Abstract
Hybrid catfish created by crossing of female channel catfish (Ictalurus punctatus) and male blue catfish (Ictalurus furcatus) are being used increasingly in foodfish aquaculture because of their fast growth and efficient food conversion. However, the availability of blue catfish males is limited, and their peak spawning is at a different time than that of the channel catfish. As such, cryopreservation of sperm of blue catfish could improve production of hybrid catfish, and has been studied in the laboratory and tested for feasibility in a commercial dairy bull cryopreservation facility. However, an approach for commercially relevant production of cryopreserved blue catfish sperm is still needed. The goal of this study was to develop practical approaches for commercial-scale sperm cryopreservation of blue catfish by use of an automated high-throughput system (MAPI, CryoBioSystem Co.). The objectives were to: 1) refine cooling rate and cryoprotectant concentration, and evaluate their interactions; 2) evaluate the effect of sperm concentration on cryopreservation; 3) refine cryoprotectant concentration based on the highest effective sperm concentration; 4) compare the effect of thawing samples at 20 °C or 40 °C; 5) evaluate the fertility of thawed sperm at a research scale by fertilizing with channel catfish eggs; 6) test the post-thaw motility and fertility of sperm from individual males in a commercial setting, and 7) test for correlation of cryopreservation results with biological indices used for male evaluation. The optimal cooling rate was 5 °C/min (Micro Digitcool, IMV) for high-throughput cryopreservation using CBS high-biosecurity 0.5-ml straws with 10% methanol, and a concentration of 1 × 109 sperm/ml. There was no difference in post-thaw motility when samples were thawed at 20 °C for 40 s or 40 °C for 20 s. After fertilization, the percentage of neurulation (Stage V embryos) was 80 ± 21%, and percentage of embryonic mobility (Stage VI embryo) was 51 ± 22%. There was a significant difference among the neurulation values produced by thawed blue catfish sperm, fresh blue catfish sperm (P = 0.010) and channel catfish sperm (P = 0.023), but not for Stage VI embryos (P ≥ 0.585). Cryopreserved sperm from ten males did not show significant variation in post-thaw motility or fertility at the neurulation stage. This study demonstrates that the protocol established for high-throughput cryopreservation of blue catfish sperm can provide commercially relevant quantities and quality of sperm with stable fertility for hybrid catfish production and provides a model for establishment of commercial-scale approaches for other aquatic species.
Keywords: blue catfish Ictalurus furcatus, high throughput, sperm cryopreservation, male, variation
Introduction
Fish sperm is, in general, different from bull sperm in many ways. For example, the length of the bull sperm head is ~9 um [17], while the length of the fish sperm head in most common teleost species is 1 to 3 um [19]. Bull sperm held at ambient temperature in the presence of carbon dioxide can remain viable for days upon ejaculation [11], while sperm of most freshwater fishes (such as of channel catfish and blue catfish) can remain motile for only 1 min or less after activation by hypoosmotic solutions (<150 mOsmol/kg) [5]. Bull sperm function in an isotonic environment, but fish sperm, due to various spawning environments, become activated and motile across osmotic pressures ranging from 25 mOsm/kg to 1000 mOsm/kg depending on species. However, despite these differences, the sperm of livestock and fishes share enough features in common to provide opportunities for adaptation of mammalian cryopreservation technology and equipment for use with aquatic species.
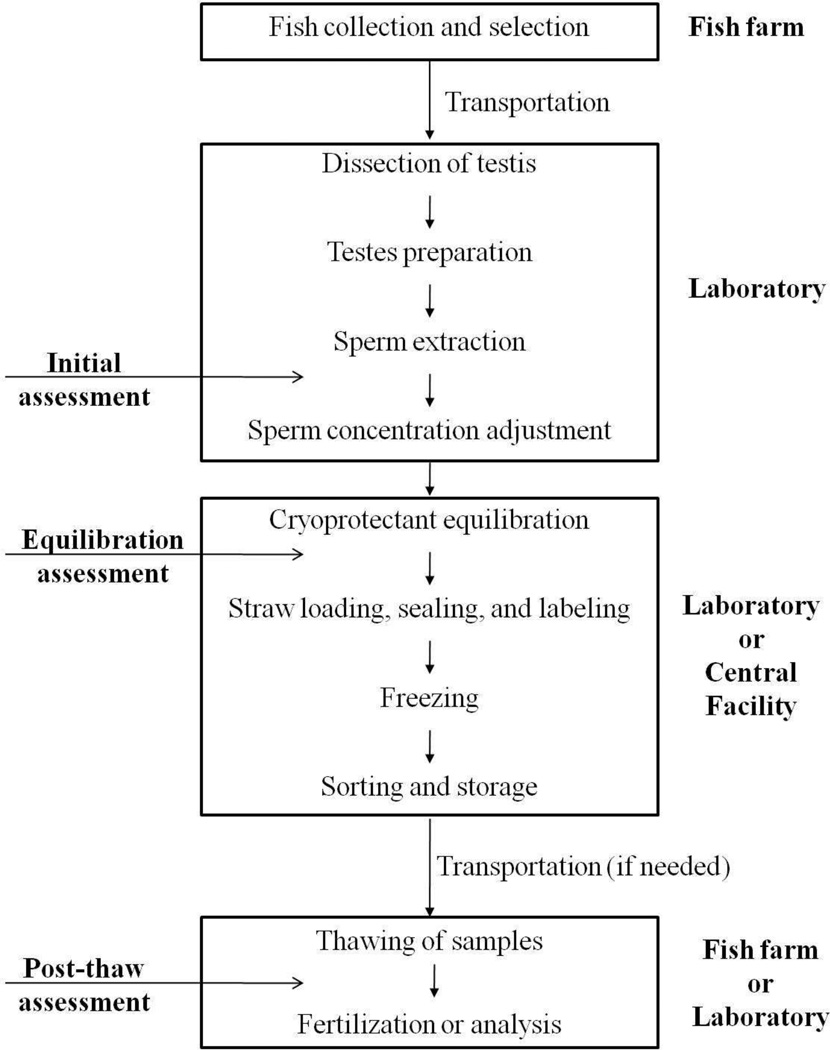
Catfish production, despite recent problems with global competition and rising prices of feed and fuel, remains the largest foodfish aquaculture industry in the United States [44]. Hybrid catfish created by crossing of female channel catfish (Ictalurus punctatus) and male blue catfish (Ictalurus furcatus) are in high demand by the industry because of their fast and uniform growth rate, disease resistance and efficient food conversion [23]. However, this hybrid is available only in limited numbers because blue catfish do not readily hybridize with channel catfish naturally [23] due to biological differences including non-coincident times of peak spawning, and limited commercial availability of blue catfish males. Because induced spawning is required to produce the hybrid, sperm cryopreservation can be used to provide blue catfish sperm when female channel catfish are in peak spawning condition. To date, sperm cryopreservation of blue catfish has been studied in the laboratory; initial studies have been done to establish the feasibility of using commercial facilities dedicated to cryopreservation of dairy bull sperm for blue catfish sperm [3; 20], and a basic approach to commercial-scale application has been generated (Fig. 1). However, to meet the large industrial demand for hybrid catfish fingerlings, an approach for high-throughput production of cryopreserved sperm is needed for use at facilities dedicated to aquatic species.
Figure 1.
Overview of the sperm cryopreservation process used for blue catfish (Ictalurus furcatus). The motility assessment points are indicated on the left, and the locations of activities are shown on the right.
High-throughput cryopreservation has been widely applied in the dairy industry for decades [28]. Cryopreserved germplasm constitutes an independent industry used for animal breeding, preservation of genetic diversity, and medical research. However, there is currently no system for large-scale production of cryopreserved germplasm of aquatic species. Recently, a survey of fish culturists revealed a high demand for genetic improvement of the type that can be provided by cryopreservation [8], especially among hatcheries that produce hybrid catfish. Therefore, this study focused on the development of a high-throughput sperm cryopreservation approach for fishes, specifically addressing the problems of blue catfish.
Development of a high-throughput cryopreservation approach for fish using existing equipment requires several modifications. For example, an automated system for loading, sealing, labeling, and reading of straws has been developed (MAPI system, CryoBioSystem Co. Paris, France) for sperm cryopreservation of livestock and humans. The sperm packaging required by the MAPI system is a specially designed 0.5-ml (CBS) straw with an external polyvinyl chloride identification jacket for labeling. The CBS straw is made from an ionomeric resin different from the traditional 0.5-ml French straw (IMV Technologies, Paris, France) which is made from polyvinyl chloride and polyethylene terephthalate glycol [32]. Thus, the cooling rate, and cryoprotectant (CPA) type and concentration, require refinement for the CBS straw because the identification jacket and other differences in thermal properties can affect heat transfer compared to the 0.5-ml French straw used in previous trials [12]. Second, sperm concentration is an extremely important factor in sperm cryopreservation [14], but cell concentration has not been reported in previous publications on blue catfish (or for the vast majority of aquatic species studied) [42]. In addition, from a commercial point of view, higher concentrations of sperm loaded into a single straw (the “product unit”), will lower the costs of packaging, storage, shipping, and use. Third, the thawing process is a critical factor that can affect the viability of cryopreserved sperm [26]. Due to potential differences in thermal properties deriving from the use of the CBS straw for the MAPI system, thawing methods need to be evaluated as well.
The overall purpose of this research is to establish general protocols for high-throughput cryopreservation for aquatic species. The goal of this study was to develop practical approaches for commercial-scale sperm cryopreservation of blue catfish by use of an automated high-throughput system (MAPI, CryoBioSystem Co.). The objectives were to: 1) refine cooling rate and cryoprotectant concentration, and evaluate their interactions; 2) evaluate the effect of sperm concentration on cryopreservation; 3) refine cryoprotectant concentration based on the highest effective sperm concentration; 4) compare the effect of thawing samples at 20 °C or 40 °C; 5) evaluate the fertility of thawed sperm at a research scale by fertilizing with channel catfish eggs; 6) test the post-thaw motility and fertility of sperm from individual males in a commercial setting, and 7) test for correlation of cryopreservation results with biological indices used for male evaluation. In this study, a practical protocol for sperm cryopreservation of blue catfish was established by use of the high-throughput system. This is the first example of true commercially relevant production of cryopreserved sperm designed specifically for aquatic species rather than by adapting facilities designed for mammals, and opens the door for potential large-scale production of cryopreserved sperm for hybrid catfish production and for aquatic species in general.
Materials and methods
Fish
The male blue catfish (D&B strain) and channel catfish (current commercial stocks) used in this study came from Baxter Land Company Fish Farm (Arkansas City, Arkansas: 33°34’58.64”N, 91°15’18.45”W). Males with readily observable secondary sexual characteristics (e.g. well-muscled head, and dark coloration) were selected at the farm. After transport to the Aquaculture Research Station of the Louisiana State University Agricultural Center (Baton Rouge, 30°22’07.32”N, 91°10’27.90”W) in an oxygenated hauler in February, 2009, the fish were maintained in aerated outdoor 0.1-acre ponds and fed commercial diets (Aquaxcel, CargillTM, 45% protein). Two days before the start of experiments in April, the fish were captured by seining and moved into indoor tanks with a recirculating system. The bubble-washed bead filters were back-flushed every 2 d. The water quality parameters were: pH 7.0 – 8.0, ammonia 0.1 – 0.8 mg/l, nitrite 0.04 – 0.30 mg/l, alkalinity 39 – 125 mg/l, hardness 44 – 126 mg/l, temperature 28 ± 1 °C, and dissolved oxygen 4.3 – 6.5 mg/l. Cryopreservation experiments were performed at the Aquaculture Research Station, and commercial-scale fertilization tests of individual male variance were performed at the hatchery of Baxter Land Co. Guidelines from the Institutional Animal Care and Use Committees (IACUC) of Louisiana State University were followed for animal care in this study.
Sperm Collection
Before dissection, fish were killed by a sharp blow to the head, and the body was rinsed with Hanks’ balanced salt solution at an osmolality of 300 mOsmol/kg (HBSS300) to prevent fresh water on the fish from inadvertently activating the sperm during dissection [1]. After measuring of the body weight and standard length, the fish were dissected and the testes were removed and placed into HBSS300 in a tared weigh boat (catalog number: 02-203-501, Fisher Scientific). After removal of blood and attached tissue, the testes were blotted with a paper towel and weighed. The anterior portion of each testis was separated and weighed for sperm collection [34]. To suspend the sperm, the anterior portion of the testis was crushed in a quart Ziploc freezer bag ™ (S.C. Johnson & Son, Inc., Racine, WI) after addition of HBSS300 with a volume (ml) of two times the mass (g) of the anterior testis [20]. The sperm suspensions were filtered through a mesh series consisting of a 7.62-cm round mesh strainer (1-mm mesh), a 15.24-cm round mesh strainer (0.5-mm mesh), and a 200-µ mesh filter. Samples from each male were processed separately. None of the samples were pooled.
Determination of sperm concentration
Cell concentrations were determined by measuring absorbance of the sperm suspensions with a microspectrophotometer (NanoDrop 1000, Thermo Scientific, Wilmington, DE), and calculation from an equation generated from our standard curve between the absorbance reading of serially diluted sperm suspensions and the sperm concentration as determined by hemocytometer [37] [14]. Measurements were made using 2-ul aliquots at a wavelength of 601 nm. The equation used was:
sperm concentration (cells/ml) = absorbance × 5.12 × 108 – 4.07 × 107 (R2 = 0.960).
Motility estimation
Sperm motility was estimated by viewing through a dark-field microscope (Olympus CX41RF, Japan) at 200-× magnification. Fresh sperm suspension (1 µl) was placed on the slide, and was activated by mixing with 20 ul of filtered deionized distilled water (25 mOsmol/kg). Motility estimation was based on an observation of 3–5 different fields within 20 s after activation, and expressed as the percentage of sperm swimming progressively forward within the sample. The motility of sperm suspensions estimated within 2 hr of testis collection (without CPA) was considered the “initial motility”, the motility of the sperm suspension at the end of the 30-min equilibration process (after addition of CPA) was the “equilibration motility” (Figure 1), and the motility of sperm after thawing was “post-thaw motility.” Sperm suspensions with an initial motility of less than 40% were not included in these experiments (based on this samples from 4 of the 21 males were excluded).
Sample preparation for cryopreservation using the automated system
After motility was estimated, the CPA was prepared in HBSS300 at twice the target concentration, and was mixed 1:1 (v/v) with the sperm samples to yield the desired final concentrations of CPA (5% and 10%) and desired sperm concentrations (ranging from 1 × 108 to 1.7 × 109 sperm/ml depending on experimental design). Upon mixing with CPA, the sperm samples were placed on the MAPI system, and filling, sealing and labeling of the straws were controlled by a proprietary computer program (SIDE, CBS). Samples were drawn into 0.5-ml CBS straws by vacuum applied from the cotton end of the straw, and the straws were continuously transferred to the sealing platform to seal both ends by application of 158 °C heat clamps. The sealed straws were labeled with alphanumeric information on the identification jacket with an ink printer (A400, Domino, IL, USA) before being transferred to the collection area for label verification, and quality control evaluation. For freezing, the straws were arrayed on horizontal racks (40 straws per rack) and placed in a commercial-scale programmable freezer (Micro Digitcool, IMV, France) with a capacity of 280 straws per freezing cycle. We did not attempt to equalize the thermal mass at every freezing (e.g. by adding “dummy” straws) and the number of straws in each freezing cycle ranged from 180 to 240 straws. At 30 min after addition of CPA to the sperm suspension, the cooling program was initiated. The cooling rate for this freezer can be programmed from 1 °C /min to 40 °C/min (based on chamber temperature) and two rates were studied for this research (detailed below). When the final target temperature (−80 °C) was reached and held for 5 min the frozen samples were removed and placed into liquid nitrogen. The individual straws were sorted under liquid nitrogen into 12-compartment storage containers (Daisy goblets, reference number: 015144, Cryo Bio System) for long-term storage in liquid nitrogen.
Egg collection and artificial fertilization
Channel catfish eggs were used for artificial fertilization. Two days before egg collection, the females were intraperitoneally injected with 100 µg/kg of luteinizing hormone-releasing hormone analog (LHRHa, Syndel Laboratories Ltd.) at the Aquaculture Research Station, or with 10 mg/kg common carp pituitary (lot numbers: 031109, 032209, 033109, Stoller Fisheries) at Baxter Land Co. based on existing techniques at each facility [1]. Eggs were collected into HBSS300 in a greased (high vacuum grease, Dow Corning®, USA) bowl by gentle squeezing of the abdominal area. Individual eggs with full yellowish yolk and limited or no blood contamination were considered to be of good quality and were selected for artificial fertilization.
Small-scale (research) studies were performed at the Aquaculture Research Station. Eggs were placed into 100-ml tri-cornered polypropylene beakers (catalog number: 14-955-111B, Fisher Scientific) to form a monolayer at the bottom (yielding ~100 eggs). Frozen straws were removed from the liquid nitrogen storage Dewar and plunged into a 40 °C water bath for 20 s, and diluted to 1 × 108 sperm/ml by addition of 4.5 ml of HBSS300, and 1 ml of this sperm suspension was used to fertilize the 100 eggs in each beaker. To activate the gametes, 10 ml of water from the hatchery system were added to the beakers producing a final sperm concentration of 9 × 106 sperm/ml, and a sperm-to-egg ratio of 1 × 108 sperm/egg. An additional 10 ml of water was added 10 min later to assist final water-hardening (expansion) of the egg chorion. After an additional 10 min, the eggs were transferred into individual fully screened cups in a recirculating system at 27 to 29 °C for incubation and development [4]. For fertilization evaluation, three beakers from each female were used for sperm samples from each individual male, and two females were used for each male.
Commercial-scale studies were performed at the Baxter Land Co. Fish Hatchery. Before sample transport, all frozen straws were sorted into Daisy goblets and grouped by males at the Aquaculture Research Station. All Daisy goblets were placed into shipping Dewars that had been filled at least twice with liquid nitrogen (AR1000, Cryoport, USA), and transported to the hatchery. During transportation, the Dewars were tightly positioned at the back of the vehicle. In the hatchery during spawning, batches of eggs collected from individual females were divided into two aliquots with a volume of 150–200 ml each (average 40 eggs/ml): one for thawed sperm, and one for fresh blue catfish sperm (used to evaluate egg quality). Frozen sperm were thawed at 40 °C for 20 s, and four straws (a total of 2 ml suspension with 1 × 109 sperm/ml) were used to fertilize each aliquot of eggs. Each day, fresh sperm samples were collected from male blue catfish that came from the same population used for the frozen sperm samples, and were stored in refrigerator (4 °C) at a concentration of 1 × 109 sperm/ml. The same volume (2 ml) of sperm was used to fertilize each aliquot of the eggs. The final sperm concentrations were therefore 1 ~ 1.3 × 107 sperm/ml, and the sperm-to-egg ratio was 2.5 ~ 3.3 × 105 sperm/egg. The eggs were held in 25-cm metal pie pans, and after addition of the sperm, water (1 L) from the hatchery system was added to the pans to activate the gametes for fertilization. After 20 min, eggs that had adhered normally into masses were moved into individual mesh baskets in a flow-through system for embryo development and incubation following routine hatchery procedures [35]. For sperm samples from each blue catfish male, eggs from 5 to 8 females were used as replicates. A total of 71 females were tested, and more than 21 L of eggs were used for fertilization trials.
At 27 to 30 hr after fertilization at 27 to 29 °C, the neurula stage covers the yolk sac and segmentation of trunk mesoderm is initiated in the embryo (Stage V) [22; 30; 33]. The neurulated embryos were counted by viewing with the naked eye and back illumination, and fertilization rate was expressed as the percentage of neurulated embryos in relation to the total number of eggs (referred to as “neurulation”). This number was determined for both aliquots of eggs from each female representing the control (fertilized with fresh sperm) and thawed sperm. The Stage VI embryos (initiation of embryonic mobility) [22; 30; 33] were counted at 48–50 hr after fertilization. Because there was no way to maintain separate groups of hatched larvae (sacfry) in the commercial hatchery, hatch rate was estimated as the percentage of Stage VI embryos in relation to the total number of eggs.
Study I: Refinement of cooling rate and cryoprotectant
Two concentrations of methanol (5 and 10 %; v/v) were tested, because methanol has previously been used in our laboratory for sperm cryopreservation of blue catfish [20] and channel catfish [12; 13]. For each concentration of methanol, two cooling rates 5 °C/min, and 40 °C/min) were used to bring the samples from 5 to −80 °C. For these experiments, the final concentration of the sperm suspensions was held within the range of 1 × 108 sperm/ml to 1 × 109 sperm/ml. Three replicates were produced from sperm of each of three individual males. Three straws from each treatment and each male were thawed in a water bath at 40 °C for 20 s and post-thaw motility was evaluated as described above.
Study II: Effect of sperm concentration on cryopreservation
In this experiment, sperm samples were frozen with 10% methanol as CPA and a cooling rate of 5 °C/min. In the first trial, two sperm concentrations (1 × 108 sperm/ml and 1 × 109 sperm/ml) were tested, using sperm for three individuals as replicates. In the second trial, two concentrations (1 × 109 sperm/ml and 1.7 × 109 sperm/ml) were tested from another three individuals as replicates. From these, three straws from each treatment were thawed as described in Study I and motility was assessed as described above.
Study III: Refinement of methanol concentration based on the maximum sperm concentration
Sperm samples from six individuals were used for this experiment with a final sperm concentration of 1 × 109 sperm/ml (based on Study II). The methanol concentrations tested were 5% (1.23 mol/L) and 10% (2.46 mol/L). All samples were frozen at a cooling rate of 5 °C/min from 5 to −80 °C. From these, three straws from each treatment were thawed as described in Study I and motility was assessed as described above.
Study IV: Effect of thawing temperature on post-thaw motility
Sperm samples from three individuals were used in this experiment at a final concentration of 1 × 109 sperm/ml and were frozen with 10% methanol as CPA and a cooling rate of 5 °C/min. To test the effect of thawing temperature, three straws from each male were thawed in a water bath at 40 °C for 20 s, or at 20 °C for 40 s. Post-thaw motility was assessed as described above.
Study V: Fertilization of cryopreserved blue catfish sperm by crossing with channel catfish eggs
Sperm from three blue catfish were cryopreserved at a final concentration of 1 × 109 sperm/ml with 10% methanol as CPA and a cooling rate of 5 °C/min from 5 °C to −80 °C. In addition, sperm from three channel catfish were frozen using the same protocol. Frozen samples from both species were thawed at 40 °C for 20 s, and used to fertilize eggs from two female channel catfish as described above for research-scale artificial fertilization at the Aquaculture Research Station.
Study VI: Effect of male-to-male variation on post-thaw motility and fertility
Sperm were collected from ten blue catfish in May, 2009. Sperm samples at a final concentration of 1 × 109 sperm/ml were cryopreserved with 10% methanol at a cooling rate of 5 °C/min from 5 °C to −80 °C. Frozen samples (N = 276 straws) were thawed at 40 °C for 20 s, and used at Baxter Land Co. to fertilize eggs collected from five to eight blue catfish females for each male as described above for artificial fertilization. The thawed samples were held at 4 °C and used within 12 hr after thawing. Previous studies have held thawed sperm of channel catfish at 4 °C for more than 7 d [13].
Study VII: Correlation within biological indices
For the 10 males used in Study VI, correlation analysis was performed among the biological characteristics (body weight, body length, testis weight, anterior testis weight), gonadosomatic index (GSI) body condition factor (BCF), initial motility of fresh sperm, post-thaw motility, and post-thaw fertility (neurulation percentage). The GSI was calculated as the percentage of testis weight (g) in relation to body weight (g) (Testis weight (g) / Body weight (g) × 100%), and the BCF was calculated as the percentage of body weight (g) in relation to the cube of the body length (cm) (Body weight (g) / Body length3 (cm) × 100%).
Data analysis
Data were organized using Microsoft Office Excel 2007, and analyzed with the GLM, CORR program and ANOVA t-test (Statistical Analysis System, version 9.0, 2002). Percentage data were arcsin-square-root transformed before analysis. Correlation analysis was performed using SAS 9.0. Differences were considered significant at P < 0.050.
Results
Study I: Refinement of cooling rate and cryoprotectant
The initial motility of fresh sperm from 5 males was 69 ± 5% (mean ± SD). There were no significant differences between initial motility and equilibration motility (after 30 min with CPA) (P = 0.915). Post-thaw motility decreased significantly compared to equilibration motility (P < 0.001). The concentration of CPA (P < 0.001) and cooling rate (P < 0.001) each significantly affected post-thaw motility. The highest post-thaw motility was achieved using 10% methanol (v/v) with a cooling rate of 5 °C/min (24 ± 8%), followed by 5% methanol at 5 °C/min (16 ± 5%), 10% methanol at 40 °C/min (4 ± 2%), and finally 5% methanol at 40 °C/min (1 ± 1%).
Study II: Effect of sperm concentration on cryopreservation
The initial motility of fresh sperm from 6 males was 59 ± 11%. In the first trial, no significant difference in post-thaw motility was found between sperm cryopreserved at 1 × 108 cell/ml (17 ± 7%) and sperm cryopreserved at 1 × 109 sperm/ml (22 ± 8%) (P = 0.240). In the second trial, post-thaw motility of sperm cryopreserved at 1 × 109 sperm/ml (30 ± 10%) was significantly higher than that at 1.7 × 109 sperm/ml (4 ± 5%) (P < 0.001). In the first trial there was no significant difference (P > 0.050) between initial motility and equilibration motility, but there was for the second trial (P = 0.041).
Study III: Refinement of methanol concentration based on the maximum sperm concentration
The initial motility of fresh sperm from 5 males was 69 ± 5%. With t he selected sperm concentration of 1 × 109 sperm/ml, post-thaw motility was higher when sperm were cryopreserved with 10% methanol (35 ± 6 %) than that with 5% methanol (16 ± 8%) (P < 0.001). There was no significant difference in equilibration motility between 5% methanol (59 ± 2%) and 10% methanol (63 ± 5%) (P = 0.160).
Study IV: Effect of thawing temperature on post-thaw motility
The initial motility of fresh sperm from 3 males was 50 ± 7%. The temperatures of 20 °C and 40 °C did not produce a significant difference in post-thaw motility of cryopreserved sperm (32 ± 9% vs. 28 ± 11%) (P = 0.415). Post-thaw motility at each thawing temperature was significantly lower than initial motility (51 ± 6%) (P < 0.001).
Study V: Fertilization by cryopreserved blue catfish sperm when crossed with channel catfish eggs
The initial motility of fresh sperm from 3 males was 52 ± 1%. Fresh blue catfish sperm yielded 17 ± 10% neurulation (Stage V), and 53 ± 37% initiation of embryo motility (Stage VI); fresh channel catfish sperm yielded 20 ± 16% neurulation, and 41 ± 32% Stage VI embryos. No differences were found between the fresh sperm of blue catfish and channel catfish, indicating that the fertilization potential of blue catfish sperm with channel catfish eggs is comparable to that of channel catfish sperm. With the selected protocol, the post-thaw motility of blue catfish sperm was 28 ± 1%. After fertilization, the percentage of neurulation was 80 ± 21%, and percentage of Stage VI embryos was 51 ± 22% after normalizing by considering the fertility with fresh sperm as 100% (not all eggs are fertilizable). For a parallel comparison, there were no differences between cryopreserved blue catfish sperm and cryopreserved channel catfish sperm in post-thaw motility (12 ± 11 %) (P = 0.155), percentage of neurulation (75 ± 21%) (P = 0.476), and percentage of Stage VI embryos (67 ± 29%) (P = 0.169).
Study VI: Effect of male-to-male variation on post-thaw motility and fertility
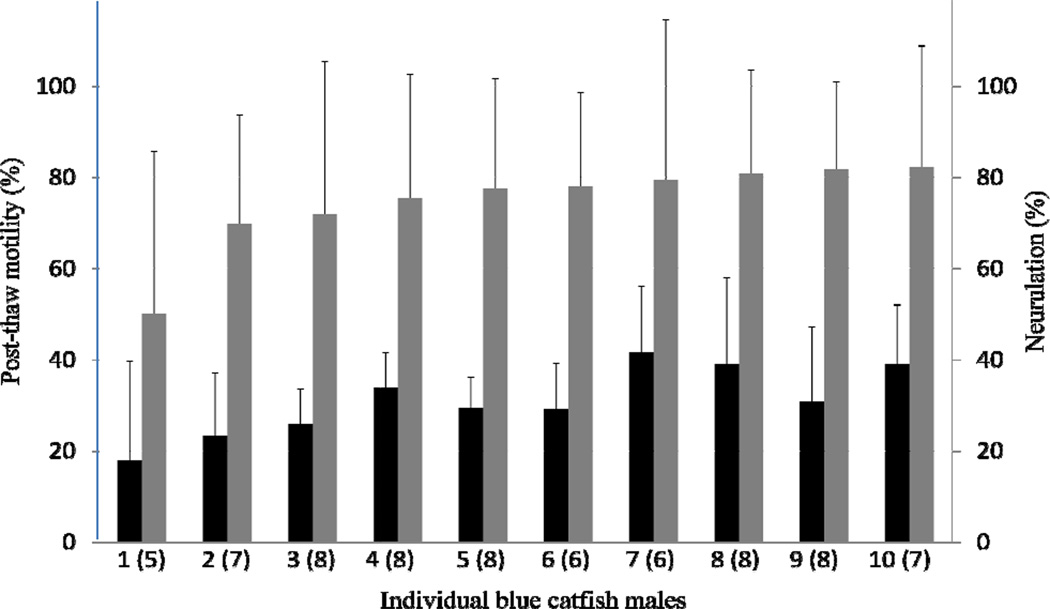
The initial motility of fresh sperm from 10 individuals was 52 ± 9% (Table 1). After thawing, the post-thaw motility was 31 ± 12% (Table 1), which was significantly lower than the initial motility (P = 0.040). Among these individual males, there was no significant difference in initial motility (P ≥ 0.560) or post-thaw motility (P ≥ 0.207). Fertilization of thawed sperm from these 10 males with eggs from channel catfish showed an average neurulation of 38 ± 17% (absolute numbers) (Fig. 2), which was significantly lower than that for the egg quality test with fresh sperm (45 ± 16 %) (P = 0.009). Among the 10 individuals, the fertility of thawed sperm (in terms of percentage of neurulation) was not different (P = 0.767).
Table 1.
Values (x̄ ± SD) and indices measured from ten blue catfish (Ictalurus furcatus) males. Body condition factor (BCF) was calculated as the percentage of body weight (g) to the cube of the body length (cm). Gonadosomatic index (GSI) was calculated as the percentage of testis weight to the body weight. The initial concentration of the sperm suspension was measured after crushing and filtering*. “Initial motility”: motility of the sperm suspension prior to addition of cryoprotectant; “equilibration motility”: motility of the sperm suspension after equilibration time with cryoprotectant (30 min); “post-thaw motility”: motility after thawing at 40 °C for 20 s; Neurulation: the percentage of Stage V embryo produced with thawed sperm normalized by comparing to the percentage of Stage V embryos produced with fresh sperm.
| Male | BCF | GSI | Initial concentration (sperm/ml) |
Initial motility |
Equilibration motility |
Post-thaw motility |
Neurulation |
|---|---|---|---|---|---|---|---|
| 1 | 0.012 | 0.288 | 1.68 × 109 | 43 % | 43 % | 30 ± 7 % | 78 ± 24 % |
| 2 | 0.011 | 0.226 | 1.72 × 109 | 55 % | 58 % | 34 ± 8 % | 75 ± 27 % |
| 3 | 0.011 | 0.196 | 9.13 × 109 | 40 % | 40 % | 18 ± 22 % | 50 ± 35 % |
| 4 | 0.012 | 0.213 | 3.73 × 109 | 45 % | 45 % | 29 ± 10 % | 78 ± 21 % |
| 5 | 0.009 | 0.205 | 3.10 × 109 | 53 % | 43 % | 39 ± 13 % | 82 ± 26 % |
| 6 | 0.011 | 0.377 | 2.15 × 109 | 63 % | 45 % | 42 ± 14 % | 79 ± 35 % |
| 7 | 0.011 | 0.191 | 1.93 × 109 | 50 % | 45 % | 26 ± 8 % | 72 ± 33 % |
| 8 | 0.011 | 0.117 | 2.42 × 109 | 55 % | 45 % | 39 ± 19 % | 81 ± 23 % |
| 9 | 0.011 | 0.243 | 2.26 × 109 | 48 % | 38 % | 23 ± 14 % | 70 ± 24 % |
| 10 | 0.011 | 0.340 | 1.15 × 109 | 68 % | 58 % | 31 ± 16 % | 82 ± 19 % |
Sperm were collected by crushing the testis in a 2:1 ratio of extender (ml):anterior testis weight (g).
Figure 2.
Sperm from ten blue catfish (Ictalurus furcatus) cryopreserved using 1 × 109 sperm/ml with 10% methanol with a 5 °C/min cooling rate. Sperm were thawed and used to fertilize eggs from 5 to 8 females, (the number of females is indicated within parentheses). Post-thaw motility (dark bars) and neurulation (light bars) were used to evaluate male-to-male variation. Among the 10 individuals, the post-thaw motility (P ≥ 0.207) and the fertility of thawed sperm (percent neurulation) (P = 0.767) were not significantly different.
Study VII: Correlations with biological indices
The correlation analysis of initial motility, post-thaw motility, fertility, and the biological indices showed significant relationships among these factors (Tables 1 and 2). Significant correlations were found between initial motility and post-thaw motility (P = 0.048), and between post-thaw motility and neurulation (P = 0.005). The initial motility and neurulation were not correlated (P = 0.059). Anterior testis weight was correlated with total testis weight (P < 0.001), and anterior testis weight (P = 0.014) and total testis weight (P = 0.005) were each correlated with body condition factor. As would be expected, body length was correlated with body weight (P < 0.001).
Table 2.
Pearson correlation coefficients among body and gonadal indices of blue catfish (Ictalurus furcatus) males (n = 10). The values listed in each cell (except for those with a single number) are correlation coefficient (above) and P value (below). BCF: body condition factor was calculated as the percentage of body weight (g) to the cube of the body length (cm); GSI: gonadosomatic index was calculated as the percentage of testis weight to the whole body weight. The initial concentration of the sperm suspensions was measured after crushing and filtering.
| weight | Length | Anterior testis |
Testis | BCF | GSI | Initial concentration |
Initial motility |
Post-thaw motlity |
Neurulation | |
|---|---|---|---|---|---|---|---|---|---|---|
| Weight | 1.000 | 0.966 <.001 * |
0.131 0.717 |
0.266 0.458 |
−0.533 0.113 |
−0.236 0.512 |
0.165 0.648 |
−0.395 0.259 |
−0.366 0.298 |
−0.393 0.261 |
| Length | 1.000 | 0.011 0.975 |
0.163 0.652 |
−0.497 0.144 |
−0.321 0.367 |
0.060 0.870 |
−0.388 0.268 |
−0.378 0.281 |
−0.490 0.151 |
|
| Anterior testis | 1.000 | 0.985 <.001* |
−0.740 0.014* |
0.362 0.304 |
0.120 0.741 |
0.515 0.127 |
0.463 0.178 |
0.336 0.343 |
||
| Testis | 1.000 | −0.807 0.005 * |
0.294 0.409 |
0.084 0.817 |
0.456 0.185 |
0.384 0.274 |
0.242 0.500 |
|||
| BCF | 1.000 | 0.162 0.655 |
−0.127 0.727 |
−0.275 0.442 |
−0.353 0.318 |
−0.130 0.721 |
||||
| GSI | 1.000 | −0.275 0.442 |
0.487 0.154 |
0.171 0.636 |
0.241 0.503 |
|||||
| Initial concentration | 1.000 | −0.112 0.757 |
0.401 0.251 |
0.489 0.152 |
||||||
| Initial motility | 1.000 | 0.635 0.048* |
0.614 0.059** |
|||||||
| Post-thaw motility | 1.000 | 0.807 0.005 * |
||||||||
| Neurulation | 1.000 |
values with P < 0.050;
values with 0.050 < P < 0.100.
Discussion
Development of protocols for high-throughput sperm cryopreservation in fish
Cryopreservation of sperm was first achieved 60 yr ago for livestock [29] and fish [6]. Since then a multi-billion dollar global industry has developed for cryopreserved livestock germplasm, but no corresponding industry exists for aquatic species which currently rely on live populations to provide genetic improvement. Specialized equipment and facilities that have been developed for high-throughput processing of livestock and human sperm are available for adoption with aquatic species.
To adapt high-throughput cryopreservation technology to fishes, the first task was to establish a reliable and practical protocol for sperm cryopreservation by use of an automated system. In this study, the system selected for sample filling, sealing, and labeling requires a specifically designed high-biosecurity straw (CBS straw) which possesses an outer identification jacket and is manufactured using different materials compared to the traditional 0.5-ml French straw (used in previous catfish publications) which results in differences in flexibility and thermal properties between the straw types. Structurally, use of the CBS straw for sperm cryopreservation interferes with heat transfer, and consequently affects the cooling and thawing rates of samples, which are two critical factors for sperm viability that are interact with CPA type and concentration [49]. Also, in comparison with previous publications addressing cryopreservation of catfish sperm [2; 3; 7; 12; 13; 18; 20; 21; 41; 45; 46], only one [41] reported the estimated sperm concentration. To develop a high-throughput protocol, sperm concentration is a required factor to consider because it controls the yield in straw production; dictates the time and space requirements for storage, shipping, and use of samples, and the sperm concentration can directly affect sperm viability during cryopreservation [15; 42]. Therefore, at a minimum, development of protocols for high-throughput sperm cryopreservation must address the cooling rate within the container (i.e. specific straw type), type and concentration of CPA, and sperm concentration.
Faced with the multi-factorial interactions among cooling rate, sperm loading concentration, and CPA concentration, we chose in this study to use previous information from our laboratory and to sequentially tease apart the interactions rather than utilize a large multifactorial experimental matrix. In this study, the cooling rate was determined first, followed by the loading concentration, and then the CPA concentration. The differences between CPA concentrations of 5% and 10% methanol became statistically significant when these results were integrated. Sperm motility values at the end of the 30-min equilibration period were reported to differentiate the effect of the freezing and thawing processes (rather than combining them with potential toxicity effects encountered before freezing). However, in this study, the lack of a significant difference between initial motility (at collection) and equilibration motility (after addition of CPA) indicated that methanol at either concentration produced little or no toxic effects (at least in regard to motility) prior to freezing.
Channel catfish and blue catfish are closely related members of the North American genus Ictalurus [27]. Both species possess 58 chromosomes [50] and the hybrid of these species is fertile and can produce F2 and subsequent generations, or back cross to either parental species [40]. Mature blue catfish are larger than channel catfish and are considered to be too large to serve as routine broodstock for catfish aquaculture. Otherwise the gametes of these species are very similar in structure and function [20; 41, and our unpublished observations]. In a previous study of channel catfish, sperm cryopreservation with 0.5-ml French straws showed that freezing at 3 °C/min and 45 °C/min each yielded satisfactory post-thaw motilities [13]. In the present study, freezing at 5 °C/min retained higher post-thaw motility than 40 °C/min with CBS straws. Also, interaction effects of CPA concentration and cooling rate were detected in this study. In a previous study, 5% methanol and French straws were used for channel catfish cryopreservation. However, the combination of 5% methanol and the CBS straw was not as effective as the combination of 10% methanol and CBS straw at either cooling rate. This could be because: 1) higher sperm concentrations were used in the present study, which required the use of a higher concentration of CPA; 2) 10% methanol would be more effective than 5% methanol in decreasing the freezing point of the solution [26], and 3) a lower freezing point provided more protection from intracellular ice formation during freezing, especially when coupled with slower cooling rates [24].
Sperm concentration is an important factor for cryopreservation, and is commonly ignored by most researchers for aquatic species [42; 14]. In this study, comparison of sperm concentrations showed significant differences in post-thaw motility. To maintain a high sample capacity for each straw, a sperm concentration of 1 × 109 sperm/ml was chosen. Also, determination of sperm concentration is a necessary component for standardizing protocols to yield reliable products especially for high-throughput production. More importantly, control and refinement of sperm number can greatly improve the efficiency of use of cryopreserved sperm during artificial fertilization. At the same time, choice of the concentration of sperm in straws depends on a balance between the biological characteristics of the species and the demands of practical utility and economics. For sperm of Pacific oyster Crassostrea gigas, the fertility decreased because of increased sperm concentration at given CPA concentrations [14], and a concentration of 1 × 108 fresh sperm/ml was chosen in that study as the maximum concentration to maintain highest biological function by avoiding sperm agglutination (head-to-head aggregations elicited by an acrosome reaction) [14]. A previously recommended sperm-to-egg ratio for production of catfish hybrids was 1.25 × 105 fresh sperm per egg [2]. In the present study, best results were obtained with 1.35 × 105 motile sperm per egg using post-thaw sperm [41]. When freezing a large volume of samples, the use of higher loading concentration in each straw is an efficient way to reduce the costs of straw packaging, storage space, and shipping. Also, because the condensed sperm can easily be diluted to a final working concentration without forming agglutination in blue catfish, a relatively high straw loading concentration (1 × 109 cell/ml) was used in this study. Most of the previous research of catfish have yielded satisfactory results in the laboratory based on estimated concentrations of around 1 × 108 sperm/ml (our estimations of the mass of testis per volume of extender reported) [41]. In this study we tested the maximum loading concentration, and found that 1 × 109 sperm/ml was not different from 1 × 108 sperm/ml in post-thaw motility, but motility dropped significantly with less than a doubling of this maximum concentration to 1.7 × 109 sperm/ml. The sperm solutions became viscous, perhaps decreasing the capacity for molecular movement [14] or simply exceeding the cryoprotectant capability per unit concentration of the available CPA. Based on these results, 1 × 109 sperm/ml was selected a s the optimal loading concentration for blue catfish sperm for high-throughput cryopreservation.
The effect of thawing temperature
Thawing rate can be as important as the cooling rate, in terms of being critical factors that affect the viability of cryopreserved sperm [13]. In previous publications on blue catfish, the thawing method used for samples in 0.5-ml French straws was 40 °C for 7 s [13; 20]. This method was originally applied for mammalian sperm [28]. In the present study, samples within CBS straws required 20 sec for thawing at 40 °C. When samples were heated in a 20 °C water bath, complete thawing required 40 s. For practical purposes, it is more convenient and economical to use city (tap) water rather than heating in a water bath for thawing. The results showed that thawing at 20 °C or 40 °C did not affect post-thaw motility, but if time is limited, 40 °C should be considered, especially when large numbers of samples need to be processed because of time constraints in the hatchery.
Quality management of sperm for high-throughput cryopreservation
Motility is a direct and convenient index to evaluate sperm quality and viability. In this study, the viability of sperm after cryopreservation was evaluated by post-thaw motility and the percentages of neurulated (Stage V) and actively mobile (Stage VI) embryos (which spin within the chorion). Although cryopreservation decreased the fertility of sperm from blue catfish and channel catfish, the difference between the species was not significant. This suggested that by using the same cryopreservation techniques for sperm for artificial fertilization, the same number of fertilized (hybrid) eggs could be produced with blue catfish sperm as when fertilizing eggs with channel catfish sperm. In addition, the estimated hatching rates (Stage VI embryos) were not different when making hybrids using cryopreserved sperm or using fresh sperm. Thus cryopreserved sperm could serve as an alternative when fresh sperm is not available, and the results demonstrate the potential commercial application of cryopreserved blue catfish sperm for production of hybrid catfish.
Male-to-male variation in post-thaw motility and fertility
Male-to-male variation for cryopreserved sperm is commonly observed in species studied thus far including mammals, fishes, and invertebrates [13; 25; 47; 48], although the underlying mechanisms remain unclear. Unlike previous reports, no significant variation was observed in post-thaw motility and fertility among the ten males used in this study to evaluate the final protocol. This is a desirable result for high-throughput technology because it can provide products with consistent and predictable output. Possible explanations for the lack of male-to-male variability in this study include: 1) a small sample size of ten males (although comparable to previous studies); 2) unknown attributes of the biology of blue catfish; 3) tight standardization of sperm concentration for each straw which limited variation in response to toxicity or cryopreservation, and 4) the setting of threshold of initial motilities (>40%) at the beginning of the process which reduced the variability of fresh sperm quality (although comparable to previous studies). Although these factors alone or in combination could explain the low observed variability we propose that the use of a tightly standardized protocol (especially for sperm concentration given that the majorly of previous studies in aquatic species did not control this critical variable) would be consistent with production of a predictable and consistent range of post-thaw quality.
Future work should address selection of broodstock for sperm collection. Broodstock blue catfish males are large (typically 3 – 20 kg) and are at least 4–5 yr old. As such, they are individually valuable and it is highly desirable to only kill males with well-developed testes. In this study, blue catfish body length and weight were highly correlated. The negative coefficient between body condition factor and testis weight indicated that long thin fish had bigger testes than did rounder fish, and perhaps there is an energetic trade-off between somatic growth and reproductive fitness. The correlations among initial motility, post-thaw motility and neurulation indicated that fresh sperm suspensions with higher percentages of motile sperm would be more tolerant to cryopreservation, and have higher post-thaw motilities and fry production rates. In a previous study of cryopreservation of channel catfish sperm [13] there was no relationship between pre-freeze motility and post-thaw motility among 50 males with initial motilities of 70% or greater (high quality for catfish sperm collected by crushing of the testis). This seems to be in conflict with the correlation found between initial and post-thaw motility in the present study. However, close examination of the methods reveals that despite using only high-quality sperm samples, the previous study used hand-processing of straws and undetermined and uncontrolled sperm concentrations. From this perspective, the conclusion from previous publications supports the results from this study: standardization of protocols, especially sperm numbers, will yield consistent and predictable quality. Further studies focusing on hatchery management for hybrid catfish could establish quantified relationships among factors such as motility before freezing and after thawing, neurulation rates, and hatching, which would be of value for commercial use in sample selection.
It is ironic that although there is considerable potential similarity between the processing of bull semen and fish sperm in principles, procedures, and technology, there has been little effort to adapt the livestock model for automated processing to aquatic species [16]. Cryopreserved bull semen has been far more widely accepted within agricultural production than cryopreserved fish sperm. Bull owners were willing to use cryopreserved sperm because they historically had breeder’s clubs for example, and shared access to high-value sires [11]. Fish breeders have typically utilized mass selection of populations or families [38] disregarding the breeding value of individuals and this has delayed application of cryopreserved products especially for species that can produce sufficient offspring each year simply by natural spawning (such as channel catfish) to meet demands for seedstock. As such, commercial-scale cryopreservation facilities do not yet exist for aquatic species, but with development of high-throughput facilities, sperm samples or fish can be transported for cryopreservation, and be shipped back to the original farm hatchery or laboratory, a third-party customer, or to germplasm repositories for storage or use [43]. The economics of establishing or integrating these capabilities into an existing fish hatchery have been investigated [9; 10], and germplasm repositories such as the USDA National Animal Germplasm Program are in operation (http://www.ars.usda.gov).
Conclusions
In summary, this study established a reliable and practical protocol for sperm cryopreservation of blue catfish by use of a commercial-scale automated straw processing system, and demonstrated the feasibility for high-throughput sperm cryopreservation for catfish. The detailed protocol was: 1) collection of sperm by crushing of dissected anterior testis in HBSS300 at a volume (ml) of 2 times the testis weight (g); 2) filtration of the sperm suspensions (down to 200-um mesh size) to remove large pieces of tissue; 3) determination of sperm concentration and adjustment to 2 × 109 sperm/ml; 4) mixing of 20% methanol in HBSS300 with an equal volume of sperm suspension to yield a final concentration of 10% methanol and 1 × 109 sperm/ml; 5) filling, sealing, and labeling of 0.5-ml CBS straws by the automated MAPI system; 6) freezing of samples in straws on horizontal racks in a programmable freezer at 5 °C/min from 4 °C to −80 °C (programmed using chamber temperature) and holding for 5 min; 7) removal of frozen samples and plunging them into liquid nitrogen for sorting into multi-compartment goblets under liquid nitrogen; 8) storage of the frozen samples in liquid nitrogen in storage Dewars; 9) transportation of the samples if necessary in vapor-phase shipping Dewars to the site of usage; 10) thawing of straws at 40 °C for 20 s in a water bath, and 11) release of thawed samples from the straws for use in fertilization or analysis within 12 hr of thawing. In addition to post-thaw motility, channel catfish eggs provide an alternative option to test cryopreserved sperm quality because blue catfish eggs are typically not available. After testing of post-thaw motility and fertilization with channel catfish eggs, thawed sperm showed fertility comparable to fresh sperm, which indicates that the high-throughput process is feasible for commercial application. The correlations among body and gonadal indices showed for sexually mature male blue catfish during the channel catfish spawning season, that the body length and weight were related and that relatively thinner fish had larger testes (and larger anterior portions of the testis). And, importantly, that sperm with higher initial motility yielded higher post-thaw motility and higher fertility when conditions such as sperm concentration were controlled.
Based on the results of this study, processing for cryopreservation based on an automated system can be used for mass production in catfish. Compared to other aquatic species, blue catfish is an atypical species, because sperm cannot be stripped from live males [3; 20]. Dissection of the testis requires extra time and labor, and usually killing of the male. Other large-bodied aquatic species that allow non-lethal sperm collection by stripping such as salmon or trout [31; 36] would be easier to adapt to high-throughput cryopreservation processes. The potential value of cryopreserved aquatic germplasm is essentially unexploited at present. Meanwhile, more studies are needed to increase the efficiency of cryopreserved sperm to equal or exceed the current potential production of using fresh sperm in commercial settings. This work would involve hatchery management and technique improvement outside of the laboratory. If cryopreserved sperm can be used to replace or serve as an alternative to live males, fish germplasm banks can be established based on this technology. In that way, hatchery operations and facilities will change; live males will be reduced or eliminated at the hatchery; fish diversity can be preserved; breeding programs will be more efficient, and in the event of disease or other catastrophic problems in the industry, specific fish populations can be reconstituted in a short time. Overall blue catfish can serve as a model fish species to develop high-throughput techniques for sperm cryopreservation including small-bodied aquarium fishes such as zebrafish used in biomedical research [39; 47]. Thus, this study can serve as a template for development of high-throughput cryopreservation technology for application across most aquatic species.
Acknowledgements
We thank A. Saale, C. Staudermann, D. Kuenz, E. Tan, J. Atilano, J. Christensen, J. Tanca, M. Doan, N. Novelo, R. Uribe, S. Harris, and L. Savolainen for technical assistance during the spawning season, data collection, and suggestions. We thank C. Green for advice during experiments and J. Daly for manuscript review. We thank Baxter Land Company for providing blue catfish males and hatchery access. This work was supported in part by funding from the Louisiana Sea Grant College Program, USDA special grants, USDA-SBIR, and the National Center for Research Resources of the National Institutes of Health. This manuscript has been approved for publication by the Director of the Louisiana Agricultural Experiment Station as number (2010-244-7314).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Avery J, Steeby J, Bosworth BG, Small BC. In: Producing hybrid catfish fry: workshop manual. USDA-ARS Catfish Genetics Research Unit; Mississippi State University National Warmwater Aquaculture Center, editor. Stoneville, MS: Mississippi State University, Delta Branch Experiment Station; 2005. [Google Scholar]
- 2.Bart AN, Dunham RA. Effects of sperm concentration and egg number on fertilization efficiency with channel catfish (Ictalurus punctatus) eggs and blue catfish (Ictalurus furcatus) spermatozoa. Theriogenology. 1996;45:673–682. doi: 10.1016/0093-691x(95)00413-3. [DOI] [PubMed] [Google Scholar]
- 3.Bart AN, Wolfe DF, Dunham RA. Cryopreservation of blue catfish spermatozoa and subsequent fertilization of channel catfish eggs. Transactions of the American Fisheries Society. 1998;127:819–824. [Google Scholar]
- 4.Bates MC, Tiersch TR. Preliminary studies of artificial spawning of channel catfish as male-females pairs or all-famale groups in recirculating systems. Journal of the World Aquaculture Society. 1998;29:325–334. [Google Scholar]
- 5.Bates MC, Wayman WR, Tiersch TR. Effect of osmotic pressure on the activation and storage of channel catfish sperm. Transactions of the American Fisheries Society. 1996;125:798–802. [Google Scholar]
- 6.Blaxter JHS. Sperm storage and cross-fertilization of spring and autumn spawning herring. Nature. 1953;172:1189–1190. [Google Scholar]
- 7.Bobe J, Labbe C. Chilled storage of sperm and eggs. In: Cabrita E, Robles V, Herraez MP, editors. Methods in reproductive aquaculture. Boca Raton, FL: CRC; 2008. pp. 222–223. [Google Scholar]
- 8.Boever BP. Agricultural Economics & Agribusiness. Baton Rouge, LA: Louisiana State University; 2006. Analysis of U.S. aquacultural producer preferences for genetic improvement and cryopreservation. [Google Scholar]
- 9.Caffey RH, Tiersch TR. Economics and marketing of cryopreservation fish sperm. In: Tiersch TR, Mazik PM, editors. Cryopreservation in aquatic species. Baton Rouge, LA: the World Aquaculture Society; 2000. pp. 388–408. [Google Scholar]
- 10.Caffey RH, Tiersch TR. Cost analysis for integrating cryopreservation into an existing fish hatchery. Journal of the World Aquaculture Society. 2000;31:51–58. [Google Scholar]
- 11.Chandler JE. Cryopreservation of sperm of dairy bulls. In: Tiersch TR, Mazik PM, editors. Cryopreservation in aquatic species. Baton Rouge, LA: the World Aquaculture Society; 2000. pp. 84–90. [Google Scholar]
- 12.Christensen JM, Tiersch TR. Cryopreservation of channel catfish spermatozoa: effect of cryoprotectant, straw size, and formulation of extender. Theriogenology. 1997;47:639–645. doi: 10.1016/s0093-691x(97)00022-8. [DOI] [PubMed] [Google Scholar]
- 13.Christensen JM, Tiersch TR. Cryopreservation of channel catfish sperm: effects of cryoprotectant exposure time, cooling rate, thawing conditions, and male-to-male variation. Theriogenology. 2005;63:2103–2112. doi: 10.1016/j.theriogenology.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Dong Q, Huang C, Tiersch TR. Control of sperm concentration is necessary for standardization of sperm cryopreservation in aquatic species: Evidence from sperm agglutination in oysters. Cryobiology. 2007;54:87–98. doi: 10.1016/j.cryobiol.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Dong Q, Eudeline B, Huang C, Tiersch TR. Standardization of photometric measurement of sperm concentration from diploid and tetraploid Pacific oysters, Crassostrea gigas (Thunberg) Aquaculture Research. 2005;36:86–93. [Google Scholar]
- 16.Dong Q, Eudeline B, Huang C, Allen, Tiersch TR. Commercial-scale sperm cryopreservation of diploid and tetraploid Pacific oysters, Crassostrea gigas. Cryobiology. 2005;50:1–16. doi: 10.1016/j.cryobiol.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Gravance GG, Vishwanath R, Pitt C, Garner DL, Casey PJ. Effects of cryopreservation on bull sperm head morphometry. Journal of Andrology. 1998;19:704–709. [PubMed] [Google Scholar]
- 18.Guest WC, Avault JW, Roussel JD. Preservation of channel catfish sperm. Transactions of the American Fisheries Society. 1976;105:469–474. [Google Scholar]
- 19.Jamieson BGM. Fish evolution and systematics: evidence from spermatozoa. Cambridge, UK: Cambridge University Press; 1991. [Google Scholar]
- 20.Lang RP, Riley KL, Chandler JE, Tiersch TR. The use of dairy protocols for sperm cryopreservation of blue catfish (Ictalurus furcatus) Journal of the World Aquaculture Society. 2003;34:66–75. [Google Scholar]
- 21.Linhart O, Rodina M, Flajshans M, Gela D, Kocour M. Cryopreservation of European catfish Silurus glanis sperm: Sperm motility, viability, and hatching success of embryos. Cryobiology. 2005;51:250–261. doi: 10.1016/j.cryobiol.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Makeeva AP, Emel'yanova NG. Early development of channel catfish, Ictalurus punctatus. Journal of Ichthyology. 1993;33:87–103. [Google Scholar]
- 23.Masser M, Dunham R. Production of hybrid catfish. Southern Regional Aquaculture Center (SRAC) 1998 [Google Scholar]
- 24.Mazur P. Principles of cryobiology. In: Fuller BJ, Lane N, Benson EE, editors. Life in the Frozen State. Boca Raton: CRC Press LLC; 2004. pp. 1–66. [Google Scholar]
- 25.Mazur P, Leibo SP, Seidel GE., Jr Cryopreservation of the germplasm of animals used in biological and medical research: importance, impact, status, and future directions. Biology of Reproduction. 2008;78:2–12. doi: 10.1095/biolreprod.107.064113. [DOI] [PubMed] [Google Scholar]
- 26.Muldrew K, Acker JP, Elliott JAW, McGann LE. The water to ice transition: implications for living cells. In: Fuller BJ, Lane N, Benson EE, editors. Life in the frozen state. Boca Raton, FL: CRC Press LLC; 2004. pp. 67–108. [Google Scholar]
- 27.Nelson JS, Crossman EJ, Espinosa-Perez H, Findley LT, Gilbert CR, Lea RN, Williams JD. Common and Scientific Names of Fishes from the United States. Bethesda, MD: Canada, and Mexico American Fisheries Society; 2004. [Google Scholar]
- 28.Pickett BW, Berndtson WE. Preservation of bovine spermatozoa by freezing in straws: a review. J. Dairy Sci. 1974;57:1287–1301. doi: 10.3168/jds.S0022-0302(74)85058-7. [DOI] [PubMed] [Google Scholar]
- 29.Polge C, Rowson LEA. Fertilizing capacity of bull spermatozoa after freezing at −79 °C. Nature. 1952;169:626–627. doi: 10.1038/169626b0. [DOI] [PubMed] [Google Scholar]
- 30.Saksena VP, Yamamoto K, Riggs CD. Early development of the channel catfish. The Progressive Fish-Culturist. 1961;23:156–161. [Google Scholar]
- 31.Scott AP, Baynes SM. A review of the biology, handling and storage of salmonid spermatozoa. Journal of Fish Biology. 1980;17:707–739. [Google Scholar]
- 32.Shaw JM, Jones GM. Terminology associated with vitrification and other cryopreservation procedures for oocytes and embryos. Human Reproduction Update. 2003;9:583–605. doi: 10.1093/humupd/dmg041. [DOI] [PubMed] [Google Scholar]
- 33.Small BC, Bates TD. Effect of low-temperature incubation of channel catfish Ictalurus punctatus eggs on development, survival, and growth. Journal of the world aquaculture society. 2001;32:189–194. [Google Scholar]
- 34.Sneed KE, Clemens HP. The morphology of the testes and accossory reproductive glands of the catfishes (Ictaluridae) American Society of Ichthyologists and Herpetologists. 1963;1963:606–611. [Google Scholar]
- 35.Steeby J, Avery J. Channel catfish broodfish and hatchery management. Stoneville, Mississippi: Southern Regional Aquaculture Center; 2005. [Google Scholar]
- 36.Stoss J. Fish gamete preservation and spermatozoa physiology. In: Hoar WS, Randall DJ, Donaldson EM, editors. Fish Physiology. New York, NY: Academic Press; 1983. pp. 305–350. [Google Scholar]
- 37.Tan E, Yang H, Tiersch TR. Determination of sperm concentration for small-bodied biomedical model fishes by use of microspectrophotometry. Zebrafish. 2010;7:233–240. doi: 10.1089/zeb.2010.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tave D. Inbreeding and brood stock management Food & Agriculture Organization of the United Nations (FAO) Rome, Italy: 2000. [Google Scholar]
- 39.Tiersch TR. Cryopreservation in aquarium fishes. Marine Biotechnology. 2001;3:S212–S223. doi: 10.1007/s10126001-0044-z. [DOI] [PubMed] [Google Scholar]
- 40.Tiersch TR, Goudie CA. Inheritance and variation of genome size in half-sib families of hybrid catfish. Journal of Heredity. 1993;84:122–125. [Google Scholar]
- 41.Tiersch TR, Goudie CA, Carmichael GJ. Cryopreservation of channel catfish sperm: storage in cryoprotectants, fertilization trials, and growth of channel catfish produced with cryopreserved sperm. Transactions of the American Fisheries Society. 1994;123:580–586. [Google Scholar]
- 42.Tiersch TR, Yang H, Jenkins JA, Dong Q. Sperm cryopreservation in fish and shellfish. In: Roldan ERS, Gomendio M, editors. Spermatology (Society of Reproduction and Fertility supplement 65) Nottingham, U.K: Nottingham University Press; 2007. pp. 493–508. [PubMed] [Google Scholar]
- 43.Tiersch TR, Wayman WR, Skapura DP, Neidig CL, Grier HJ. Transport and cryopreservation of sperm of the common snook, Centropomus undecimalis (Bloch) Aquaculture Research. 2004;35:278–288. [Google Scholar]
- 44.U.S. Department of Agriculture, editor. USDA-NASS. 2005 Census of aquaculture. USDA National Agricultural Statistics Service; 2005. [Google Scholar]
- 45.Viveiros ATM, So N, Komen J. Sperm cryopreservation of African catfish: cryoprotectants, freezing rates and sperm:egg dilution ratio. Theriogenology. 2000;54:1395–1408. doi: 10.1016/s0093-691x(00)00462-3. [DOI] [PubMed] [Google Scholar]
- 46.Withler FC. Cryopreservation of spermatozoa of some freshwater fishes cultured in South and Southeast Asia. Aquaculture. 1982;26:395–398. [Google Scholar]
- 47.Yang H, Carmichael C, Varga ZM, Tiersch TR. Development of a simplified and standardized protocol with potential for high-throughput for sperm cryopreservation in zebrafish Danio rerio. Theriogenology. 2007;68:128–136. doi: 10.1016/j.theriogenology.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang H, Hazlewood L, Walter RB, Tiersch TR. Sperm cryopreservation of a live-bearing fish, Xiphophorus couchianus: male-to-male variation in post-thaw motility and production of F1, hybrid offspring. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 2009;149:233–239. doi: 10.1016/j.cbpc.2008.10.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang H, Norris M, Winn R, Tiersch TR. Evaluation of cryoprotectant and cooling rate for sperm cryopreservation in the euryhaline fish medaka Oryzias latipes. Cryobiology. 2010 doi: 10.1016/j.cryobiol.2010.07.006. In Press, Accepted Manuscript. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Q, Cooper RK, Tiersch TR. Overview of Ictalurus genomes: nuclear DNA content, diploid chromosome features, and physical mapping of genes. American Fisheries Society Symposium. 1999;24:257–262. [Google Scholar]