I. Introduction
This chapter will not provide a detailed description of patch clamp methodology, since this information can be found in many other sources. The most comprehensive description of these techniques is likely to be Single Channel Recording (Neher and Sakmann, 1983). Other useful reference works include volumes edited by Standen et al. (1987), Conn (1991), Kettenmann and Gratyn (1992), and Bean (1992). We describe how these techniques can be applied to the study of Ca2+ currents as well as some of the advantages and potential pitfalls of the various recording configurations.
The first indication of the presence of Ca2+ channels came from studies on crustacean muscle by Fatt and Katz (1953). Our awareness of different types of Ca2+ channels has expanded rapidly; a useful subdivision based on some properties of voltage-dependent Ca2+ channels was proposed by Nowycky et al. (1985). These authors proposed three groups of channels—T-, L-, and N-types—based on biophysical and pharmacological properties of Ca2+ channels in chick dorsal root ganglion neurons. Subsequent studies on central nervous system neurons revealed at least one additional class, P-type, found at high concentrations in cerebellar Purkinje neurons (Llinas et al., 1989). Biophysical studies also have suggested the presence of voltage-independent metabolically regulated Ca2+ channels of the G-type (Rojas et al., 1990; Cena el al., 1991).
As summarized in Fig. 1, parallel biochemical studies have revealed the presence of multiple subunits in Ca2+ channel structures (for review, see Catterall, 1988). The application of molecular biological techniques has revealed an increasing number of subunit isotypes (for review, see Tsien et al., 1991; Miller, 1992). These types of study have been particularly successful in the investigation of intracellular Ca2+ channels such as the inositol trisphosphate (IP3) receptor family and the ryanodine receptor family, which have a tetrameric structure that has been reviewed by Berridge (1993). This tetrameric structure contrasts with data on voltage-sensitive Ca2+ channels, which appear to be composed of five different subunits (for review, see Catterall, 1988; Fig. 1). Despite this pentameric structure, α1 subunits alone appear to form functional voltage-dependent Ca2+ channels (Mikami et al., 1989; Perez-Reyes et al., 1989). In addition to the heterogeneous structure of Ca2+ channels, individual channels are able to exhibit multiple modes of activity (e.g., Hess et al., 1984; Delcour et al., 1993). This diversity of channel structure and behavior requires investigators to be cautious in the interpretation of data from Ca2+ channels and highlights the need to record for reasonable periods of time to distinguish between the presence of more than one type of channel and possible shifts in mode of activity.
Fig. 1.
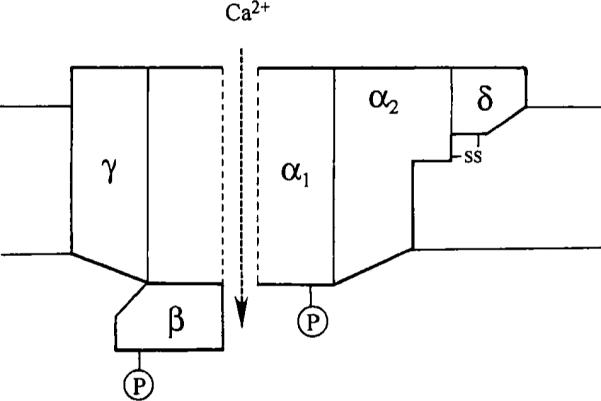
Subunit structure of a voltage-dependent Ca2+ channel. The α1 subunit, which forms the pore, has a molecular mass of 175 kDa; α2 is 143 kDa and is disulfide linked to δ (27 kDa); the subunit is 54, Da and γ is 30 kDa. (Reproduced with permission from Catterall, 1988.)
II. Pipettes and Solutions
Numerous considerations must be borne in mind when fabricating pipettes. Most cells seem to have a preference for particular glass types, which will, as often as not, be the deciding factor in making a choice. However, being aware that different glass types have been reported to influence channel behavior, although not directly for Ca2+ channels is important. Cota and Armstrong (1988) reported that, in rat pituitary cells, the use of certain types of soft glass with low (0.5 mM) ethylene glycol bis(β-amino ethylether)-N,N,N1,N1-tetraacetic acid (EGTA) buffer concentrations could induce fast inactivation of potassium channels. These effects were prevented with high (20 mM) EGTA concentrations or by using hard glass pipettes. The inactivation was suggested to result from channel block by di- or multivalent ions leeching from the glass. Zuazaga and Steinacker (1990) also reported variations in channel behavior attributable to differences in glass type, as observed in studies of acetylcholine-gated channels in patches from Xenopus myocytes. In this case, hard glass pipettes caused the preferential dropout of 40 pS channels compared with 60 pS channels. These investigators also showed that longer openings of the smaller channel dropped out first, distorting open time distributions.
Patch clamp techniques have been applied to a large number of cells from different sources, although the type of glass used to fabricate pipettes often is omitted from methods sections of papers. However, capillaries usually can be bought in small quantities, at least for the more common type of glass, and the process of trial and error can resolve the choice between hard- and soft-type glass reasonably quickly. Detailed discussion of the properties of different types of glass can be found in Corey and Stevens (1983). The majority of, if not all, electrode pullers can be adapted to pull patch pipettes. Pipettes conventionally are pulled in two stages to produce a more steeply tapered electrode shank, although a single-stage pull can be used. The heating coil temperature is usually the main determinant of the pipette tip size, the next factor that must be considered. Choice of tip size may be restricted by the ability to form and maintain a stable seal. Whole-cell recording generally employs a lower resistance pipette than is used for single channel experiments. The lower the resistance of the pipette, the lower the access resistance to the cell whereas, for single channel records, higher resistance electrodes which, as a rule of thumb, are likely to give a smaller patch of membrane may reduce the possibility of multiple channels being present in the patch. However, no simple direct relationship exists between tip size, pipette resistance, and patch area (Sakmann and Neher, 1983). Some characteristics of membrane patches sealed into pipettes and the influence of transmembrane pressure gradients are discussed by Sokabe and Sachs (1990).
Single Ca2+ channels often have a relatively small conductance, especially under physiological ion gradients, as well as brief openings. These properties require low noise recording at reasonably high bandwidths, conditions that can be achieved more easily by coating the shank of the electrode with one of several materials. The most commonly used material is Sylgard 184 (Dow-Corning), a silicone polymer. This compound can be premixed, partially cured to a thick stringy consistency, and then aliquoted and stored in a freezer for several weeks. The Sylgard is painted onto the pipette, close to the tip and up the shank to a point above the solution level in the bath, and cured by heating in a coil or by oven baking. The design of a jig suitable for this procedure is given by Corey and Stevens (1983). The pipette tip usually must be fire-polished after Sylgard coating to remove any material from the tip, a process that may not be necessary with uncoated pipettes. A modest coat of Sylgard can reduce recording noise substantially. Some laboratories have noted a qualitative improvement in ease of seal formation but a reduction in seal stability using Sylgard coated pipettes; whether this effect is the result of incomplete curing or reflects some property of fully cured material is unclear.
Ca2+ currents can be enhanced by elevation of extracellular [Ca2+]. For some types of Ca2+ channel substitution of other ions, commonly barium, also can enhance currents, although not all Ca2+ channels have a greater permeability to Ba2+ than to Ca2+. Most experiments are performed at elevated concentrations of permeant ion, commonly raised to levels between 10 times the physiological concentration and isotonic solutions. Ca2+ currents can be enhanced further by designing salines to block other ionic currents, especially K+ currents in cells that contain voltage- and Ca2+-activated K+ channels. These solutions must be tailored to the cell under investigation to suit the complement of channels in that cell type, which can be very variable.
III. Patch Configurations
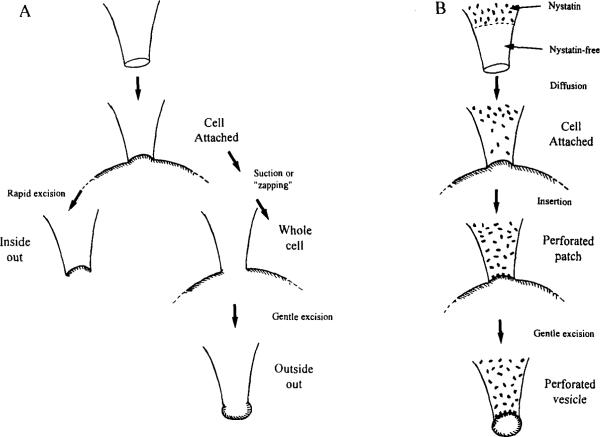
The different patch configurations (Hamill et al., 1981; see Fig. 2) have been described in many reviews and will be familiar to most readers. Descriptions of how to monitor seal formation and obtain these preparations are provided in the handbooks supplied with commercial patch amplifiers and will not be described here, since these details are hardware dependent.
Fig. 2.
Different patch configurations. (A) When the recording pipette is sealed onto the cell, a cell-attached patch is achieved. Rapid excision results in an inside-out patch (left). Suction results in a whole-cell patch (right) where gentle excision results in an outside-out patch. (B) Use of nystatin in a procedure similar to the one shown in A results in formation of a perforated patch or, with gentle excision, a perforated vesicle.
A. Cell-Attached Recording
The first patch configuration to be obtained is the cell-attached mode, in which the recording pipette is sealed onto the cell. This mode allows control of the patch membrane potential relative to the resting potential of the cell; the exact value of the resting potential is unknown unless measured or estimated independently. A common technique is to bathe the cell in isotonic KCI to set the membrane potential to zero artifically, a procedure that also can eliminate artifacts caused by action currents in excitable cells. The membrane potential also can be estimated if the cell contains a type of channel with defined properties. For example, if a channel is known to activate at −40 mV and is seen to be activated by a step of 20 mV, the cell resting potential is −60 mV. Despite this limitation, the cell-attached patch is useful because the cell is not disrupted and its normal intracellular environment is maintained. Calcium channels are known to be modulated by intracellular second messenger systems and these mechanisms are not disturbed. The effects of agents that act through second messenger systems also can be studied by application of these agents to the cell membrane outside the patch or by inclusion of such agents in the pipette filling solution.
Successful recordings in the cell-attached mode (and in the two whole-cell modes described in the next section) require a stable physical relationship between the pipette tip and the cell. Given the use of a suitable manipulator and vibration isolation, one common source of electrode drift appears to be the joint between the pipette and the electrode holder. Patch pipette holders have a rubber gasket to seal the shank of the pipette into the holder, which allows the application of pressure to the inside of the pipette. These gaskets appear to age and their regular replacement aids stability.
B. Whole Cell and Perforated Patch
Whole-cell and perforated-patch configurations permit the same type of macroscopic current recording from a cell but are distinctly different. The whole-cell method involves the physical disruption of the patch membrane from the cell-attached mode, which can be achieved by suction or by electronic “zapping”—application of a brief large-amplitude voltage pulse to the pipette. The choice of method sometimes is dictated by the cell type; some cells do not respond reliably to suction as a means of gaining electrical access, that is, the seal breaks more often than the membrane. Once the patch has been disrupted, the membrane potential of the cell can be measured directly (in current clamp) or can be controlled (for suitable cells, under voltage clamp). The cell is dialyzed with the pipette solution in whole-cell recording, that is, the cell contents are reasonably well known (however, see subsequent discussion) and various compounds can be introduced to the cell interior. The disadvantage is that normal second messenger systems also can be dialyzed out, and some types of Ca2+ current are very susceptible to wash-out. The pipette tip appears to form the major barrier to diffusion and equilibration of the pipette and intracellular solutions (see, for example, Mathias et al., 1990). Under conditions of active transport across the cell membrane, by Na+/K+ pumps or Na+/Ca2+ exchangers, the intracellular ion concentrations may not be equal to pipette concentrations, especially in regions close to the plasma membrane.
The perforated-patch method was introduced by Horn and Marty (1988). This method involves briefly dipping the pipette tip in normal pipette solution and then back-filling with solution to which, most commonly, nystatin has been added. Other antibiotics such as amphotericin B also have been used (Rae et al., 1991). The duration of the tip dipping will depend on the geometry of the pipette; we use 5–10 sec for soft glass pipettes with a resistance of ~4 MΩ. The nystatin solution we use is prepared by dissolving 6 mg nystatin in 100 μl dimethylsulfoxide (DMSO) by vortexing, then holding for 30 sec in a bath sonicator. From this stock solution, 20 μl is added to 5 ml filtered pipette solution, vortexed, and sonicated for 30 sec. This final solution should be used within 2–3 hr. Once the pipette has been back-filled with the nystatin solution, a seal must be obtained on the cell as quickly as possible. Seal formation becomes more difficult, if not impossible, once the nystatin has diffused to the pipette tip. Some laboratories recommend using positive pressure as the pipette is passed through the air–saline interface; others do not. We tend not to use positive pressure, especially when using nystatin, and routinely do not have difficulty forming seals. Once the seal has formed and the nystatin diffuses to the pipette tip, it begins to insert in the patch membrane. This process can be observed as a slow decrease in the access resistance and increase of the cell capacitance. For a given pipette, the series resistance will be higher than under standard whole-cell conditions, so particular care must be taken to compensate for this difference during experiments to investigate voltage-dependent Ca2+ channels. The time required to obtain good electrical access can vary from 1–2 min to 30 min. These delays can put people off from using this technique, but for the study of Ca2+ channels the advantages are numerous. Nystatin pores are permeant to monovalent ions, but not to divalents; hence normal intracellular Ca2+ buffering is maintained. Second messenger systems also are not washed out; nystatin pores are impermeant to compounds with a molecular weight of more than ~200. These factors allow the study of Ca2+ currents that are lost rapidly during standard whole-cell recording. For example, Kaczmarek (1986) showed that phorbol esters that activate protein kinase C induced activity of a second type of Ca2+ channel in cell-attached patches from Aplysia bag cells. This activation was lost if the phorbol ester was applied after dialysis of the cell in the whole-cell mode.
C. Outside-Out and Perforated Vesicle
Outside-out and perforated vesicle methods allow the recording of single channel activity from a small area of membrane with the external face of the membrane exposed to the bath solution. Both methods allow the bath application of experimental solutions to the external face of the patch and investigation of the effects of physiological modulators, or pharmacological agents, on channel function. The outside-out patch is obtained from a cell initially under whole-cell recording conditions and the perforated vesicle (Levitan and Kramer, 1990) from a cell under perforated-patch conditions; each has advantages and disadvantages similar to those of the initial configurations. The amount, and content, of the cytoplasm held within the perforated vesicle will be quite variable, and the presence or absence of wash-out of Ca2+ channels will be similarly variable. Internal perfusion of patch electrodes is used rarely, if ever, with outside-out patches since these tend to be quite fragile (bath perfusion of these patches also requires extra care). The nystatin-permeated membrane of the perforated vesicle also would limit the range of compounds that could be introduced to the cytoplasm of the patch.
D. Inside-Out Patch
The inside-out patch configuration permits the recording of single channels and bath perfusion of the cytoplasmic face of the membrane. Potential problems include wash-out of channel activity. Patch excision also has been shown to affect channel kinetics, at least for ligand-gated channels (Trautmann and Siegelbaum, 1983; Covarrubias and Steinbach, 1990). Perfusion of the cytoplasmic face of the membrane allows the investigation of how various agents can reverse run-down or influence inactivation of the channels. Inside-out patches can be remarkably stable and perfusing the pipette is possible (Cull-Candy et al., 1980), although few laboratories routinely use this technique. The remarkable stability (at least for some preparations) is demonstrated also by Sokabe and Sachs (1990), who showed that inside-out patches, which often show an Ω-profile inside the pipette, could be everted from the pipette tip under pressure.
E. Loose Patch
The loose-patch method is applied to tissues for which the tight seals between pipette and membrane required for standard cell-attached recording are not possible, for example, to avoid the extensive enzyme treatment sometimes required to “clean” the membrane. Commercial amplifiers are available for standard loose-patch recording; these generally have analog compensation for the lack of seal resistance (Stuhmer et al., 1983). A more advanced form of loose-patch was described by Almers et al., (1984); this technique uses concentric electrodes to eliminate the problem of potential gradients across the rim of the recording pipette. These concentric electrodes are, however, difficult to produce and the technique is used infrequently.
The primary use of the loose-patch method is mapping currents along large cells, the same pipette being used to take many recordings. The method is noninvasive to the cell and, hence, has similar advantages to cell-attached recording in terms of maintenance of normal intracellular mechanisms. The pipettes used generally have a larger tip diameter than for tight seal configurations, and records are typically from several hundred channels. As with cell-attached recording, the membrane potential is not known unless measured or estimated independently.
IV. Reconstitution of Purified Channel Proteins
Ca2+ channels are abundant in locations not amenable to direct patch clamp recording. To access these channels, membrane vesicle preparations can be made or channel proteins can be purified biochemically. Much work using reconstitution methods has been directed at investigation of intracellular organelles involved in Ca2+ release, for example, the sarcoplasmic reticulum (SR), and at the study of the transverse tubular system (T tubules) of vertebrate skeletal muscle, which contains high concentrations of dihydropyridine-sensitive Ca2+ channels.
Two configurations commonly are used to record from reconstituted channels: the planar lipid bilayer and microelectrode-based recording from giant liposomes or bilayers formed directly onto the pipette by tip dipping. The bilayer method is well known and has been used for single channel recording of a number of types of channel. Single Ca2+ channel records from lipid bilayers have been reported by several groups (e.g. Smith et al., 1986) from a number of preparations. Giant liposome preparations also have been described extensively (e.g., Tank and Miller, 1983) but have not been applied as extensively as bilayers to the study of single channel Ca2+ currents. Liposome suspensions with Ca2+ channels incorporated have, however, been widely used for flux studies.
The advantage of lipid bilayers for the study of Ca2+ channels lies in the ability to change solutions on either side of the membrane easily; this ability can be helpful since the insertion of channels into the membrane may not always be in the same orientation. Under these circumstances, the ability to manipulate the ion gradient across the membrane by changing either side can be advantageous. Although perfusing the interior of patch pipettes is possible when recording from a patch excised from a liposome, we have noticed that these patches tend to be more fragile than native membranes. We also find that liposomes seem to lift quite readily when attempting to excise patches. One additional advantage of the bilayer seems to be that the membrane can be monitored until the Ca2+ channels become incorporated, whereas the channel is either present or not in traditional microelectrode patches. Attempts to balance the lipid : protein ratio of liposomes to optimize the probability of hitting a channel over getting multiple channels can be frustrating. In practice, the bilayer has remained the method of choice for recording single channel currents through Ca2+ channels.
Whichever method is used, another choice also must be made. The Ca2+ channel can be in a native membrane vesicle or can be purified and reconstituted into artificial vesicles. The use of native membrane vesicles requires less manipulation and possibly reduces the risk of losing channel activity, but the vesicle may contain other types of channel. The use of purified components allows investigation of the action of combinations of individual subunits. However, the risk of denaturation or the absence of some component required for the normal interaction of subunits remains, although this problem does not seem to be major.
Two main methods are available for generating giant liposomes suitable for patch clamp recording. The first is the freeze–thaw method (Kasahara and Hinkle, 1977; Tank and Miller, 1983) and the second is dehydration/rehydration (Criado and Keller, 1987). Which method works (best) depends on the mix of lipids used in the liposomes. For example, we find that phosphatidylserine-based liposomes reliably fuse with dehydration/rehydration, but not with freeze–thaw.
With the increasing use of molecular biology to isolate genes and express channel subunits, either alone or in combination, the use of reconstitution may become less important at least for Ca2+ channels found in the plasma membrane, since conventional patch technology can be applied readily to many cells used for expression studies. Reconstitution studies will remain useful for direct investigation of electrophysiological properties of intracellular Ca2+ channels which, when expressed, may be directed for insertion into intracellular membranes.
V. Expression Cloning of Channel Subunits
The use of Xenopus oocytes for studies directed at the cDNA cloning and functional expression of Ca2+ channel subunits has been reviewed by Snutch (1988). Detailed methodology regarding preparation of Ca2+ channel subunit mRNA (or cRNA) for purposes of microinjection is found in Snutch and Mandel (1992). Methodology detailing the construction of mammalian cell lines (CHO cells, HEK293 cells, mouse L cells) that stably express L- and N-type Ca2+ channel subunit cDNAs is found in the studies by Bosse et al. (1992), Williams et al. (1992), and Lacerda et al. (1991). The construction of a Ca2+ channel subunit cDNA expression vector for transfection of primary cell cultures of adrenal chromaffin cells is described by Ma et al. (1992). In general, these approaches have allowed coexpression studies using two electrode voltage clamps which, for example, have demonstrated that the β subunits derived from skeletal muscle accelerate not only the activation, but also the inactivation of Ca2+ channels formed by the α1 subunits (Varardi et al., 1991; Perez-Reyes et al., 1992). In addition, more detailed macroscopic current analysis of the L-type α1 subunit has revealed a voltage-sensor sequence (S4 region) and an activation domain (Tanabe et al., 1991). Although the application of patch clamp methodology to the analysis of Ca2+ channels derived by recombinant DNA technology is still in its infancy, clearly only such an approach at the single channel level will allow the identification of functional domains in the channel that influence transitions between different forms of modal gating behavior.
VI. Pancreatic β Cell: A Model System for Analysis of Ca2+ Signaling
To illustrate the usefulness of an integrated approach to the study of intracellular Ca2+ signaling, we have selected the pancreatic β cell, a specialized endocrine cell that secretes the hormone insulin in a Ca2+-dependent fashion (reviewed by Ashcroft and Rorsman, 1989; Rajan et al., 1990; Boyd, 1992). β Cells are noteworthy because insulin secretion is a tightly regulated process that is subject to stimulatory and inhibitory modulation by a remarkably large number of nutrients (glucose, amino acids), hormones (glucagon-like peptides, arginine vasopressin, gastric inhibitory peptide), and neurotransmitters (norepinephrine, somatostatin, galanin). The combined application of patch clamp (e.g., Holz et al., 1993) and Ca2+-imaging analysis (e.g., Grapengiesser et al., 1991; Valdeolmillos et al., 1992) to the study of signaling pathways that mediate these modulatory influences is one approach currently being used by investigators seeking to define the subcellular basis for stimulus–secretion coupling in this system.
The primary physiological stimulus that induces insulin secretion from pancreatic β cells is the rise in blood glucose concentration that results after ingestion of a meal (reviewed by Holz and Habener, 1992). Glucose stimulates insulin secretion via a sequence of events that requires its metabolism by aerobic glycolysis to generate ATP (Ashcroft et al., 1984) and cyclic ADP–ribose (Takasawa et al., 1993), two metabolites that act via a signaling cascade to depolarize β cells and to increase the concentration of cytosolic Ca2+ (see Fig. 3). Since vesicular insulin secretion generally is recognized to result from Ca2+-dependent exocytosis, considerable attention has focused on exactly how the glucose-induced rise in intracellular Ca2+ is achieved. In this regard, the patch clamp technique has proven a very useful tool for analysis of Ca2+ channels that mediate the glucose-induced entry of Ca2+ across the plasma membrane. Perforated-patch studies (see Sala et al., 1991, for a review of this technique as it applies to β cells) have revealed the existence of voltage-gated Ca2+ channels that open in response to glucose-induced depolarization (Falke et al., 1989); these channels correspond to the dihydropyridine-sensitive (L-type) channels, as demonstrated by cell-attached patch recordings of unitary Ca2+ currents (Smith et al., 1989). Rat β cells also contain a second type of voltage-dependent Ca2+ channel that is not susceptible to wash-out (Hiriart and Matteson, 1988). Interestingly, the β cell also expresses low conductance voltage-independent Ca2+ channels that open in response to glucose and have been classified as G channels by Rojas and co-workers (Rojas et al., 1990).
Fig. 3.
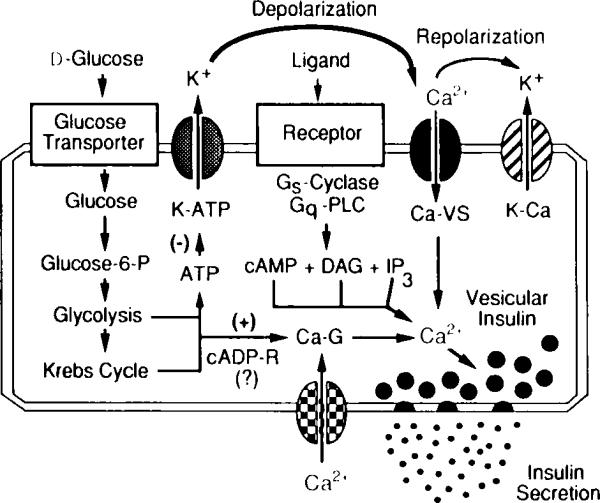
Signaling systems that regulate intracellular Ca2+ and insulin secretion from pancreatic β cells. Illustrated are essential features of the glucose signaling system (left); the hormonally regulated 3′,5′-cyclic adenosine monophosphate (cAMP), inositol trisphosphate IP3), and diacylglycerol (DAG) signaling systems (middle); and the cyclic ADP–ribose signaling system (bottom) that act in concert to trigger insulin secretion. The initial uptake of glucose is facilitated by the type-2 glucose transporter, whereas the conversion of glucose to glucose 6-phosphate is catalyzed by glucokinase. Stimulation of aerobic glycolysis generates multiple signals, one of which is an increased ratio of intracellular ATP relative to ADP. Binding of ATP to ATP-sensitive potassium channels (K–ATP) induces closure of the channels and membrane depolarization, which is necessary for the opening of L-type voltage-sensitive Ca2+ channels (Ca–VS). The glucose-induced rise in Ca2+ also results from the action of glucose-derived metabolites to open G-type voltage-independent Ca2+ channels (Ca–G). In this figure, one such metabolite is proposed to be cyclic ADP–ribose (cADP-R). Entry of Ca2+ across the plasma membrane triggers vesicular insulin secretion by Ca2+-dependent exocytosis. Repolarization results from the action of cytosolic Ca2+ to activate Ca2+-dependent potassium channels (K–Ca) and to inhibit voltage-sensitive Ca2+ channels. Also illustrated are hormonally regulated second messenger signaling systems that act through GTP-binding protein (Gs,Gq)-coupled receptors to stimulate production of cAMP (a messenger generated by adenylyl cyclase) and IP3 or DAG (messengers generated by phospholipase C, PLC). These messengers increase cytosolic Ca2+ by facilitating the opening of Ca–VS and by mobilizing Ca2+ from intracellular Ca2+ stores.
Patch clamp analysis also has been applied to the study of second messenger pathways that mediate the infuence of hormones and neurotransmitters on β-cell Ca2+ channels. Arginine vasopressin, a stimulator of insulin secretion and an activator of protein kinase C, was reported to facilitate L-type Ca2+ currents in a transformed β cell line (Thorn and Peterson, 1991). Conversely, inhibitors of insulin secretion such as norepinephrine, somatostatin, and galanin reportedly inhibit β-cell L-type Ca2+ currents via a signaling pathway that involves a pertussis toxin-sensitive GTP-binding protein (Keahey et al., 1989; Homaidan et al., 1991; Hsu et al., 1991; Schmidt et al., 1991). Significantly, the facilitatory and inhibitory modulation of L-type Ca2+ currents by these transmitters was observed under conditions of whole-cell dialysis and recording, as expected if these responses are mediated by membrane-delimited signaling pathways that do not involve cytosolic second messengers. This situation contrasts with that which has been described for the facilitatory action of glucose on the L-type Ca2+ current. As mentioned earlier, the L-type Ca2+ channels in β cells are known to open in response to glucose-induced cellular depolarization. In addition, a second more direct facilitatory action of glucose has been described that involves modulation of the channels through influences on the kinetics of channel gating (Smith et al., 1989). Under conditions of whole-cell dialysis and recording, both responses to glucose are abolished (“wash-out’), as expected given that the β-cell glucose signaling system requires the functional integrity of numerous cytosolic enzymes, cofactors, and substrates. Only under conditions of perforated-patch recording are these actions of glucose observed, a finding that underscores the importance of carefully considering which patch clamp configuration to employ when designing an experimental study.
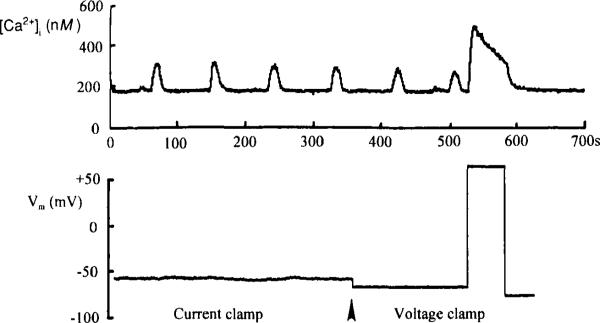
An important methodological advance that is generally relevant to the study of stimulus–secretion coupling is the attempt by investigators (e.g., Rorsman et al., 1992) to combine the fura-2 Ca2+-imaging technique (Grynkiewicz et al., 1985) with perforated-patch clamp analysis. The advantage of this approach is illustrated clearly by studies conducted in our laboratory using perforated-patch and fura-2 recordings. As illustrated in Fig. 4, fura-2 recordings obtained from β cells exposed to a near-threshold stimulatory concentration of glucose (7.5 mM) revealed large periodic oscillations in the concentration of intracellular Ca2+. Simultaneous perforated-patch recordings revealed that the oscillations were independent of detectable changes in membrane potential, and also were observed when the membrane potential was voltage clamped and held at −70 mV. The oscillations are, therefore, unlikely to result from influx of Ca2+ through voltage-gated (L-type) Ca2+ channels. Instead, the oscillations may result from the mobilization of intracellular Ca2+ stores and/or the activity of voltage-independent Ca2+ channels (G channels). Using this combined patch clamp and imaging approach, we can begin to assess the relative contribution of voltage-dependent and -independent processes that govern intracellular Ca2+ homeostasis.
Fig. 4.
One final example illustrates the impressive flexibility of patch clamp recording for analysis of Ca2+-dependent processes. The perforated-patch configuration was reported to be suitable for measurement of changes in cellular capacitance that are attributable to exocytotic fusion of secretory vesicles with the plasma membrane (Gillis and Misler, 1992). Although this capacitance tracking methodology has yet to be exploited fully, it should allow an assessment of the temporal pattern of vesicle fusion in response to chemical stimuli (glucose, hormones, neurotransmitters) or voltage clamp stimuli (step depolarizations). Certainly the full potential of this technique will be realized in future studies that combine Ca2+-imaging analysis with perforated-patch recordings of Ca2+ currents and cellular capacitance.
References
- Almers W, Roberts WM, Ruff RL. Voltage clamp of rat and human skeletal muscle. Measurements with an improved loose-patch technique. J. Physiol. 1984;347:751–768. doi: 10.1113/jphysiol.1984.sp015094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman F. Electrophysiology of the pancreatic β-cell. Prog. Biophys. Mol. Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Harrison DE, Ashcroft SJH. Glucose induces closure of single potassium channels in isolated rat pancreatic β-cells. Narure (London) 1984;312:446–448. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Bean BP. Whole-cell recording of calcium channel currents. Meth. Enzymol. 1992;207:181–193. doi: 10.1016/0076-6879(92)07013-e. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Inositol triphosphate and calcium signalling. Nature (London) 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Bosse E, Bottlender R, Kleppisch T, Hescheler J, Welling A, Hofman F, Flockerzi V. Stable and functional expression of the calcium channel α1, from smooth muscle in somatic cell lines. EMBO J. 1992;11(6):2033–2038. doi: 10.1002/j.1460-2075.1992.tb05260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd AE., III The role of ion channels in insulin secretion. J. Cell. Biochem. 1992;48:234–241. doi: 10.1002/jcb.240480303. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and function of voltage-sensitive ion-channels. Science. 1988;242:50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Cena V, Brocklehurst KW, Pollard HB, Rojas E. Pertussis toxin stimulation of catecholamine release from adrenal medullary chromaffin cells: Mechanism may be by direct activation of L-type and G-type calcium channels. J. Membrane Biol. 1991;122:23–31. doi: 10.1007/BF01872736. [DOI] [PubMed] [Google Scholar]
- Conn PM, editor. Methods in Neurosciences. Vol. 4. Academic Press; San Diego: 1991. Electrophysiology and Microinjection. [Google Scholar]
- Corey DP, Stevens CF. Science and technology of patch-recording electrodes. In: Neher E, Sakmann B, editors. Single Channel Recording. Plenum Press; New York: 1983. pp. 53–68. [Google Scholar]
- Cota G, Armstrong CM. Potassium channel “inactivation” induced by soft-glass patch pipettes. Biophys. J. 1988;53:107–109. doi: 10.1016/S0006-3495(88)83071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias M, Steinbach JH. Excision ofmembrane patches reduces the mean open time of nicotinic acetylcholine receptors. Pflügers Arch. 1990;416:385–392. doi: 10.1007/BF00370744. [DOI] [PubMed] [Google Scholar]
- Criado M, Keller BU. A membrane fusion strategy for single channel recordings of membranes usually non-accessible to patch-clamp electrodes. FEBS Lett. 1987;224:172–176. doi: 10.1016/0014-5793(87)80442-8. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Miledi R, Parker I. Single glutamate-activated channels recorded from locust muscle fibres with perfused patch-clamp electrodes. J. Physiol. 1980;321:195–210. doi: 10.1113/jphysiol.1981.sp013979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcour AH, Lipscombe D, Tsien RW. Multiple modesof N-type calcium channel activity distinguished by differences in gating kinetics. J. Neurosci. 1993;13:181–194. doi: 10.1523/JNEUROSCI.13-01-00181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke LC, Gillis KD, Pressel DM, Misler S. “Perforated patch” recording allows long-term monitoring of metabolite-induced electrical activity and voltage-dependent Ca currents in pancreatic β-cells. FEBS Lett. 1989;251:167–172. doi: 10.1016/0014-5793(89)81448-6. [DOI] [PubMed] [Google Scholar]
- Fatt P, Katz B. The electrical properties of crustacean muscle fibres. J. Physiol. 1953;120:171–204. doi: 10.1113/jphysiol.1953.sp004884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis WD, Misler S. Single cell assay of exocytosis from pancreatic β-cells. Pflügers Arch. 1992;420:121–123. doi: 10.1007/BF00378654. [DOI] [PubMed] [Google Scholar]
- Grapengiesser E, Gylfe E, Hellman B. Cyclic AMP as a determinant for glucose-induction of fast Ca2+ oscillations in isolated pancreatic β-cells. J. Biol. Chem. 1991;266:12207–12210. [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien R. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording in cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hess P, Lansman JB, Tsien RW. Different modes of Ca-channel gating behaviour favoured by dihydropyridine Ca-agonists and antagonists. Nature (London) 1984;311:538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hiriart M, Matteson DR. Na channels and two types of Ca2+ channels in rat pancreatic β-cells identified with the reverse hemolytic plaque assay. J. Gen. Physiol. 1988;91:617–639. doi: 10.1085/jgp.91.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Habener JF. Signal transduction crosstalk in the endocrine system: Pancreatic β-cells and the glucose competence concept. Trends Biochem. Sci. 1992;7:388–393. doi: 10.1016/0968-0004(92)90006-u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Kuhtreiber WM, Habener JF. Pancreatic β-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7–37) Nature (London) 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homaidan FR, Sharp GW, Nowak LM. Galanin inhibits a dihydropyridine-sensitive Ca2+ current in the RINm5f cell line. Proc. Natl. Acad. Sci. U.S.A. 1991;88:8744–8788. doi: 10.1073/pnas.88.19.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J. Gen. Physiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu WH, Xiang HD, Rajan AS, Kunze DL, Boyd AE. Somatostatin inhibits insulin secretion by a G-protein-mediated decrease in Ca entry through voltage-dependent Ca channels in the beta-cell. J. Biol. Chem. 1991;266:837–843. [PubMed] [Google Scholar]
- Kaczmarek LK. Phorbol esters, protein phosphorylation and the regulation of ion-channels. J. Exp. Biol. 1986;124:375–392. doi: 10.1242/jeb.124.1.375. [DOI] [PubMed] [Google Scholar]
- Kasahara M, Hinkle PC. Reconstitution and purification of the d-glucose transporter from human erythrocytes. J. Biol. Chem. 1977;252:7384–7390. [PubMed] [Google Scholar]
- Keahey H, Boyd AE, Kunze DL. Catecholamine modulation of calcium currents in clonal pancreatic β-cells. Am. J. Physiol. 1989;257:C1171–C1176. doi: 10.1152/ajpcell.1989.257.6.C1171. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Gratyn R, editors. Practical Electrophysiological Methods. A Guide for In Vitro Studies in Vertebrate Neurobiology. Wiley-Liss; New York: 1992. [Google Scholar]
- Lacerda A-E, Kim H-S, Ruth P, Perez-Reyes E, Flockerzi V, Hofmann F, Birnbaumer L, Brown A-M. Normalization of current kinetics by interaction between the α1 and β subunits of skeletal muscle dihydropyridine-sensitive Ca2+ channel. Nature (London) 1991;352:521–530. doi: 10.1038/352527a0. [DOI] [PubMed] [Google Scholar]
- Levitan ES, Kramer RH. Neuropeptide modulation of single calcium and potassium channels detected with a new patch clamp configuration. Nature (London) 1990;348:546–547. doi: 10.1038/348545a0. [DOI] [PubMed] [Google Scholar]
- Llinas R, Sugimori M, Lin JW, Cherksey B. Blocking and isolation of a calcium channel from neurones in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc. Natl. Acad. Sci. U.S.A. 1989;86:1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WJ, Holz RW, Uhler MD. Expression of a cDNA for a neuronal calcium channel alpha 1 subunit enhances secretion from adrenal chromaffin cells. J. Biol. Chem. 1992;267:22728–22732. [PubMed] [Google Scholar]
- Mathias RT, Cohen IS, Oliva C. Limitations of the whole cell patch-clamp technique in the control of intracellular concentrations. Biophys. J. 1990;58:759–770. doi: 10.1016/S0006-3495(90)82418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature (London) 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Miller RJ. Voltage-sensitive Ca2+ channels. J. Biol. Chem. 1992;267:1403–1406. [PubMed] [Google Scholar]
- Neher E, Sakmann B. Single Channel Recording. Plenum Press; New York: 1983. [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature (London) 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Kim HS, Lacerda AE, Horne W, Wei X, Rampe D, Campbell KP, Brown AM, Birnbaumer L. Induction of calcium currents by the expression of the α1-subunit of the dihydropyridine receptor from skeletal muscle. Nature (London) 1989;340:233–236. doi: 10.1038/340233a0. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Castellano A, Kim HS, Bertrand P, Baggstrom E, Lacerda AE, Wei XY, Birnbaumer L. Cloning and expression of a cardiac/brain beta subunit of the L-type calcium channel. J. Biol. Chem. 1992;267:1792–1797. [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J. Neurosci. Meth. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Rajan AS, Aguilar-Bryan L, Nelson DA, Yaney GC, Hsu WH, Kunze DL, Boyd AE. Ion channels and insulin secretion. Diabetes Care. 1990;13:340–363. doi: 10.2337/diacare.13.3.340. [DOI] [PubMed] [Google Scholar]
- Rojas E, Hidalgo J, Carroll PB, Li MX, Atwater I. A new class of calcium channel activated by glucose in human pancreatic β-cells. FEES Lett. 1990;261:265–270. doi: 10.1016/0014-5793(90)80568-4. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Ammala C, Berggren P-O, Bokvist K, Larsson O. Cytoplasmic calcium transients due to single action potentials and voltage-clamp depolarizations in mouse pancreatic β-cells. EMBO J. 1992;11:2877–2884. doi: 10.1002/j.1460-2075.1992.tb05356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B, Neher E. Geometric parameters of pipettes and membrane patches. In: Neher E, Sakmann B, editors. Single Channel Recording. Plenum Press; New York: 1983. pp. 37–51. [Google Scholar]
- Sala S, Parsey RV, Cohen AS, Matteson DR. Analysis and use of the perforated patch technique for recording ionic currents in pancreatic β-cells. J. Membrane Biol. 1991;122:177–187. doi: 10.1007/BF01872640. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hescheler J, Offermanns S, Spicher K, Hinsch KD, Klinz FJ, Codina J, Birnbaumer L, Gausepohl H, Frank R, Schultz G, Rosenthal W. Involvement of pertussis toxin-sensitive G-proteins in the hormonal inhibition of dihydropyridine-sensitive Ca2+ currents in an insulin-secreting cell line (RINm5F) J. Biol. Chem. 1991;266:18025–18033. [PubMed] [Google Scholar]
- Smith JS, Coronado R, Meissnes G. Single channel measurements of the calcium release channel from skeletal muscle sarcoplasmic reticulum. Activation by Ca2+ and ATP and modulation by Mg2+ J. Gen. Physiol. 1986;88:573–588. doi: 10.1085/jgp.88.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PA, Rorsman P, Ashcroft FM. Modulation of dihydropyridine-sensitive Ca2+ channels by glucose metabolism in mouse pancreatic β-cells. Narure (London) 1989;342:550–553. doi: 10.1038/342550a0. [DOI] [PubMed] [Google Scholar]
- Snutch TP. The use of Xenopus oocytes to probe synaptic communication. Trends Neurosci. 1988;11:250–256. doi: 10.1016/0166-2236(88)90102-6. [DOI] [PubMed] [Google Scholar]
- Snutch TP, Mandel G. Tissue RNA as a source of ion channels and receptors. Merh. Enzymol. 1992;207:297–309. doi: 10.1016/0076-6879(92)07019-k. [DOI] [PubMed] [Google Scholar]
- Sokabe M, Sachs F. The structure and dynamics of patch-clamped membranes: A study using differential interference contrast light microscopy. J. Cell Biol. 1990;111:599–606. doi: 10.1083/jcb.111.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standen NB, Gray PTA, Whitaker MJ, editors. Microelectrode Techniques. The Plymouth Workshop Handbook. The Company of Biologists; Cambridge: 1987. [Google Scholar]
- Stuhmer W, Roberts WM, Almers W. The loose-patch technique. In: Neher E, Sakmann B, editors. Single Channel Recording. Plenum Press; New York: 1983. pp. 123–132. [Google Scholar]
- Takasawa S, Nata K, Yonekura H, Okamoto H. Cyclic ADP-ribose in insulin secretion from pancreatic β-cells. Science. 1993;259:370–373. doi: 10.1126/science.8420005. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Adams BA, Numa S, Beam KG. Repeat 1 of the dihydropyridine receptor is critical in determining calcium channel activation kinetics. Nature (London) 1991;352:800–803. doi: 10.1038/352800a0. [DOI] [PubMed] [Google Scholar]
- Tank DW, Miller C. Patch-clamped liposomes. Recording reconstituted ion-channels. In: Neher E, Sakmann B, editors. Single Channel Recording. Plenum Press; New York: 1983. pp. 91–105. [Google Scholar]
- Thorn P, Peterson OH. Activation of voltage-sensitive Ca2+ currents by vasopressin in an insulin-secreting cell line. J. Membrane Biol. 1991;124:63–71. doi: 10.1007/BF01871365. [DOI] [PubMed] [Google Scholar]
- Trautmann A, Siegelbaum SA. The influence of membrane patch isolation on single acetylcholine-channel current in rat myotubes. In: Neher E, Sakmann B, editors. Single Channel Recording. Plenum Press; New York: 1983. pp. 473–480. [Google Scholar]
- Tsien RW, Ellinor PT, Horne WA. Molecular diversity of voltage-dependent Ca2+-channels. Trends Pharmacol. Sci. 1991;12:349–354. doi: 10.1016/0165-6147(91)90595-j. [DOI] [PubMed] [Google Scholar]
- Valdeolmillos M, Nadal A, Contreras D, Soria B. The relationship between glucose-induced K-ATP channel closure and the rise in Ca2+ in single mouse pancreatic β-cells. J. Physiol. (London) 1992;455:173–186. doi: 10.1113/jphysiol.1992.sp019295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varardi G, Lory P, Schultz D, Varadi M, Schwartz A. Acceleration of activation and inactivation by the beta subunit of the skeletal muscle calcium channel. Nature (London) 1991;352:159–162. doi: 10.1038/352159a0. [DOI] [PubMed] [Google Scholar]
- Williams ME, Brust PF, Feldman DH, Patthi S, Simerson S, Maroufi A, McCue AF, Velicelebi G, Ellis SB, Harpold MM. Structure and functional expression of an omega-conotoxin-sensitive human N-type calcium channel. Science. 1992;257:389–395. doi: 10.1126/science.1321501. [DOI] [PubMed] [Google Scholar]
- Zuazaga C, Steinacker A. Patch-clamp recording of ion channels: Interfering effects of patch pipette glass. News Physiol. Sci. 1990;5:155–158. [Google Scholar]