We read with interest the article “Human Circulating MicroRNA-1 and MicroRNA-126 as Potential Novel Indicators for Acute Myocardial Infarction” by Guangwen Long et al 1. The authors gathered blood samples from 17 patients and 25 healthy adults at Tongji Hospital between October 2009 and May 2010. They found the ability of the miR-1 score to differentiate the AMI group from the control group, and by using different threshold scores, they achieved both high sensitivity and specificity for the diagnosis of AMI patients. The results of miR-126 score were similar. They came to the conclusion that miR-1 and miR-126 had potential utility as novel biomarkers for clinic diagnosis of AMI.
This is indeed a remarkable study and the results hold a lot of truth in clinical practice, especially the good correlation between the plasma concentration of miR-1/miR-126 and cTnI 2. What concerns us the most is whether miR-1 and miR-126 demonstrate potential utility for clinic diagnosis of AMI. The control group was composed of 25 healthy adult volunteers with normal electrocardiogram and no history of cardiovascular diseases. The difference between AMI patients and healthy people is meaningful. Nevertheless, the identification of the AMI patients with asymptomatic healthy people in the control group is clinical irrelevant.
Diagnostic tests and comparative studies are substantially different. The evaluation indicators for diagnostic tests are sensitivity and specificity. However, the objective of a comparative study is usually to detect the correlation between exposures and outcomes, avoiding possible confounders and bias, which is totally different from that of a diagnostic test.
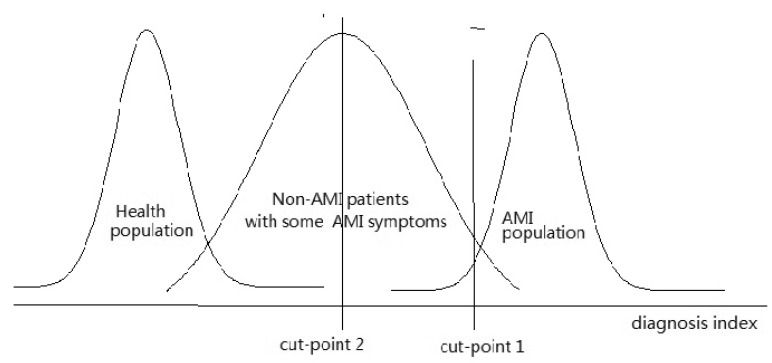
When normal and healthy people are selected as subjects of the control group, the specificity of the diagnostic test will be significantly elevated. For instance, if the subjects in the study are divided into the AMI group and non-AMI group, patients with diagnosis index below cut point 1 should be diagnosed as non-AMI and those with index higher than cut point 1 as AMI (figure 1). However, if the subjects belong to the AMI group and healthy group, cut point 2 should be selected. In clinical practice, those whose seek for diagnosis often suffer from various diseases, many of which would also leave some impact on the potential indicators. We can learn from figure 1 that many of those who with diagnosis index higher than cut point 2 are non-AMI. Therefore, the specificity would decrease substantially, which will result in great increase of the probability of false positive.
Fig 1.
Different cut points for different study population
Generally speaking, if objective is screening AMI, the control should be normal people; otherwise, the control should be non-AMI. Non-AMI patients should be used instead of the control group made up of healthy volunteers in this case, in that the objective is to identify patients of target disease among all patients who might seek for diagnosis. These patients are definitely different from normal people. Similar mistakes are common in scientific researches, which call for attention from every researcher. If normal people are selected as control group, the result can't be applied to the population of non-AMI patients. This always results in the high specificity in clinical researches but low specificity in clinical practice. As a result, we consider that the sensitivity of miR-1 and miR-126 as novel potential biomarkers for clinic diagnosis of AMI is dubious, while the specificity is questionable and might be potentially overestimated. In conclusion, the subjects in the control group should be carefully selected to make sure that they are identical with the population that the potential method might be applied to.
Abbreviations
- miRNAs
microRNAs
- AMI
acute myocardial infarction
- cTnI
cardiac troponin I.
References
- 1.Guangwen Long, Feng Wang, Quanlu Duan, Fuqiong Chen, Shenglan Yang. et al. Human Circulating MicroRNA-1 and MicroRNA-126 as Potential Novel Indicators for Acute Myocardial Infarction. Int J Biol Sci. 2012;8(6):811–818. doi: 10.7150/ijbs.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ji X, Takahashi R, Hiura Y, Hirokawa G, Fukushima Y, Iwai N. Plasma miR-208 as a biomarker of myocardial injury. Clin Chem. 2009;55:1944–9. doi: 10.1373/clinchem.2009.125310. [DOI] [PubMed] [Google Scholar]