Abstract
Background
Up to 50 % of patients do not take medications as prescribed. Interventions to improve adherence are needed, with an understanding of which patients benefit most.
Objective
To test the effect of two low-literacy interventions on medication adherence.
Design
Randomized controlled trial, 2 × 2 factorial design.
Participants
Adults with coronary heart disease in an inner-city primary care clinic.
Interventions
For 1 year, patients received usual care, refill reminder postcards, illustrated daily medication schedules, or both interventions.
Main Measures
The primary outcome was cardiovascular medication refill adherence, assessed by the cumulative medication gap (CMG). Patients with CMG < 0.20 were considered adherent. We assessed the effect of the interventions overall and, post-hoc, in subgroups of interest.
Key Results
Most of the 435 participants were elderly (mean age = 63.7 years), African-American (91 %), and read below the 9th-grade level (78 %). Among the 420 subjects (97 %) for whom CMG could be calculated, 138 (32.9 %) had CMG < 0.20 during follow-up and were considered adherent. Overall, adherence did not differ significantly across treatments: 31.2 % in usual care, 28.3 % with mailed refill reminders, 34.2 % with illustrated medication schedules, and 36.9 % with both interventions. In post-hoc analyses, illustrated medication schedules led to significantly greater odds of adherence among patients who at baseline had more than eight medications (OR = 2.2; 95 % CI, 1.21 to 4.04) or low self-efficacy for managing medications (OR = 2.15; 95 % CI, 1.11 to 4.16); a trend was present among patients who reported non-adherence at baseline (OR = 1.89; 95 % CI, 0.99 to 3.60).
Conclusions
The interventions did not improve adherence overall. Illustrated medication schedules may improve adherence among patients with low self-efficacy, polypharmacy, or baseline non-adherence, though this requires confirmation.
KEY WORDS: coronary heart disease, medical adherence, medication management
INTRODUCTION
Despite the importance of cardiovascular risk factor control in patients with coronary heart disease (CHD),1 only 50–80 % of patients remain adherent to medical therapy.2–4 This contributes to poor disease control,5 as well as excess health care utilization, costs, and mortality.6,7 Patients may be non-adherent for many reasons, including forgetfulness, lack of motivation, and barriers such as high drug costs.8,9 Inadequate health literacy,2,10–12 poor understanding of the medication regimen,13 low self-efficacy,14 and medication regimen complexity can also adversely affect adherence.15
A number of complex interventions have demonstrated moderate success in enhancing medication adherence.16 However, in multimodal programs, it is difficult to know which components were most effective.16 Moreover, analyses rarely show how patient characteristics affected the success of the intervention.16 Interventions should be expected to achieve different results across different types of patients, as their reasons for non-adherence vary. Simpler interventions, along with enhanced understanding of patients most likely to benefit, are needed. This would enable more targeted and cost-effective implementation of adherence interventions.
National and international organizations have called for interventions to improve medication management, with attention to low health literacy,8,17–19 which affects approximately 36 % of adult Americans.20 Among the possible approaches, better patient education with the use of illustrated teaching aids appears promising. In two systematic reviews, illustrated materials improved recall, comprehension, and adherence in patients of various literacy levels.21,22 However, most of the studies were small and involved simulated medication regimens, rather than patients’ actual medications.
We conducted a randomized controlled trial to evaluate the effect of illustrated medication schedules and refill reminder postcards on medication adherence during a 1 year period. We also examined the effect of certain characteristics, including low health literacy, self-efficacy, and medication regimen complexity, on the effect of the interventions.
METHODS
Design Overview
The study, Improving Medication Adherence through Graphically Enhanced interventions in Coronary Heart Disease (IMAGE-CHD), randomized patients in a 2 × 2 factorial design, with concealment of allocation and blinding of outcome assessors. The study was approved by the university Institutional Review Board (IRB) and the health system Research Oversight Committee. Participants provided written consent and HIPAA authorization.23
Setting and Participants
Enrollment took place in the primary care clinics of an urban, safety net health system in Atlanta, GA, between March 2004 and March 2005. Clinics are staffed by Internal Medicine faculty from an affiliated university, who supervise medical residents and nurse practitioners. The majority of clinic patients are African-American, female, and of low socio-economic status. All are more than 18 years of age.
Patients with established CHD (i.e., angiographically proven CHD, positive non-invasive stress test, or history of myocardial infarction or revascularization) were eligible. Only patients who regularly filled their prescriptions in the health system pharmacy, and intended to continue doing so, were enrolled. At the time of the study, medications were dispensed in person, in a 30-day supply. Most patients had a $2.00 co-pay (range $0.50 to $5.00, based on income).
Patients were excluded for: routinely receiving caregiver assistance with medication management, already using a medication chart similar to the intervention, corrected visual acuity worse than 20/60, inability to communicate in English, no telephone, no mailing address, police custody, lack of cooperation, psychiatric illness (schizophrenia, bipolar disorder, or schizoaffective disorder), delirium or severe dementia (disoriented to person, place, or time), being too ill to participate, or previous enrollment in the study.
Randomization and Interventions
Potential subjects with CHD were identified in advance by chart screening, and recruited from the clinic waiting room on the day of scheduled primary care appointments. Consenting patients completed a 30–45 minute interviewer-assisted questionnaire and received $5.00 compensation. They were then randomized to receive usual care, refill reminder postcards, illustrated medication schedules, or both interventions for 1 year. Randomization was performed in advance using a computerized random number generator. Each treatment assignment was sealed in an opaque envelope for concealment of treatment allocation. Outcome assessors were blinded; patients and treating physicians were not.
Patients assigned to the illustrated medication schedules received, upon completion of the primary care visit, a visual depiction of their prescribed medication regimen.24 It contained the name, indication, and dosing instructions for each medication in plain language, a color image of each medication, and an icon to indicate its purpose. A grid showed how the regimen should be administered across four times of the day (Fig. 1); this format has been called the Universal Medication Schedule.25 The patient education tool had been developed and pilot tested in the clinic to ensure patient comprehension. Because study patients filled their prescriptions in the health system pharmacy, the medication images that corresponded to the pharmacy supplies were used. If suppliers changed, images were updated. When patients received their first medication schedule on the day of enrollment, a pharmacist or study coordinator (when the pharmacist was unavailable) met with the patient for approximately 5 minutes to provide orientation to the tool and briefly review the medications. Patients confirmed comprehension through teach-back. Patients were encouraged to contact the study coordinator for an updated medication schedule when their prescriptions or medication appearance changed. The coordinator also updated patients’ medication schedule quarterly based on pharmacy records, mailed this to patients with a short letter that summarized changes, and followed up with a brief phone call to verify changes and answer questions.
Figure 1.
Illustrated medication schedule. Copyright © Emory University.
The health system pharmacy always dispensed chronic medications in a 30-day supply. Therefore, patients randomized to reminder postcards had the postcards mailed to their home approximately 25 days after their last medication fill, as a behavioral cue.26 At the time of the study in this inner-city health system, mailings were a preferred format for reminders. Each postcard displayed the refill date as well as other important reminders (Fig. 2). If patients did not refill their medications, another reminder was sent every 2 weeks.
Figure 2.
Refill reminder postcard.
Data Collection
Upon enrollment, patients completed the Self-Efficacy for Appropriate Medication Use Scale (SEAMS), which measures confidence in following the regimen.27 The SEAMS is a validated, reliable (Cronbach’s α = 0.89), 13-item instrument; scores may range from 13 (low self-efficacy) to 39 (high self-efficacy). The validated 4-item Morisky Medication Adherence Scale (MMAS) assessed baseline adherence on a scale of 4 (poor) to 8 (good).28 Health literacy was measured using the Rapid Estimate of Adult Literacy in Medicine (REALM), a 66-item word pronunciation test that provides a valid and reliable assessment of literacy in the healthcare setting.29 The enrollment questionnaire also included demographics and cognitive function.30 Selected comorbidities and the number of prescription medications on the day of enrollment were abstracted from clinic charts.
The primary outcome, adherence to cardiovascular medication refills during the 1-year follow-up period, was determined by calculation of the cumulative medication gap (CMG),31 using refill data from the health system pharmacy. CMG is a valid measure of medication refill adherence that represents the proportion of time that patients did not have their medications available. For each medication, CMG is calculated as the number of days in which the medication was not available (gap), divided by the number of days between the first and last prescription fill during the study period. A medication must have been filled at least twice during the study period in order to provide sufficient data for calculation of the CMG. CMG values may range from 0 to 1, with 0 indicating no gaps or 100 % refill adherence. The prescriptions of interest here included all regularly scheduled oral medications used in the treatment or prevention of CHD, including antiplatelet therapy, antianginals, lipid-lowering medications, antihypertensives, and oral hypoglycemics; drugs taken as needed (e.g., nitroglycerine) or on different schedules (e.g., warfarin) were excluded. After calculating CMG for each cardiovascular medication, a weighted average was constructed for each patient. Weights for each medication were determined by the number of days between the first and last fill. For example, if drug A had a CMG of 0.3 and a duration of 300 days, and drug B had a CMG of 0.5 and a duration of 100 days, then the weighted average CMG for the patient would be [(0.3 × 300) + (0.5 × 100)]/400 = 0.35.2 A priori, a weighted average CMG score < 0.20, equivalent to having medications at least 80 % of the time, was considered adherent.5
Statistical Analysis
For sample size calculations, we assumed 50 % baseline adherence,4,9 alpha = 0.05, and beta = 0.20. Prior interventions had achieved absolute improvements in adherence of 15 % to 21 %.32,33 In order to detect a 20 % difference in adherence between the control and intervention groups, we determined that we needed 90 patients in each group. To account for loss to follow-up, we enrolled 440 subjects.
Descriptive statistics summarized the sample characteristics, baseline measures, and CMG. We compared baseline characteristics using ANOVA and chi-square tests. We determined the effect of treatment assignment on the primary outcome using a chi-square test. We then used logistic regression to compute the odds of adherence, including each intervention as a predictor. Post-hoc, to test whether important baseline characteristics altered the effect of each intervention, we ran models stratified by age, cognitive function, education, health literacy, self-reported adherence, medication self-efficacy, or number of prescribed medications. For the stratified models, we dichotomized MMAS at 8, REALM at 44, and self-efficacy and number of medications near the median. Models were initially constructed including an interaction term between treatments, but since no evidence of interaction was found, the results from simpler and more statistically efficient models without the interaction term are presented here. Analyses were performed by intention to treat, using SPSS 17.0 for Windows.
The study was supported by a grant from the American Heart Association, which had no role in the design of the study; the collection, analysis, and interpretation of the data; or the decision to approve publication of the finished manuscript.
RESULTS
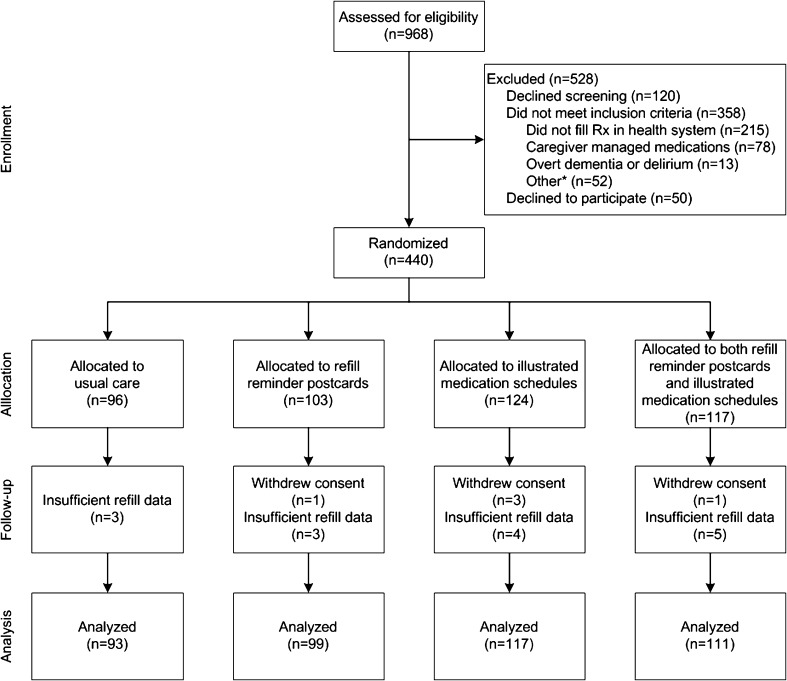
Chart screening revealed 968 patients with CHD, 490 of whom were eligible (Fig. 3). Initially, 440 patients provided consent and enrolled, although five later withdrew consent, leaving 435 patients in the study. Among them, 96 patients were assigned to receive usual care, 102 to refill reminder postcards, 121 to illustrated medication schedules, and 116 to both interventions.
Figure 3.
Study flow. *Other includes: already using medication chart (n = 11), incomplete eligibility screen (n = 10), inability to communicate in English (n = 9), visual acuity >20/60 (n = 7), no telephone or mailing address (n = 6), police custody (n = 2), lack of cooperation (n = 2), too ill to participate (n = 2), previous enrollment (n = 2), or psychiatric illness (n = 1).
Most patients were women (55.6 %) and African-American (91.0 %) (Table 1). The mean age was 63.7 years. Patients had, on average, 10.9 years of education. On the REALM, 45.1 % of subjects had a literacy level ≤ 6th grade. The median number of prescription medications was eight. The median score on the SEAMS was 32 out of 39. Half of patients were classified as having good baseline adherence by the MMAS. No significant differences in baseline characteristics were present across treatment groups (Table 1).
Table 1.
Baseline Patient Characteristics Overall and by Treatment Assignment
| Characteristic | Overall (n = 435) | Usual Care (n = 96) | Postcard Reminder (n = 102) | Illustrated Schedule (n = 121) | Both Interventions (n = 116) |
|---|---|---|---|---|---|
| Age, mean (SD) | 63.7 (10.4) | 63.7 (9.3) | 64.9 (10.4) | 63.6 (11.3) | 62.8 (10.0) |
| Female | 242 (55.6) | 53 (55.2) | 53 (52.0) | 66 (54.5) | 70 (60.3) |
| Race, African-American | 396 (91.0) | 88 (91.7) | 93 (91.2) | 106 (87.6) | 109 (94.0) |
| Yrs education, mean (SD) | 10.9 (3.1) | 10.9 (3.2) | 10.8 (3.1) | 10.8 (3.5) | 11.1 (2.6) |
| REALM | |||||
| ≤6th grade | 196 (45.1) | 43 (44.8) | 53 (52.0) | 51 (42.1) | 49 (42.2) |
| ≥7th grade | 239 (54.9) | 53 (55.2) | 49 (48.0) | 70 (57.9) | 67 (57.8) |
| MMSE, mean (SD) | 24.7 (3.2) | 24.9 (2.8) | 24.6 (3.4) | 24.7 (3.1) | 24.7 (3.5) |
| Number of prescription medications, median (IQR) | 8 (7-10) | 9 (7-10) | 8.5 (7-10) | 8 (7-11) | 8 (7-11) |
| Hypertension | 429 (98.6) | 96 (100.0) | 100 (98.0) | 119 (98.3) | 114 (98.3) |
| Hypercholesterolemia | 380 (87.4) | 87 (90.6) | 89 (87.3) | 107 (88.4) | 97 (83.6) |
| Diabetes | 196 (45.1) | 42 (43.8) | 46 (45.1) | 56 (46.3) | 52 (44.8) |
| SEAMS, median (IQR) | 32 (26-37) | 33 (26-37) | 33 (27-37) | 33 (26-37) | 30 (25-35) |
| Adherent by MMAS | 217 (50) | 53 (55.2) | 53 (52.0) | 62 (51.7) | 49 (42.2) |
Values are given as N (%) unless otherwise noted. SD = standard deviation. IQR = interquartile range
REALM = Rapid Estimate of Adult Literacy in Medicine. MMSE = Mini Mental Status Examination. SEAMS = Self-Efficacy for Appropriate Medication Use Scale. MMAS = Morisky Medication Adherence Scale
Medication Adherence
Sufficient pharmacy data to permit CMG calculations were available on 420 patients (96.6 %). No meaningful differences were present between patients with and without refill data. Values for the weighted average CMGs ranged from 0 to 0.83, with a mean of 0.32 (SD = 0.20) and median of 0.31. Overall, 32.9 % of patients (138 of 420) had CMG < 0.20 and were considered adherent to cardiovascular medications during the 1 year of follow-up.
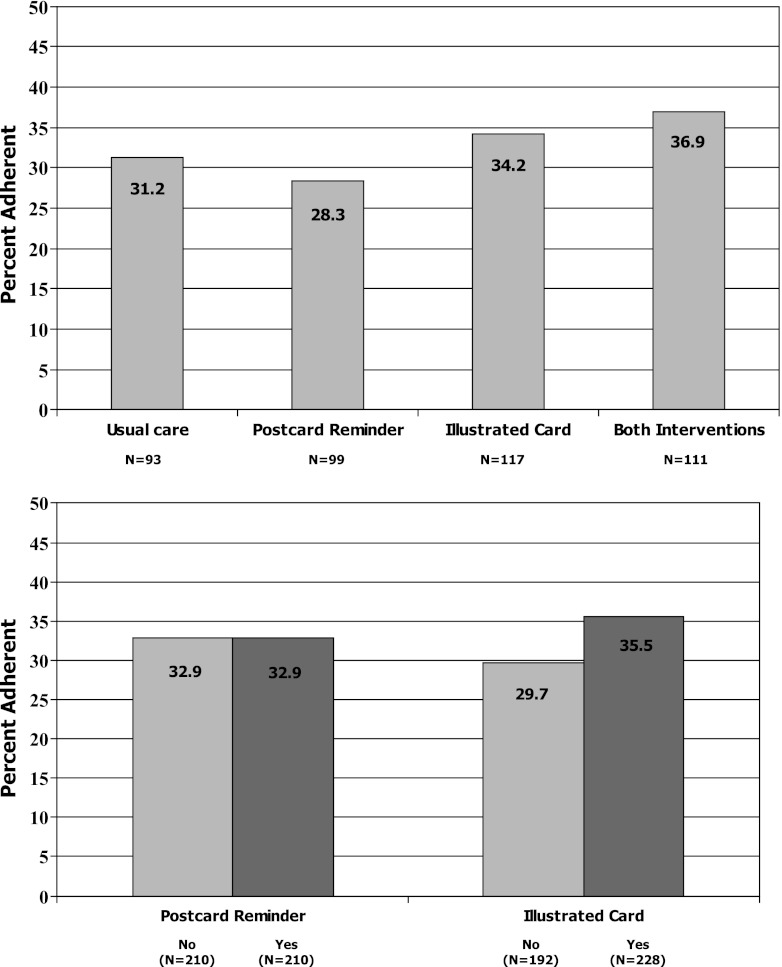
The rate of adherence (i.e., CMG < 0.20) among patients assigned to receive both interventions was 36.9 %, compared to 34.2 % in the illustrated medication schedule group, 28.3 % in the refill reminder postcard group, and 31.2 % in usual care (Fig. 4, Panel a). These differences were not statistically significant, p = 0.58. The test for interaction between treatment arms was not significant, p = 0.73.
Figure 4.
Medication adherence by study group (Panel a) and intervention (Panel b).
Analysis by intervention type showed that 35.5 % of patients (81 of 228) assigned to receive the illustrated medication schedule were adherent to refills, compared to 29.7 % of patients (57 of 192) who were not assigned to this intervention, p = 0.20 (Fig. 4, Panel b). Among the 210 patients assigned to receive the refill reminder postcards, 32.9 % (N = 69) were adherent; the same level of adherence was observed among the 210 patients not assigned to receive these reminders (N = 69 of 210 patients, 32.9 %), p = 1.0.
Overall, patients assigned to receive illustrated medication schedules had slightly higher odds of adherence (OR = 1.31; 95 % CI, 0.87-1.97), though this effect was not statistically significant (Table 2). In post-hoc subgroup analyses, illustrated medication schedules led to significantly greater odds of adherence among patients who at baseline had more than eight medications (OR = 2.21; 95 % CI, 1.21 to 4.04) or low self-efficacy for managing medications (OR = 2.15; 95 % CI, 1.11 to 4.16); a trend was present among patients who reported non-adherence at baseline (OR = 1.89; 95 % CI, 0.99 to 3.60).
Table 2.
Odds of Medication Adherence by Treatment Assignment, Overall and by Important Patient Subgroups
| Illustrated medication schedule | Postcard reminders | |
|---|---|---|
| Overall (N = 420) | 1.31 (0.87-1.97) | 1.01 (0.67-1.52) |
| Age | ||
| ≥65 (N = 203) | 1.40 (0.73-2.70) | 0.92 (0.49-1.76) |
| <65 (N = 217) | 1.29 (0.75-2.22) | 1.36 (0.66-1.96) |
| Cognition (MMSE) | ||
| <24 (N = 156) | 0.97 (0.49-1.91) | 0.92 (0.47-1.81) |
| ≥24 (N = 264) | 1.56 (0.92-2.62) | 1.06 (0.63-1.78) |
| Education | ||
| <12 yrs (N = 201) | 0.98 (0.54-1.80) | 0.73 (0.40-1.33) |
| ≥12 yrs (N = 219) | 1.64 (0.93-2.90) | 1.33 (0.76-2.34) |
| Health literacy (REALM) | ||
| ≤6th grade (N = 191) | 1.05 (0.57-1.91) | 0.81 (0.44-1.47) |
| ≥7th grade (N = 229) | 1.59 (0.90-2.82) | 1.20 (0.69-2.10) |
| Adherence (MMAS) | ||
| <8 (N = 207) | 1.89 (0.99-3.60)* | 0.77 (0.42-1.44) |
| =8 (N = 212) | 1.09 (0.62-1.90) | 1.27 (0.73-2.22) |
| Self-efficacy (SEAMS) | ||
| <32 (N = 195) | 2.15 (1.11-4.16)** | 0.93 (0.50-1.73) |
| ≥32 (N = 225) | 0.95 (0.55-1.66) | 1.03 (0.59-1.80) |
| Number of medications | ||
| >8 (N = 215) | 2.21 (1.21-4.04)† | 0.91 (0.50-1.64) |
| ≤8 (N = 202) | 0.81 (0.46-1.44) | 1.10 (0.62-1.96) |
Values are presented as (95 % CI)
MMSE = Mini Mental Status Examination. REALM = Rapid Estimate of Adult Literacy in Medicine. MMAS = Morisky Medication Adherence Scale. SEAMS = Self-Efficacy for Appropriate Medication Use Scale
*p = 0.055, **p = 0.023, †p = 0.010
Patients assigned to receive refill reminder postcards did not have better adherence overall (OR = 1.01; 95 % CI, 0.67 to 1.52) or in any of the tested subgroups (Table 2).
DISCUSSION
The IMAGE-CHD study tested two low-literacy interventions that sought to improve medication adherence in an underserved population with CHD and other comorbidities. Mailed refill reminders did not improve adherence overall or in any subgroups of interest. Illustrated medication schedules, initially accompanied by a brief orientation and review of the medication list, did not significantly improve adherence overall. In post-hoc subgroup analyses, we found that illustrated medication schedules may improve adherence among patients with low medication self-efficacy, polypharmacy, or baseline non-adherence. These results should be considered hypothesis-generating and put in the context of other investigations.
A systematic review of the literature showed that illustrated medication instructions are generally effective in improving patient satisfaction, comprehension, recall, and, in two small studies, adherence to short-term regimens such as antibiotics.22 Many of the studies summarized in that 2006 review involved simulated regimens or were conducted internationally among populations with a high prevalence of illiteracy. More recent studies from the United States, evaluating the effect of illustrated instructions on patients’ adherence and health outcomes, are limited. Among the largest studies was a randomized trial of patients with heart failure, in which illustrated materials as part of a pharmacist intervention enhanced adherence and reduced healthcare utilization and costs.34 An intervention that consisted of patient counseling and pictogram-based instruction sheets for liquid medications reduced dosing errors and improved adherence.35 Machtinger and colleagues demonstrated that brief counseling and a visual medication schedule improved warfarin regimen concordance and anticoagulation control, but only among patients whose self-reported regimen was discordant at baseline with the regimen stated by their clinician.36 At hospital discharge, a pictorial medication tool alone did not improve self-reported adherence,37 though others found that a more comprehensive intervention including pictorial instructions did reduce unplanned healthcare utilization.38 One possibility is that the combination of pictorial instructions and more intensive patient counseling is effective, but this requires further study.
It is interesting that, in the study by Machtinger, a visual medication schedule was beneficial only when uncertainty existed about the medication. However, when patients and providers were already clear about the anticoagulation regimen, illustrating the instructions provided little additional value. Similarly, in our post-hoc analyses, patients who at baseline reported less than average confidence about managing their medications appeared to benefit from having them depicted in an illustrated medication schedule. Moreover, because we studied patients’ entire medication regimen, our findings extend those of Machtinger (who studied warfarin only) by also showing possible benefit among patients with polypharmacy. However, these findings require confirmation.
Although the IMAGE-CHD interventions were designed to accommodate to the needs of patients with low health literacy,39 we found in stratified analysis that the treatment effect did not differ significantly according to patients’ health literacy. One possible explanation is that, although health literacy may be indirectly associated with medication management,2,40–43 other factors such as medication understanding and self-efficacy are more closely related to adherence behavior.27 Our stratified analyses suggest that, rather than targeting patients on the basis of low health literacy, adherence interventions should be directed toward patients with low medication self-efficacy, a complex medication regimen, or evidence of non-adherence.
Strengths of this randomized controlled trial include its focus on medication adherence in an underserved patient population, as well as high participation and follow-up rates. Notably, the interventions tested in this study were relatively simple. A review of the adherence literature reveals that successful adherence interventions often involve multiple components.16 However, when studies are positive, it is difficult to ascertain which elements were most beneficial. Moreover, intensive interventions have high resource requirements. Simple interventions are more feasible to implement and sustain, particularly in underserved settings where the needs may be greatest. More research is needed to rigorously examine the effect of simple adherence interventions such as those tested in IMAGE-CHD.
Several limitations are present. First, this study was conducted at a single institution, and the results may not be generalizable. Second, although refill adherence is considered a valid and objective measure which correlates highly with pill count and electronic monitoring,31,44,45 it is a surrogate outcome and does not directly capture daily medication taking behavior, which was a target of the illustrated medication schedule intervention. Third, refill adherence may be underestimated if patients use other pharmacies from which data are not available. Study participants all regularly used the health system pharmacy from which we obtained data, but they could have used other pharmacies during the study. This would have reduced the observed adherence rate, which was lower than that seen in other studies,2,46 but we have no reason to believe that this differed by treatment arm to bias the observed treatment effect. Fourth, the study was powered to detect a difference of 20 percentage points in adherence, a relatively large effect size that had been seen in several successful previous adherence studies.34,35 If illustrated medication schedules have an effect on adherence, the effect size is likely much smaller. Larger studies that include cost-effectiveness analyses are needed to determine the efficacy and value of interventions with a small effect size. These are worth pursuing because even small improvements in adherence (e.g., 5 percentage points) are important on a population level, particularly for prevalent conditions such as CHD.47 Fifth, the stratified analyses were conducted post-hoc, in order to better understand the effect of the interventions in subgroups of interest. Larger prospective studies with a priori hypotheses, alternate measures of daily adherence, and clinical outcome assessment are needed.
These limitations notwithstanding, the IMAGE-CHD study adds to the adherence literature a test of two simple interventions in a vulnerable population. Neither intervention significantly improved medication refill adherence overall. Illustrated medication schedules may improve adherence in certain at-risk subgroups, and this merits further study.
Acknowledgements
We wish to thank Jessica Praska, PharmD, and Akilah Strawder, PharmD, for their assistance in intervention delivery. We thank Junling Ren for her assistance in outcome assessment, as well as Courtney Cawthon, MPH, and Abby Myers for their help with manuscript preparation.
This research was supported by a grant from the American Heart Association.
Prior Presentation
Kripalani S, Robertson RS, Schmotzer B, Jacobson TA. Improving Medication Adherence through Graphically Enhanced interventions in Coronary Heart Disease: The IMAGE-CHD Study. J Gen Intern Med 2007; 21(S1):151. Poster at American Heart Association grantee symposium 2006. Oral abstract at SGIM 2007.
Kripalani S Jacobson TA. Illustrated medication schedules improve medication adherence in at-risk patients with coronary heart disease. J Gen Intern Med 2010; 25(S3):S301. Oral abstract at SGIM 2010.
Conflict of Interest
Dr. Kripalani serves as a consultant to and holds equity in PictureRx, LLC, which makes patient education tools to improve medication management. PictureRx did not provide materials or funding for this study. The terms of this arrangement were reviewed and approved by Emory University and Vanderbilt University in accordance with their conflict of interest policies. Dr. Kripalani also has served as a consultant to Pfizer, Inc. Dr. Jacobson and Mr. Schmotzer have no relevant conflicts of interest to report. The statistical analysis was performed independently by Mr. Schmotzer, who was not affiliated with or compensated by PictureRx.
Footnotes
Figure 1 was published previously in Kripalani et al. Development of an illustrated medication schedule as a low-literacy patient education tool. Pt Educ Counsel 2007; 66:386-77. Emory University retained the copyright to this figure.
REFERENCES
- 1.Smith SC, Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113(19):2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 2.Gazmararian J, Kripalani S, Miller MJ, Echt KV, Ren J, Rask KJ. Factors associated with medication refill adherence in cardiovascular-related diseases: a focus on health literacy. J Gen Intern Med. 2006;21:1215–1221. doi: 10.1111/j.1525-1497.2006.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho PM, Magid DJ, Shetterly SM, et al. Medication nonadherence is associated with a broad range of adverse outcomes in patients with coronary artery disease. Am Heart J. 2008;155(4):772–779. doi: 10.1016/j.ahj.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 4.DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 5.Ho PM, Bryson CL, Rumsfeld JS. Medication adherence: its importance in cardiovascular outcomes. Circulation. 2009;119(23):3028–3035. doi: 10.1161/CIRCULATIONAHA.108.768986. [DOI] [PubMed] [Google Scholar]
- 6.Simpson SH, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ. 2006;333(7557):15. doi: 10.1136/bmj.38875.675486.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43(6):521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Adherence to Long-Term Therapies: Evidence for Action (2003). Available at: http://www.who.int/chp/knowledge/publications/adherence_report/en/index.html. Accessed June 1, 2012.
- 9.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 10.Murray MD, Tu W, Wu J, Morrow D, Smith F, Brater DC. Factors associated with exacerbation of heart failure include treatment adherence and health literacy skills. Clin Pharm Ther. 2009;85(6):651–658. doi: 10.1038/clpt.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis TC, Wolf MS, Bass PF, III, et al. Literacy and misunderstanding prescription drug labels. Ann Intern Med. 2006;145(12):887–894. doi: 10.7326/0003-4819-145-12-200612190-00144. [DOI] [PubMed] [Google Scholar]
- 12.Wolf MS, Davis TC, Shrank W, et al. To err is human: patient misinterpretations of prescription drug label instructions. Patient Educ Couns. 2007;67(3):293–300. doi: 10.1016/j.pec.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Kripalani S, Henderson LE, Chiu EY, Robertson R, Kolm P, Jacobson TA. Predictors of medication self-management skill in a low-literacy population. J Gen Intern Med. 2006;21(8):852–856. doi: 10.1111/j.1525-1497.2006.00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandura A. Self-efficacy: The exercise of control. New York: Freeman; 1997. [Google Scholar]
- 15.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. doi: 10.1016/S0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 16.Kripalani S, Yao X, Haynes RB. Interventions to enhance medication adherence in chronic medical conditions: a systematic review. Arch Intern Med. 2007;167(6):540–550. doi: 10.1001/archinte.167.6.540. [DOI] [PubMed] [Google Scholar]
- 17.National Council on Patient Information and Education. Enhancing Prescription Medicine Adherence: A National Action Plan (2007). Available at: http://www.talkaboutrx.org/. Accessed June 1, 2012.
- 18.Health literacy. A prescription to end confusion. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 19.Joint Commission. “What Did the Doctor Say?:” Improving Health Literacy to Protect Patient Safety (2007). Available at www.jointcommission.org. Accessed June 1, 2012.
- 20.Kutner M, Greenberg E, Jin Y, Paulsen C. The health literacy of America's adults: Results from the 2003 National Assessment of Adult Literacy (NCES 2006-483) Washington, DC: U.S. Department of Education, National Center for Education Statistics; 2006. [Google Scholar]
- 21.Houts PS, Doak CC, Doak LG, Loscalzo MJ. The role of pictures in improving health communication: a review of research on attention, comprehension, recall, and adherence. Patient Educ Couns. 2006;61(2):173–190. doi: 10.1016/j.pec.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Katz MG, Kripalani S, Weiss BD. Use of pictorial aids in medication instructions: a review of the literature. Am J Health-Syst Pharm. 2006;63:2391–2397. doi: 10.2146/ajhp060162. [DOI] [PubMed] [Google Scholar]
- 23.Kripalani S, Bengtzen R, Henderson LE, Jacobson TA. Clinical research in low-literacy populations: using teach-back to assess comprehension of informed consent and privacy information. IRB: Ethics & Human Research. 2008;30(2):13–19. [PubMed] [Google Scholar]
- 24.Kripalani S, Robertson R, Love-Ghaffari MH, et al. Development of an illustrated medication schedule as a low-literacy patient education tool. Patient Educ Couns. 2007;66(3):368–377. doi: 10.1016/j.pec.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Standardizing medication labels: Confusing patients less, workshop summary. Washington, DC: The National Academies Press; 2008. [Google Scholar]
- 26.Schlenk EA, Burke LE, Rand C. Behavioral strategies to improve medication-taking compliance. In: Burke LE, Ockene IS, editors. Compliance in healthcare and research. Armonk: Futura Publishing Company, Inc; 2001. pp. 57–70. [Google Scholar]
- 27.Risser J, Jacobson TA, Kripalani S. Development and psychometric evaluation of the Self-Efficacy for Appropriate Medication Use Scale (SEAMS) in low-literacy patients with chronic disease. J Nurs Meas. 2007;15(3):203–219. doi: 10.1891/106137407783095757. [DOI] [PubMed] [Google Scholar]
- 28.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Davis TC, Crouch MA, Long SW, et al. Rapid assessment of literacy levels of adult primary care patients. Fam Med. 1991;23(6):433–435. [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. "Mini-Mental State" A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 31.Steiner JF, Prochaska AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50:105–116. doi: 10.1016/S0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 32.Raynor DK, Booth TG, Blenkinsopp A. Effects of computer generated reminder charts on patients' compliance with drug regimens. BMJ. 1993;306(6886):1158–1161. doi: 10.1136/bmj.306.6886.1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newell SA, Bowman JA, Cockburn JD. A critical review of interventions to increase compliance with medication-taking, obtaining medication refills, and appointment-keeping in the treatment of cardiovascular disease. Prev Med. 1999;29:535–548. doi: 10.1006/pmed.1999.0579. [DOI] [PubMed] [Google Scholar]
- 34.Murray MD, Young J, Hoke S, et al. Pharmacist intervention to improve medication adherence in heart failure: a randomized trial. Ann Intern Med. 2007;146(10):714–725. doi: 10.7326/0003-4819-146-10-200705150-00005. [DOI] [PubMed] [Google Scholar]
- 35.Yin HS, Dreyer BP, van Schaick L, Foltin GL, Dinglas C, Mendelsohn AL. Randomized controlled trial of a pictogram-based intervention to reduce liquid medication dosing errors and improve adherence among caregivers of young children. Arch Pediatr Adoles Med. 2008;162(9):814–822. doi: 10.1001/archpedi.162.9.814. [DOI] [PubMed] [Google Scholar]
- 36.Machtinger EL, Wang F, Chen L-L, Rodriguez M, Wu S, Schillinger D. A visual medication schedule to improve anticoagulation control: a randomized, controlled trial. Jt Comm J Qual Patient Saf. 2007;33(10):625–635. doi: 10.1016/s1553-7250(07)33072-9. [DOI] [PubMed] [Google Scholar]
- 37.Cordasco KM, Asch SM, Bell DS, et al. A low-literacy medication education tool for safety-net hospital patients. Am J Prev Med. 2009;37(6 Suppl 1):S209–216. doi: 10.1016/j.amepre.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 38.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med. 2011;155(2):97–107. doi: 10.7326/0003-4819-155-2-201107190-00005. [DOI] [PubMed] [Google Scholar]
- 40.Gazmararian J, Jacobson KL, Pan Y, Schmotzer B, Kripalani S. Effect of a pharmacy-based health literacy intervention and patient characteristics on medication refill adherence in an urban health system. Ann Pharmacother. 2010;44(1):80–87. doi: 10.1345/aph.1M328. [DOI] [PubMed] [Google Scholar]
- 41.Kalichman S, Ramachandran B, Catz S. Adherence to combination antiretroviral therapies in HIV patients of low health literacy. J Gen Intern Med. 1999;14:267–273. doi: 10.1046/j.1525-1497.1999.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chew LD, Bradley KA, Flum DR, Cornia PB, Koepsell TD. The impact of low health literacy on surgical practice. Am J Surgery. 2004;188(3):250–253. doi: 10.1016/j.amjsurg.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Golin CE, Liu H, Hays RD, et al. A prospective study of predictors of adherence to combination antiretroviral medication. J Gen Intern Med. 2002;17(10):756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choo PW, Rand CS, Inui TS, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care. 1999;37(9):846–857. doi: 10.1097/00005650-199909000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43(3):413–422. doi: 10.1345/aph.1L496. [DOI] [PubMed] [Google Scholar]
- 46.Vermeire E, Hearnshaw H, Van Royen P, Denekens J. Patient adherence to treatment: three decades of research. A comprehensive review. J Clin Pharm Ther. 2003;26:331–342. doi: 10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- 47.Miller NH, Hill M, Kottke T, Ockene IS. for the Expert Panel on Compliance. The multilevel compliance challenge: recommendations for a call to action. A statement for healthcare professionals. Circulation. 1997;95:1085–1090. doi: 10.1161/01.CIR.95.4.1085. [DOI] [PubMed] [Google Scholar]