Abstract
Objective
To assess the construct validity of a milk consumption Stages of Change (SOC) algorithm among adolescent survivors of childhood cancer ages 11 – 21 years (n = 75).
Methods
Baseline data from a randomized controlled trial designed to evaluate a health behavior intervention were analyzed. Assessments included a milk consumption SOC algorithm and hypothesized theoretical and behavioral predictors of SOC.
Results
Compared with survivors who expressed no readiness to change, those expressing readiness to change behavior for both 2 or 4 daily servings of milk reported more frequent milk consumption (p <; .001), greater dietary calcium intake (p = .006), and were more likely to meet age-specific recommendations for daily calcium intake (p = .01).
Conclusion and Implications
Results provide support for the construct validity of the milk consumption SOC algorithm relative to behavioral criteria. Research is needed to further examine algorithm validity with respect to theoretical predictors of SOC.
Keywords: cancer, pediatrics, survivors, milk consumption, bone health, stages of change
INTRODUCTION
Bone health problems are a common late effect of cancer treatment among childhood cancer survivors, including suboptimal bone density and clinical signs of osteopenia.1 These problems are linked to stunted growth and increased risk for osteoporosis and fractures in adulthood.1 Risk for bone health problems may be exacerbated by the fact that few survivors meet recommendations for bone health behaviors, such as consuming recommended levels of calcium daily.2
Although clinical signs of bone health problems do not typically appear until adulthood,1 interventions encouraging bone health-promoting behaviors among young cancer survivors may help prevent or delay onset of bone health morbidities. A comprehensive approach to promoting bone health among young survivors includes consuming a balanced diet and engaging in healthful physical activity.1 Increasing calcium and vitamin D intake through diet and supplementation are effective methods of improving bone density3 and may reduce risk for fracture in high-risk groups.4,5 For childhood cancer survivors, follow-up care guidelines encourage meeting age-specific recommended levels of calcium for optimal bone health.1 Milk is a leading source of dietary calcium,6,7 and interventions targeting milk consumption may be one important element of comprehensive approaches to reduce long-term risk for bone health problems in this vulnerable population.1,8
Recent data support the efficacy of a bone health behavior intervention for young cancer survivors, including milk consumption.9 However, the impact could be enhanced by tailoring content to address survivors’ individual needs for behavior change.10 The Transtheoretical Model (TTM) provides a framework for tailoring interventions to an individual’s readiness to change, positing that health behavior change occurs via five stages of change (SOC) from Precontemplation (no intention to change behavior within next 6 months) to Maintenance (performance of the recommended behavior for ≥ 6 months).11 Transition between stages is dynamic and includes progression towards maintenance and relapse to earlier stages.11
The SOC approach has been applied to the study of dietary behaviors among young people, including milk consumption.12-15 SOC is typically assessed using a staging algorithm, which characterizes individuals based on current behavioral practices, duration of practices, and intention to take action to change practices.11 It is critical to first establish the construct validity of SOC algorithms designed to characterize SOC for the recommended health behavior. However, no such milk consumption algorithm has been validated among adolescent cancer survivors. We examined the construct validity of a SOC algorithm for consuming 2 and 4 daily servings of milk among adolescent survivors of childhood cancer. The algorithm is designed to provide a flexible tool to assess milk consumption and can be used with other sources of dietary information to tailor bone health interventions among young cancer survivors.
METHODS
Procedures
The Survivor Health and Resilience Education (SHARE) Program was a randomized controlled trial to test the efficacy of a multiple health behavior intervention among adolescent survivors of childhood cancer. Study methods and bone health behavior outcomes were reported previously.9 The study included adolescents ages 11-21 years who were treated for cancer, ≥ 1 year post treatment and ≥ 1 year cancer-free. Participants were recruited from 2 local pediatric cancer centers; the Georgetown University institutional review board approved all procedures. Following participant assent and parental consent, participants completed a baseline assessment via 2 consecutive 30-minute telephone interviews.
Measures
Demographic characteristics included age, gender, race, household composition, and school performance (mostly A’s or B’s or other grades). Clinical characteristics included diagnosis of Leukemia (the primary cancer diagnosis) or another form of cancer, and years since diagnosis.
Theoretical predictors were derived from the TTM. Bone health knowledge was assessed as a proxy for consciousness-raising, the process of change that is critical to move individuals from Precontemplation to Contemplation by providing new information supporting behavior change.11 Using 6 multiple choice items adapted from the National Bone Health Campaign for children16 and prior research,17,18 knowledge was operationalized as a continuous variable indicating the number of items answered correctly (range 0 -6). Self-efficacy is an individual’s confidence to engage in a behavior and is central to TTM.11 Calcium consumption self-efficacy was assessed using 11 Likert-type items adapted from prior research.19 Responses were summed to create a total score with higher values reflecting greater self-efficacy (range 0 – 55; Cronbach’s α = 0.86).
Milk consumption behaviors were assessed using 2 items adapted from the National Bone Health Campaign.16 Milk consumption frequency was measured by asking “How often would you say you drink milk?” Responses ranged from (1) ‘never’ to (4) ‘always’ and a continuous variable was created for analyses. The second item asked “During a normal day, do you drink at least 4 glasses of milk?”
Calcium intake was measured with the 5-Step Multiple Pass 24-hour recall method.20 This method asked participants to describe everything that s/he ate/drank for a 24-hour period, when and where foods were eaten, and details of each food, and reviews information to confirm accuracy. Calcium intake in milligrams [mg] was derived using NutritionistPro, a third-party software program that converts dietary data into nutritional information. We analyzed a continuous variable for overall calcium intake and a dichotomous variable indicating whether participants met age-specific dietary calcium guidelines.21
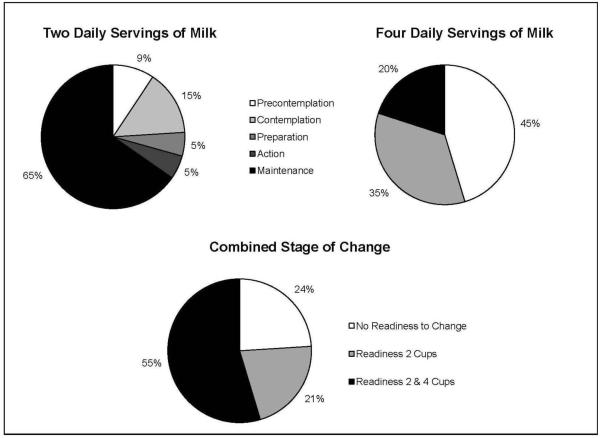
An algorithm originally developed among adult women was used to assess SOC for consuming 2 and 4 daily servings of milk (see supplemental file).22 The algorithm assessed criteria for each of the TTM stages,11 defining a standard serving of milk as one cup.23 Questions assessed participants’ current behaviors and readiness to consume 2 and 4 daily servings of milk. We created a 3-level variable with the following categories: (1) No readiness to change: Precontemplation or Contemplation for 2 cups and Precontemplation for 4 cups; (2) Readiness 2 cups: Preparation, Action, or Maintenance for 2 cups and Precontemplation for 4 cups; (3) Readiness 2 and 4 cups: Preparation, Action, or Maintenance for 2 cups and Contemplation, Preparation, Action, or Maintenance for 4 cups. These categories were created based on the distribution of participants across SOC (Figure 1) and to reflect levels of readiness to change for both behaviors. For example, participants in Precontemplation or Contemplation for 2 cups and Precontemplation for 4 cups expressed little or no readiness to change either behavior. In comparison, those in Preparation, Action, or Maintenance for 2 cups and Contemplation or beyond for 4 cups expressed some readiness to change both behaviors, or were achieving behavioral goals.
Figure 1.
Milk consumption stages of change among adolescent survivors of childhood cancer (n = 75)
Analyses
Construct validity is established by examining associations between the focal construct and related predictors.24 To examine the algorithm’s construct validity, we used bivariate analyses to assess relationships among SOC and clinical and demographic characteristics, and behavioral and theoretical predictors. None of the clinical and demographic characteristics were associated with SOC, therefore our final analyses were unadjusted bivariate F-tests and χ2 tests of associations between SOC and behavioral and theoretical predictors of interest.
RESULTS
Participant Characteristics
Table 1 displays participant characteristics, and Figure 1 illustrates survivors’ SOC for two and four daily servings of milk. For the combined outcome, 24% of survivors (n = 18) expressed no readiness to change (Figure 1). Sixteen (21%) expressed readiness to change behavior for 2 daily servings of milk, and 55% (n = 41) expressed readiness to change behavior for both 2 and 4 daily servings of milk.
Table 1.
Sample characteristics and associations with milk consumption stages of change
| Sample | Combined Stage of Change | ||||
|---|---|---|---|---|---|
| (n = 75) | No Readiness To Change (n = 18) |
Readiness 2 Cups (n = 16) |
Readiness 2 & 4 Cups (n = 41) |
P- value |
|
| Demographics | |||||
| Age (years; Mean [SD]) | 14.2 (2.4) | 14.9 (2.9) | 13.3 (2.4) | 14.2 (2.2) | 0.16 |
| Race | 0.17 | ||||
| White | 75%, 56 | 61%, 11 | 69%, 11 | 83%, 34 | |
| Non-White | 25%, 19 | 39%, 7 | 31%, 5 | 17%, 7 | |
| Gender | 0.13 | ||||
| Male | 48%, 36 | 50%, 9 | 69%, 11 | 39%, 16 | |
| Female | 52%, 39 | 50%, 9 | 31%, 5 | 61%, 25 | |
| Household Composition | 0.75 | ||||
| 2 Parent Home | 83%, 62 | 78%, 14 | 88%, 14 | 83%, 34 | |
| Other | 17%, 13 | 22%, 4 | 12%, 2 | 17%, 7 | |
| School Achievement | 0.52 | ||||
| Mostly A’s/B’s | 76%, 57 | 67%, 12 | 75%, 12 | 81%, 33 | |
| Other | 24%, 18 | 33%, 6 | 25%, 4 | 19%, 8 | |
| Clinical Characteristics | |||||
| Primary Diagnosis | 0.95 | ||||
| Leukemia | 52%, 39 | 50%, 9 | 50%, 8 | 46%, 19 | |
| Other Type | 48%, 36 | 50%, 9 | 50%, 8 | 54%, 22 | |
| Years Since Diagnosis (Mean [SD]) |
9.0 (3.4) | 8.2 (3.5) | 9.4 (3.3) | 9.1 (3.4) | 0.57 |
| Theoretical Predictors | |||||
| Bone Health Knowledge (Mean, [SD]) |
2.9 (0.9) | 2.1 (1.2) | 2.3 (1.1) | 2.3 (1.1) | 0.72 |
| Calcium Self-Efficacy (Mean, [SD]) |
38.9 (7.9) | 36.6 (8.0) | 39.1 (6.5) | 39.7 (8.2) | 0.37 |
| Behavioral Predictors | |||||
| Milk Consumption Frequency (Mean, [SD])a |
2.1 (0.89) | 2.4 (0.85)1 | 2.7 (0.95)1 | 3.3 (0.74)2 | <.001 |
| Normal Day ≥ 4 Glasses of Milk |
28%, 21 | 6%, 1 | 19%, 3 | 42%, 17 | 0.01 |
| Meets Recommended Calcium Intakeb |
27%, 20 | 6%, 1 | 19%, 3 | 39%, 16 | 0.02 |
| Calcium Intake (mg) (Mean, [SD])a |
991.0 (509.0) |
702.5 (346.4)1 |
945.4 (502.1)1,2 |
1135.4 (523.6)2 |
0.006 |
Note. Unless noted, cells display % and n. SD = Standard Deviation.
Means with different superscript numbers differ at p < 0.05 after adjustment for multiple comparisons.
P-value from Fisher’s exact χ2
Predictors of Stage of Change
Compared with survivors who expressed no readiness to change and those who only expressed readiness to change for 2 daily servings of milk, those who reported readiness to change for both 2 and 4 servings reported significantly more frequent milk consumption (p < 0.001, Table 1). Similarly, survivors who expressed readiness to change for 2 and 4 servings were the most likely to report consuming ≥ 4 servings of milk on a normal day (n = 17, 42%, p = 0.01) and to meet dietary calcium recommendations (n = 16, 39%, p = 0.01; Table 1). Finally, survivors who reported readiness to change for both 2 and 4 servings of milk consumed significantly greater milligrams of dietary calcium (Mean = 1135.4 mg, SD = 523.6 mg), compared with those who expressed no readiness to change milk consumption behavior (Mean = 702.5 mg, SD = 346.4 mg, p = 0.006).
DISCUSSION
Adolescent survivors of childhood cancer are at an increased risk for bone-related morbidity,1 but evidence supporting interventions to promote bone health behaviors for this population, such as milk consumption, remains limited.9 The TTM has been widely used to inform behavioral interventions targeting dietary behaviors25 and the impact of interventions to promote milk consumption among young survivors could be enhanced by tailoring content to individual survivors’ SOC.10 Our findings provide preliminary support for the construct validity of a milk consumption SOC algorithm based on behavioral criteria, and highlight important topics for research.
Adolescent survivors who expressed readiness to change for consuming 2 and 4 daily servings of milk reported more frequent milk consumption, were more likely to drink 4 servings of milk a day, and consumed more dietary calcium. These findings are consistent with prior studies that sought to validate the algorithm for use in other populations, such as college-age young adults.14,15 We used the 24-hour recall method to measure dietary calcium consumption which is a significant methodological improvement over prior studies using less robust measures of calcium intake.14,15,22 The algorithm also incorporates several validity-enhancing strategies, including using simple behavioral goals, applying a clear SOC classification scheme, and specifying objective behaviors to avoid misclassification.26
We did not observe associations between SOC and knowledge or self-efficacy. This finding may be because our measures for these constructs were not based directly on the TTM, but were developed to capture broader bone health constructs.27 We also evaluated validity based on a combined SOC outcome for 2 and 4 daily servings of milk, rather than examining these behaviors separately. This may have affected our ability to detect differences based on theoretical constructs. It is critical that future studies demonstrate the validity of the algorithm relative to constructs derived more specifically from TTM, such as processes of change and decisional balance.26 Future studies can build from this work by examining milk consumption behaviors independently.
Our findings highlight other important considerations for future studies seeking to develop bone health behavior interventions targeting young cancer survivors. While milk is a primary source of dietary calcium, survivors may consume a variety of calcium-rich foods to improve bone health (e.g., leafy green vegetables). Young survivors in later SOC were more likely to meet recommendations for daily calcium consumption, but just over one quarter met recommendations. A comprehensive approach to bone health interventions that motivates young survivors to consume a variety of bone health-promoting foods and engage in healthful physical activity appears needed to reduce survivors’ risk for bone health morbidity.9 Future studies in this area may also warrant comparing self-report behavioral assessments to objective measures of bone health (e.g., bone density scans). That our assessment did not differentiate between types of milk (e.g., non-fat milk, whole milk) is an important consideration to address in future studies, as some commonly-consumed forms of milk may have negative health effects (e.g., milk flavored with sugar).7
Our findings should be interpreted in light of study limitations. The small sample restricted our ability to analyze construct validity separately for both milk consumption behaviors and limits generalizability of findings. The broad age range of survivors may have spanned distinct developmental periods, which could influence our findings. We relied on self-report assessments, some of which were not developed based on the TTM or were brief and adapted from prior work (e.g., milk consumption frequency).
Implications for Research and Practice
Despite study limitations, our findings provide preliminary support for the validity of the milk consumption SOC algorithm among adolescent survivors of childhood cancer relative to behavioral criteria. Additional research is needed to evaluate the construct validity of the algorithm relative to TTM constructs (e.g., processes of change) among larger, more diverse samples. Interventions that take a broad approach by motivating young survivors to consume milk and a variety of other healthful foods and engage in other bone health-promoting behaviors may be ideal to reduce the risk for bone health morbidity in this vulnerable population and could be informed using this SOC algorithm.
Supplementary Material
Acknowledgements
This research was supported by grants from the American Cancer Society, the Lance Armstrong Foundation, and the National Cancer Institute (CA091831) to Kenneth P. Tercyak, PhD. The project was also supported in part by Award Number P30CA051008 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Milk consumption SOC algorithm
References
- 1.Wasilewski-Masker K, Kaste SC, et al. Bone mineral density deficits in survivors of childhood cancer: long-term follow-up guidelines and review of the literature. Pediatrics. 2008;121:e705–e713. doi: 10.1542/peds.2007-1396. [DOI] [PubMed] [Google Scholar]
- 2.Demark-Wahnefried W, Aziz NM, et al. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huncharek M, Muscat J, Kupelnick B. Impact of dairy products and dietary calcium on bone-mineral content in children: results of a meta-analysis. Bone. 2008;43:312–321. doi: 10.1016/j.bone.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Rizzoli R, Bianchi ML, Garabedian M, et al. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Tang BM, Eslick GD, Nowson C, et al. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370:657–666. doi: 10.1016/S0140-6736(07)61342-7. [DOI] [PubMed] [Google Scholar]
- 6.Black RE, Williams SM, Jones IE, Goulding A. Children who avoid drinking cow milk have low dietary calcium intakes and poor bone health. Am J Clin Nutr. 2002;76:675–680. doi: 10.1093/ajcn/76.3.675. [DOI] [PubMed] [Google Scholar]
- 7.Rafferty K, Watson P, Lappe JM. The selection and prevalence of natural and fortified calcium food sources in the diets of adolescent girls. J Nutr Educ Behav. 2011;43:96–102. doi: 10.1016/j.jneb.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKean H, Looker S, Hartmann LC, et al. Are cancer survivors/patients knowledgeable about osteoporosis? Results from a survey of 285 chemotherapy-treated cancer patients and their companions. J Nutr Educ Behav. 2008;40:144–148. doi: 10.1016/j.jneb.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Mays D, Black JD, Mosher RB, et al. Efficacy of the Survivor Health and Resilience Education (SHARE) Program to Improve Bone Health Behaviors Among Adolescent Survivors of Childhood Cancer. Ann Behav Med. 2011;42:91–98. doi: 10.1007/s12160-011-9261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull. 2007;133:673–693. doi: 10.1037/0033-2909.133.4.673. [DOI] [PubMed] [Google Scholar]
- 11.Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. 1997;12:38–48. doi: 10.4278/0890-1171-12.1.38. [DOI] [PubMed] [Google Scholar]
- 12.Buchanan H, Coulson NS. Consumption of carbonated drinks in adolescents: a transtheoretical analysis. Child Care Health Dev. 2007;33:441–447. doi: 10.1111/j.1365-2214.2006.00691.x. [DOI] [PubMed] [Google Scholar]
- 13.Di Noia J, Prochaska JO. Mediating variables in a transtheoretical model dietary intervention program. Health Educ Behav. 2010;37:753–762. doi: 10.1177/1090198109334897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snelling AM, Adams TB, Korba C, Tucker L. Stages of change algorithm for calcium intake by male college students. J Am Diet Assoc. 2006;106:904–907. doi: 10.1016/j.jada.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Tucker LJ, Snelling AM, Adams TB. Development and validation of a stages of change algorithm for calcium intake for college female students. J Am Coll Nutr. 2002;21:530–535. doi: 10.1080/07315724.2002.10719251. [DOI] [PubMed] [Google Scholar]
- 16.U.S. Department of Health and Human Services [Accessed August 1, 2011];Best Bones Forever! Quizzes. www.bestbonesforever.gov/fun/quizzes.cfm.
- 17.Martin JT, Coviak CP, Gendler P, et al. Female adolescents’ knowledge of bone health promotion behaviors and osteoporosis risk factors. Orthop Nurs. 2004;23:235–244. doi: 10.1097/00006416-200407000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Rydell SA, French SA, Fulkerson JA, et al. Use of a Web-based component of a nutrition and physical activity behavioral intervention with Girl Scouts. J Am Diet Assoc. 2005;105:1447–1450. doi: 10.1016/j.jada.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 19.Horan ML, Kim KK, Gendler P, et al. Development and evaluation of the Osteoporosis Self-Efficacy Scale. Res Nurs Health. 1998;21:395–403. doi: 10.1002/(sici)1098-240x(199810)21:5<395::aid-nur3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the U.S. Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77:1171–1178. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- 21.Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011;14:938–939. doi: 10.1017/S1368980011000565. [DOI] [PubMed] [Google Scholar]
- 22.Gulliver P, Horwath C. Women’s readiness to follow milk product consumption recommendations: design and evaluation of a ‘stage of change’ algorithm. J Hum Nutr Diet. 2001;14:277–286. doi: 10.1046/j.1365-277x.2001.00295.x. [DOI] [PubMed] [Google Scholar]
- 23.Gebhardt SE, Thomas RG. Nutritive Value of Foods. Vol. 72. USDA, Home and Garden Bulletin; [Accessed August 1, 2011]. 2002. www.nal.usda.gov/fnic/foodcomp/Data/HG72/hg72_2002.pdf. [Google Scholar]
- 24.DiIorio CK. Measurement in Health Behavior: Methods for Research and Evaluation. Jossey-Bass Publishers; San Francisco, CA: 2005. [Google Scholar]
- 25.Spencer L, Wharton C, Moyle S, Adams T. The transtheoretical model as applied to dietary behaviour and outcomes. Nutr Res Rev. 2007;20:46–73. doi: 10.1017/S0954422407747881. [DOI] [PubMed] [Google Scholar]
- 26.Horwath CC. Applying the transtheoretical model to eating behaviour change: challenges and opportunities. Nutr Res Rev. 1999;12:281–317. doi: 10.1079/095442299108728965. [DOI] [PubMed] [Google Scholar]
- 27.Schmiege SJ, Aiken LS, Sander JL, Gerend MA. Osteoporosis prevention among young women: psychosocial models of calcium consumption and weight-bearing exercise. Health Psychol. 2007;26:577–587. doi: 10.1037/0278-6133.26.5.577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.