Abstract
Adulterants “cut into” street heroin are common and often not detected by standard urine toxicology screening; however, their unwitting co-injection may have clinical consequences. We report a case of accelerated atrioventricular junctional arrhythmia that we determined to have been caused by quinine/quinidine cut into heroin. While identification and discontinuation of the offending agent helps confirm the diagnosis and is the treatment of choice, this is often complicated by the individual’s dependence on the street drug in which the adulterant is mixed. This case highlights the need for clinicians to be aware of common adulterants, to know how to test for them, and to consider them as possible causes of medical complications in individuals who use drugs.
KEY WORDS: arrhythmias, heroin, cocaine, adulterants
INTRODUCTION
Adulterants are often added to (“cut into”) street heroin during packaging to increase profit by increasing product quantity, enhancing desirable/expected drug effects, or mimicking other drug characteristics. According to the Drug Enforcement Administration, heroin seized in Baltimore between January 2009 and March 2010 had a purity of 0–36 %; adulterants found included acetaminophen, caffeine, diphenhydramine, methorphan, alprazolam, quetiapine, chloroquine, diltiazem, cocaine, procaine, lidocaine, quinine/quinidine, phenacetin, and thiamine (personal communication with DEA Special Testing and Research Laboratory, March 23, 2010). Some of these adulterants have their own cardiac and other medical implications that can add to those of heroin.
CASE REPORT
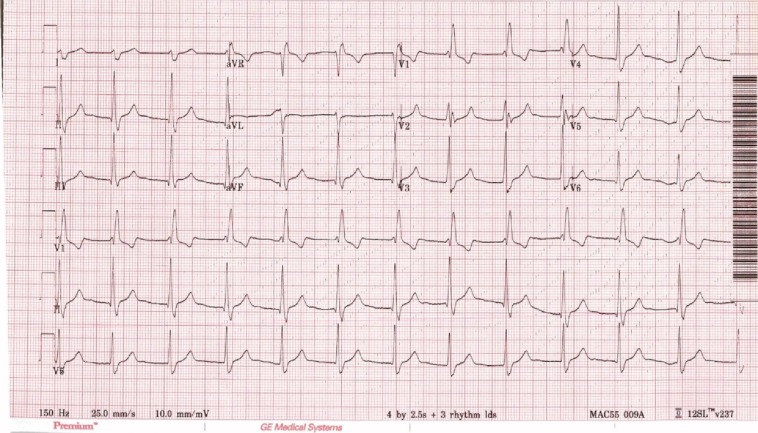
An asymptomatic 31-year-old male participating in a clinical research study was found to have an accelerated atrioventricular (AV) junctional rhythm on routine electrocardiogram obtained per protocol (Fig. 1). He reported no recent change in his level of exertion or stress. His past medical history included intravenous heroin dependence, hepatitis C virus, and tobacco dependence. Previous electrocardiograms reviewed by a cardiologist showed sinus bradycardia with a QTc of 443 ms and right bundle branch block. His prescribed medications included methadone 100 mg PO daily. On review of systems, he denied palpitations, fatigue, poor exercise tolerance, dyspnea, and presyncope. His vital signs were normal, and a physical exam was unremarkable. Laboratory workup was negative for abnormalities in electrolytes, glucose, thyroid hormones, and erythrocyte sedimentation rate. Screening urine toxicology confirmed heroin metabolites (codeine/morphine) and methadone. Transthoracic echocardiogram showed an estimated ejection fraction of 50–55 % and mild tricuspid regurgitation with RSVP 26 mmHg. The left ventricular size and wall thickness were normal, as were the right ventricular size and function. There was borderline left atrial enlargement and normal right atrial size. Trace mitral regurgitation and trace pulmonary regurgitation were also present. Having ruled out common metabolic and structural causes of an accelerated junctional rhythm, we considered inadvertent drug-induced causes. Gas chromatography/mass spectromic (GC/MS) analysis of the urine specimen collected at the time of the detected ECG abnormality identified codeine/morphine, methadone, acetaminophen, and quinine/quinidine.
Figure 1.
Accelerated AV junctional rhythm @75 bpm with right bundle branch block and probable retrograde P waves in lead V2.
DISCUSSION
An AV junctional escape rhythm is a narrow QRS complex at the rate of 40–60 beats per minute (bpm) and is a normal escape-rhythm response when the sino-atrial rate falls below the typical AV junctional rate or when AV heart block is present. Junctional escape rhythms can occur at any age and are equally common in males and females; they are especially common in younger and/or athletic individuals during periods of increased vagal tone (e.g., sleep). Junctional escape rhythms may be symptomatic or asymptomatic (usually determined by heart rate). Prominent jugular venous pulsations from cannon “a” waves may also be present because of the contraction of the right atrium against a closed tricuspid valve.1
Accelerated junctional rhythms, however, are less common and potentially more problematic. An accelerated AV junctional rhythm is an automatic tachycardia, usually with narrow uniform QRS complexes, rate >60 bpm, and variable retrograde P-wave activation. The most common cause of an accelerated junctional rhythm is digitalis toxicity. Other causes include sick sinus syndrome, recent cardiac surgery (typically valve replacement), acute myocardial infarction (especially acute inferior infarction involving the posterior descending artery, the origin of the atrioventricular nodal branch), isoproterenol infusion, acute inflammatory processes (e.g., acute rheumatic fever, Lyme disease), metabolic states with increased adrenergic tone, diphtheria, and other drugs that cause bradycardia (e.g., beta-blockers, calcium blockers, etc.), none of which were present in this patient.
In this patient, the most notable findings from the workup were the presence of acetaminophen and quinine/quinidine in the urine. In 2009–2010, the three most common heroin adulterants in Baltimore were: (1) caffeine (83 % of samples), (2) quinine/quinidine (64 % of samples), and (3) acetaminophen (40 % of samples) (personal communication with DEA Special Testing and Research Laboratory, March 23, 2010).
The use of adulterants in drugs of abuse is common. Adulterants are deliberately added to increase bulk, enhance or mimic a pharmacological effect, or to facilitate drug delivery, and they have the potential to cause serious health issues.2 In 1995, 370 instances of intoxication by heroin adulterated with scopolamine were reported to poison-control centers in New York City, New Jersey, the Delaware Valley, and Maryland. In these cases, 55 % of patients presented with signs and symptoms of heroin toxicity, but then became severely agitated with anticholinergic symptoms when naloxone was used to reverse respiratory depression. Three percent of patients developed seizures, 90 % required admission, and half were admitted to a critical care unit.3,4
In a 4-month period in 2005, 26 individuals in 5 states reported to poison-control centers and public-health agencies with atypical reactions after heroin use. The reactions included tachycardia and palpitations, which were thought to be due to clenbuterol cut into the heroin and identified on urine GC/MS.5 Clenbuterol is a β2 adrenergic receptor agonist with rapid onset and a long duration of action, approved for limited veterinary use in the US. Clinical manifestations of clenbuterol intoxication include hypokalemia, hyperglycemia, agitation, tachycardia, and hypotension.6 In its investigation, the CDC concluded that the 26 cases likely represented a fraction of the actual cases of clenbuterol poisoning, as individuals may have not sought medical evaluation for fear of repercussions, and misdiagnosis and underreporting were probable.5
In 2009, levamisole was identified in at least 70 % of adulterated cocaine in the US.7 Originally FDA-approved as an immunomodulator, a chemotherapy adjuvant, and an antihelminthic medication, levamisole can cause agranulocytosis,8 lichenoid rash,9 bilateral necrosis of the ears, positive perinuclear antineutrophil cytoplasmic antibodies, and laboratory findings of antiphospholipid syndrome, such as anticardiolipin antibodies and/or lupus anticoagulant.10 In our clinical research setting in a 19-month period from 2009 to 2011, we detected levamisole in 62 % of cocaine-positive urine samples tested with GC/MS.
The presence of adulterants with possibly harmful effects is not limited to drugs of abuse. Synthetic steroidal and non-steroidal anti-inflammatory drugs have been found in ayurvedic/herbal healthcare products.11 Adulterants such as pesticides, heavy metals, anorectics, antidepressants, diuretics, and phosphodiesterase-5 inhibitors, all of which have potential cardiovascular effects, have also been found in herbal supplements.12
In heroin, quinine and quinidine are often used as adulterants because their bitter taste mimics that of heroin and their hypotensive effect can mimic the heroin “rush.”13 Quinine is derived from the cinchona tree, was originally discovered by the Quechua Indians of Peru and Bolivia, and has antipyretic, antimalarial, analgesic, and anti-inflammatory properties. Quinidine is a stereoisomer of quinine; it is used as a type IA antiarrhythmic. Quinine and quinidine have similar effects and have been successfully used interchangeably.14,15 Both drugs can prolong QTc, with similar rates of elimination from the cardiac conduction “effect” compartment.16 However, when given intravenously, quinidine has a greater QTc prolongation effect.17–19 Quinine and quinidine both slow the rapid upstroke of the cardiac action potential by blocking the inward sodium current (INa), slowing depolarization, and thereby widening the QRS complex;20 they are negatively inotropic, similar to other class 1 antiarrhythmic drugs.20 Their cardiovascular toxicity may initially be manifested as slowed sinus or AV node conduction. Urine GC/MS is unable to differentiate between quinine and quinidine, and thus they are reported together on urine toxicology screens. Given that the stereoisomers have similar cardiac effects (although quinidine’s are more pronounced),19 distinguishing them from each other in this clinical setting is not necessary. The only way to differentiate between quinine and quinidine is by a specific serum test, which was not obtained in our patient.
Methadone package inserts acknowledge possible cardiac-related side effects, such as bradycardia, palpitations, hypotension, faintness, and syncope.21 Methadone has also been shown to prolong the QTc interval; however, the incidence of clinically significant arrhythmias is often accompanied by concurrent predisposing factors such as electrolyte abnormalities, structural heart abnormalities, or concomitant use of other drugs.22,23 Accelerated atrioventricular junctional arrhythmias have not been reported with methadone or with heroin.
While our patient did not stop using heroin, he did decrease his use. Repeat ECG 3 days later showed sinus bradycardia with a period of atrioventricular dissociation due to sinus bradycardia plus slight junctional acceleration. The junctional acceleration in this ECG occurred at a slower rate than in earlier ECGs; the cardiologist confirmed that this change reflected an improvement in the rate and rhythm. The patient’s decreased heroin use presumably resulted in decreased unwitting co-injection of quinine/quinidine, accompanied by a dose-related improvement in ECG parameters.
CONCLUSION
This case illustrates an accelerated junctional rhythm that may have resulted from a drug or drugs unwittingly co-injected during intravenous heroin use. Drug adulterants may produce arrhythmias or other medical complications, as well as toxicity from unsuspected drug-drug interactions (quinidine alone has over 135 major interactions) (Table 1). When working with patients who use illicit drugs, clinicians should look beyond the known drugs of abuse, and consider adulterants and diluents to determine possible etiologies of cardiac and other medical complications. Clinicians should also be aware that standard urine toxicologies do not detect diluents and adulterants and that, if the clinical picture warrants it, urine GC/MS should be performed.
Table 1.
Clinical Effects of Common Adulterants Found in Heroin and Cocaine
| Drug of abuse | Adulterant | Possible clinical effects |
|---|---|---|
| Heroin | Acetaminophen | Hepatic failure, irritability, seizure, coma |
| Caffeine | Respiratory distress, arrhythmia, mental status change | |
| Chloroquine | Blindness, arrhythmias, tinnitus, seizures | |
| Clenbuterol | Tachycardia, hypotension, hypokalemia, hyperglycemia | |
| Cocaine | Tachycardia, arrhythmia, myocardial ischemia | |
| Diltiazem | Hypotension, bradycardia | |
| Diphenhydramine | Tachycardia, agitation, blurred vision, delirium, seizures | |
| Fentanyl | Respiratory depression, tachycardia, seizure | |
| Lidocaine | Arrhythmias, dizziness, confusion | |
| Methorphan | Seizures, tachycardia, hallucinations, coma | |
| Phenacetin | Renal failure, hemolytic anemia, methemoglobinemia | |
| Quetiapine | Respiratory failure, tachycardia, syncope | |
| Scopolamine | Anticholinergic toxicity | |
| Cocaine | Atropine | Anitcholinergic toxicity |
| Benzocaine | Methemoglobinemia | |
| Caffeine | Respiratory distress, arrhythmia, mental status change | |
| Diltiazem | Hypotension, bradycardia | |
| Hydroxyzine | Tachycardia, palpitations, hypotension, shortness of breath | |
| Levamisole | Agranulocytosis, lichenoid rash | |
| Lidocaine | Arrhythmia, seizure | |
| Methylephedrine | Arrhythmia, hypertension, respiratory distress | |
| Phenacetin | Renal failure, hemolytic anemia, methemoglobinemia |
Key points are:
Adulterants cut into street drugs such as heroin and cocaine can have clinical consequences beyond that of the street drug itself.
Standard urine toxicology testing does not detect diluents and adulterants.
Urine testing by gas chromatography/mass spectroscopy (GC/MS) does detect many diluents and adulterants, and can be a useful tool if such compounds are suspected etiologies of the clinical condition.
Acknowledgements
Thanks to Mr. (Arnold) Keith Adkins and Ms. Sini Panicker, DEA Special Testing and Research Lab, for providing the detailed information on confiscated heroin in Baltimore. This research was supported by the National Institute on Drug Abuse, Intramural Research Program, National Institutes of Health. This research was presented at the SGIM Mid-Atlantic Meeting in March 2011.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Key Points
• Adulterants cut into street drugs such as heroin and cocaine can have clinical consequences beyond that of the street drug itself.
• Standard urine toxicology testing does not detect diluents and adulterants.
• Urine testing by gas chromatography/mass spectroscopy (GC/MS) does detect many diluents and adulterants, and can be a useful tool if such compounds are suspected etiologies of the clinical condition.
References
- 1.Kelley's Textbook of Internal Medicine. 4th ed: Lippincott Williams & Wilkins; 2000.
- 2.Cole C, Jones L, McVeigh J, Kicman A, Syed Q, Bellis M. Adulterants in illicit drugs: a review of empirical evidence. Drug Test Anal. 2011;3:89–96. doi: 10.1002/dta.220. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton RJ, Perrone J, Hoffman R, et al. A descriptive study of an epidemic of poisoning caused by heroin adulterated with scopolamine. J Toxicol Clin Toxicol. 2000;38:597–608. doi: 10.1081/CLT-100102008. [DOI] [PubMed] [Google Scholar]
- 4.Perrone J, Shaw L, De Roos F. Laboratory confirmation of scopolamine co-intoxication in patients using tainted heroin. J Toxicol Clin Toxicol. 1999;37:491–6. doi: 10.1081/CLT-100102441. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention Atypical reactions associated with heroin use-Five states, January-April 2005. MMWR. 2005;54:793–6. [PubMed] [Google Scholar]
- 6.Brambilla G, Cenci T, Franconi F, et al. Clinical and pharmacological profile in a clenbuterol epidemic poisoning of contaminated beef meat in Italy. Toxicol Lett. 2000;114:47–53. doi: 10.1016/S0378-4274(99)00270-2. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention (CDC) Agranulocytosis associated with cocaine use—four States, March 2008-November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381–5. [PubMed] [Google Scholar]
- 8.Chang A, Osterloh J, Thomas J. Levamisole: A Dangerous New Cocaine Adulterant. Clin Pharmacol Ther. 2010;88:408–11. doi: 10.1038/clpt.2010.156. [DOI] [PubMed] [Google Scholar]
- 9.Kirby JD, Black M, McGibbon D. Levamisole-induced lichenoid eruptions. J R Soc Med. 1980;73:208–11. doi: 10.1177/014107688007300311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de la Hera I, Sanz V, Cullen D, et al. Necrosis of ears after use of cocaine probably adulterated with levamisole. Dermatology. 2011;223:25–8. doi: 10.1159/000329436. [DOI] [PubMed] [Google Scholar]
- 11.Savaliya AA, Prasad B, Raijada DK, Singh S. Detection and characterization of synthetic steroidal and non-steroidal anti-inflammatory drugs in Indian ayurvedic/herbal products using LC-MS/TOF. Drug Test Anal. 2009;1:372–81. doi: 10.1002/dta.75. [DOI] [PubMed] [Google Scholar]
- 12.Cohen PA, Ernst E. Safety of herbal supplements: a guide for cardiologists. Cardiovasc Ther. 2010;28:246–53. doi: 10.1111/j.1755-5922.2010.00193.x. [DOI] [PubMed] [Google Scholar]
- 13.Dillmann JM. Bogus heroin. J Emerg Nurs. 1997;23:457–9. doi: 10.1016/S0099-1767(97)90145-8. [DOI] [PubMed] [Google Scholar]
- 14.Bhavnani SM, Preston SL. Monitoring of intravenous quinidine infusion in the treatment of Plasmodium-falciparum malaria. Ann Pharmacother. 1995;29:33–5. doi: 10.1177/106002809502900107. [DOI] [PubMed] [Google Scholar]
- 15.Sheldon R, Duff H, Koshman ML. Antiarrhythmic activity of quinine in humans. Circulation. 1995;92:2944–50. doi: 10.1161/01.CIR.92.10.2944. [DOI] [PubMed] [Google Scholar]
- 16.Karbwang J, Davis TME, Looareesuwan S, Molunto P, Bunnag D, White NJ. A comparison of the pharmokinetic and pharmacodynamic properties of quinine and quinidine in healthy Thai males. Br J Clin Pharmacol. 1993;35:265–71. doi: 10.1111/j.1365-2125.1993.tb04165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White NJ, Looareesuwan S, Warrell DA. Quinine and quinidine—A comparison of ECG effects during the treatment of malaria. J Cardiovasc Pharmacol. 1983;5:173–5. doi: 10.1097/00005344-198303000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Phillips RE, Warrell DA, White NJ, Looareesuwan S, Karbwang J. Intravenous quinidine for the treatment of severe Falciparum-malaria—Clinical and pharmokinetic studies. New Engl J Med. 1985;312:1273–8. doi: 10.1056/NEJM198505163122001. [DOI] [PubMed] [Google Scholar]
- 19.White NJ. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis. 2007;7:549–58. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- 20.Grace AA, Camm J. Quinidine. New Engl J Med. 1998;338:35–45. doi: 10.1056/NEJM199801013380107. [DOI] [PubMed] [Google Scholar]
- 21.Roxane. Methadone Hydrochloride Tablets USP [package insert]. Columbus, OH: Roxane Laboratories Inc.; Revised October 2006.
- 22.Butler B, Rubin G, Lawrance A, Batey R, Bell J. Estimating the risk of fatal arrhythmia in patients in methadone maintenance treatment for heroin addiction. Drug Alcohol Rev. 2011;30:173–80. doi: 10.1111/j.1465-3362.2010.00213.x. [DOI] [PubMed] [Google Scholar]
- 23.Mayet S, Gossop M, Lintzeris N, Markides V, Strang J. Methadone maintenance, QTc and torsade de pointes: Who needs an electrocardiogram and what is the prevalence of QTc prolongation? Drug Alcohol Rev. 2011;30:388–96. doi: 10.1111/j.1465-3362.2010.00237.x. [DOI] [PubMed] [Google Scholar]