Abstract
The actions of serotonin (5-HT) were studied in the isolated frog spinal cord and dorsal root ganglion preparations.
In the spinal cord, 5-HT increased the spontaneous activity recorded from dorsal roots, facilitated evoked spinal reflexes and produced fast and slow primary afferent depolarization (PAD).
A direct action of 5-HT on primary afferent terminals is likely since 5-HT induced PAD remained in the presence of 1 µM tetrodotoxin and 2 mM Mn2+.
The direct action of 5-HT on primary afferent terminals was blocked by methysergide and attenuated by concentrations of Mn2+ in excess of that required to block transmitter release.
Cell bodies of the dorsal root ganglion were also depolarized by 5-HT. A slow hyperpolarization occasionally followed the initial depolarization. The depolarizing action of 5-HT in the dorsal root ganglion was also attenuated by treatment with Mn2+.
It is concluded that 5-HT acts directly on frog primary afferents and that this influence may involve a calcium sensitive process. The dorsal root ganglion response to 5-HT appears to be a suitable model of the afferent terminal response.
INTRODUCTION
Somatosensory processing in the mammalian spinal cord is influenced by supraspinal serotonergic centers originating within brainstem raphe nuclei, descending in the dorsal lateral and ventral lateral funiculi and terminating in the spinal grey (Dahlstrom and Fuxe, 1964, 1965). Activation of this system is accompanied by facilitation of the monosynaptic reflex conveying the stretch reflex (Banna and Anderson, 1968; Barasi and Roberts, 1974; Ellaway and Trott, 1975), inhibition of flexor reflex afferent transmission (Engberg et al., 1968) and inhibition of nociception (Yaksh et al., 1981). Part of these effects may be mediated by a direct presynaptic action of serotonin (5-HT) on primary afferent terminals which alters afferent polarization, excitability and transmission.
The demonstration of such a presynaptic action of 5-HT requires direct recordings from primary afferent endings under conditions in which interneuronal influences are blocked. Unfortunately, intracellular microelectrode studies of serotonin’s action have yet to be accomplished due to the small size of primary afferent terminals. In addition, no certain method of selectively blocking interneuronal transmission is possible using in vivo preparations. For these reasons, simplified in vitro preparations have been employed for studying serotonin’s effects.
Extracellular recordings from primary afferents using isolated spinal cord preparations have yielded conflicting results regarding the site of serotonin’s action. Tebecis and Phillis (1967) and Phillis and Kirkpatrick (1979) reported a tetrodotoxin (TTX) and Mg2+ resistant 5-HT depolarization of the attached dorsal roots of isolated anuran spinal cords. Since TTX or Mg2+ treatments minimize interneuronal influences on primary afferents, this 5-HT induced primary afferent depolarization (PAD) was attributed to serotonin’s direct action on afferent nerve terminals. In contrast, Shirasawa and Koketsu (1977) reported Mg2+ blockade of the depolarizing action of 5-HT on anuran dorsal roots, an observation suggesting an interneuronal mediation of this response. Therefore, we have re-examined serotonin’s purported effect on anuran primary afferents in an attempt to more accurately define its site of action. Using an intra-arterially perfused spinal cord, a superfused hemisected spinal cord and an isolated dorsal root ganglion preparation from the frog, we have observed multiple effects of 5-HT which may aid the interpretation of studies examining serotonergic influences on primary afferents. We report here a direct action of 5-HT on primary afferent terminals and cell bodies and demonstrate that, since this effect is Mn2+ sensitive at both sites, it may represent a calcium sensitive process affected by 5-HT. Additionally, we propose that the 5-HT responses recorded from the frog sensory neuron cell body are suitable models of the action of 5-HT on primary afferent terminals.
METHODS
Winter and summer bullfrogs (Rana catesbiana, 5–6 in, either sex) were anesthetized in ice whereupon the dorsal aspect of the vertebral column was removed by laminectomy. The dura was reflected and spinal roots 2–11 (nomenclature of Gaupp as described by Kudo, 1978) were sectioned at their exit points from the vertebral column. The spinal cord was transected just rostral to the anterior intumescentia, removed and either hemisected with a razor blade or cannulated and perfused through the ventral spinal artery using a fine glass pipette as described by Matsura et al. (1969). Dorsal root ganglia with attached dorsal roots (DR 9 or 10) were removed and pinned in a sylgard lined dish. The ganglia were desheathed so as to expose the cell bodies to the superfusion stream. All preparations were placed in a chamber of 1 ml volume through which chilled superfusate (14–16°C) flowed at a rate of 1 ml/min. The superfusate/perfusate consisted of: NaCl, 100.0 mM: KCl, 2.4 mM; NaHCO3, 9.5 mM; CaCl2, 1.9 mM; TRIZMA–HCl, 2.1 mM; TRIZMA–Base, 7.9 mM; and dextrose, 5.6 mM. This and all test solutions were continuously bubbled with a 95/5 mixture of O2/CO2 to maintain a pH of 7.4 ± 0.1.
With intra-arterially perfused spinal cords, DC recordings of spontaneous and evoked activity were obtained from dorsal and ventral roots of segments 9 or 10 using calomel suction electrodes positioned immediately adjacent to the root entry zone. When recording from a dorsal root, the dorsal root–dorsal root potential and reflex (DR–DRP, DR–DRR) were evoked by stimulation of the peripheral end of an adjacent segment’s dorsal root using a suction electrode. During ventral root recordings, the dorsal root-ventral root potential and reflex (DR–VRP, DR–VRR) were evoked through stimulation of the same segment’s dorsal root.
With hemisected spinal cords and dorsal root ganglia, highly sensitive measurements of polarization changes in primary afferents were obtained using the sucrose gap technique. The preparation’s attached dorsal root (DR 9 or 10) was extended across a flowing isosmotic sucrose stream into a chamber containing isotonic KCl. Intermixing of the three solutions was prevented by drawing the dorsal root through rubber membranes in which fine holes had been melted using a hot wire. Agar salt bridges served to connect the superfusate and KCl chambers with calomel ground and recording electrodes respectively (Corning type 16-639-79). Slow potentials were amplified and displayed on an oscilloscope and on a strip chart recorder with curvilinear pen excursions. The signal was also filtered to remove slow potentials, and action potentials counted using a window discriminator and frequency meter. Spinal reflexes were evoked by a square wave pulse applied to the peripheral end of DR 10 stretched across Ag wire electrodes immersed in mineral oil. Evoked reflexes were computer averaged.
Bath application of drugs was accomplished by switching between superfusates or perfusates containing normal or test solutions. An alternative mode of administration involved microinjection of a small bolus of concentrated test solution directly into the superfusion stream. This minimized rapid desensitization and tachyphylaxis to 5-HT. Drugs used were tetrodotoxin (Calbiochem-Behring), serotonin creatinine sulfate (Sigma), gamma-amino-butyric-acid (GABA, Sigma) and methysergide maleate (Sandoz). Neuronal firing was blocked using superfusates containing 1 µM TTX. Calcium dependent transmitter release was blocked using superfusates containing 2 or 4 mM MnCl2 with no added calcium.
RESULTS
Both intra-arterially perfused and hemisected superfused spinal cords were used for the studies on spinal cord reflexes and spontaneous activity. In general, we observed that intra-arterial perfusion produced preparations with a higher level of spontaneous neuronal activity, and greater sensitivity to 5-HT. However, the hemisected cord produced more stable and reproducible responses.
Serotonin’s effects on spontaneous and evoked spinal activity
The intra-arterially perfused spinal cord exhibited high levels of spontaneous activity characterized by spontaneous dorsal and ventral root potentials (s-DRPs and s-VRPs). As illustrated in the oscilloscope tracings in Figs 1(A) and (B), these s-DRPs and s-VRPs were synchronized in time, with each s-DRP preceding the s-VRP by about 5 msec. A dorsal root discharge (DRD), characterized by a brief burst of antidromic firing, appeared near the peak of each s-DRP and a synchronous ventral root discharge (VRD) accompanied each s-VRP. Treatment with TTX or Mn2+ blocked this spontaneous activity.
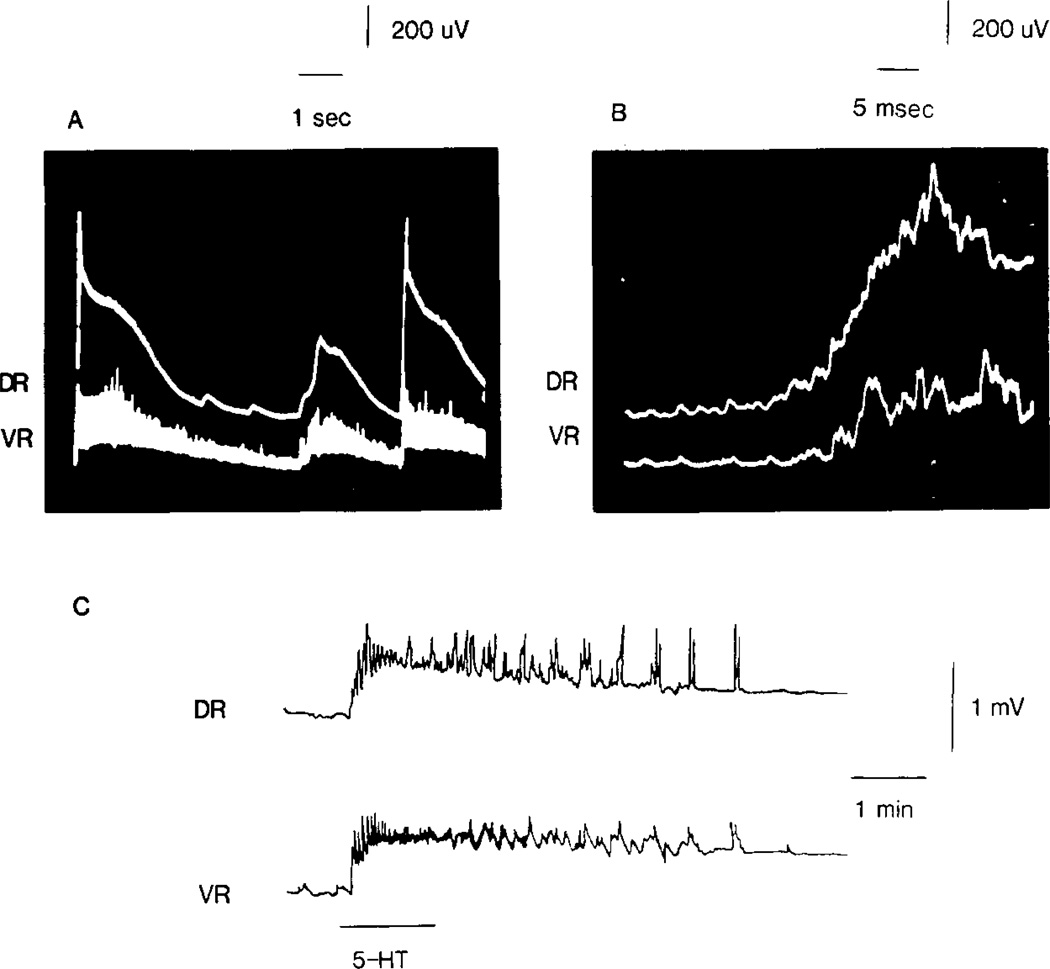
Fig. 1.
Spontaneous dorsal and ventral root potentials recorded simultaneously from an intra-arterially perfused spinal cord. In (A) an oscilloscope tracing reveals a series of s-DRPs (top) and s-VRPs (bottom). Expansion of the time base in (B) demonstrates that the s-DRP precedes the s-VRP. A synchronous antidromic firing of primary afferents rides the s-DRP. In the polygraph tracing (C), perfusion of 5-HT (100 µM) induced s-DRPs and s-VRPs in a preparation which initially exhibited little spontaneous activity. Depolarization upward, here and in succeeding figures.
The introduction of 5-HT into the perfusate markedly increased all spontaneous activity (s-DRPs, s-VRPs, DRD, VRD) as well as producing both dorsal and ventral root depolarization. These effects of 5-HT were dose dependent. Concentrations as low as 10 nM produced detectable increases in spontaneous activity. In concentrations of 0.1–50 µM, 5-HT also affected evoked activity by enhancing the early components of the dorsal and ventral root reflexes, shortened the time course of the DRP, but had little effect on root polarization (Figs 2A and B). With higher concentrations of 5-HT, prolonged dorsal and ventral root depolarization was observed which was usually accomplished by marked increases in spontaneous discharges (Fig. 1C). However, with concentrations greater than 100 µM of 5-HT, a strong depolarization of the roots was observed with diminished spontaneous and evoked reflex activity probably due to occlusion.
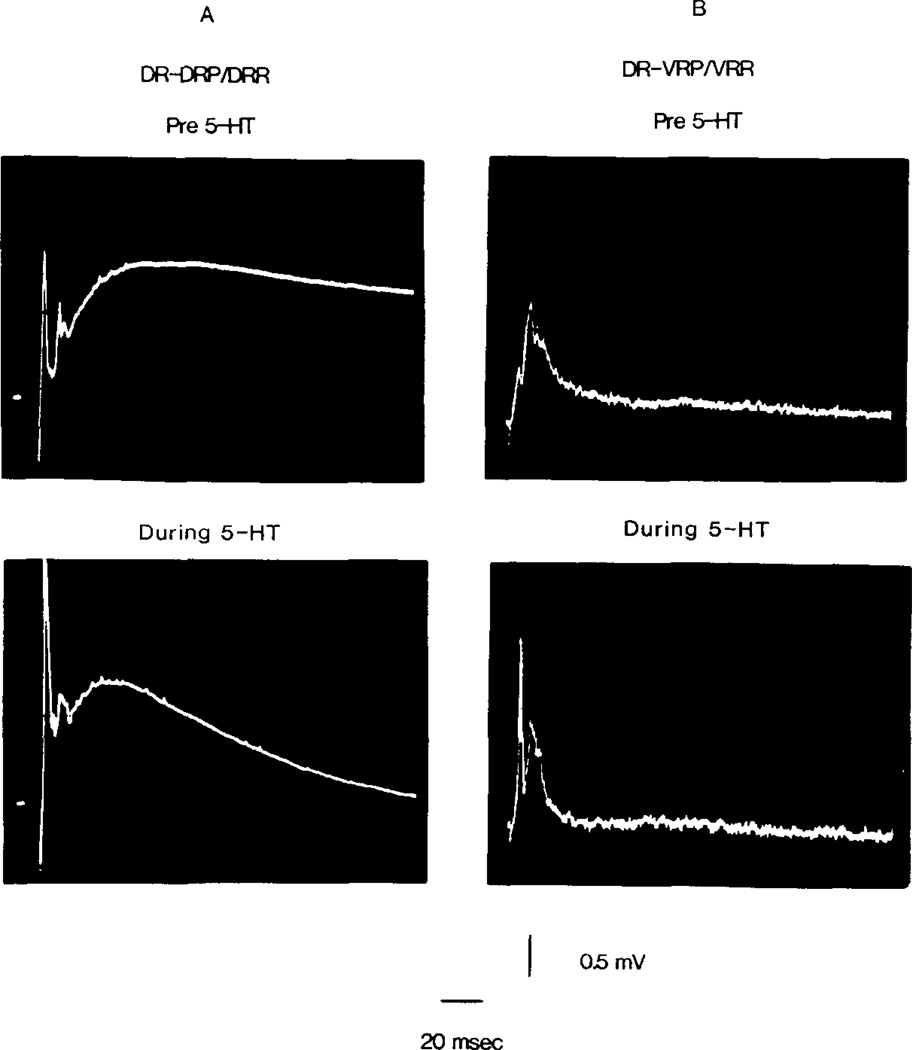
Fig. 2.
Alteration of spinal reflexes during intra-arterial perfusion of 5-HT. In (A) the effect of 5-HT (0.1 µM for 90 sec) on the DR-DRP/DRR is shown. In (B) signal averaged DR-VRP/VRR responses (7 reflexes per photo) are shown prior to and during 5-HT (10 µM for 240 sec). Supramaximal stimulation, 0.2 msec duration.
The hemisected spinal cord preparation exhibited less spontaneous activity and less sensitivity to 5-HT. Since superfusion of 5-HT resulted in marked desensitization to subsequent applications, tachyphylaxis was minimized by bolus injection of 5-HT into the superfusion stream. In the hemisected preparation, 5-HT-induced PAD was frequently associated with an augmented level of spontaneous antidromic DRD. This is shown in Fig. 3(A), which correlates the 5-HT-induced depolarization with a rate meter record of DRD spike frequency. An oscilloscope tracing of this effect is presented in Fig. 3(B). The 5-HT-induced DRD differed from the spontaneous DRD observed in the intra-arterially perfused cord in that it occurred independent of spontaneous dorsal root potentials. Although not shown, 5-HT also increased the frequency of the VRD.
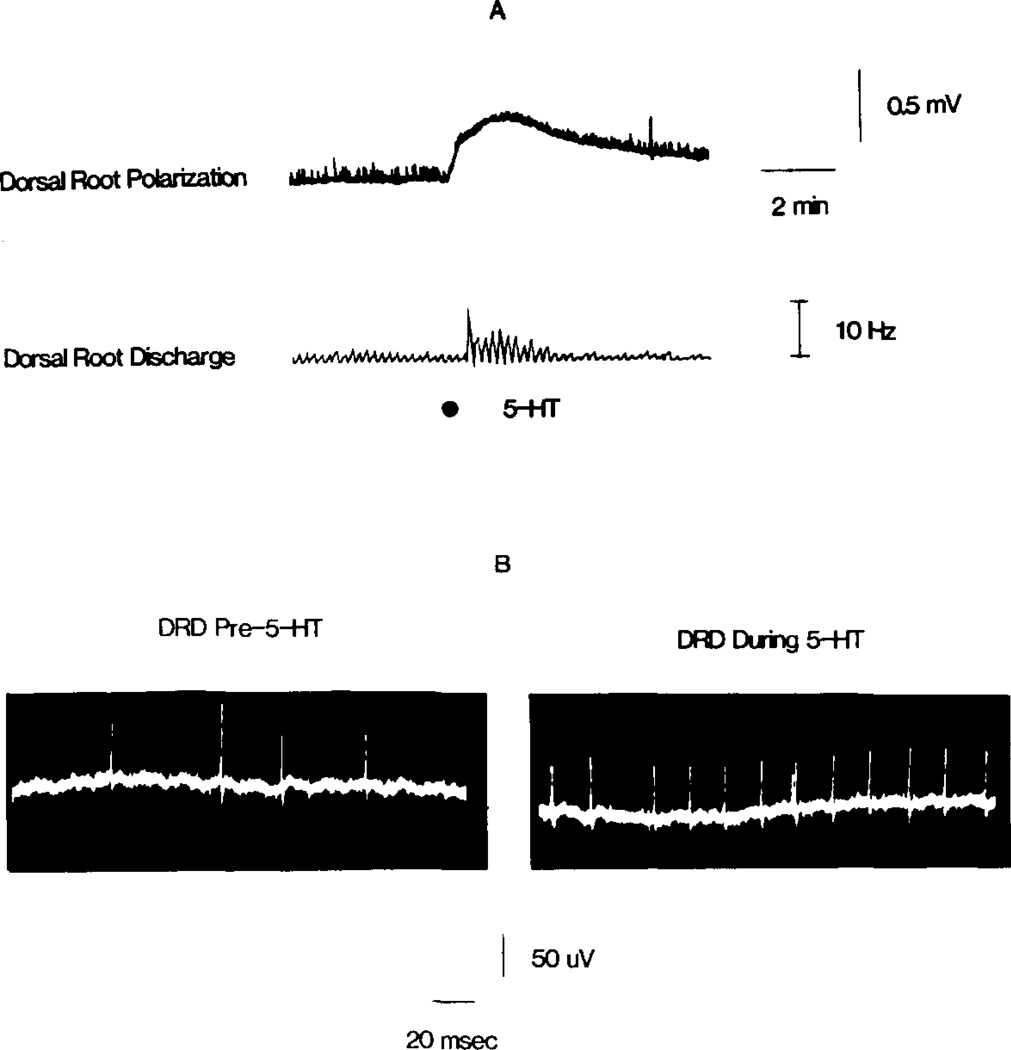
Fig. 3.
Sucrose gap recordings from the dorsal root of a hemisected spinal cord illustrate the effect of 5-HT on the DRD. In (A), injection of 5-HT (10 µl of 5 mM) into the superfusate depolarized the dorsal root (top) and increased the frequency of spontaneous antidromic firing in primary afferents (bottom rate meter record). The upward deflections in the top trace are small s-DRPs. (B) Illustrates the DRD prior to and during the peak of 5-HT-induced depolarization of the dorsal root.
5-HT and GABA induced PAD
A comparison of 5-HT- and GABA-induced PAD in the hemisected spinal cord is illustrated in Figs 4(A) and (B). Equal volumes of equieffective concentrations of 5-HT or GABA were injected into the superfusion stream. The first injection of 5-HT consistently led to a biphasic PAD with fast and slow components (Fig. 4A), whereas GABA injections produced only a fast monophasic depolarization (Fig. 4B). The rate of onset of 5-HT and GABA depolarizations were comparable, but the duration of the 5-HT response lasted 5–10-fold longer than the GABA response. Repeated exposure to 5-HT produced pronounced desensitization with elimination of the fast depolarizing phase, and a reduction in amplitude of the slow phase. In addition, the 5-HT response became more prolonged and frequently failed to fully recover. This necessitated a 40–60 min interval between applications to avoid desensitization. In contrast to the response following serotonin’s injection into the superfusate, bath application of 5-HT produced only a monophasic dorsal root depolarization. This response was concentration dependent over a range of 1.0–100 µM 5-HT.
Fig. 4.
5-HT- and GABA-induced PAD recorded from the dorsal root of a sucrose gapped hemisected spinal cord. In (A) and (B) the upward deflections are s-DRPs. In this preparation 5-HT (10 µl of 5 mM) injected into the superfusate produced biphasic PAD (A) while GABA (10 µl of 10 mM) produced monophasic PAD (B). In (C) a biphasic 5-HT response is shown in a different preparation following pretreatment with 1 µM TTX (arrow indicates the fast phase following injection of 100 µl of 20 mM 5-HT).
5-HT-indueed PAD is TTX resistant
The above results suggest that 5-HT-induced PAD may result, in part, from altered interneuronal influences on primary afferents. To test this hypothesis, hemisected spinal cords were superfused with a solution containing 1 µM TTX. This concentration blocked all recordable evoked and spontaneous activity, indicating a blockade of interneuronal transmission. In TTX treated preparations, bath application of 5-HT led to a slowly developing, concentration dependent PAD (0.1–1.0 mV; EC50 14 µM) which also exhibited pronounced tachyphylaxis. A TTX resistant depolarization of the ventral root was also observed (not shown). Although the TTX resistant action of 5-HT on the dorsal root was monophasic following bath application, a biphasic response was occasionally observed upon injection of 5-HT into the superfusate (Fig. 4C). The magnitude of the 5-HT response after TTX was 65 ± 5.5% (mean ± SEM; N = 6 spinal cords) of that seen in the preparation prior to TTX treatment. This decrement in response to 5-HT could indicate the contribution of interneuronal pathways to 5-HT induced PAD.
5-HT-induced PAD is methysergide sensitive
The TTX resistant depolarizing action of 5-HT on primary afferent terminals was completely blocked in six of six cases by pretreatment of spinal cords with 1–10 µM methysergide. This blockade was prolonged and could not be reversed even after a washout period of two hours. In two of two cases, pretreatment with 250 nM methysergide irreversibly reduced the 5-HT response by 50%, while 100 nM methysergide had no effect. Since 100 µM methysergide, itself, did not depolarize the dorsal roots in the presence of TTX, this effect may result from methysergide’s antagonism of serotonin’s direct action. To confirm that this was antagonism and not deterioration of the preparation, the spinal cord was hemisected and one-half of the cord superfused in a solution containing 1 µM TTX and 50 µM methysergide. Serotonin (10 µl of 5 mM) was then injected into the superfusate. No response to 5-HT could be observed in any of the 3 hemisected spinal cords tested even though GABA induced PAD remained. We then tested the second half of the spinal cord in a solution containing 1 µM TTX without methysergide. In all 3 spinal cords tested, injection of 5-HT into the superfusate depolarized the dorsal root.
The influence of Mn2+ on 5-HT-induced PAD
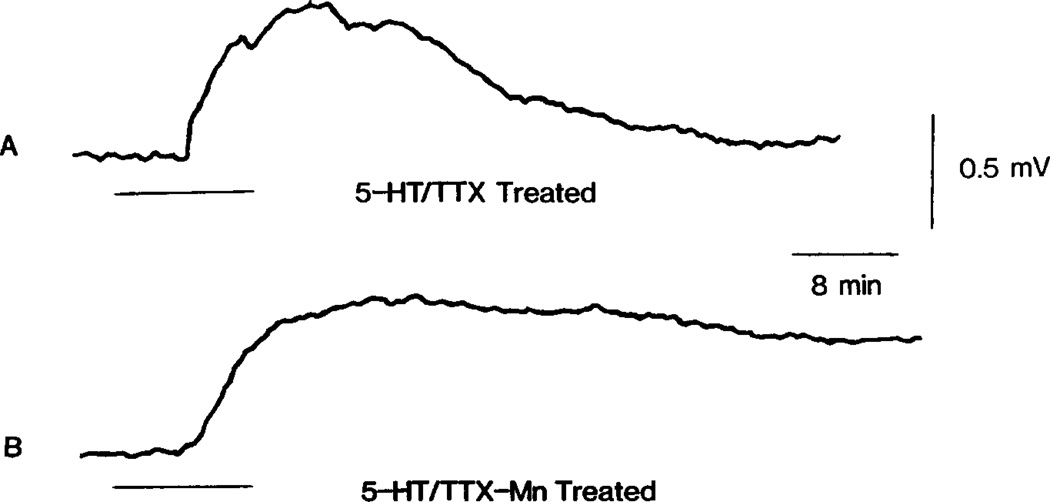
The TTX resistant response illustrated in Fig. 5(A) might result from a direct action of 5-HT on primary afferent terminals. Alternatively, it might be due to an indirect action via the release of a depolarizing neurotransmitter from adjacent neurons depolarized by 5-HT. This possibility was examined by testing the sensitivity of the 5-HT response to concentrations of Mn2+ sufficient to minimize Ca2+ dependent transmitter release. Superfusion of hemisected spinal cords with solutions containing 2 mM MnCl2 blocked all recordable spontaneous and evoked activity, suggesting a blockade of synaptic transmission. However, as shown in Fig. 5(B), the size of the 5-HT response, recorded during 2 mM MnCl2 superfusion, remained identical to that observed after treatment with TTX (Fig. 5A), although recovery was incomplete. Increasing the concentration of Mn2+ to 4 mM led to a gradual 4–6 mV depolarization of the dorsal root and attenuation of the 5-HT but not GABA response. This is shown in Fig. 6(A–C), which compares the 5-HT response of a preparation first treated with TTX, then treated with 4 mM MnCl2 (no TTX included) and finally returned to the original TTX containing superfusate. Note that the 5-HT response recorded in the presence of 4 mM Mn2+, superimposed on a slow Mn2+ induced depolarization, was markedly smaller than the TTX resistant response.
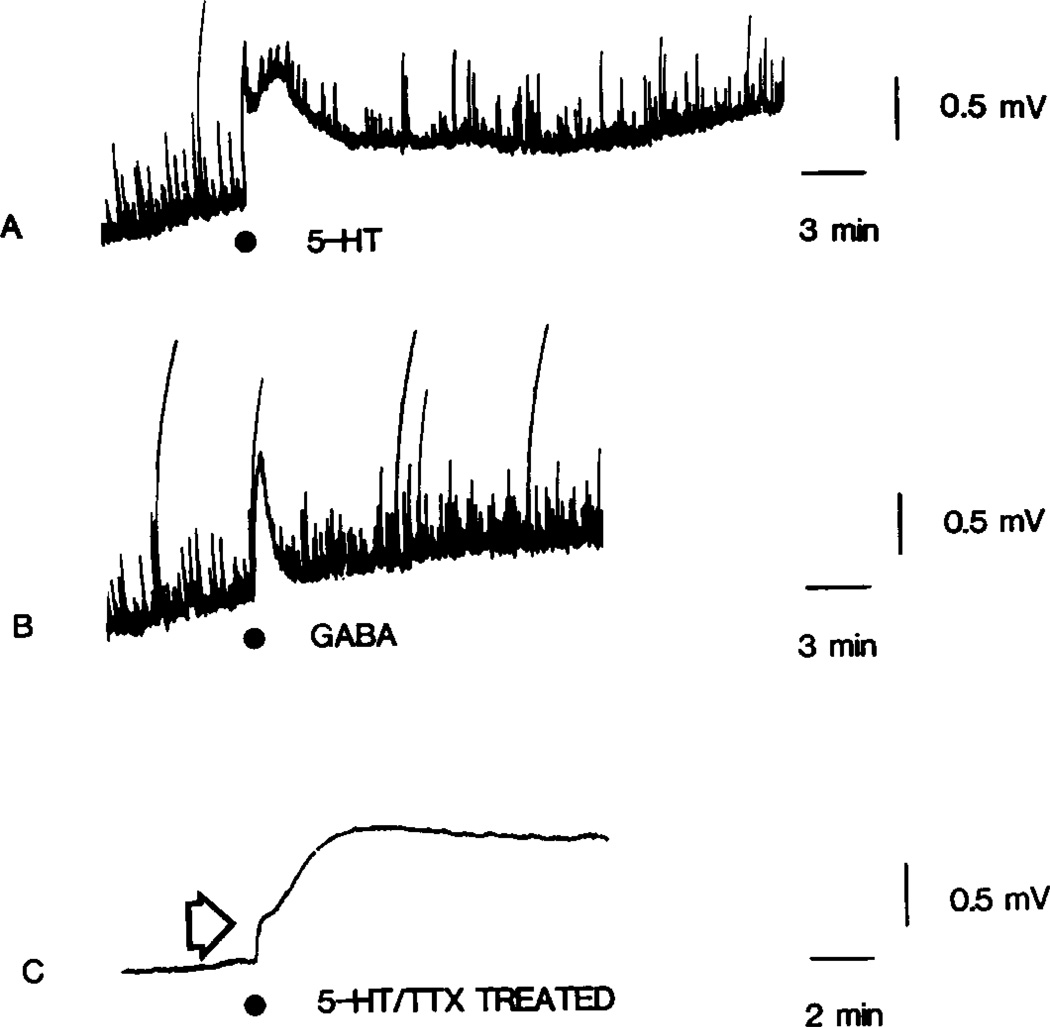
Fig. 5.
5-HT induced PAD which is TTX and Mn2+ resistant. (A) Illustrates the action of bath applied 5-HT (100 µM) as recorded from the dorsal root of a sucrose gapped hemisected spinal cord pretreated with 1 µM TTX. The superfusate was then switched to a solution containing 1 µM TTX and 2 mM MnCl2. After exposure to this solution for 40 min, the response to 5-HT (100 µM) was again determined and is shown in (B).
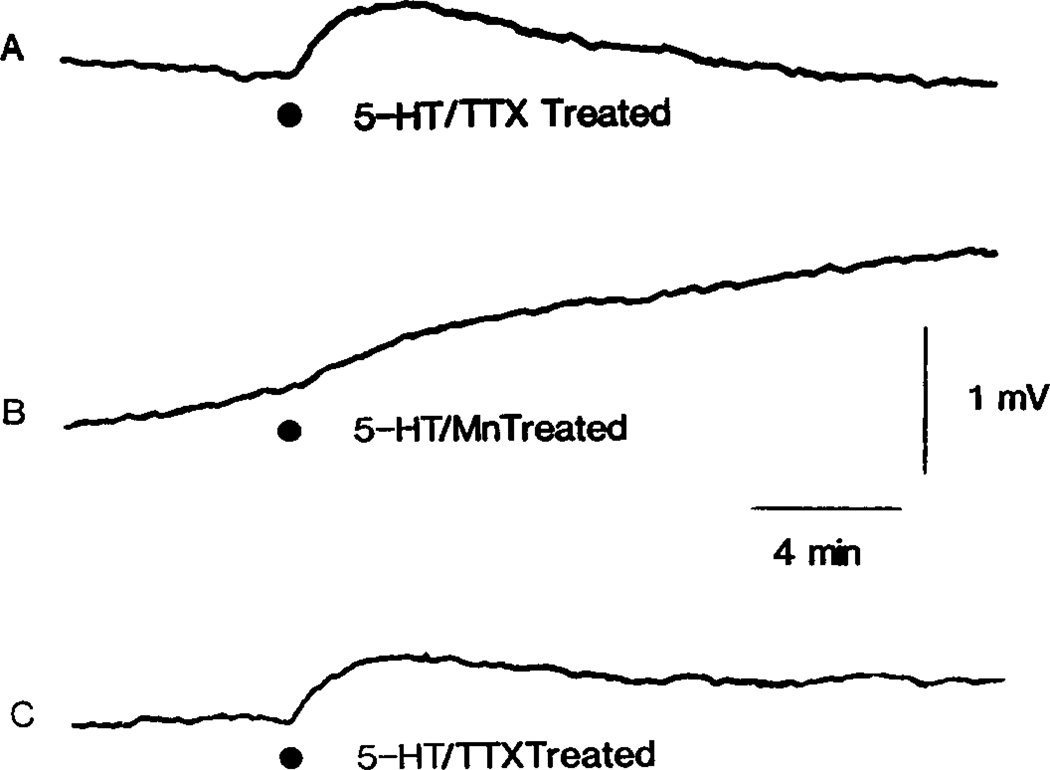
Fig. 6.
Attenuation of 5-HT induced PAD by high levels of Mn2+. (A–C) Illustrates 5-HT responses recorded from a sucrose gapped hemisected spinal cord. In (A) the 1 µM TTX resistant 5-HT depolarization is shown. The superfusate was then switched to a solution containing 4 mM MnCl2, with no added TTX, and the 5-HT response again tested after 60 min exposure as shown in (B). Next the composition of the superfusate was returned to the original TTX containing solution and 5-HT once again tested 60 min after washout of Mn2+ as shown in (C). At each of the indicated points, 5-HT(30 µl of 2 mM) was injected into the superfusate.
Dorsal root ganglion responses to 5-HT
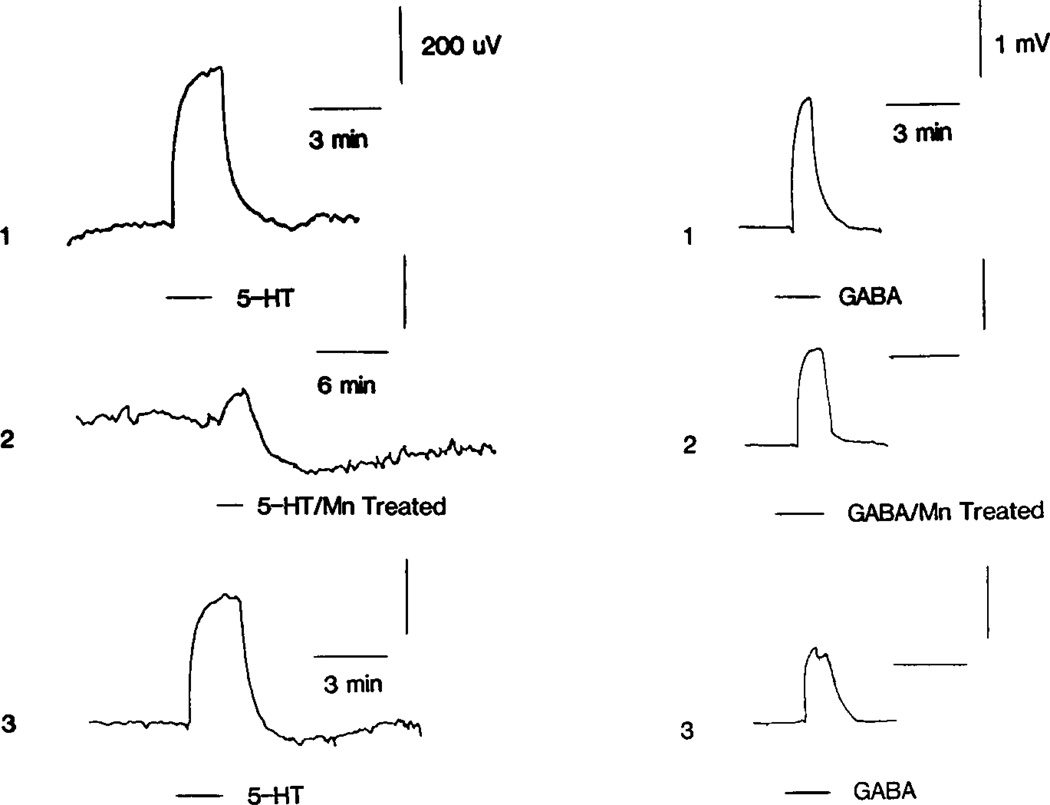
Attenuation of the 5-HT response by 4 mM Mn2+ suggests a calcium sensitive basis for this response. Although a serotonergic influence on calcium dependent interneuronal transmitter release cannot be ruled out completely, Mn2+ could act directly on primary afferents to alter their sensitivity to 5-HT. These two possibilities are extremely difficult to differentiate in the spinal cord preparation where axo-axonic synapses occur on primary afferents (Szekely and Kosaras, 1977). A simpler system to investigate is the primary afferent cell body, which, in the frog, is not known to receive synaptic contacts. As shown in Fig. 7(A–C), frog primary afferent cell bodies were also depolarized by 5-HT, lending further support for a direct action of 5-HT on afferent neurons. In 10 of 14 cases, the DRG response to 5-HT was solely depolarizing, while in four cases the response included a late hyperpolarization. The 5-HT response also exhibited pronounced tachyphylaxis and tended to be smaller in amplitude (0.1–0.4 mV) than the afferent terminal response in the cord preparation. The depolarizing response was irreversibly blocked by 2 mM Mn2+ in four of six cases, while in two cases full reversibility occurred. In one case of reversible attenuation, shown in Fig. 7(A–C), Mn2+ superfusion reduced the depolarizing component to one-third of its original size while enhancing the late hyperpolarization. Return to a calcium containing superfusate allowed full recovery of the response. These effects of Mn2+ were not due to a non-specific cell depression since responses to GABA were never reduced by more than 30% by any Mn2+ treatment.
Fig. 7.
Attenuation of the dorsal root ganglion response to 5-HT by Mn2+. Sucrose gap recordings from the dorsal root of the DRG. In No. 1, the 5-HT and GABA responses, recorded in a normal superfusate, are shown in the left- and right-hand columns, respectively. The superfusate composition was then switched so as to contain 2 mM MnCl2. After 2.5 hr exposure, the 5-HT and GABA responses were again determined as shown in No. 2. Note the slow after-hyperpolarizing phase of the 5-HT response which is accentuated here by slowing the time base. Recovery of the 5-HT response is shown in No. 3, 45 min after return to the original solution containing no MnCl2. At the indicated points 1 mM 5-HT and 1 mM GABA were bath applied.
DISCUSSION
This study demonstrates serotonin’s direct depolarizing action on anuran primary afferents and therefore confirms the original finding of Tebecis and Phillis (1967). To reveal this direct action, spinal cords were pretreated with 1 µM TTX and 2 mM MnCl2. These treatments minimize influences on primary afferents conveyed by neuronal conduction and calcium dependent transmitter release. The efficacy of these treatments was demonstrated by their ability to block all recordable spontaneous and evoked activity. Our observation that 5-HT retained most of its depolarizing action in the presence of TTX suggests that 5-HT acts directly on afferent nerve terminals. Since injection of 5-HT into superfusates of TTX treated spinal cords produced fast and slow PAD, a dual action of 5-HT directly on afferent terminals is suggested and is supported by the differential desensitization of these two processes. A 5-HT receptor probably mediates these depolarizations since methysergide pretreatment blocked serotonin’s TTX resistant action.
A TTX resistant 5-HT depolarization is not, however, sufficient proof of serotonin’s direct action. Even in the presence of TTX, 5-HT might indirectly influence primary afferents by inducing the release of a depolarizing transmitter from nerve endings synapsing on afferent terminals. However, this seems unlikely since 2 mM MnCl2 did not attenuate serotonin’s TTX resistant action. Still, since 4 mM MnCl2 did reduce the 5-HT depolarization, one might argue that concentrations of MnCl2 in excess of 2 mM may be required to block 5-HT-induced transmitter release.
To further substantiate the direct action on 5-HT on primary afferents, we examined the 5-HT sensitivity of afferent cell bodies of the dorsal root ganglion on the premise that this preparation is free from synaptically mediated influences. The 5-HT depolarization of afferent cell bodies reported here lends additional support for serotonin’s direct action. However, in this preparation, 2 mM MnCl2 attenuated the 5-HT depolarization. This is significant since an electron microscope study has reported the presence of a few small synapses on cat DRG cells (Kayahara et al., 1981). Since it is possible that such synapses have been missed in the frog, the action of 5-HT might still be explained by its ability to indirectly influence primary afferents through an induction of transmitter release.
In considering these possible mechanigms of serotonin’s action, additional data must be discussed. As noted here, superfusion of the TTX-treated spinal cord with Mn2+ containing solutions depolarized the dorsal root. A similar effect has also been noted upon exposure to Co2+ (Padjen and Smith, 1980). Therefore, attenuation of 5-HT depolarizations by Mn2+ may involve this ion’s direct influence on an electrogenic process occurring on primary afferent membranes. Indeed, 5-HT induces a depolarizing inward current in molluscan cells and this action is blocked by Co2+, an effect possibly due to the ability of Co2+, itself, to induce a depolarizing inward current (Paupardin-Tritsch et al., 1981). The direct 5-HT depolarization reported here may therefore involve a conductance affected by Mn2+. Alternatively, Mn2+ may affect an intracellular process, an electrogenic pump, 5-HT receptor sensitivity or membrane surface charge. Further studies are required to identify which of these possibilities occur. In the meantime, it must be realized that the observation of Shirasawa and Koketsu (1977), that Mg2+ blocks 5-HT depolarizations of primary afferents, should not simply be interpreted as indicating an interneuronal site of serotonin’s action. It may be that both Mg2+ and Mn2+ block the direct 5-HT depolarization of afferent terminals.
This study also examined serotonin’s actions on spontaneous and evoked activity. Intra-arterial perfusion of 5-HT increased the frequency of s-DRPs and s-VRPs and in low concentrations augmented evoked DR and VR reflexes. Since the s-DRP preceded the s-VRP, the dorsal root depolarization produced by this 5-HT-induced spontaneous activity cannot result solely from an excitatory action of 5-HT on motoneurons generating intraspinal recurrent PAD. Rather, a facilitatory action of 5-HT on interneuronal systems may trigger these s-DRPs and s-VRPs. Such a facilitatory influence might also lead to an excitability increase in reflex pathways. This action, in addition to serotonin’s direct depolarizing actions may raise the excitability of afferent terminals and motoneurons and favor their reflexive firing.
Serotonin also induced repetitive firing in spinal cord dorsal roots. This dorsal root discharge represents antidromic tiring in primary afferents since frog dorsal roots do not contain efferents (Wilhelm and Coggeshall, 1981). In the hemisected spinal cord preparation, the 5-HT-induced increase in the DRD occurred independent of any effect on s-DRPs but closely correlated with 5-HT induced PAD. Therefore, in this preparation, the 5-HT-induced increase in the DRD may largely result from serotonin’s direct depolarization of afferent terminals which increased their excitability and allowed ongoing interneuronal activity to more effectively induce antidromic firing.
Serotonin’s actions may now be summarized. In the spinal cord, 5-HT depolarized afferent terminals through direct and interneuronally mediated influences. The depolarization consisted of fast and slow phases and was accompanied by the repetitive antidromic firing of these sensory neurons. In the DRG, the 5-HT response consisted of depolarizing and hyperpolarizing components. Finally, in both preparations, Mn2+ pretreatment attenuated serotonin’s direct depolarizing action.
What functional consequences do serotonin’s actions suggest? Presynaptic inhibition of anuran primary afferent transmission may result from the direct and interneuronally mediated 5-HT depolarization of afferent terminals as originally proposed by Carels (1962). However, depolarization per se cannot be equated with presynaptic inhibition since the Mn2+ sensitivity of serotonin’s direct action suggests an effect of 5-HT on a calcium sensitive process. An influence of 5-HT on calcium entry or mobilization could lead to presynaptic inhibition, as has been proposed for cultured chick DRG cell 5-HT responses (Dunlap and Fischbach, 1978; Fischback et al., 1981), or presynaptic facilitation as has been proposed for molluscan ganglion cell (Klein and Kandel, 1978), and lobster neuromuscular junction (Glusman and Kravitz, 1982) 5-HT responses. Additionally, the repetitive firing of primary afferents during 5-HT depolarizations suggests that transmission in afferent terminal arborizations may be altered by serotonin’s influence on spike generation. Finally, the hyperpolarizing component of serotonin’s action in the DRG suggests the possibility of a similar 5-HT hyperpolarization of afferent terminals.
In conclusion, primary afferent terminal and cell body 5-HT responses share important similarities. These include a direct 5-HT depolarization, pronounced tachyphylaxis upon repeated administrations, and reversible attenuation of the depolarizing response following Mn2+ treatment. Such similarities suggest that valid inferences may be drawn regarding serotonin’s action on afferent nerve terminals through an analysis of its action on afferent cell bodies. We are presently employing this model system for an intra-cellular study of serotonin’s actions in the DRG.
REFERENCES
- Banna NR, Anderson EG. The effects of 5-hydroxytryptamine antagonists on spinal neuronal activity. J. Pharmac. exp. Ther. 1968;162:319–325. [PubMed] [Google Scholar]
- Barasi S, Roberts MHT. The modification of lumbar motor-neurone excitability by stimulation of a putative 5-hydroxytryptamine pathway. Br. J. Pharmac. 1974;52:339–348. doi: 10.1111/j.1476-5381.1974.tb08601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carels G. Depression par la sérotonine de la transmission monosynaptique dans la moelle isolée de la grenouille. Arch. int. Pharmacodyn. Thér. 1962;138:326–328. [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine containing neurons in the central nervous system—I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta. physiol. scand. 1964;62(Suppl. 232):1–55. [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for the existence of monoamine containing neurons in the central nervous system—II. Experimentally induced changes in the extra-neuronal amine levels of bulbospinal neuron systems. Acta. physiol. scand. 1965;64(Suppl. 247):1–36. [PubMed] [Google Scholar]
- Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium component of sensory neuron action potentials. Nature, Lond. 1978;276:837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- Ellaway PH, Trott JR. The mode of action of 5-hydroxytryptophan in facilitating a stretch reflex in the spinal cat. Exp. Brain Res. 1975;22:145–162. doi: 10.1007/BF00237685. [DOI] [PubMed] [Google Scholar]
- Engberg I, Lundberg A, Ryall RW. Is the tonic decerebrate inhibition of reflex paths mediated by monoaminergic pathways? Acta. physiol. scand. 1968;72:123–133. doi: 10.1111/j.1748-1716.1968.tb03834.x. [DOI] [PubMed] [Google Scholar]
- Fischbach GD, Dunlap K, Mudge A, Leeman S. Peptide and amine transmitter effect on embryonic chick sensory neurons in vitro. Adv. Biochem. Psychopharmac. 1981;28:175–188. [PubMed] [Google Scholar]
- Glusman S, Kravitz EA. The action of serotonin on excitatory nerve terminals in lobster nerve-muscle preparations. J. Physiol. 1982;325:223–241. doi: 10.1113/jphysiol.1982.sp014147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayahara T, Takimoto T, Sakashita S. Synaptic junctions in the cat spinal ganglion. Brain Res. 1981;216:277–290. doi: 10.1016/0006-8993(81)90130-x. [DOI] [PubMed] [Google Scholar]
- Klein M, Kandel ER. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization of Aplysia californica. Proc. natn. Acad. Sci., U.S.A. 1978;77:6912–6916. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y. The pharmacology of the amphibian spinal cord. Prog. Neurobiol. 1978;11:1–76. doi: 10.1016/0301-0082(78)90007-2. [DOI] [PubMed] [Google Scholar]
- Matsuura S, Kawaguchi S, Ichiki M, Sorimachi M, Kataoka K, Inouye A. Perfusion of frog’s spinal cord as a convenient method for neuropharmacological studies. Eur. J. Pharmac. 1969;6:13–16. doi: 10.1016/0014-2999(69)90058-2. [DOI] [PubMed] [Google Scholar]
- Padjen AL, Smith PA. Divalent ions and amino acid responses in frog spinal cord. Abst., Soc. Neurosci. 1980;10:54.1. [Google Scholar]
- Paupardin-Tritsch D, Deterre P, Gerschenfeld HM. Relationship between two voltage dependent serotonin responses of molluscan neurons. Brain Res. 1981;217:201–206. doi: 10.1016/0006-8993(81)90201-8. [DOI] [PubMed] [Google Scholar]
- Phillis JW, Kirkpatrick JR. Action of biogenic amines on the isolated toad spinal cord. Gen. Pharmac. 1979;10:115–119. doi: 10.1016/0306-3623(79)90045-4. [DOI] [PubMed] [Google Scholar]
- Shirasawa Y, Koketsu K. Action of 5-hydroxytryptamine on isolated spinal cord of bullfrogs. Jap. J. Pharmac. 1977;27:23–29. doi: 10.1254/jjp.27.23. [DOI] [PubMed] [Google Scholar]
- Székely G, Kosaras B. Electron microscopic identification of postsynaptic dorsal root terminals: a possible substrate of dorsal root potentials in the frog spinal cord. Exp. Brain Res. 1977;29:531–539. doi: 10.1007/BF00236190. [DOI] [PubMed] [Google Scholar]
- Tebecis AK, Phillis JW. The effects of topically applied biogenic monoamines on the isolated toad spinal cord. Comp. Biochem. Physiol. 1967;23:553–563. doi: 10.1016/0010-406x(67)90407-0. [DOI] [PubMed] [Google Scholar]
- Wilhelm GB, Coggeshall RE. An electron microscopic analysis of the dorsal root in the Frog. J. comp. Neurol. 1981;196:421–429. doi: 10.1002/cne.901960306. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Hammond DL, Tyce GM. Functional aspects of bulbospinal monoaminergic projections in modulating processing of somatosensory information. Fedn Proc. Fedn Am. Socs. exp. Biol. 1981;40:2786–2794. [PubMed] [Google Scholar]