ABSTRACT
BACKGROUND
Adults at high risk for diabetes may have reduced health-related quality of life (HRQoL).
OBJECTIVE
To assess changes in HRQoL after interventions aimed at diabetes risk reduction.
DESIGN, SETTING, AND PARTICIPANTS
A randomized clinical trial, the Diabetes Prevention Program, was conducted in 27 centers in the United States, in 3,234 non-diabetic persons with elevated fasting and post-load plasma glucose, mean age 51 years, mean BMI 34 Kg/m²; 68 % women, and 45 % members of minority groups.
INTERVENTIONS
Intensive lifestyle (ILS) program with the goals of at least 7 % weight loss and 150 min of physical activity per week, metformin (MET) 850 mg twice daily, or placebo (PLB).
MEASUREMENTS
HRQoL using the 36-Item Short-Form (SF-36) health survey to evaluate health utility index (SF-6D), physical component summaries (PCS) and mental component summaries (MCS). A minimally important difference (MID) was met when the mean of HRQoL scores between groups differed by at least 3 %.
RESULTS
After a mean follow-up of 3.2 years, there were significant improvements in the SF-6D (+0.008, p = 0.04) and PCS (+1.57, p < 0.0001) scores in ILS but not in MET participants (+0.002 and +0.15, respectively, p = 0.6) compared to the PLB group. ILS participants showed improvements in general health (+3.2, p < 0.001), physical function (+3.6, p < 0.001), bodily pain (+1.9, p = 0.01), and vitality (+2.1, p = 0.01) domain scores. Treatment effects remained significant after adjusting sequentially for baseline demographic factors, and for medical and psychological comorbidities. Increased physical activity and weight reduction mediated these ILS treatment effects. Participants who experienced weight gain had significant worsening on the same HRQoL specific domains when compared to those that had treatment-related (ILS or MET) weight loss. No benefits with ILS or MET were observed in the MCS score.
CONCLUSION
Overweight/obese adults at high risk for diabetes show small improvement in most physical HRQoL and vitality scores through the weight loss and increased physical activity achieved with an ILS intervention.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-012-2122-5) contains supplementary material, which is available to authorized users.
KEY WORDS: quality of life, lifestyle, metformin, diabetes risk, weight loss
INTRODUCTION
Healthy People 2020 outlines numerous public health goals, as set forth by the US Department of Health and Human Services, including the reduction of diabetes and the economic burden of the disease, and improvement in the quality of life for all persons who are at risk for or who have diabetes.1
Diabetes has not only been associated with increased morbidity and mortality, but also with a poor health-related quality of life (HRQoL).2,3 Studies have also shown that people with diabetes-related complications have a reduced HRQoL compared to those without complications.4–6
There is limited information available on changes in HRQoL associated with prediabetes or the development of type 2 diabetes (T2D). A cross-sectional study examined the association of HRQoL with glucose tolerance status in an Australian population.7 While those with previously diagnosed diabetes were at a significantly greater risk of being in the lowest quartile of each domain of the 36-Item Short-Form (SF-36) scale, there was also evidence of reduced HRQoL on some SF-36 domains among those with newly diagnosed diabetes, and in those with impaired glucose tolerance (IGT). More recently, a study in Canadian individuals with prediabetes showed that those who were more physically active had better physical and mental HRQoL than those who were inactive.8
The Diabetes Prevention Program (DPP) is a large, multi-center, randomized trial that demonstrated the benefits of an intensive lifestyle intervention (ILS) and of pharmacotherapy with metformin (MET) on the reduction of T2D risk in overweight or obese adults with IGT.9Previous analyses in DPP participants using the Self-Administered Quality of Well-Being scale showed that participants randomized to ILS or MET intervention groups accrued 0.07 and 0.02 more quality-adjusted life-years (QALYs),respectively, over 3 years, than participants randomized to the placebo (PLB) intervention group.10 There are limited prospective data on the impact that changes in known predictors of diabetes and glucose tolerance status have in each physical and mental HRQoL domain. Therefore, we tested the hypotheses that DPP interventions lead to better HRQoL in subjects at high risk for T2D, and that those randomized to intensive lifestyle will have a greater improvement in HRQoL than those in the metformin group.
METHODS
Participants
The eligibility criteria, design, and methods of the DPP have been reported elsewhere.9,11 In brief, selection criteria included: age ≥25 years, body mass index ≥24 kg/m² (≥22 kg/m² in Asian Americans), fasting plasma glucose levels between 95 and 125 mg/dl, and IGT (2-hour post-load glucose of 140–199 mg/dl). People were excluded if they were taking medications known to alter glucose tolerance, or if they had illnesses that could seriously reduce their life expectancy or their ability to participate in the trial.
The current report and analyses includes 3,234 participants seen at baseline, who were randomly assigned to one of the three treatment arms investigated. Written informed consent was obtained from all participants before screening, consistent with the Declaration of Helsinki and the guidelines of each center’s institutional review board.
DPP Study Interventions
The ILS intervention was a goal-based diet and physical activity intervention designed to achieve and maintain modest weight reduction.12 Goals were to achieve and maintain at least 150 min per week of moderate physical activity and to reduce weight by 7 % from baseline. Participants in both MET and PLB arms received standard lifestyle recommendations in the form of written information, and an annual 20–30-min individual session that emphasized the importance of a low-fat diet and regular physical activity to achieve modest weight reduction. Treatment with MET was increased over 1 month to a full dose of 850 mg taken twice daily. The PLB group received a matching placebo tablet.
Procedures
Standardized interviewer-administered questionnaires were used to obtain demographic and clinical data. Physical activity was measured by the Modifiable Activity Questionnaire.13,14 Blood pressure and anthropometrics were measured using standard techniques. All analytical measurements for glucose, insulin, and lipids were performed at the Central Biochemistry Laboratory (University of Washington, Seattle). Insulin secretion was estimated with the corrected insulin response (CIR).15 Insulin resistance (IR) was estimated using the insulin sensitivity index (ISI), reciprocal of the IR-homeostasis model assessment.16 Development of diabetes was determined by an annual oral glucose tolerance test and semiannual fasting plasma glucose (FPG) tests, and required confirmation by a second test, using the criteria of the American Diabetes Association and the World Health Organization.17,18
Generic quality of life assessments were collected annually using the SF-36.19 Extensive psychometric data support the reliability and validity of the SF-36.20The SF-36 was administered from the start of the DPP and is available for 3,206 participants. The SF-36 consists of two composite scores: the physical component summary (PCS) and mental health component summary (MCS) scores. Higher scores on the two SF-36 component summaries indicate more favorable quality of life. The SF-36 is divided into eight domains, measuring two main components: physical (general health, physical functioning, role limitation–physical, and bodily pain) and mental (mental health, social functioning, role limitation–emotional, and vitality). Scores in the SF-36 domains could be between 0 (worst) and 100 (best). The health utility index SF-6D (scale 0.0 to 1.0, with 1.0 indicating "full health") is a preference-based health state classification developed from the SF-36.21 The six dimensions captured by the SF-6D are physical functioning, role limitations, social functioning, pain, mental health, and vitality.
A minimally important difference (MID), defined as the smallest score difference that the patient perceives as beneficial or deleterious,22 was met when the mean of HRQoL scores between groups differed by at least 3 %. This is equivalent to the mean change in the SF-6D that has been cited in the literature, including reported change in general health in nine patient groups.23 This value is also approximately equivalent to an estimate of the MID for the EuroQoL (EQ-5D);24 and it was used in a population-based study that compared healthier individuals with older patients with T2D.25 Others have proposed alternative definitions of MID as being 5 %.26 Therefore, we will consider small HRQoL changes as those <3 %, modest those changes between 3 % and <5 %, moderate between 5 % and <10 %, and large for those achieving changes 10 % and above.
Statistical Analysis
This analysis is based on data collected from the start of DPP (June 1996) through July 31, 2001, after which the participants were unmasked to the study results and treatment assignment. To test our hypothesis, we performed the analysis following the intention-to-treat principle in the three main DPP treatment groups. SAS was used for all analyses (version 8.2; SAS Institute, Cary, NC).
Mixed-effects models with the assumption of normally distributed errors were used to assess mean differences over time in the scores for the SF-6D, PCS, MCS, and eight SF-36 domains, and were adjusted for their baseline values, participant demographic characteristics, baseline weight and physical activity, and the presence of medical (hypertension, dyslipidemia, myocardial infarction, stroke) or psychological (depression, anxiety) co-morbidities at baseline. With controls for these baseline measures, additional models were obtained to assess the associations of HRQoL with factors that might account for the effect of treatment on HRQoL, including time-dependent changes in physical activity level, weight, fasting and 2-hour plasma glucose, IR, and beta cell function. Analyses were conducted to evaluate whether treatment group differences in HRQoL remain significant after accounting for the effect of converting to diabetes. In addition, comparisons in HRQoL scores within and across DPP treatment groups were performed among those who reverted to NGT, or progressed to diabetes.
RESULTS
Quality of Life at Baseline
Baseline demographic and clinical characteristics of DPP participants are shown in Table A in the appendix (available on line). The overall HRQoL scores, as well as the eight SF-36 domains, were similar across treatment groups at the time of randomization. Most scores are similar or slightly better than those reported in other populations with prediabetes. 7
Quality of Life Change over Follow-up in Each Treatment Group
During DPP follow-up, HRQoL summary scores worsened in all three treatment arms, but the decline for SF-6D (p < 0.05) and PCS (p < 0.01) was slower in ILS participants compared to the changes in those in PLB or MET groups; however, none reached the MID of 3 % (Table 1). During the first year of intervention, physical function, general health (Fig. 1a, b), and vitality scores (Figure A-2 available on line) improved significantly in ILS participants, reaching MID when compared to those treated with PLB or MET. Subsequently, all scores decreased in the follow-up years but remained better in ILS than in PLB for the following specific domains: physical function, body pain, general health, and vitality (with the first two reaching MID). After adjusting for demographic factors, baseline weight and physical activity, and medical and psychiatric comorbidities, these small HRQoL benefits with ILS intervention remained significant when compared with the PLB group (Table 2, Model 1).
Table 1.
Changes in Health-Related Quality of Life over 3.2 years (Study Duration) in Lifestyle and Metformin Participants Compared to Placebo
| Measurement | Lifestyle (n = 1048) | Metformin (n = 1043) |
|---|---|---|
| Short Form-6D | 0.0084(0.0041)† | 0.0019(0.0041) |
| Physical component summary | 1.57 (0.30)‡ | 0.15(0.30) |
| Mental component summary | −0.29(0.32) | 0.22(0.32) |
| Physical function | 3.58(0.71)‡* | 0.13(0.71) |
| Role physical | 1.86(0.99) | 1.32(0.99) |
| Body pain | 1.93(0.78)‡ | 0.50(0.78) |
| General health | 3.23(0.66)‡* | 0.06(0.66) |
| Vitality | 2.05(0.77)‡ | 0.09(0.76) |
| Social functioning | 0.97(0.66) | 0.81(0.66) |
| Role emotional | 0.20(0.95) | 0.78(0.95) |
| Mental health | −0.50(0.57) | 0.32(0.57) |
Data presented as mean (SD); * reached minimally important difference of 3 %; †p < 0.05; ‡p < 0.01
Figure 1.
Changes in physical function (1a) and general health (1b) scores across treatment groups. ILS, intensive lifestyle; PLB, Placebo; MET, Metformin. Data available in study participants decreased overtime from baseline (n = 3206) to year 1 (n = 3143), year 2 (n = 2988), year 3 (n = 2941), and year 4 (n = 1859).
Table 2.
Effect of Lifestyle on Health Related Quality of Life after Adjustments for Covariates
| Score | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Short form-6D | 0.009(0.14)‡ | 0.81(0.11)† | 0.10(0.00) | 0.07(0.00) |
| Physical component summary | 1.39 (0.57) ‡ | 1.31 (0.49) ‡ | 0.41 (0.06) | 0.43 (0.06) |
| Physical function | 3.37(0.60)‡ | 3.22(0.53)‡ | 1.25(0.07)† | 1.13(0.06)¶ |
| Body pain | 1.61(0.11)‡ | 1.48(0.09)† | 0.06(0.00) | 0.08(0.00) |
| General health | 3.35(0.79)‡ | 3.21(0.69)‡ | 1.31(0.11)‡ | 1.45(0.16)‡ |
| Vitality | 2.44(0.31)‡ | 2.24(0.25)‡ | −0.07(0.00) | 0.15(0.01) |
Data presented as the mean difference for the score ILS vs. placebo (adjusted R²). †p < 0.05; ‡p < 0.01; ¶p = 0.05; Model 1: age, sex, race/ethnicity, baseline weight and physical activities, medical and psychiatric comorbidities; Model 2: model 1 + changes in physical activity; Model 3: model 2 + weight loss; Model 4: model 3 + insulin secretion and insulin resistance
Mediating Factors for the Effects of Interventions on Quality of Life
We tested a series of factors that could mediate the HRQoL benefits of ILS intervention. A series of explanatory factors were added sequentially in regression models starting with those related to behavioral goals (i.e., changes in physical activity in model 2 and weight loss in model 3) and following with metabolic factors related to diabetes development (i.e., CIR and ISI at each time point in model 4). As shown in Table 2, the effect of ILS on summary and specific domain scores remained after adjusting for changes in physical activity; however, the adjustment for weight loss partially (physical function and general health) or fully (SF-6D, PCS, body pain, and vitality) accounted for these HRQoL benefits. No additional changes were observed after adjustments were made for CRI or ISI.
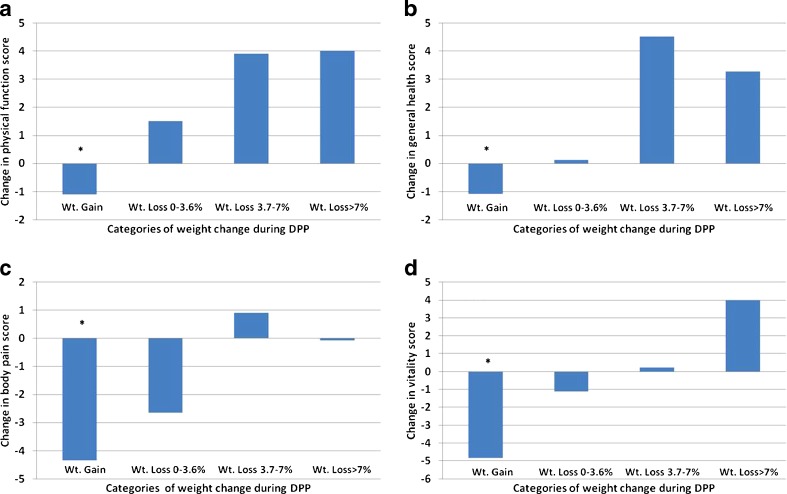
In an additional analysis, we evaluated changes in specific SF-36 domains across the following categories of weight change in ILS participants: weight gain (n = 362), minimal weight loss (up to 3.6 %, n = 196), moderate weight loss (between 3.7 and 7 %, n = 201), and major weight loss (greater than 7 %, n = 347). Those who experienced weight gain had significant worsening on specific HRQoL scores when compared to those that had weight loss, with the following differences reaching MID (Fig. 2a-d): more than 5 points in physical function (vs. those with moderate or major weight loss); more than 4 points in general health and body pain (vs. those with moderate or major weight loss); and more than 8 points in vitality (vs. those with major weight loss). A similar approach was used across categories of weight change in MET participants: weight gain (n = 396), minimal weight loss (n = 282), moderate weight loss (n = 157), and major weight loss (n = 183) (Fig. 3a-d). MET participants who had weight gain reported a similar pattern of worsening of HRQoL scores when compared to those who achieved major weight loss: approximately 5-point difference on physical function, general health and body pain, but only 3-point difference in vitality.
Figure 2.
Changes in physical function (2a), general health (2b), body pain (2c), and vitality (2d) scores among lifestyle intervention participants over the DPP follow-up period and according to weight (Wt) change categories. *P < 0.001 for a comparison of the Wt. gain category with any of the Wt. loss categories.
Figure 3.
Changes in physical function (3a), general health (3b), body pain (3c), and vitality (3d) scores among metformin intervention participants over the DPP follow-up period and according to weight (Wt) change categories. *P < 0.01 for a comparison between the Wt. gain category and the major Wt. loss category.
Quality of Life and Progression to Diabetes
We further studied the patterns of HRQoL when participants experienced change in glucose-tolerance status. Progression to diabetes was associated with worsening in the PCS (−1.1, p < 0.05), physical function (−2.6, p < 0.05), and general health (−2.6, p < 0.01) scores.
DISCUSSION
Subjects at high risk for T2D experienced a significant, but small, improvement in the overall and specific HRQoL scores through increased physical activity and weight loss achieved with an ILS intervention. In general, HRQoL scores of DPP participants decreased over the follow-up period, but were better in the ILS intervention. The findings of this study support the hypothesis that participation in an ILS behavior modification intervention for weight management is associated with relative improvement in HRQoL, as measured by the PCS, physical function, general health, body pain, and vitality scores of the SF-36. No clear overall benefits on HRQoL were observed in participants receiving MET intervention, except in those that experienced major (>7 %) weight loss. These results are aligned with one of the Healthy People 2020 goals: to reduce diabetes burden and improve HRQoL in those with or at high risk for diabetes.1
The DPP demonstrated considerable differences in lifestyle behavior change and weight reduction in ILS participants, compared with those in the PLB-control group. Accordingly, we found a modest but significant improvement in the SF-6D and PCS scores with ILS, when compared with PLB after an average of 3.2 years of follow-up. In a previous analysis, we have shown small improvements in HRQoL over the first year of the study, associated with weight loss.27 DPP participants with a baseline BMI ≥ 35 kg/m² showed improvement in the PCS and SF-6D scores over one year of ILS intervention.27 Similarly, baseline BMI moderated the beneficial effect of the first year of lifestyle intervention on the PCS score in patients with T2D in the Look AHEAD trial.28 Furthermore, we found that compared to those who experienced weight gain, HRQoL scores in three physical domains (physical function, general health, and body pain) and in one mental domain (vitality) reached MID in those who achieved moderate (3.7–7 %) or major (>7 %) weight loss. A previous report from the Women Health Initiative (WHI) has also shown that heavier weight negatively affects physical HRQoL (bodily pain, physical functioning, general health, and role limitations due to physical health), but not mental health.29 In fact, weight-relevant physical health factors (such as diabetes and osteoarthritis) have a greater impact on this weight-HRQOL association than do emotional/ psychological factors. Similarly, a report from an urban population of Belgrade showed that increased BMI has much greater impact on physical that on mental health HRQoL.30
To our knowledge, this study is one of the first to evaluate the potential mediation of treatment effects of lifestyle intervention or MET on HRQoL in those at high risk for T2D. We found that weight loss in the ILS group was the most important factor associated with these HRQoL benefits, in models that also accounted for the effects of changes in physical activity, insulin secretion, and insulin resistance. Similar analyses in the Look AHEAD trial showed that the improvement in the PCS score, associated with lifestyle intervention in subjects with T2D, was only partially mediated by the improvements in body weight and physical fitness and by the reduction in physical symptoms.28 Other factors related to the ILS intervention (i.e., improved metabolic variables, improved functional abilities, counseling and group support, changes in the social and/or family environment) may account for the HRQoL benefits associated with weight management in overweight and obese adults at high risk for T2D. A similar pattern of the effect of weight changes and HRQoL was observed in MET participants. A previous study in women with polycytic ovary syndrome (PCOS) reported improvements in vitality, social function, emotional role and mental health, which were correlated with reduction in body weight and more pronounced in those who achieved normalization in menstrual disturbances.31 Future studies may provide additional insights on mediators of the ILS- and MET-specific benefits on improving physical function and reducing pain and fatigue (i.e., improving vitality).
We also found that changes in body weight among ILS participants were associated with clinically significant improvements (i.e., reaching MID) in two physical SF-36 domains (physical function and general health), and with statistically significant but smaller improvements in two other domains (bodily pain and vitality). These findings suggest that weight loss in overweight or obese adults with IGT is likely to result in small to modest direct benefits on HRQoL. A recent systematic review found mixed effects of weight loss interventions on various generic and obesity-specific measures of HRQoL.32 However, this review was not designed to distinguish between changes in HRQoL mediated by weight loss and those resulting from other intervention effects unrelated to weight change.
Overweight and obesity in middle age are associated with lower HRQoL in older age, as shown in a prospective cohort study that followed up on men and women free of diabetes or myocardial infarction at baseline, for a mean of 26 years.33 A recent analysis in the Longitudinal Australian Diabetes, Obesity and Lifestyle (AusDiab) study has shown that obesity is associated with deterioration in HRQoL over 5 years for seven of the eight SF-36 domains in women (excluding mental health), and six out of eight SF-36 domains in men (not on role–emotional and mental health).34 In our study, we found that participants in both intervention groups (slightly more pronounced with ILS than with MET) who experienced weight gain had significant worsening on physical function, general health, body pain, and vitality, compared to those who achieved major weight loss. It is possible that ILS participants who gained weight, despite the specific goals for increased physical activity and weight loss set up for them, may have experienced a more negative reaction (i.e. worsening in HRQoL) than those in the MET group who were not asked to adopt new behaviors, except taking medication. However, patients taking oral antihyperglycemic agents who experience weight gain may also lower their HRQoL, as shown in a study using an internet-based survey and the EQ-5D.35
Our study has some limitations worth mentioning. First, our population was a highly selected group of overweight and obese adults at high risk for diabetes. Therefore, these findings should not be generalized to other (less highly selected) populations. However, baseline SF-6D utility score was 0.801, which is consistent with a previously reported score in a general population of adults aged 45–54.36 Second, HRQoL measurements used were not disease-specific (i.e., not for subjects at risk for diabetes or with newly diagnosed diabetes). It is also possible that our results would have differed if we had used an HRQoL instrument other than the SF-36. The relative emphasis of various specific domains differs across instruments. Third, the MID in SF-6D score and specific SF-36 physical and mental domains were based on comparison to other global health indicators, and not related to health outcomes or cost-effectiveness analysis. Fourth, although we used data from a large randomized clinical trial, it is possible that unmeasured confounders could have impacted our results. Finally, it is possible that the HRQoL benefits resulted from the so-called "regression to the mean," where individuals with worse HRQoL at baseline get the greatest benefits from the weight management intervention, as previously reported.28,37 In our study, we adjusted for baseline values of each HRQoL measurement, in addition to the participant demographic characteristics, baseline weight and physical activity, and the presence of medical and psychological co-morbidities.
In summary, our study demonstrates that lifestyle modification characterized by intentional weight loss and increased physical activity has an independent but small to modest association with better HRQoL in overweight or obese participants at high risk for T2D. Future work is needed to determine whether these changes in HRQoL persist over much longer periods, as well as to replicate our findings in other study populations and to use different measures of HRQoL. Studies should explore whether lifestyle intervention may lead to greater and sustained HRQoL benefits in certain high-risk groups, such as those with greater obesity and the elderly.
Electronic supplementary materials
(DOC 182 kb)
Acknowledgements
The Investigators gratefully acknowledge the commitment and dedication of the participants of the DPP. The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health provided funding to the clinical centers and the Coordinating Center for the design and conduct of the study; and collection, management, analysis, and interpretation of the data. The Southwestern American Indian Centers were supported directly by the NIDDK and the Indian Health Service. The General Clinical Research Center Program, National Center for Research Resources, supported data collection at many of the clinical centers. Funding for data collection and participant support was also provided by the Office of Research on Minority Health, the National Institute of Child Health and Human Development, the National Institute on Aging, the Centers for Disease Control and Prevention, the Office of Research on Women’s Health, and the American Diabetes Association. Bristol-Myers Squibb and Parke-Davis provided medication. This research was also supported, in part by the intramural research program of the NIDDK. LifeScan Inc., Health O Meter, Hoechst Marion Roussel, Inc., Merck-Medco Managed Care, Inc., Merck and Co., Nike Sports Marketing, Slim Fast Foods Co., and Quaker Oats Co. donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices Corp., Matthews Media Group, Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center. The opinions expressed are those of the investigators and do not necessarily reflect the views of the Indian Health Service or other funding agencies. A complete list of Centers, investigators, and staff can be found in the online appendix.
The work was also supported in part by the VA-GRECC/ University of Miami CTSI program and HHS/NIH grant 1R18AE000049-01[HF].
Conflict of Interest
The authors declare that they do not have a conflict of interest related to this manuscript.
Footnotes
Christopher D. Saudek deceased
Trial registration
DPP is registered in www.clinicaltrials.gov (NCT00004992).
REFERENCES
- 1.US Department of Health and Human Services (DHSS). In: Healthy people 2020. Washington; 2010. Available at http://healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicId=8 Accessed May 18, 2012.
- 2.Goldney RD, Phillips PJ, Fisher LJ, Wilson DH. Diabetes, depression, and quality of life: a population study. Diabetes Care. 2004;27(5):1066–1070. doi: 10.2337/diacare.27.5.1066. [DOI] [PubMed] [Google Scholar]
- 3.Manuel DG, Schultz SE. Health-related quality of life and health-adjusted life expectancy of people with diabetes in Ontario, Canada, 1996–1997. Diabetes Care. 2004;27(2):407–414. doi: 10.2337/diacare.27.2.407. [DOI] [PubMed] [Google Scholar]
- 4.U.K. Prospective Diabetes Study Group Quality of life in type 2 diabetic patients is affected by complications but not by intensive policies to improve blood glucose or blood pressure control (UKPDS 37) Diabetes Care. 1999;22(7):1125–1136. doi: 10.2337/diacare.22.7.1125. [DOI] [PubMed] [Google Scholar]
- 5.Redekop WK, Koopmanschap MA, Rutten GE, Wolffenbuttel BH, Stolk RP, Niessen LW. Resource consumption and costs in Dutch patients with type 2 diabetes mellitus. Results from 29 general practices. Diabet Med. 2002;19(3):246–253. doi: 10.1046/j.1464-5491.2002.00654.x. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd A, Sawyer W, Hopkinson P. Impact of long-term complications on quality of life in patients with type 2 diabetes not using insulin. Value Health. 2001;4(5):392–400. doi: 10.1046/j.1524-4733.2001.45029.x. [DOI] [PubMed] [Google Scholar]
- 7.Tapp RJ, Dunstan DW, Phillips P, Tonkin A, Zimmet PZ, Shaw JE, AusDiab Study Group Association between impaired glucose metabolism and quality of life: results from the Australian diabetes obesity and lifestyle study. Diabetes Res Clin Pract. 2006;74(2):154–161. doi: 10.1016/j.diabres.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Taylor LM, Spence JC, Raine K, Plotnikoff RC, Vallance JK, Sharma AM. Physical activity and health-related quality of life in individuals with prediabetes. Diabetes Res Clin Pract. 2010;90(1):15–21. doi: 10.1016/j.diabres.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Knowler WC, Barrett-Connor E, Fowler SE, Diabetes Prevention Program Research Group et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diabetes Prevention Program Research Group Within-trial cost-effectiveness of lifestyle intervention or metformin for the primary prevention of type 2 diabetes. Diabetes Care. 2003;26(9):2518–23. doi: 10.2337/diacare.26.9.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diabetes Prevention Program Research Group The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care. 1999;22(4):623–34. doi: 10.2337/diacare.22.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabetes Prevention Program (DPP) Research Group The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kriska AM, Knowler WC, LaPorte RE, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care. 1990;13(4):401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 14.Pereira MA, FitzerGerald SJ, Gregg EW, et al. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc. 1997;29(6 Suppl):S1–S205. [PubMed] [Google Scholar]
- 15.Phillips DI, Clark PM, Hales CN, Osmond C. Understanding oral glucose tolerance: comparison of glucose or insulin measurements during the oral glucose tolerance test with specific measurements of insulin resistance and insulin secretion. Diabet Med. 1994;11(3):286–292. doi: 10.1111/j.1464-5491.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report on the Expert Committee on Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 18.Alberti K, Zimmet P, for the WHO consultation Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. . Med Care. 1992;30(6):473–483. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 20.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1):40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ. 2002;21(2):271–292. doi: 10.1016/S0167-6296(01)00130-8. [DOI] [PubMed] [Google Scholar]
- 22.Jaeschke R, Singer J, Guyatt GH. Measurement of health status.Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 23.Walters SJ, Brazier JE. What is the relationship between the minimally important difference and health state utility values? The case of the SF-6D. Health Qual Life Outcomes. 2003;1:4–11. doi: 10.1186/1477-7525-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan PW, Lawrence WF, Ghushchyan V. A national catalog of preference-based scores for chronic conditions in the United States. Med Care. 2005;43(7):736–749. doi: 10.1097/01.mlr.0000172050.67085.4f. [DOI] [PubMed] [Google Scholar]
- 25.Kontodimopoulos N, Pappa E, Papadopoulos AA, Tountas Y, Niakas D. Comparing SF-6D and EQ-5D utilities across groups differing in health status. Qual Life Res. 2009;18(1):87–97. doi: 10.1007/s11136-008-9420-8. [DOI] [PubMed] [Google Scholar]
- 26.Wyrwich KW, Tierney WM, Babu AN, Kroenke K, Wolinsky FD. A comparison of clinically important differences in health-related quality of life for patients with chronic lung disease, asthma, or heart disease. Health Serv Res. 2005;40:577–591. doi: 10.1111/j.1475-6773.2005.0l374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ackermann RT, Edelstein SL, Narayan KM, Diabetes Prevention Program Research Group et al. Changes in health state utilities with changes in body mass in the Diabetes Prevention Program. Obesity. 2009;17(12):2176–2181. doi: 10.1038/oby.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williamson DA, Rejeski J, Lang W, Van Dorsten B, Fabricatore AN, Toledo K, Look AHEAD Research Group Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med. 2009;169(2):163–171. doi: 10.1001/archinternmed.2008.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch CP, McTigue KM, Bost JE, Tinker LF, Vitolins M, Adams-Campbell L, Sarto GE, Hays-Grudo J, Manson JE, Kuller LH. Excess weight and physical health-related quality of life in postmenopausal women of diverse racial/ethnic backgrounds. J Womens Health. 2010;19:1449–1458. doi: 10.1089/jwh.2009.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasiljevic N, Ralevic S, Marinkovic J, Kocev N, Maksimovic M, Milosevic GS, Tomic J. The assessment of health-related quality of life in relation to the body mass index value in the urban population of Belgrade. Health Qual Life Outcomes. 2008;6:106. doi: 10.1186/1477-7525-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hahn S, Benson S, Elsenbruch S, Pleger K, Tan S, Mann K, Schedlowski M, van Halteren WB, Kimmig R, Janssen OE. Metformin treatment of polycystic ovary syndrome improves health-related quality-of-life, emotional distress and sexuality. Hum Reprod. 2006;21:1925–1934. doi: 10.1093/humrep/del069. [DOI] [PubMed] [Google Scholar]
- 32.Maciejewski ML, Patrick DL, Williamson DF. A structured review of randomized controlled trials of weight loss showed little improvement in health-related quality of life. J Clin Epidemiol. 2005;58(6):568–578. doi: 10.1016/j.jclinepi.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Daviglus ML, Liu K, Yan LL, Pirzada A, Garside DB, Schiffer L, Dyer AR, Greenland P, Stamler J. Body mass index in middle age and health-related quality of life in older age: the Chicago heart association detection project in industry study. Arch Intern Med. 2003;163(20):2448–2455. doi: 10.1001/archinte.163.20.2448. [DOI] [PubMed] [Google Scholar]
- 34.Cameron AJ, Magliano DJ, Dunstan DW, Zimmet PZ, Hesketh K, Peeters A, Shaw JE. A bi-directional relationship between obesity and health-related quality of life: evidence from the longitudinal AusDiab study. Int J Obes (Lond) 2012;36:295–303. doi: 10.1038/ijo.2011.103. [DOI] [PubMed] [Google Scholar]
- 35.Marrett E, Stargardt T, Mavros P, Alexander CM. Patient-reported outcomes in a survey of patients treated with oral antihyperglycaemic medications: associations with hypoglycaemia and weight gain. Diabetes Obes Metab. 2009;11(12):1138–1144. doi: 10.1111/j.1463-1326.2009.01123.x. [DOI] [PubMed] [Google Scholar]
- 36.Petrou S, Hockley C. An investigation into the empirical validity of the EQ-5D and SF-6D based on hypothetical preferences in a general population. Health Econ. 2005;14(11):1169–1189. doi: 10.1002/hec.1006. [DOI] [PubMed] [Google Scholar]
- 37.Engel SG, Crosby RD, Kolotkin RL, Hartley GG, Williams GR, Wonderlich SA, Mitchell JE. Impact of weight loss and regain on quality of life: mirror image or differential effect? Obes Res. 2003;11(10):1207–1213. doi: 10.1038/oby.2003.166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 182 kb)