Abstract
Background
Opioids are the drugs of choice for management of breathlessness in advanced disease, but acute episodic breathlessness remains difficult to manage. New routes of opioid applications with quicker onset of action seem attractive for the management of episodic breathlessness.
Objective
This study aimed to determine the acceptability and preference of different routes of opioid applications in patients suffering from breathlessness due to advanced disease.
Design
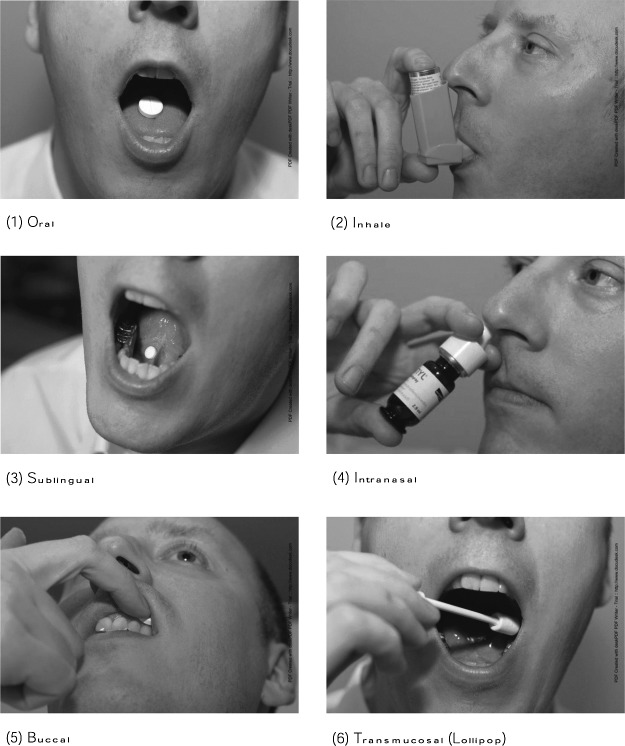
The study consisted of structured face-to-face interviews with patients suffering from breathlessness due to lung cancer (LC), chronic obstructive pulmonary disease (COPD), chronic heart failure (CHF), and motor neurone disease (MND). Images and explanation were used to illustrate six application forms (oral, inhaled, sublingual, intranasal, buccal, transmucosal).
Results
Participants numbered 119 (UK n=48, Germany n=71), 60% male, mean age 67.7 years (SD 9.9); 50% suffered from COPD. Inhaled was the most accepted (87%) and preferred (68%) route of application, followed by sublingual (45%/13%) and intranasal (42%/8%). The oral was least accepted (24%) and least preferred (9%) although nearly all participants had previous experiences with it (97%). Ratings were similar in both countries but different for preferences of sublingual (UK>Germany) and intranasal (Germany>UK). In general, participants from the UK rated more often “yes” for acceptability of all routes compared to Germany.
Conclusion
Inhaled was the most accepted and preferred route of application, but no route seemed to be acceptable to all patients. Therefore, individual patient preferences should be explored before drug prescription to enhance compliance and convenience.
Introduction
Breathlessness is one of the most common and distressing symptoms in advanced cancer and nonmalignant diseases.1,2 It has been defined as “a subjective experience of breathing discomfort that consists of qualitatively distinct sensations that vary in intensity.”3
The prevalence of breathlessness ranges from 78% for patients with lung cancer, 60% to 88% for chronic heart failure (CHF), 90% to 95% for chronic obstructive lung disease (COPD), to nearly 100% for motor neuron disease (MND).2,4,5 In particular, episodes of severe or acute breathlessness are burdensome for patients and their carers and cause panic and terror with fear of dying or suffocating.1
In an observational study with 70 cancer patients, 81% suffered from episodic breathlessness, a “clinically significant aggravation of dyspnoea in patients with continuous dyspnoea or occurring intermittently.”6 Severe, incident, acute, or breakthrough breathlessness are equivalent terms used to express episodic breathlessness, often compared with breakthrough pain (BTP) because of similarities like quick onset, severe intensity, and short duration of each attack.7–9
Current pharmacological interventions include opioids, benzodiazepines, other anxiolytics, and oxygen.10 However, sufficient evidence exists only for the use of opioids with oral immediate-release morphine (IRM) often used for acute breathlessness.11,12 Fentanyl, a potent opioid with fast onset, could be a new management option for episodic breathlessness but has not been adequately tested yet. Case studies, uncontrolled studies, and one randomized controlled trial indicate that there could be a positive effect of nebulized and intranasal fentanyl for the management of breathlessness.13–15 Recently, a variety of new routes of fentanyl applications (sublingual, intranasal, buccal, lollipop) have been introduced for pain management that could potentially be used for treatment of breathlessness as well.16
Acceptability and preference for type of drug application are important factors to enhance compliance in drug use, but little is known in this area. Two studies on the management of BTP showed that acceptability and preference for routes of application were influenced by previous use and pain intensity. Walker and colleagues reported that for severe BTP the most accepted route of application is the oral route and the least accepted, rectal.17 According to Davies and colleagues the oral transmucosal and subcutaneous routes were most accepted in pain.9 There is some evidence that patients' preferences vary between countries, e.g., patients from the UK were more likely to accept any route compared to patients from Scandinavian countries or Germany.9 In order to tailor breathlessness management to patients' needs and preferences, information on their views on different routes of application is necessary.
Therefore, this study aimed to determine the acceptability and preference for six different routes of drug application for acute breathlessness in patients with advanced diseases.
Methods
Study design
This study is a cross-sectional descriptive survey, with four structured questions using illustrating material (images) in a face-to-face interview asking patients about the potential use of different routes of administration. We adapted the questions from a previous study about acceptability of different routes of administration in pain17 and added two questions on preferences. We asked patients about the most common and also latest noninvasive routes of opioid application: oral, inhaled, sublingual, intranasal, buccal, and transmucosal. According to the pain literature, patients' views seem to differ between countries. In order to see whether this also applies to patients with breathlessness, we compared patients from two countries, the UK and Germany. In both countries the study was embedded in a larger study: in the UK it was part of a qualitative study about patients' experiences with episodic breathlessness; in Germany, it was embedded in the baseline cross-sectional data collection of a longitudinal study assessing symptoms of COPD and cancer patients over time.
The questions were first developed in English and then translated into German by two experienced researchers and physicians. The questions were piloted with three healthy volunteers in each country evaluating the understanding and feasibility of the questions and images of application forms.
Participants and recruitment
We included adult patients suffering from breathlessness due to four different advanced diseases: (1) primary and secondary lung cancer (LC) at all stages and mesothelioma (UK and Germany), (2) COPD stage III or IV according to the GOLD (Global Initiative on Obstructive Lung Disease) classification (UK and Germany),18 (3) CHF stages II to IV NYHA (New York Heart Association) classification (UK only),19 and (4) MND (UK only). Patients did not need to be on opioids to participate in this study.
Exclusion criteria were a lack of capacity to give informant consent or to be interviewed, and a limited comprehension of the predominant language of each country.
In the UK, patients were recruited from two large teaching hospitals (inpatients and outpatients) in South London in 2010. In Germany, recruitment took place in two hospitals and two outpatient clinics in 2010.
Data collection
The procedure of the interview was identical in all centers. Eligible patients were recruited by their physician and written consent obtained before the interview. After a short introduction about the aim and the process of the study, the participant was asked to imagine that the different application forms contain the same ingredient with the same efficacy and same time of onset action. Photographs of each route of drug administration were shown on laminated cards in a fixed order and the interviewer briefly explained each application. The following questions were asked for each route:
(1) Is this route acceptable when you are severely breathless?
(2) Have you used this route before?
The experience was asked for all indications. Answer options were Yes, No, Possible, and I don't know for the first question and Yes or No for the second. The participants were asked (3) to rank their three favorite routes of administration and (4) to determine the least acceptable (worst) route. Patients' comments were noted by the interviewer. Demographic and medical information, ethnicity, education, functional status, characteristics of breathlessness, and use of oxygen were elicited from patient records after consent.
Analysis
Descriptive analysis included total numbers and percentages, mean, and standard deviation (SD), median, and range as applicable. Descriptive analysis (including descriptive fourfold tables) was used for comparison between countries and disease groups, and for analysis of potential influencing factors. As the study was not powered for differences, we did not intend to test the results for significance. The preferences for routes of application were ranked, giving three points to the first choice, two points to the second choice, and one point to the third choice. The numbers of each route of application were summed up.
Content analysis was used for the additional comments and quotes to describe the main comments.
Results
Participants
A total of 123 participants were recruited, with 119 participants providing data for analyses. Four participants had to be excluded, as they didn't complete the interview or they misunderstood the questions.
Sixty percent (71/119) of participants were male; mean age was 67.7 years (SD 9.9). Participants' characteristics, gender, age, Karnofsky, and breathlessness severity, were similar between countries. The UK sample was more ethnically diverse, and participants in Germany had lower levels of education (see Table 1).
Table 1.
Characteristics of Participants
| Total (n=119) n (%) | UK (n=48) n (%) | Germany (n=71) n (%) | |
|---|---|---|---|
| Age years, mean (SD) | 67.7 (9.9) | 68.1 (11.9) | 67.4 (8.3) |
| Range | 39.4–92.0 | 39.4–92.0 | 45.5–85.1 |
| Male | 71 (60) | 28 (58) | 43 (61) |
| Disease | |||
| Lung Cancer | |||
| - Primary | 10 (8) | 6 (13) | 4 (6) |
| - Secondary | 22 (18) | 1 (2) | 21 (30) |
| - Mesothelioma | 4 (3) | 4 (8) | - |
| COPD | 59 (50) | 13 (27) | 46 (65) |
| CHD | 15 (13) | 15 (31) | - |
| MND | 9 (8) | 9 (19) | - |
| Education | |||
| Higher education (high school, university) | 26 (22) | 16 (33) | 10 (14) |
| Lower education (primary, secondary school) | 93 (78) | 32 (67) | 61 (86) |
| Ethnicity | |||
| White | 108 (91) | 37 (77) | 71 (100) |
| Indian | 4 (3) | 4 (8) | - |
| Black African | 3 (3) | 3 (6) | - |
| Black Caribbean | 4 (3) | 4 (8) | - |
| Smoking | |||
| Never smoked | 22 (18) | 16 (33) | 6 (8) |
| Smoked but stopped | 77 (65) | 29 (60) | 48 (68) |
| Still smoking | 14 (12) | 3 (6) | 11 (15) |
| Pack years, mean (SD) | 47.1 (14.4) | 48.7 (20.5) | 46.7 (13.5) |
| Missing data | 6 (5) | - | 6 (8) |
| O2 supply | 40 (34) | 12 (25) | 28 (39) |
| Karnofsky (median, range) | 70 (30–90) | 60 (40–90) | 70 (30–90) |
| Breathlessness severity measured on a Borg Scale 0–10, mean (SD), *missing data | |||
| Average 24 h | 2.8 (1.9) *8 | 3.0 (1.3) *7 | 2.7 (2.2) *1 |
| Worst | 4.1 (2.8) *8 | 4.8 (2.3) *7 | 3.6 (3.0) *1 |
| Now | 1.5 (1.5) *7 | 1.4 (1.3) *6 | 1.5 (1.7) *1 |
| At rest | 1.2 (1.3) *9 | 1.2 (1.2) *8 | 1.2 (1.4) *1 |
| On exertion | 5.2 (2.4) *9 | 5.3 (2.1) *8 | 5.1 (2.5) *1 |
Values are numbers unless otherwise indicated.
CHD, Chronic Heart Disease; COPD, Chronic Obstructive Pulmonary Disease; MND, Motor Neuron Disease; SD, standard deviation.
Experience
Most participants had previous experience with oral and inhaled applications (see Table 2). In contrast, only few participants have previously used transmucosal lollipops or the buccal route. Different experiences between the two countries were reported for the sublingual and nasal route; participants from the UK had more experience with the sublingual route, whereas German participants used the nasal application form more often.
Table 2.
Experience and Acceptability with Six Drug Application Forms in the United Kingdom and Germany
| Route of administration | Response to question | Both countries (n=119) | United Kingdom (n=48) | Germany (n=71) | |
|---|---|---|---|---|---|
| Oral | Experience | 116 (97) | 48 (100) | 68 (96) | |
| Acceptability | Yes | 28 (24) | 14 (29) | 14 (20) | |
| No | 82 (69) | 28 (58) | 54 (76) | ||
| Possible | 6 (5) | 6 (13) | 0 (0) | ||
| I don't know | 3 (3) | 0 (0) | 3 (4) | ||
| Inhaled | Experience | 104 (87) | 42 (88) | 62 (87) | |
| Acceptability | Yes | 103 (87) | 37 (77) | 66 (93) | |
| No | 7 (6) | 5 (10) | 2 (3) | ||
| Possible | 5 (4) | 5 (10) | 0 (0) | ||
| I don't know | 4 (3) | 1 (2) | 3 (4) | ||
| Sublingual | Experience | 35 (30) | 21 (44) | 14 (20) | |
| Acceptability | Yes | 54 (45) | 34 (71) | 20 (28) | |
| No | 48 (40) | 7 (15) | 41 (58) | ||
| Possible | 7 (6) | 7 (15) | 0 (0) | ||
| I don't know | 10 (8) | 0 (0) | 10 (14) | ||
| Intranasal | Experience | 79 (66) | 26 (54) | 53 (75) | |
| Acceptability | Yes | 50 (42) | 24 (50) | 26 (37) | |
| No | 51 (43) | 19 (40) | 32 (45) | ||
| Possible | 4 (3) | 3 (6) | 1 (1) | ||
| I don't know | 14 (12) | 2 (4) | 12 (17) | ||
| Buccal | Experience | 10 (8) | 5 (10) | 5 (7) | |
| Acceptability | Yes | 34 (29) | 21 (44) | 13 (18) | |
| No | 61 (51) | 16 (33) | 45 (63) | ||
| Possible | 9 (8) | 9 (19) | 0 (0) | ||
| I don't know | 15 (13) | 2 (4) | 13 (18) | ||
| Lollipop | Experience | 2 (2) | 2 (4) | 0 (0) | |
| Acceptability | Yes | 43 (36) | 21 (44) | 22 (31) | |
| No | 48 (40) | 18 (38) | 30 (42) | ||
| Possible | 4 (3) | 4 (8) | 0 (0) | ||
| I don't know | 24 (20) | 5 (10) | 19 (27) | ||
Values are given as absolute numbers and proportions in brackets (%).
Acceptability
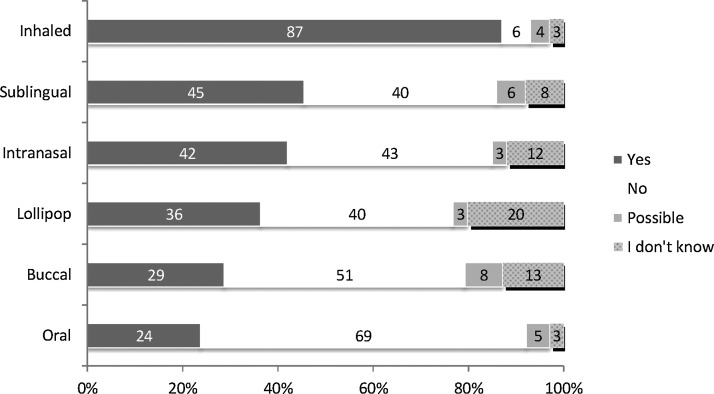
In total, 103/119 participants (87%) would accept the inhaled application form for a situation of severe breathlessness. In contrast, only 28/119 participants (24%) would accept use of the oral route of administration. Sublingual (54/119; 45%) and intranasal (50/119; 42%) application were judged about equal, whereas the transmucosal lollipop (43/119; 36%) and the buccal route (34/119; 29%) were less acceptable (see Figure 1).
FIG. 1.
Acceptability of six different drug application forms (all participants).
Acceptability of oral and inhaled application was similar in both countries (see Table 2). In the UK, buccal and sublingual routes were more favored compared to Germany. There were similar judgments regarding the nasal application and the lollipop. In general, UK participants complied more often with an application form, whereas German patients stated more often that they didn't know which one was acceptable.
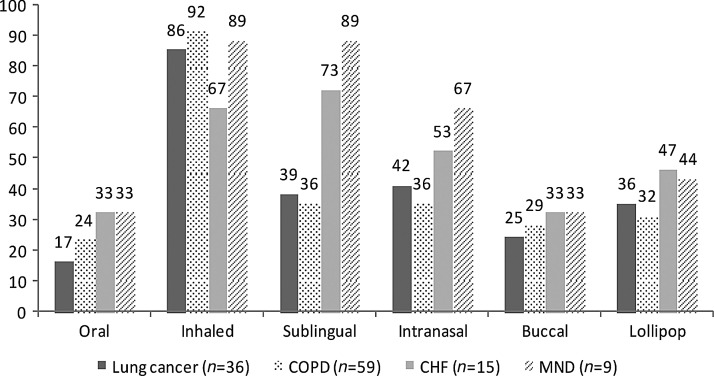
Comparing the disease groups, there was a higher acceptability for the sublingual and intranasal route by CHF and MND patients (see Figure 2). The CHF group showed less acceptance of the otherwise well-accepted inhaled application.
FIG. 2.
Acceptability (Yes) of participants divided into disease groups. Numbers are (%). COPD, chronic obstructive lung disease; CHF, chronic heart failure; MND, motor neuron disease.
Previous experience of using the application form seems not to be related to acceptability (see Table 2). For example, the oral route (nearly known by all participants (97%) had low acceptability (24%), but transmucosal (lollipop) application (experienced by only two participants) was acceptable for 36% of participants.
Preferences
Asked about their preferences for the six routes of application, 81/119 participants (68%) chose the inhaled route as first choice, 15/119 (13%) the sublingual route, 11/119 (9%) the oral route, and 9/119 (8%) the nasal route.
The inhaled application was ranked highest both in the UK and in Germany (see Table 3). Preference for route differed between sublingual, nasal, and lollipop.
Table 3.
Ranking of Preferences
| Rank | Both countries | United Kingdom | Germany | |||
|---|---|---|---|---|---|---|
| 1 | Inhaled | 278a | Inhaled | 93 | Inhaled | 185 |
| 2 | Intranasal | 90 | Sublingual | 69 | Intranasal | 46 |
| 3 | Sublingual | 88 | Intranasal | 44 | Lollipop | 38 |
| 4 | Oral | 60 | Oral | 31 | Oral | 29 |
| 5 | Lollipop | 56 | Lollipop | 18 | Sublingual | 19 |
| 6 | Buccal | 17 | Buccal | 15 | Buccal | 2 |
Sums calculated by adding three points to the first choice, two points to the second choice, and one point to the third choice.
Overall, 38/119 participants (32%) stated that the least acceptable application form would be the oral route (see Table 4).
Table 4.
Answers to Question about Least Accepted Application Form
| Total (n=119) | United Kingdom (n=48) | Germany (n=71) | |
|---|---|---|---|
| Oral | 38 (32)a | 13 (27) | 25 (35) |
| Intranasal | 28 (24) | 15 (31) | 13 (18) |
| Buccal | 20 (17) | 10 (21) | 10 (14) |
| Lollipop | 14 (12) | 6 (13) | 8 (11) |
| Sublingual | 3 (3) | 2 (4) | 1 (1) |
| Inhaled | 2 (2) | 2 (4) | 0 (0) |
| Missing data | 14 (12) | 0 (0) | 14 (20) |
Values are given as absolute numbers and (%). Ordered by importance of total.
Patients' comments
Patients' comments related to the administration of the drug, effects on breathing, previous experience with the route of application, adverse effects, comfort, and speed of action of the drug (see Table 5). Suffocation and choking were often mentioned as problems for oral application, and dental prosthesis for the buccal route. Participants often considered the potential ease of administration for their carers. The sublingual, lollipop, and intranasal routes were deemed to be more appropriate.
Table 5.
Comments of Patients about Various Routes of Application
| Oral | Inhaled | Sublingual | Intranasal | Buccal | Transmucosal | |
|---|---|---|---|---|---|---|
| Administration | “Hard without water” | “Difficult to get it right, especially for older people” | “Mechanically easy” | “It is difficult to use if you have your oxygen on” | “More difficult than the sublingual tablet” | “Easy handling for yourself” |
| Effect on breathing | “Swallow is difficult when you're breathless” | “Difficult to hold your breath while you are breathless” | “Doesn't affect the breathing” | “It affects the breathing” You still have your mouth to gape for air” |
- | “You can breathe parallel” |
| Previous experience | “Don't like tablets” | “Very good experiences” | “Good experiences with the ‘heartspray’ under the tongue” | - | - | - |
| Adverse effects | “Choking” “Vomiting” “Fear of suffocation” |
- | “Fear of ulcers” Falls out of the mouth” |
“Affects the nasal mucosa” | “Falls out” | “Vomit” “Collects germs” |
| Comfort/ discomfort | “Socially discreet” | - | “Easy handling for carers” | “Easy for relatives and carers” | “Difficult for patients with dental prosthesis” | “Much easier for carers” “Calming just because of the procedure” |
| Speed of action | “Take too long” | “It acts quickly” | “Have to dissolve until it acts” | - | “Dissolving takes too long” | - |
Participants mentioned several alternative routes of application for managing acute breathlessness: subcutaneous injection, liquid drug, suppository, gel-like fluid that can be licked from the finger, or sublingual spray under the tongue.
Discussion
This descriptive study aimed to determine acceptability and preferences among patients for the potential use of different routes of opioid application in a situation of acute breathlessness in advanced disease. The inhaled route was most accepted by patients, followed by the sublingual and intranasal. Drugs applied orally, although known to nearly all participants, were the least accepted route of application. We found similarities and differences between the UK and Germany. Except for the inhaled application, the tested application routes were not acceptable for a considerable number of German participants, while their UK counterparts judged all applications but the oral one to be acceptable.
There could be various reasons why the inhaled route is most preferred by patients: previous experiences with inhalers, the belief that the drug is applied directly at the site of action, or the inability to swallow something when breathless. Inhalers are widely used in the treatment of COPD, and therefore it is not surprising that COPD patients would prefer this route for other drugs as well. However, lung cancer and MND patients rated this route similarly high. Dysphagia is a well-known complication in MND, making any mode of application that circumvents swallowing more attractive. These results differ slightly from preferences of cancer patients with BTP, where in one study, 75% found the inhaled route acceptable for severe pain, but only 39% had prior experience17 and in a second study only 46% found this an acceptable route.9 Comparison of these results with our study is limited, because not only were two different symptoms compared but also different disease groups. A small group of participants was not in favor of the inhaled application form, mainly because of fearing the difficult handling, in particular for older and severely impaired patients.
For the oral application, many participants mentioned a big fear of choking and suffocation, and being unable to swallow a tablet when breathless. In contrast, this route is well accepted by cancer patients for treatment of BTP where 97% of patients would accept oral drug delivery.17
The sublingual route was accepted by nearly half of all participants, with a clear difference regarding acceptability and previous experience between the UK and Germany. Taking a tablet under the tongue seems to not affect the breathing in the imagination of participants, and is easy to apply for carers. Even participants who had never used the sublingual application imagined the advantages of this route. However, some participants feared that the sublingual tablets might not dissolve or might fall out of the mouth. The latter view is also suspected regarding the buccal application. Little is known about the acceptability of this application for the management of BTP, but there is some evidence that buccal fentanyl shows efficiency in the management of BTP9 and that this application of fentanyl is more often preferred by participants than is nasal fentanyl.20
As with pain patients,9 participants with breathlessness were divided about the intranasal application, with strong votes for both preferring and for rejecting it. Participants were concerned about irritation of the nasal mucosa and effects on breathing, especially when receiving oxygen. Although acceptability was similar in both countries, German participants had more previous experience. Easy handling of intranasal drug delivery was seen as an advantage by patients and for their carers.
Participants imagined alternative routes of application that we did not include in our interview. These suggestions occurred from spontaneous imagination during the interview or from previous experience. It shows that patients are interested in improving drug delivery during acute breathlessness and to make it more comfortable.
The divergence between the UK and Germany could be based on cultural differences: The diversity of ethnicity is higher for UK participants than for the German participants. Nevertheless, our results are similar to pain patients asked about their preferences for drug application with even higher disparity between the UK and Germany regarding the acceptability of the intranasal and inhaled route.9 To explore cultural differences further, a comparison of more countries is needed.
Strengths and limitations
One of the strengths of this study is that we included patients with several conditions involving breathlessness during the course of their disease, not only patients suffering from cancer or COPD.
We were able to compare data from two countries. Resource constraints prevent us from running the survey in more than two countries.
One limitation to this study is that we asked patients to imagine severe breathlessness and acceptability of the routes but did not test them in a real situation of severe breathlessness. Imagination and understanding of the situation might vary between patients, but we felt it is neither feasible nor ethically justifiable to test acceptability and preference about routes of application during acute breathlessness. So we offered patients explanations and standardized photographs to focus their imagination and provide both an oral and a visual aid.
A second limitation is the bias by patients prejudice of each application form regarding efficacy and time to onset of dyspnea relief. We aim to reduce the bias by reassuring the patients at the beginning and during the interview that all application forms would contain the same drug with identical efficacy and time of onset.
A third limitation is that we asked participants to rank their preference for routes of application they might not have used before. Requiring previous experience with all administration forms would limit the inclusion of participants to the study. Using photographs and demonstration of forms of administration seemed a good way to provide the necessary information.
Implication for clinical practice and research
Patients suffering from breathlessness clearly show different preferences. Therefore, clinicians should not make any assumptions about the preferred route of application but should incorporate individual patient choice for drug application in decision making about treatment of breathlessness. Clinicians also need to be familiar with the different routes of application and the related dosages to choose the best suitable form for the patient.
The study supports the approach of including questions on patients' preference in drug trials, as most BTP studies did.16 Future interventional studies on dyspnea should include these questions. Inhaled spray or a sublingual spray might be additional routes of application to develop to meet patients' preferences best.
Although a variety of opioid applications are available in analgesia and have great potential for breathless patients, only the oral and nebulized routes have been tested in RCTs. Intervention studies evaluating other routes of application are needed to demonstrate the safety and effectiveness of these forms.
Appendix. Photographs of the Size Application Form
Acknowledgments
We thank Dr. Werner Jackstädt Foundation, Wuppertal, Germany, and Cicely Saunders International, London, UK, whose funding made this research possible. Special thanks to all patients who participated in this study. We would like to thank Birgit Kannenberg-Otremba, Verena Gerdes, Nicole Sowinsky, and Christine Scheve for their supportive recruitment of patients for this study.
The UK study obtained ethics approval from the Joint UCL/UCLH Committees of the Ethics of Human Research (09/H0715/81) and was registered on the National Institute for Health Research Clinical Research Network Portfolio (NIHR CRN Study ID 7859) and on www.clinicaltrials.gov (NCT01138358). The German study received ethics approval from the Board of Physicians of Lower Saxony (Bo/20/2009) and was registered on the German Clinical Trials Register (DRKS00000312).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Booth S. Silvester S. Todd C. Breathlessness in cancer and chronic obstructive pulmonary disease: Using a qualitative approach to describe the experience of patients and carers. Palliat Support Care. 2003;1(4):337–344. doi: 10.1017/s1478951503030499. [DOI] [PubMed] [Google Scholar]
- 2.Solano JP. Gomes B. Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. J Pain Symptom Manage. 2006;31(1):58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Parshall MB. Schwartzstein RM. Adams L. Banzett RB. Manning HL. Bourbeau J, et al. An Official American Thoracic Society Statement: Update on the Mechanisms, Assessment, and Management of Dyspnea. Am J Respir Crit Care Med. 2012;185(4):435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edmonds P. Karlsen S. Khan S. Addington-Hall J. A comparison of the palliative care needs of patients dying from chronic respiratory diseases and lung cancer. Palliat Med. 2001;15:287–295. doi: 10.1191/026921601678320278. [DOI] [PubMed] [Google Scholar]
- 5.Tripodoro VA. De Vito EL. Management of dyspnea in advanced motor neuron diseases. Curr Opin Support Palliat Care. 2008;2(3):173–179. doi: 10.1097/SPC.0b013e32830c9049. [DOI] [PubMed] [Google Scholar]
- 6.Reddy SK. Parsons HA. Elsayem A. Palmer JL. Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med. 2009;12(1):29–36. doi: 10.1089/jpm.2008.0158. [DOI] [PubMed] [Google Scholar]
- 7.Zeppetella G. O'Doherty CA. Collins S. Prevalence and characteristics of breakthrough pain in cancer patients admitted to a hospice. J Pain Symptom Manage. 2000;20(2):87–92. doi: 10.1016/s0885-3924(00)00161-5. [DOI] [PubMed] [Google Scholar]
- 8.Portenoy RK. Hagen NA. Breakthrough pain: Definition, prevalence and characteristics. Pain. 1990;41(3):273–281. doi: 10.1016/0304-3959(90)90004-W. [DOI] [PubMed] [Google Scholar]
- 9.Davies A. Zeppetella G. Andersen S. Damkier A. Vejlgaard T. Nauck F, et al. Multi-centre European study of breakthrough cancer pain: Pain characteristics and patient perceptions of current and potential management strategies. Eur J Pain. 2011;15(7):756–763. doi: 10.1016/j.ejpain.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Booth S. Moosavi SH. Higginson IJ. The etiology and management of intractable breathlessness in patients with advanced cancer: A systematic review of pharmacological therapy. Nat Clin Pract Oncol. 2008;5(2):90–100. doi: 10.1038/ncponc1034. [DOI] [PubMed] [Google Scholar]
- 11.Jennings AL. Davies AN. Higgins JP. Broadley K. Opioids for the palliation of breathlessness in terminal illness. Cochrane Database Syst Rev. 2001;(4) doi: 10.1002/14651858.CD002066. CD002066. [DOI] [PubMed] [Google Scholar]
- 12.Abernethy AP. Currow DC. Frith P. Fazekas BS. McHugh A. Bui C. Randomised, double blind, placebo controlled crossover trial of sustained release morphine for the management of refractory dyspnoea. BMJ. 2003;327(7414):523–528. doi: 10.1136/bmj.327.7414.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyne PJ. Viswanathan R. Smith TJ. Nebulized fentanyl citrate improves patients' perception of breathing, respiratory rate, and oxygen saturation in dyspnea. J Pain Symptom Manage. 2002;23(2):157–160. doi: 10.1016/s0885-3924(01)00391-8. [DOI] [PubMed] [Google Scholar]
- 14.Sitte T. Bausewein C. Intranasal fentanyl for episodic breathlessness. J Pain Symptom Manage. 2008;36(6):e3–6. doi: 10.1016/j.jpainsymman.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Jensen D. Alsuhail A. Viola R. Dudgeon DJ. Webb KA. O'Donnell DE. Inhaled fentanyl citrate improves exercise endurance during high-intensity constant work rate cycle exercise in chronic obstructive pulmonary disease. J Pain Symptom Manage. 2012;43(4):706–719. doi: 10.1016/j.jpainsymman.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Davis MP. Fentanyl for breakthrough pain: A systematic review. Expert Rev Neurother. 2011;11(8):1197–1216. doi: 10.1586/ern.11.63. [DOI] [PubMed] [Google Scholar]
- 17.Walker G. Wilcock A. Manderson C. Weller R. Crosby V. The acceptability of different routes of administration of analgesia for breakthrough pain. Palliat Med. 2003;17(2):219–221. doi: 10.1191/0269216303pm755oa. [DOI] [PubMed] [Google Scholar]
- 18.Rabe KF. Hurd S. Anzueto A. Barnes PJ. Buist SA. Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 19.Heart Failure Society of America. New York Heart Association classification. 2002. www.abouthf.org/questions_stages.htm. [Feb 6;2012 ]. www.abouthf.org/questions_stages.htm
- 20.Portenoy RK. Taylor D. Messina J. Tremmel L. A randomized, placebo-controlled study of fentanyl buccal tablet for breakthrough pain in opioid-treated patients with cancer. Clin J Pain. 2006;22(9):805–811. doi: 10.1097/01.ajp.0000210932.27945.4a. [DOI] [PubMed] [Google Scholar]