Abstract
A monogenic dominant mutant of white clover (Trifolium repens L.), designated Mortal, which is defective in the formation of adventitious nodal roots, is described. Mortal plants grown at temperatures ranging from 10 to 25°C do not initiate nodal root primordium development. However, all other aspects of plant development are normal, including the formation of lateral roots and wound-induced adventitious roots. In some genetic backgrounds, the Mortal mutation has a temperature-sensitive conditional phenotype. Mortal plants shifted from growing conditions of 20 to 30°C for 2 to 3 d form nodal root meristems. However, new nodes that develop after plants are returned to 20°C exhibit the mutant phenotype. The capacity to form nodal roots on cuttings placed in water is also influenced by the genetic background of the Mortal mutation. Genetic analysis established that the physiological reversion of Mortal to nodal root formation is controlled by at least two separate dominant genetic loci, one for Nodal water response (Now) and one for Nodal temperature response (Not); the Now locus has a dominant epistatic interaction with the Not locus. The conditional nature of Mortal should provide opportunities for the identification of genetic and physiological mechanisms that influence the development of nodal roots.
Whereas the basic structure of angiosperms is established during embryogenesis, most organs are formed by postembryonic development (Esau, 1977). Generally, all of the shoot structures (leaves, nodes, internodes, axillary shoot meristems, and flowers) are derived from the primary shoot apical meristem. However, adventitious shoot-borne roots are an exception because they develop endogenously from differentiated parenchyma cells close to the vascular tissues (Lovell and White, 1986).
Little is known about the genes that control adventitious shoot-borne root morphogenesis, despite their importance for anchorage, nutrient acquisition, and water uptake from the soil in a wide range of plant species. One approach to understanding the genetic mechanisms that underlie adventitious root initiation and development is to identify and characterize mutants altered in the process. At present, few mutants with defects in adventitious root development are known (Schiefelbein and Benfey, 1991). There are mutants of tomato that produce few or no adventitious roots (Butler, 1954; Zobel, 1991) and mutants of maize that are defective in the formation of lateral seminal roots, crown roots, or both lateral seminal and crown roots (Jenkins, 1930; de Miranda, 1980; Hetz et al., 1996).
The general unpredictability in the formation of secondary roots on shoots complicates the analysis of the genetic and molecular mechanisms controlling adventitious root development. This can be minimized by characterizing the genetic control of the formation of adventitious nodal root primordia. In some plant species, adventitious root primordia arise in a precise and ordered manner during node development. One such example is the nodal roots that form on the prostrate stolons of white clover (Trifolium repens L.; Thomas, 1987). In T. repens, the nodes of each stolon alternate in orientation so that successive nodes produce leaves and axillary buds on opposite sides of the stolon (Erith, 1924).
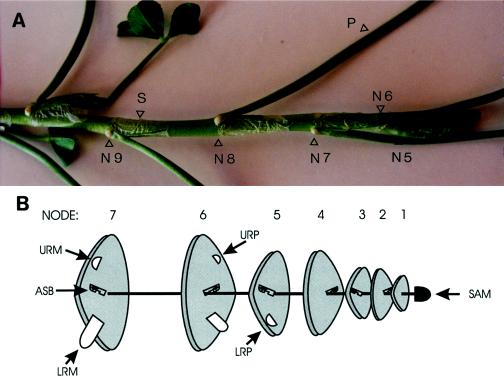
The organization of nodes and nodal roots in white clover is illustrated in Figure 1. Root primordia are typically absent from the first four nodes of wild-type white clover stolons, and nodes bearing the first five leaf primordia are enclosed within the leaf sheath of the first visible node (Erith, 1924; Thomas, 1987). The first nodal root primordium is initiated below the axillary shoot bud of the fifth node, and a second primordium forms above the axillary shoot of the sixth node. In the seventh node, the lowermost of each pair of nodal root primordia matures into a root apical meristem that grows out through both the stolon epidermis and the stipular sheath to form a visible root, whereas development of the uppermost nodal root meristem is normally arrested such that it remains within the stipule. Further growth of the uppermost nodal root meristem usually occurs only in very moist conditions.
Figure 1.
Stolon morphology of a wild-type white clover plant. A, Underside view of the apical portion of a stolon showing the nodes (N), leaf stipule (S), and petiole (P). B, Schematic representation of nodal development. SAM, Shoot apical meristem; LRP, lower root primordium; URP, upper root primordium; LRM, lower root meristem; URM, upper root meristem; and ASB, axillary shoot bud.
White clover (2n = 4× = 32) is predominantly an obligate outcrossing species with disomic inheritance. Therefore, populations are a heterogeneous mixture of highly heterozygous individuals. This heterogeneity and the associated plasticity in environmental response complicates genetic analysis of some developmental traits in white clover. However, there are dominant self-compatible alleles of the gametophytic S locus system of sexual incompatibility, which can be used to self plants for the genetic analysis of traits (Williams, 1987).
To determine the genetic control of adventitious root formation in white clover, we have identified and characterized a spontaneous mutant, designated Mortal, which is defective in nodal root primordium initiation. When grown at 20°C, Mortal plants lacked nodal root primordia but were normal in other aspects of shoot and root morphology. However, in some genetic backgrounds, Mortal was conditional, responding to either a temperature shift to 30°C or to the placing of stolon cuttings in water, by developing nodal roots. Here we describe Mortal and provide a genetic model for responses of the mutant to these temperature-shift and water treatments.
MATERIALS AND METHODS
A single plant of white clover (Trifolium repens L.) with a defect in nodal root formation was identified among seedlings grown in the controlled environment rooms of the National Climate Laboratory in Palmerston North, New Zealand. This spontaneous mutation was designated Mortal. The mutant genotype was crossed with a wild-type genotype, and mutant plants were identified from progeny. Three cycles of recurrent selection were conducted, in which only those plants that exhibited the nonnodal rooting phenotype for at least 6 months were retained. Mutant and wild-type plants were grown in individual pots containing a peat-sand mixture, under greenhouse conditions in which temperatures ranged from 10 to 30°C.
Both mutant plants and a wild-type plant (10F) were vegetatively propagated by rooting shoot (stolon) tip cuttings in a nutrient solution and then growing the rooted cuttings in a peat-sand mixture. The stolon cuttings, which included the first three to four visible nodes with leaves removed from all but the terminal node, were propagated by immersing the basal three nodes in one-half-strength Hoagland solution in the bottom, light-proofed portion of a two-chamber plastic container. The upper transparent portion of this chamber, containing the stolon tip and leaves, had small vent holes to allow air exchange while maintaining high humidity. These containers were placed in a growth cabinet (Temperzone, Temperzone Ltd., Auckland, New Zealand) set at 20°C, with a 12-h photoperiod of 800 μE m−2 s−1 PPFD.
Histology
The first three visible nodes of wild-type and Mortal stolons (nodes 5, 6, and 7) were excised, vacuum infiltrated with 50% ethanol, 5% acetic acid, and 3.7% formaldehyde for 15 min, and then fixed overnight in fresh formaldehyde at atmospheric pressure. Samples were dehydrated using ethanol, with an overnight step in 95% (v/v) ethanol and 0.1% (w/v) eosine, cleared in Histoclear (National Diagnostics, Atlanta, GA), and embedded in Paraplast (Oxford Labware, St. Louis, MO), as described by Cox and Goldberg (1988). Ten-micrometer sections were made using a rotary microtome (model RM 2045, Jung, Nusslock, Germany) and stained with a 1% aqueous solution of safranin-O (BDH, Dorset, UK). Whole transverse sections of nodes were photographed using a stereomicroscope (model Wild M3Z, Leica) and color print film (Kodak Gold III).
Temperature Treatments
Plants with 10- to 20-cm-long stolons were transferred from the greenhouse to a growth cabinet and acclimatized for 7 to 14 d at 20°C. Elevated temperature treatments for 8 to 72 h were then conducted by transferring plants to a second cabinet set at 30°C. Both cabinets had 12-h photoperiods with a PPFD of 800 μE m−2 s−1 (6× 375 W HPI/T mercury iodide high-pressure lamps and 2× 1000 W tungsten halogen lamps, Philips, Eindhoven, The Netherlands) and 50% RH. Stolon nodal rooting response was assessed 2 d after the plants had been returned to the cabinet set at 20°C. Effects of cumulative 8-h periods of 30°C interspersed with 16-h periods at 20°C were tested by growing plants in a cabinet (Conviron, Asheville, NC) with a 12-h photoperiod, 350 μE m−2 s−1 PPFD (12× 115 W cool-white fluorescent tubes, Sylvania; 8× 60 W lamps, Performer, Italy). Six temperature cycles were completed (a total of 48 h at 30°C), followed by 2 d at 20°C, before nodal rooting frequency was recorded. Each of the above experiments was replicated twice with four plants. At least two stolons from each treated plant were assessed for nodal rooting response.
Genetic Analysis
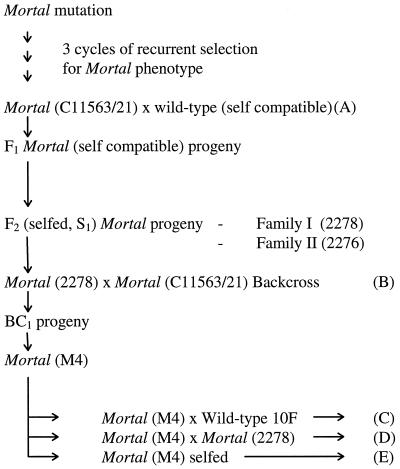
A flow chart of inheritance studies, conducted to analyze the genetic segregation of the Mortal phenotype, is given in Figure 2. The plants used in this study were derived from progeny obtained from a cross between a mutant plant (C11563/21) taken from the third cycle of recurrent selection and a wild-type genotype containing a gene for self-compatibility (A). Segregation of mutant and wild-type nodal rooting phenotypes were recorded for the F1 progeny over a 4-month period under greenhouse conditions. S1 (F2) Mortal plants 2276 and 2278 were individuals taken from two separate S1 families that were produced by self-fertilization of F1 Mortal plants.
Figure 2.
Flow chart of Mortal inheritance studies. A to E, Cross- or self-pollinated plants analyzed for genetic segregation of the Mortal mutation and interacting modifier loci.
Plants with distinctive leaf markings (Brewbaker and Carnahan, 1956; Davies, 1963) were used in the BC1 genetic analysis (B) to guard against pollen contamination. The partially inbred S1 mutant plant 2278, which had a red-fleck leaf marking but no white V marking (Rf Rf v v), was backcrossed to the original Mortal plant used in this study (C11563/21), which had a white V leaf marking at a separate locus but no red-flecking (rf rf V V). The recipient plant was emasculated prior to hand pollination. Backcross progeny were germinated and grown at 20°C in a growth cabinet and scored for both nodal rooting and leaf markings when the stolons exceeded 10 cm in length. This method of scoring the Mortal phenotype was adopted for all subsequent progeny analysis. One of the Mortal BC1 progeny plants (M4), which did not form nodal roots in response to either temperature or water treatments, was chosen for further genetic segregation analysis. Mortal (M4) was outcrossed to a wild-type plant (C), backcrossed to Mortal (2278; D), and selfed (E), all by hand pollination. Progeny from generations B to E were all assessed for the Mortal phenotype, and progeny from generations B, D, and E were also scored for response to both water and temperature-shift treatments.
Auxin Treatment
Individual nodes, including 2 to 3 mm of internode on either side, were excised from wild-type 10F and Mortal 2278 plants, incubated in Petri dishes containing 90-mm-diameter filter paper (no. 1, Whatman), and soaked in sterile water with or without 1 μm IAA, 10 μm IAA, 40 μm indole, or 80 μm indole. Twenty nodal segments of each genotype were tested with each solution. The dishes were placed in a growth cabinet at 20°C for 14 d with a 12-h photoperiod and then scored for the number of nodes forming roots and the number of roots per node.
RESULTS
Definitions of root primordia and root apical meristem given by Scheres et al. (1996) were used. The initial phase of root formation when all of the precursor cells are dividing is referred to as the root primordium, whereas when some of the cells forming the root become terminally differentiated, as in the formation of vascular tissue, the remaining mitotically active cells at the root tip are termed the root apical meristem.
Nodal Root Formation
Wild Type
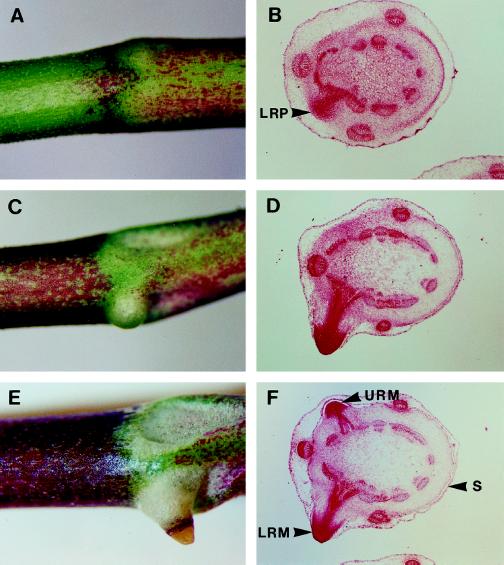
Visible nodes where nodal root development occurs were numbered proximal to the shoot apical meristem (as node 5, node 6, and node 7) and assessed for root development. Development of the upper and lower nodal root meristems was asynchronous in wild-type plants (Fig. 3). Whereas the lowermost root primordium was initiated in node 5 (Fig. 3B) and developed into a root meristem at node 6, formation of the uppermost root primordium was not initiated until node 6 (Fig. 3D). Both nodal root primordia were formed in the cortex tissue adjacent to one of the axial vascular bundles of the stolon and produced a vascular connection to that bundle (Fig. 3F). The nodal root meristem also had a vascular connection to the axillary shoot bud vascular system (not shown). Typically, the uppermost nodal root meristem was arrested in its development and remained within the stipular sheath (Fig. 3F).
Figure 3.
Nodal root development of wild-type plants. Morphology of nodes 5 (A), 6 (C), and 7 (E), and histological transverse sections of nodes 5 (B), 6 (D), and 7 (F). Positions of the leaf stipule (S), lowermost nodal root primordium (LRP), lowermost nodal root meristem (LRM), and uppermost nodal root meristem (URM) are marked with arrowheads.
Mortal
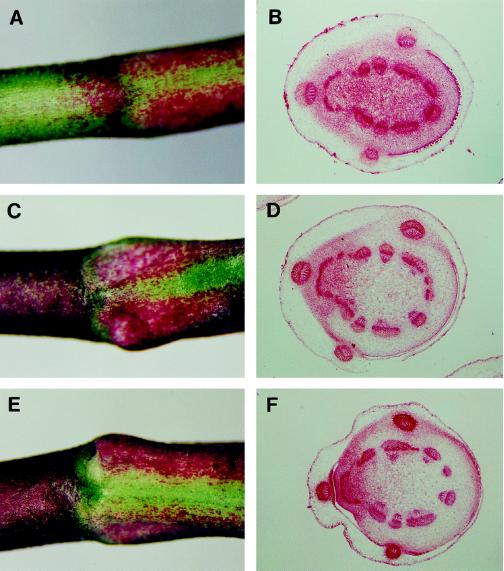
Plants of the Mortal genotypes 2276 and 2278 (from separate F2 families), grown at temperatures ranging from 10 to 25°C, were defective in nodal root primordia formation (Fig. 4). Plants with these genotypes had no external sign of nodal root formation on any of the visible nodes, and serial sectioning of nodes 5 to 7 demonstrated that even the first phase of nodal root primordium formation was absent (Fig. 4, B, D, and F). Other aspects of stolon growth and development did not appear to have been disrupted by the defect in nodal root primordia initiation. In Mortal plants grown in pots under greenhouse or growth cabinet conditions, stolon branching, leaf emergence, and flowering were all identical to that of wild-type plants.
Figure 4.
Mortal plants fail to develop nodal root primordia. Morphology of mutant nodes 5 (A), 6 (C), and 7 (E), and histological transverse sections of nodes 5 (B), 6 (D), and 7 (F).
To determine whether Mortal had retained the capacity to form either wound-induced adventitious or nodal roots, an attempt was made to root stolon tip cuttings. Stolon tip cuttings taken from wild-type genotype 10F established roots from existing nodal root primordia within 3 d when these cuttings were placed in nutrient solution (Table I). Some of these cuttings also formed adventitious roots from the wounded internode of the stolon. Surprisingly, stolon cuttings of Mortal genotype 2278 formed nodal roots after 7 to 21 d in nutrient solution (Table I). These cuttings also formed adventitious roots from cut internodes. Mortal genotype 2276 did not form nodal roots on cuttings placed in solution within a 35-d period (Table I). This genotype did, however, form adventitious roots from the wounded internode of cuttings. The data shown in Table I indicate an influence of genotype on the propensity of Mortal plants to form nodal roots in water. Therefore, a single genotype (2278) that readily formed nodal roots on stolon cuttings was selected to determine the effect of the conditional water response on the Mortal mutation (see “Genetic Analysis”).
Table I.
Nodal root formation on Mortal and wild-type plants in response to water and temperature-shift treatments
| Genotype | Rooting on Stolon
Cuttings
|
Nodal Root Frequency on 30°C-Treated Plants
|
||
|---|---|---|---|---|
| Nodal | Wound-induced | No. of roots/node | n | |
| Mortal 2278 | + | + | 2.0 | 14 |
| Mortal 2276 | − | + | 0.0 | 8 |
| Wild-type 10F | + | + | 1.4 | 10 |
Stolon cuttings (20 from each genotype) were placed in nutrient solution at 20°C and assessed for the presence (+) or absence (−) of nodal and wound-induced roots. In the temperature-shift treatment, Mortal and wild-type plants were incubated for 48 h at 30°C. Nodal root frequencies are means of the number of stolons indicated (n).
Mortal Genotype 2278 Develops Nodal Roots at 30°C
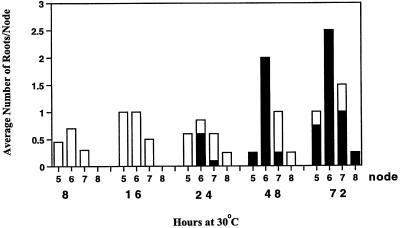
During periods in the summer when the maximum daily greenhouse temperature regularly exceeded 25°C, occasional nodal roots were observed on plants of Mortal 2278. In subsequent cooler periods the new nodes that formed on these plants were of the nonnodal rooting mutant phenotype. Mortal genotype 2276 did not form nodal roots when grown under the same conditions. This observation was confirmed by determining the nodal rooting response of mutant and wild-type genotypes treated for 2 d at 30°C (Table I). The results suggested the presence of modifier genetic loci in Mortal genotype 2278 that might interact with the mutation to give a temperature-sensitive phenotype. To characterize this phenotype further, clones of Mortal 2278 were grown in a controlled environment growth cabinet at 20°C for 2 weeks and then treated at 30°C for 8, 16, 24, 48, or 72 h before being returned to 20°C (Fig. 5).
Figure 5.
Mortal plants develop nodal root primordia in response to a temperature-shift treatment. Mutant plants grown at 20°C were shifted to 30°C for periods of 8 to 72 h and were assessed for nodal root development after being returned to growth at 20°C. Open bars, Arrested root meristems; solid bars, normal nodal root meristems. Each experiment was replicated twice with four plants.
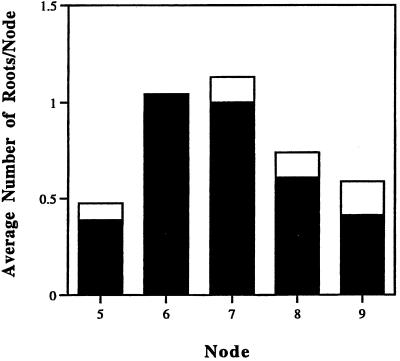
Two forms of nodal rooting response to the temperature-shift treatment were observed: (a) fully developed nodal root apical meristems equivalent to the lowermost meristem found in wild-type nodes 6 and 7, and (b) an arrested form of nodal root meristem that was visible from the outside but did not protrude through the leaf stipule. The fully formed nodal root meristems were able to develop into mature roots when placed in water at 20°C. However, the arrested form of nodal root meristem remained arrested. Mortal 2278 plants treated at 30°C for 8 or 16 h developed only arrested meristems, whereas longer periods (48 and 72 h) of high-temperature induction resulted in nodal root meristem formation. Rooting was restricted to nodes 5 to 8, with the greatest response being at node 6. Treatment of Mortal 2278 at 30°C for 48 or 72 h resulted in the formation of more than one root meristem per node on node 6 (2 and 2.5 average roots/node, respectively). The 72-h treatment resulted in some of the node 6 samples forming three root meristems. Transverse sections taken through nodes containing three roots at a node showed that all of the meristems were fully formed and connected to a stolon axial vascular bundle (data not shown).
Cumulative Nodal Root Primordia Development
Mortal plants of genotype 2278 grown in the greenhouse during the summer were not exposed to the continuous 48 h at 30°C, which was required to induce mature nodal root formation on growth-cabinet-grown plants. Instead, the periods of exposure to temperatures above 25°C in the greenhouse were most likely to have been for daily durations of fewer than 6 to 8 h. When tested, these shorter periods of elevated temperature treatment resulted only in the formation of arrested nodal root meristems incapable of further development at 20°C. This suggested that mature nodal root formation on greenhouse-grown Mortal plants might occur by the cumulative development of meristems due to successive exposure to short periods of higher temperature interspersed with longer periods at lower temperatures.
To test this hypothesis, Mortal 2278 plants were subjected to six 8-h cycles at 30°C, alternated with 16 h at 20°C (i.e. a cumulative total of 48 h at 30°C). These plants responded by producing normal nodal roots (Fig. 6) instead of the arrested root meristems that were obtained when the treatment was for a single 8-h period at 30°C (Fig. 5). However, the nodes that responded to a cumulative treatment of 48 h at 30°C, given in periods of 8 h, differed from those that responded when the elevated temperature was given as a continuous treatment. Nodal root meristems formed on node 9 of the Mortal plants receiving the cumulative treatment, whereas node 9 of plants continuously exposed to 30°C for 48 h did not undergo any form of nodal root primordia initiation. This result may be explained by the ongoing growth that occurred during the prolonged cumulative treatment. When grown for 50 d at 20°C in a growth room, both wild-type and Mortal plants had a node production rate of approximately 0.4 nodes per day (D.W.R. White and B. Campbell, unpublished data). Plants produced approximately 0.45 nodes per day when grown for 14 d at 30°C.
Figure 6.
Effect of cumulative temperature-shift treatments on nodal root development in Mortal plants. Mutant plants grown at 20°C were treated with six cycles of 30°C for 8 h, interspersed with 20°C for 16 h, and then scored for nodal root development. Each experiment was replicated twice with four plants. Open bars, Arrested root meristems; solid bars, normal nodal root meristems.
Mortal Cannot Be Rescued by Auxin Treatment
Plant growth regulators, particularly auxins, influence the initiation, growth, and development of secondary roots. To determine whether the defect in nodal root primordia initiation was due to an inadequate supply of auxin, excised nodal segments of mutant plant 2278 and wild-type plant 10F were treated with either water, IAA, or the IAA precursor indole. Incubation of Mortal node explants in water, IAA (1 or 10 μm), or indole (40 or 80 μm) for 14 d did not stimulate development of root primordia, whereas soaking wild-type nodal segments in water, IAA, or indole resulted in the outgrowth of the existing lowermost and uppermost root meristems. Therefore, the defect in nodal root primordia initiation caused by the Mortal mutation is not due to a shortage of auxin or an auxin precursor.
Genetic Analysis
Genetic analysis of the Mortal mutation was initially based on segregation for the presence or absence of nodal roots among F1 and BC1 progeny (Table II). The 1:1 segregation ratio observed in the F1 generation indicated that the mutation was possibly monogenic and dominant. To test this hypothesis, a Mortal F2 (S1) plant (2278) was backcrossed to the original Mortal (C11563/21) parent plant (Fig. 2). The 3:1 segregation of the mutant phenotype observed in the BC1 generation is consistent with the hypothesis. To confirm this conclusion, we identified a true-breeding genotype of Mortal (M4) from among the BC1 progeny plants. When Mortal M4 was outcrossed to wild type, selfed, or backcrossed to Mortal 2278, all of the progeny examined had the mutant phenotype. Some of the progeny obtained from the inbred generations D and E were stunted and slow to develop. It is likely that these stunted phenotypes were due to inbreeding depression. Such plants were not included in the analysis. In summary, the results of the segregation of the mutant phenotype among progeny, as described in Table II, indicate that Mortal is inherited as a monogenic, dominant trait.
Table II.
Genetic segregation of the Mortal phenotype
| Generation | Mortal | Wild Type | Segregation Ratio | χ2 |
|---|---|---|---|---|
| no. | ||||
| (A) Mortal (C11563/21) × Wild-type sc F1 | 23 | 25 | 1 :1 | 0.083a |
| (B) Mortal S1 (2278) × Mortal (C11563/21) BC1 | 58 | 23 | 3 :1 | 0.497a |
| (C) Mortal BC1 (M4) × Wild-type 1OF | 39 | 0 | ∞ :0 | 0a |
| (D) Mortal BC1 (M4) Selfed | 23 | 0 | ∞ :0 | 0a |
| (E) Mortal BC1 (M4) × Mortal 2278 | 37 | 0 | ∞ :0 | 0a |
No significant difference at P > 0.05.
The genetic background of the Mortal mutation appeared to influence the conditional nodal rooting response obtained from both temperature-shift and water treatments (Table I). To examine the possibility that this conditional nodal rooting response of Mortal was due to the presence of one or more modifier genes, the progenies of generations B, D, and E (Fig. 2) were tested for their response to water and temperature-shift treatments. The 1:2:1 segregation of nodal water response among the BC1 mutant progeny (Table III) indicated that this trait was controlled by a dominant modifier gene, which we have designated Now (for Nodal water response). This 1:2:1 segregation ratio would be expected if both parents were heterozygous for the locus (Now/now) and homozygous progeny had a greater penetrance for the character than heterozygous progeny. The modifier locus that appeared to control the conditional temperature shift was designated Not (for Nodal temperature response).
Table III.
Genetic segregation of nodal water response phenotype among Mortal progeny
| Generation | Nodal Rooting Response
Phenotype
|
Segregation Ratio | χ2 | ||
|---|---|---|---|---|---|
| All Now | Partial Now | now | |||
| no. | |||||
| (B) Mortal (2278) × Mortal (C11563/21) | 15 | 27 | 14 | 1:2:1 | 0.107a |
Six stolon cuttings from each genotype were tested. Three classes of response, all (All Now), some (Partial Now), or no (now) cuttings forming nodal roots, were observed.
No significant difference at P > 0.05.
Segregation analyses of both the nodal water response and the nodal temperature-response phenotypes among the mutant plants in the progeny of generations B, D, and E are given in Table IV. Mortal progeny from the backcross generation Mortal 2278 × Mortal C11563/21 segregated 12:3:1 for the phenotypes, nodal water and temperature response:temperature response only:no response to either treatment. The segregation ratio observed indicated a possible epistatic interaction between two dominant modifier loci. To obtain the observed 12:3:1 segregation ratio, both parents would have to be heterozygous for both the Now and Not genetic loci. To confirm this interpretation of the data, mutant progeny from the backcross Mortal M4 × Mortal 2278 were also analyzed for segregation of the Now and Not loci. Segregation analysis indicated that both modifier loci were absent from the M4 parent used in this cross. The 2:1:1 segregation ratio of Now/Not to now/Not to now/not observed among the mutant progeny of generation D (Table IV) supports the hypothesis that Now has a dominant epistatic interaction with the not allele of the nodal temperature response locus (i.e. in the presence of Now and the absence of Not Mortal plants formed nodal roots in response to a temperature-shift treatment).
Table IV.
Genetic segregation of modifier loci among Mortal progeny
Mortal progeny plants were scored for the formation of nodal roots in response to a temperature-shift (Not) treatment or the response of three stolon cuttings to a water (Now) treatment.
| Generation | Nodal Rooting
Response Phenotype
|
Segregation Ratio | χ2 | ||
|---|---|---|---|---|---|
| Now/Not | now/Not | now/not | |||
| no. | |||||
| (B) Mortal (2278) × Mortal (C11563/21) | 42 | 11 | 3 | 12:3:1 | 0.095a |
| (D) Mortal (M4) × Mortal (2278) | 19 | 7 | 11 | 2:1:1 | 0.892a |
| (E) Mortal (M4) selfed | 0 | 0 | 23 | 0:∞ | 0a |
Not significant at P > 0.05.
DISCUSSION
We report here the identification and initial characterization of a white clover mutant defective in the formation of nodal root primordium. To our knowledge, the only other defect specifically disrupting nodal root formation that has been described in detail is the recessive rtcs mutation of maize (Hetz et al., 1996). The Mortal mutation had no apparent pleiotropic effects on the shoot morphology of white clover plants. Furthermore, the Mortal mutation exclusively affected nodal root primordium formation; neither lateral root development nor wound-induced adventitious rooting on cuttings was altered. Smith and Fedoroff (1995) postulated that the paucity of mutants exclusively affecting secondary root development is due to the genes involved having duplicate functions.
It is noteworthy that the HRGPnt3 gene of tobacco and the LRP1 gene isolated from Arabidopsis, which are both expressed during the early phases of lateral root primordium development, are also expressed in adventitious root primordia (Vera et al., 1994; Smith and Fedoroff, 1995). However, the presence of the Mortal phenotype of white clover and the rtcs mutant phenotype in maize indicates that there are genetic aspects of secondary root primordium initiation and development that are unique to the node. Because nodal root primordia are regularly initiated in an invariant pattern on the stolons of wild-type white clover (Thomas, 1987), we were able to determine by serial sectioning of nodes that the blockage in development caused by the Mortal mutation prevented the initial divisions that contribute to nodal root primordium development. The rcts mutation in maize also prevents the initiation of nodal root primordia (Hetz et al., 1996).
An unusual feature of Mortal in some genetic backgrounds is its response to a shift in growing temperature from 20 to 30°C, which rescues nodal root primordium development. It is more common for temperature-sensitive mutants to adopt the mutant phenotype when shifted to a higher temperature rather than revert from mutant to normal development. An example is the temperature-sensitive mutants of Arabidopsis isolated by Baskin et al. (1992), which are normal when grown at 18°C but have radial swelling of the root apex when transferred to 31°C. There are only a few cases in which expression of a mutation in plant development occurs at low temperature and development is normal at high temperature. The recessive Arabidopsis mutant fab2, which overproduces the fatty acid stearate (Lightner et al., 1994), recessive sweetclover mutants defective in chlorophyll production (Bevins et al., 1993), and a recessive, temperature-dependent shooty mutant of tobacco (Samuelsen et al., 1997) are specific examples.
The Mortal mutation is distinct because the temperature-sensitive response requires the presence of a separate, dominant-modifier genetic locus. The temperature-shift effect on Mortal was rapidly reversible because further development of the partially formed primordia or meristems induced by an 8-h treatment at 30°C was blocked when the plants were returned to growth at 20°C. This arrest may parallel development of the uppermost nodal root meristem of wild-type plants, which is interrupted before maturity, and further examination of conditions that lead to continued development of the uppermost nodal root meristem of wild-type plants may provide insight into the normal function of the Mortal gene. It is noteworthy that some of the nodes on wild-type plants treated at 30°C for 48 h responded by outgrowth of the uppermost nodal root meristem (Table I). Also, further development of the uppermost nodal root meristem can be induced when the intact stolon is immersed in water. Both inhibition and reactivation of nodal meristem development may therefore be a normal feature of uppermost nodal root formation in white clover.
Numerous experimental results indicate that phytohormones play an important role in the regulation of secondary root primordium development. There are secondary root development mutants that have either elevated levels of auxin (Boerjan et al., 1995; King et al., 1995) or can be rescued by an exogenous supply of auxin (Celenza et al., 1995). Haissig (1972) demonstrated that both the level of endogenous auxin and applied GA3 influence the number of cells in developing nodal root primordia of brittle willow. However, the lack of a response to the addition of IAA or indole to nodal explants suggests that the Mortal mutation is not due to a defect in auxin biosynthesis. This conclusion does not eliminate the possibility that altered auxin homeostasis may be involved in the disruption of nodal rooting.
The genetic background of the Mortal mutation also influenced the ability of a genotype to form nodal roots on cuttings placed in nutrient solution (Table I). This genetic background effect on both temperature and water responses indicates that modifier genes activate a signaling pathway between environmental conditions and Mortal gene function. Genetic analysis determined that the defect in nodal root development designated Mortal was due to a monogenic dominant mutation. Furthermore, results from the genetic analysis support a model in which physiological reversion of Mortal to nodal root primordium development is determined by at least two independently segregating, naturally occurring, dominant modifier loci (Now for water response and Not for the temperature-shift response). The presence of the Now locus is sufficient to allow a nodal rooting response on mutant plants to both water and temperature-shift treatments, but the Not locus confers nodal rooting only in response to the temperature-shift treatment. This dominant epistatic interaction between the modifier loci suggests that a complex signaling pathway controls nodal root development and maturation in white clover.
Any model to explain the Mortal mutation has to include a dominant loss-of-function alteration and an inhibition of development that can occur at any phase between nodal root primordium initiation and formation of the meristem. Our working hypothesis is that Mortal is due to the expression of a product that inhibits both the initiation and continued division of those cells that would normally constitute the nodal root primordium.
The conditional nature of Mortal will provide a means of identifying the genetic and molecular mechanisms that control the development of adventitious root meristems. Because mutant plants can be treated to provide material arrested at various stages of nodal root primordium development, methods of mRNA comparison can be used to identify gene expression specific to different phases of adventitious root development. Furthermore, Mortal plants provide useful material with which to study the ecological implications of nodal root formation for plants such as white clover, which have a clonal vegetative growth form, and to deduce the signaling mechanisms that control nodal root development.
ACKNOWLEDGMENTS
The authors would like to thank Roy Meeking and Thomas Berryman of the D.W.R. White laboratory for their technical contributions and Greg Cousins of the Plant Improvement Group for some of the crossing of mutant white clover plants. We are grateful to Warren Williams and Bruce Campbell for comments on the manuscript.
Footnotes
This work was supported by the New Zealand Foundation for Research, Science and Technology (grant no. C10-405 to D.W.R.W. and grant no. C10-310 to J.R.C. and D.R.W.).
LITERATURE CITED
- Baskin TI, Betzner AS, Hoggart R, Cork A, Williamson RE. Root morphology mutants in Arabidopsis thaliana. Aust J Plant Physiol. 1992;19:427–437. [Google Scholar]
- Bevins MA, Madhavan S, Markwell JW. Two sweetclover (Melilotus alba Desr.) mutants temperature sensitive for chlorophyll expression. Plant Physiol. 1993;103:1123–1131. doi: 10.1104/pp.103.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerjan W, Cervera M-T, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inze D. superroot, a recessive mutant in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–1419. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewbaker JL, Carnahan JL. Leaf marking alleles in white clover—uniform nomenclature. J Hered. 1956;47:103–104. [Google Scholar]
- Butler L. Two new mutants in the tomato—propeller and rosette. J Hered. 1954;45:25–27. [Google Scholar]
- Celenza JL, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 1995;9:2131–2142. doi: 10.1101/gad.9.17.2131. [DOI] [PubMed] [Google Scholar]
- Cox KH, Goldberg RB (1988) Analysis of plant gene expression. In CH Shaw, ed, Plant Molecular Biology, A Practical Approach. IRL Press, New York, pp 1–34
- Davies WE (1963) Leaf markings in Trifolium repens. In CD Darlington, AD Bradshaw, eds, Teaching Genetics. Oliver & Boyd, London, pp 94–98
- de Miranda LT. Inheritance and linkage of root characteristic from Pueblo maize. Maize Genet Coop Newslett. 1980;54:18–19. [Google Scholar]
- Erith AG (1924) White Clover (Trifolium repens L.). Duckworth, London
- Esau K. Anatomy of Seed Plants, Ed 2. New York: John Wiley & Sons; 1977. [Google Scholar]
- Haissig BE. Meristematic activity during adventitious root primordium development: influences of endogenous auxin and applied gibberellic acid. Plant Physiol. 1972;49:886–892. doi: 10.1104/pp.49.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz W, Hochholdinger F, Schwall M, Feix G. Isolation and characterization of rtcs, a maize mutant deficient in the formation of nodal roots. Plant J. 1996;10:845–857. [Google Scholar]
- Jenkins MT. Heritable characters of maize. XXXIV. Rootless. J Hered. 1930;21:79–80. [Google Scholar]
- King JJ, Stimart DP, Fisher RH, Bleecker AB. Plant Cell. 1995;7:2023–2037. doi: 10.1105/tpc.7.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner J, James DW, Jr, Dooner HK, Browse J. Altered body morphology is caused by increased stearate levels in a mutant of Arabidopsis. Plant J. 1994;6:401–412. [Google Scholar]
- Lovell PH, White J (1986) Anatomical changes during adventitious root formation, In MB Jackson, ed, New Root Formation in Plants and Cuttings. Martinus Nijhoff Publishers, Dordrecht, The Netherlands, pp 111–140
- Samuelsen AI, Rickson FR, Mok DWS, Mok MC. A temperature-dependent morphological mutant of tobacco. Planta. 1997;201:303–310. doi: 10.1007/s004250050071. [DOI] [PubMed] [Google Scholar]
- Scheres B, McKhann HI, van den Berg C. Roots redefined. Anatomical and genetic analysis of root development. Plant Physiol. 1996;111:959–964. doi: 10.1104/pp.111.4.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW, Benfey PN. The development of plant roots: new approaches to underground problems. Plant Cell. 1991;3:1147–1154. doi: 10.1105/tpc.3.11.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Federoff NV. Plant Cell. 1995;7:735–745. doi: 10.1105/tpc.7.6.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RG (1987) Vegetative growth and development. In MJ Baker, WM Williams, eds, White Clover. CAB International, Wallingford, UK, pp 31–55
- Vera P, Lamb CJ, Doerner PW. Cell-cycle regulation of hydroxyproline-rich glycoprotein HRGPnt3 gene expression during the initiation of lateral root meristems. Plant J. 1994;6:717–727. [Google Scholar]
- Williams WM (1987) Genetics and breeding. In MJ Baker, WM Williams, eds, White Clover. CAB International, Wallingford, UK, pp 343–419
- Zobel RW (1991) Genetic control of root systems. In Y Waisel, A Eshel, U Kafkafi, eds, Plant Roots: The Hidden Half. Marcel Dekker, New York, pp 21–30