Abstract
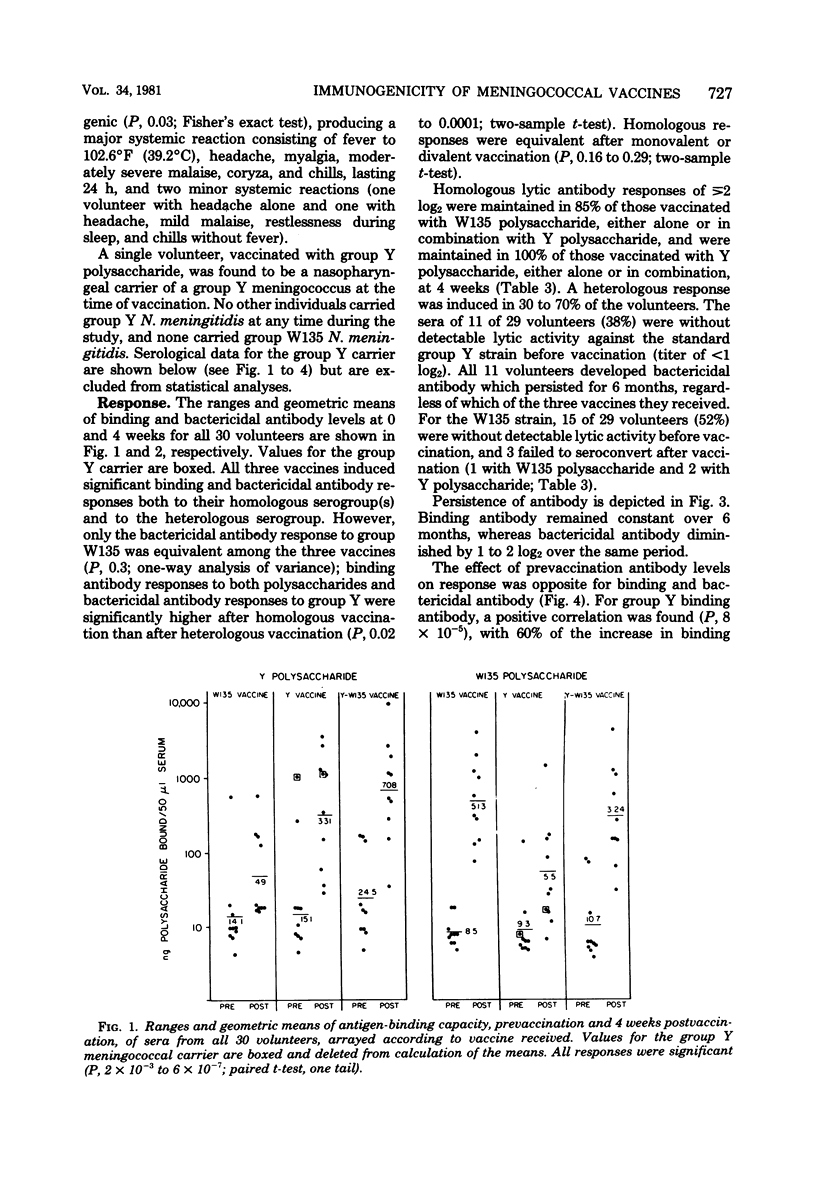
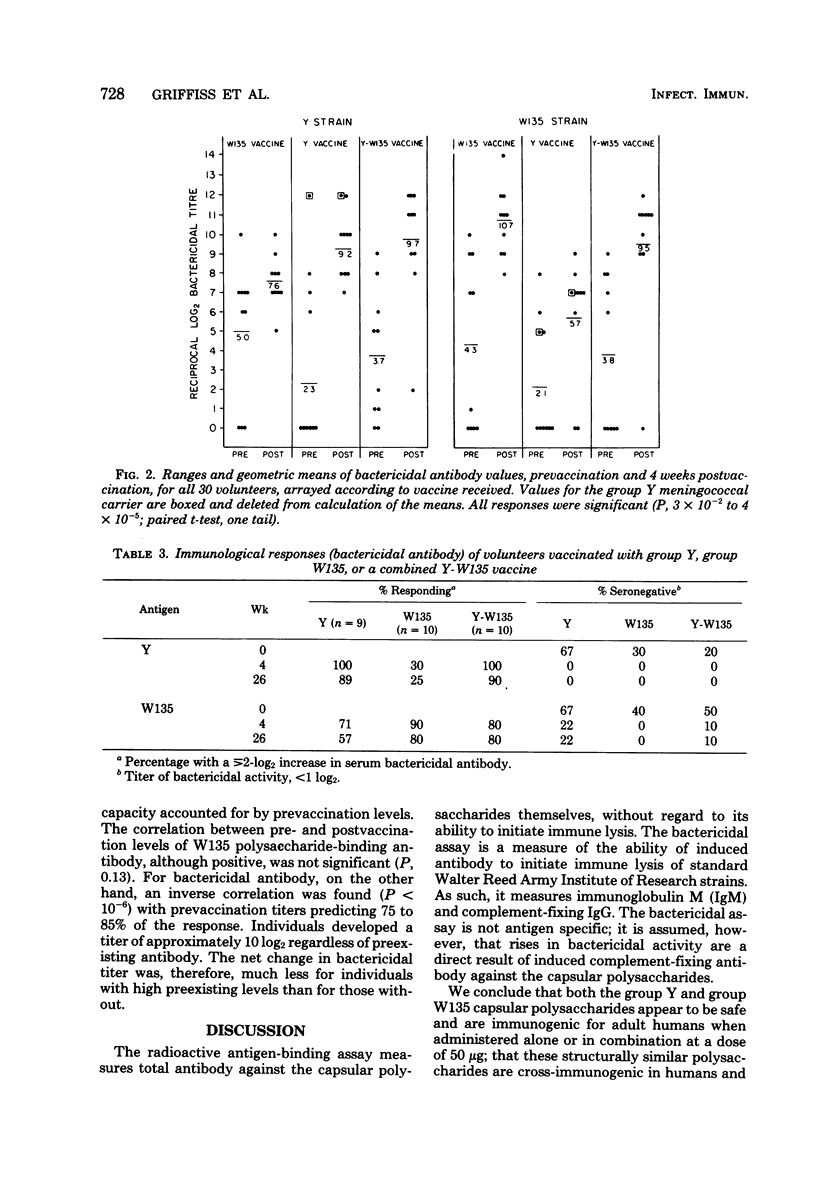
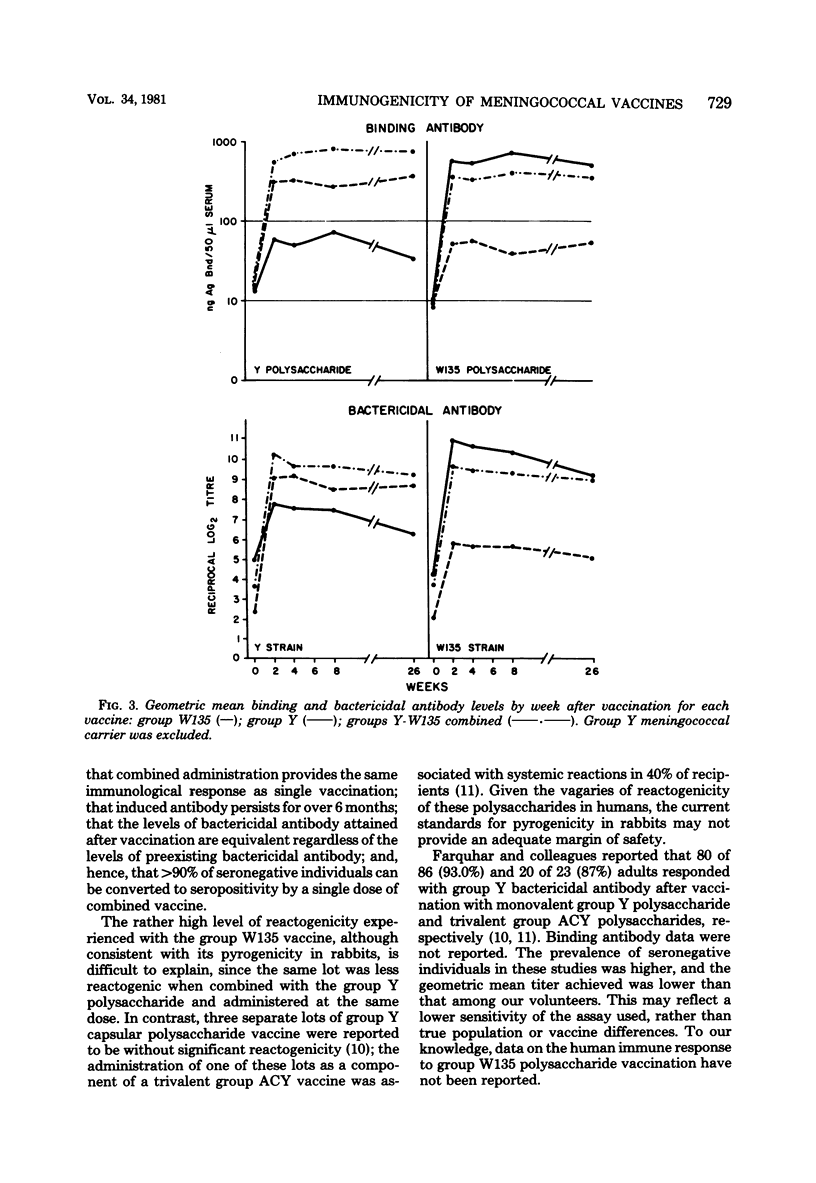
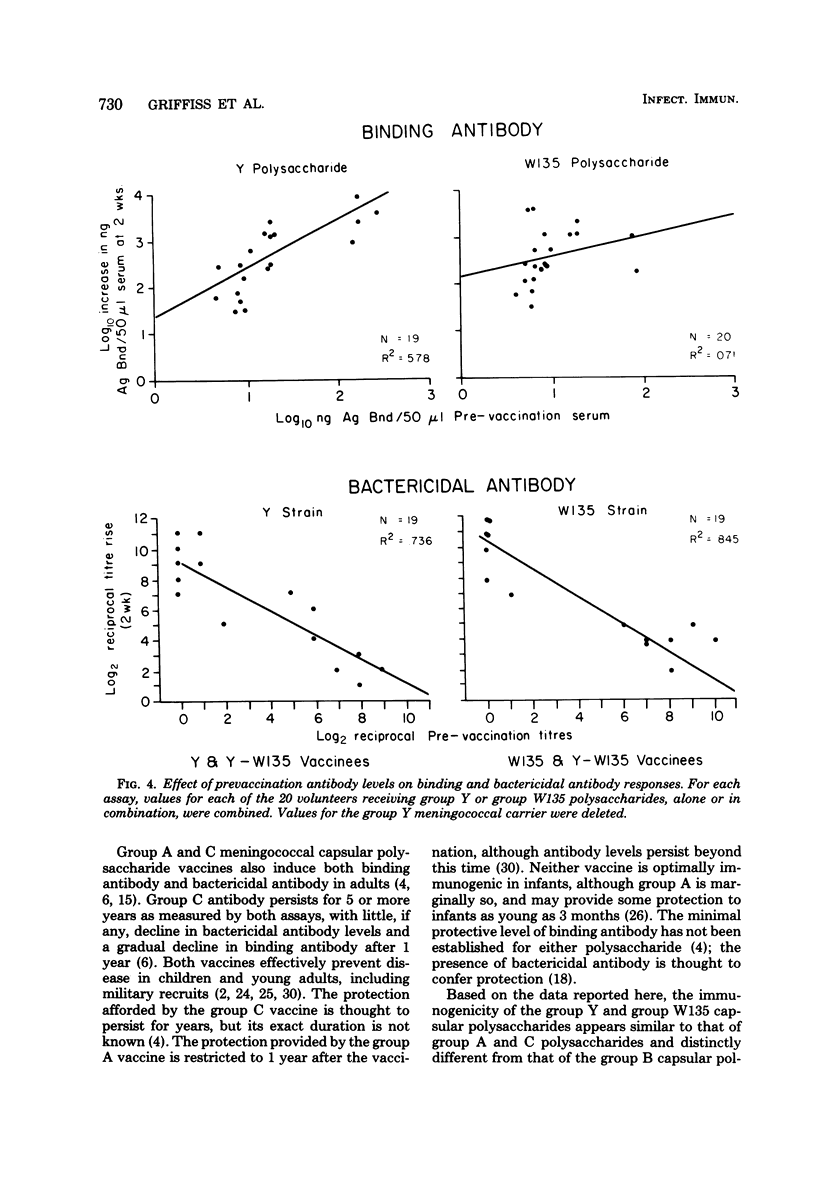
Serogroup Y and W135 Neisseria meningitidis capsular polysaccharide vaccines were tested as monovalent and divalent preparations in groups of 10 adult human volunteers at a dose of 50 (monovalent) or 100 micrograms (divalent) injected subcutaneously. Reactogenicity was low for the group Y vaccine and the group Y-W135 combined vaccine; 3 of 10 volunteers developed systemic reactions after group W135 vaccination. All three vaccines induced significant homologous and heterologous binding and bactericidal antibody. Except for group W135 bactericidal antibody, homologous responses exceeded heterologous responses, and divalent and monovalent vaccines induced equivalent homologous responses. Homologous bactericidal antibody responses were maintained for 4 weeks in 85% of group W135 vaccinates and in 100% of group Y vaccinates. Bactericidal antibody was induced in 11 of 11 group Y and 12 of 15 group W135 volunteers without preexisting respective bactericidal activities, regardless of which vaccine they received. For all three vaccines, antibody levels declined only slightly over 6 months. Prevaccination antibody levels positively affected postvaccination binding antibody levels, but not bactericidal levels.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artenstein M. S., Gold R., Zimmerly J. G., Wyle F. A., Schneider H., Harkins C. Prevention of meningococcal disease by group C polysaccharide vaccine. N Engl J Med. 1970 Feb 19;282(8):417–420. doi: 10.1056/NEJM197002192820803. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Jennings H. J., Kenny C. P., Martin A., Smith I. C. Structural determination of the polysaccharide antigens of Neisseria meningitidis serogroups Y, W-135, and BO1. Can J Biochem. 1976 Jan;54(1):1–8. doi: 10.1139/o76-001. [DOI] [PubMed] [Google Scholar]
- Brandt B. L., Artenstein M. S., Smith C. D. Antibody responses to meningococcal polysaccharide vaccines. Infect Immun. 1973 Oct;8(4):590–596. doi: 10.1128/iai.8.4.590-596.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B. L., Smith C. D., Artenstein M. S. Immunogenicity of serogroup A and C Neisseria meningitidis polysaccharide vaccines administered together in humans. J Infect Dis. 1978 Feb;137(2):202–205. doi: 10.1093/infdis/137.2.202. [DOI] [PubMed] [Google Scholar]
- Counts G. W., Petersdorf R. G. 'The wheel within a wheel': meningococcal trends. JAMA. 1980 Nov 14;244(19):2200–2201. [PubMed] [Google Scholar]
- Evans J. R., Artenstein M. S., Hunter D. H. Prevalence of meningococcal serogroups and description of three new groups. Am J Epidemiol. 1968 May;87(3):643–646. doi: 10.1093/oxfordjournals.aje.a120854. [DOI] [PubMed] [Google Scholar]
- Farquhar J. D., Hankins W. A., DeSanctis A. N., DeMeio J. L., Metzgar D. P. Clinical and serological evaluation of purified polysaccharide vaccines prepared from Neisseria meningitidis group Y. Proc Soc Exp Biol Med. 1977 Sep;155(4):453–455. doi: 10.3181/00379727-155-39828. [DOI] [PubMed] [Google Scholar]
- Farquhar J. D., Hankins W. A., Desanctis A. N., Demeio J. L., Metzgar D. P. Clinical and serological evaluation of a meningococcal polysaccharide vaccine groups A, C, and Y. Proc Soc Exp Biol Med. 1978 Jan;157(1):79–82. doi: 10.3181/00379727-157-39995. [DOI] [PubMed] [Google Scholar]
- Galaid E. I., Cherubin C. E., Marr J. S., Schaefler S., Barone J., Lee W. Meningococcal disease in New York City, 1973 to 1978. Recognition of groups y and W-135 as frequent pathogens. JAMA. 1980 Nov 14;244(19):2167–2171. [PubMed] [Google Scholar]
- Gotschlich E. C., Rey M., Etienne J., Sanborn W. R., Triau R., Cvjetanović B. The immunological responses observed in field studies in Africa with group A meningococcal vaccines. Prog Immunobiol Stand. 1971;5:485–491. [PubMed] [Google Scholar]
- Griffiss J. M. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J Immunol. 1975 Jun;114(6):1779–1784. [PubMed] [Google Scholar]
- Griffiss J. M., Brandt B. L. Disease due to serogroup W135 Neisseria meningitidis. Pediatrics. 1979 Aug;64(2):218–221. [PubMed] [Google Scholar]
- Griffiss J. M., Broud D. D., Silver C. A., Artenstein M. S. Immunoepidemiology of meningococcal disease in military recruits. I. A model for serogroup independency of epidemic potential as determined by serotyping. J Infect Dis. 1977 Aug;136(2):176–186. doi: 10.1093/infdis/136.2.176. [DOI] [PubMed] [Google Scholar]
- Koppes G. M., Ellenbogen C., Gebhart R. J. Group Y meningococcal disease in United States Air Force recruits. Am J Med. 1977 May;62(5):661–666. doi: 10.1016/0002-9343(77)90867-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mäkelä P. H., Käyhty H., Weckström P., Sivonen A., Renkonen O. V. Effect of group-A meningococcal vaccine in army recruits in Finland. Lancet. 1975 Nov 8;2(7941):883–886. doi: 10.1016/s0140-6736(75)92125-x. [DOI] [PubMed] [Google Scholar]
- Peltola H., Mäkelä H., Käyhty H., Jousimies H., Herva E., Hällström K., Sivonen A., Renkonen O. V., Pettay O., Karanko V. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med. 1977 Sep 29;297(13):686–691. doi: 10.1056/NEJM197709292971302. [DOI] [PubMed] [Google Scholar]
- Peltola H., Mäkelä P. H., ELO O., Pettay O., Renkonen O. V., Sivonen A. Vaccination against meningococcal group A disease in Finland 1974-75. Scand J Infect Dis. 1976;8(3):169–174. doi: 10.3109/inf.1976.8.issue-3.09. [DOI] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Zolyomi S., Sadoff J. C. Isolation and characterization of a high-molecular-weight polysaccharide from the slime of Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):908–918. doi: 10.1128/iai.22.3.908-918.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siber G. R., Schur P. H., Aisenberg A. C., Weitzman S. A., Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980 Jul 24;303(4):178–182. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- Smilack J. D. Group-y meningococcal disease. Twelve cases at an army training center. Ann Intern Med. 1974 Dec;81(6):740–745. doi: 10.7326/0003-4819-81-6-740. [DOI] [PubMed] [Google Scholar]
- Wahdan M. H., Sallam S. A., Hassan M. N., Abdel Gawad A., Rakha A. S., Sippel J. E., Hablas R., Sanborn W. R., Kassem N. M., Riad S. M. A second controlled field trial of a serogroup A meningococcal polysaccharide vaccine in Alexandria. Bull World Health Organ. 1977;55(6):645–651. [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Barrera O., Sutton A., May J., Hochstein D. H., Robbins J. D., Robbins J. B., Parkman P. D., Seligmann E. B., Jr Standardization and control of meningococcal vaccines, group A and group C polysaccharides. J Biol Stand. 1977;5(3):197–215. doi: 10.1016/s0092-1157(77)80005-x. [DOI] [PubMed] [Google Scholar]
- Zollinger W. D., Mandrell R. E., Griffiss J. M., Altieri P., Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Invest. 1979 May;63(5):836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]


