doi: 10.1042/bj0760610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banck G., Forsgren A. Many bacterial species are mitogenic for human blood B lymphocytes. Scand J Immunol. 1978;8(4):347–354. doi: 10.1111/j.1365-3083.1978.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Ciorbaru R., Petit J. F., Lederer E., Zissman E., Bona C., Chedid L. Presence and subcellular localization of two distinct mitogenic fractions in the cells of Nocardia rubra and Nocardia opaca: preparation of soluble mitogenic peptidoglycan fractions. Infect Immun. 1976 Apr;13(4):1084–1090. doi: 10.1128/iai.13.4.1084-1090.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clagett J. A., Engel D. Polyclonal activation: a form of primitive immunity and its possible role in pathogenesis of inflammatory diseases. Dev Comp Immunol. 1978 Apr;2(2):235–241. doi: 10.1016/s0145-305x(78)80066-4. [DOI] [PubMed] [Google Scholar]
- Damais C., Bona C., Chedid L., Fleck J., Nauciel C., Martin J. P. Mitogenic effect of bacterial peptidoglycans possessing adjuvant activity. J Immunol. 1975 Jul;115(1):268–271. [PubMed] [Google Scholar]
- Damais C., Parant M., Chedid L. Nonspecific activation of murine spleen cells in vitro by a synthetic immunoadjuvant (N-acetyl-muramyl-L-alanyl-D-isoglutamine). Cell Immunol. 1977 Nov;34(1):49–56. doi: 10.1016/0008-8749(77)90228-3. [DOI] [PubMed] [Google Scholar]
- Dziarski R., Dziarski A., Levinson A. I. Mitogenic responsiveness of mouse, rat and human lymphocytes to Staphylococcus aureus cell wall, teichoic acid, and peptidoglycan. Int Arch Allergy Appl Immunol. 1980;63(4):383–395. doi: 10.1159/000232654. [DOI] [PubMed] [Google Scholar]
- Dziarski R., Dziarski A. Mitogenic activity of staphylococcal peptidoglycan. Infect Immun. 1979 Mar;23(3):706–710. doi: 10.1128/iai.23.3.706-710.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziarski R. Relationships between adjuvant, immunosuppressive, and mitogenic activities of staphylococcal peptidoglycan. Infect Immun. 1979 Nov;26(2):508–514. doi: 10.1128/iai.26.2.508-514.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Shimono T., Narita T., Kato K. Immunoadjuvant activities of cell walls, their water-soluble fractions and peptidoglycan subunits, prepared from various gram-positive bacteria, and of synthetic n-acetylmuramyl peptides. Z Immunitatsforsch Exp Klin Immunol. 1975 Jul;149(2-4):302–319. [PubMed] [Google Scholar]
- Möller E., Ström H., Al-Balaghi S. Role of polyclonal activation in specific immune responses. Relevance for findings of antibody activity in various diseases. Scand J Immunol. 1980;12(3):177–182. doi: 10.1111/j.1365-3083.1980.tb00057.x. [DOI] [PubMed] [Google Scholar]
- Nauciel C., Fleck J. Adjuvant activity of bacterial peptidoglycans. Z Immunitatsforsch Exp Klin Immunol. 1975 Jul;149(2-4):349–353. [PubMed] [Google Scholar]
- Räsänen L., Karhumäki E., Arvilommi H. Bacteria induce lymphokine synthesis polyclonally in human B lymphocytes. J Immunol. 1978 Aug;121(2):418–420. [PubMed] [Google Scholar]
- Räsänen L., Karhumäki E., Majuri R., Arvilommi H. Polyclonal activation of human lymphocytes by bacteria. Infect Immun. 1980 May;28(2):368–372. doi: 10.1128/iai.28.2.368-372.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayegani M., Hisatsune K., Mudd S. Cell Wall Component Which Affects the Ability of Serum to Promote Phagocytosis and Killing of Staphylococcus aureus. Infect Immun. 1970 Dec;2(6):750–756. doi: 10.1128/iai.2.6.750-756.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada H., Tsujimoto M., Kotani S., Kusumoto S., Inage M., Shiba T., Nagao S., Yano I., Kawata S., Yokogawa K. Mitogenic effects of bacterial cell walls, their fragments, and related synthetic compounds on thymocytes and splenocytes of guinea pigs. Infect Immun. 1979 Aug;25(2):645–652. doi: 10.1128/iai.25.2.645-652.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


