Abstract
Objective
Our aim was to determine the effects of fetal exposure to propoxur and pyrethroids, on child neurodevelopment at 2 years of age.
Patients and Methods
Mothers were prospectively recruited during mid-pregnancy in Bulacan, Philippines where multiple pesticides including propoxur, cyfluthrin, chlorpyrifos, cypermethrin, pretilachlor, bioallethrin, malathion, diazinon and transfluthrin are used. To detect prenatal exposure to these pesticides, maternal hair and blood, infant’s hair, cord blood, and meconium were analyzed for the pesticides by gas chromatography/mass spectrometry. Infants were examined at 2 years of age with 95.1% follow up rate and their neurodevelopment outcome was assessed by the Griffiths Mental Developmental Scale (N=754).
Results
Meconium analysis was the most sensitive method to detect fetal exposure to pesticides and exposure was highest for propoxur (21.3%) and the grouped pyrethroids (2.5% - bioallethrin, transfluthrin, cyfluthrin and cypermethrin). Path analysis modeling was performed to determine the effects of fetal exposure to propoxur and pyrethroids on the child’s neurodevelopment at 24 months of age while controlling for confounders. Only singletons and those with complete data for the path analysis were included (N=696). Using a path analysis model, there was a significant negative (β= −0.14, p<0.001) relationship between prenatal pesticide exposure to propoxur and motor development at 2 years of age after controlling for confounders, e.g., infant gender, socioeconomic status, maternal intelligence, home stimulation (HOME), postnatal exposure to propoxur and blood lead level at 2 years of age.
Conclusion
At 2 years of age, prenatal exposure to propoxur was associated with poorer motor development in children.
Introduction
There is such widespread use of pesticides that human exposure to these compounds is inevitable (USEPA, 2009; Waliszewski et al., 1998). Pesticide exposure in women while pregnant is a major concern, since most pesticides are neurotoxicants and the brain of the fetus and newborn infants are highly vulnerable to these toxicants due to the rapid growth and development of their brain (Bruckner, 2000; Eriksson, 1997; Barone et al., 2000), higher dose of pesticides per body weight (Weiss 2000) and lower activity and levels of enzymes that detoxify the pesticides (Holland et al. 2006). Although the recognizable effects of maternal exposure to low doses of environmental pesticides are minimal, serious concerns have been raised about their adverse effects on the fetus, particularly on subsequent neurodevelopmental, learning and behavioral difficulties in the children. A number of studies on prenatal exposure to organophosphate pesticides have been found to be associated with increased number of abnormal reflexes in newborn infants as assessed by the Brazelton Neonatal Behavioral Assessment Scale (Engel et al. 2007; Young et al. 2005). In young children, prenatal and/or postnatal exposure to organophosphates have been associated with decreased scores on the Stanford-Binet copying test, mean reaction time, poorer short term memory, executive function and lower MDI and PDI scores on the Bayley Scales of Infant Development term memory (Eskenazi et al. 2007; Handal et al. 2008; Rauh et al. 2006, Bouchard et al. 2010; Grandjean et al. 2006; Ruckart et al. 2004; Eskenazi 2007). The carbamates are another class of pesticides that have been increasingly used worldwide. Carbamates, e.g., propoxur, are similar to organophosphates in their action of inhibiting acetylcholinesterase, but unlike organophosphates, the carbamates are transient cholinesterase inhibitors and are hydrolyzed from the cholinesterase enzymatic site within 48 hours (Bjorling et al., 2008). Thus, carbamate toxicity tends to be of shorter duration compared to the organophosphates, although the mortality rates associated with exposure to these chemical classes are still similar (Rotenberg et al., 1995). Due to their lower toxicity and shorter half life, carbamates have been used widely in place of organophosphates, particularly for home pesticides. However, despite their widespread use, there has been no information on the reproductive or developmental effects of propoxur in humans, particularly on the fetus, newborn infant and young children (USEPA 1989; USEPA 1999) In a few animal studies on rats orally exposed to propoxur, fetotoxic effects e.g., decreased numbers of pups, and depressed fetal weight have been reported. Most studies on the adverse developmental effect of fetal or early childhood exposure to cholinesterase inhibitor pesticides have been on the organophosphates (Rosas & Eskenazi 2007). On the other hand, a review of the literature through PubMed has revealed no study dealing with the adverse effects of pre- and postnatal exposure to propoxur on the children’s neurodevelopment. In the Philippines, the use of carbamate is widespread, particularly for home pesticides. The aim of this study is to report on the prevalence of prenatal and early childhood exposure to the carbamate pesticide, propoxur and its adverse effect on the neurodevelopment of children at 2 years of age.
Materials and Methods
Pesticide
Exposure Pregnant women were prospectively enrolled into the study at midgestation at the Bulacan Provincial Hospital (BPH) outpatient clinic. Bulacan is an agro-industrial province of the Philippines. A preliminary survey of the study area showed predominant use of the following pesticides at home or in the farm: cyfluthrin/propoxur (73%), chlorpyrifos (37%), cypermethrin (31%), pretilachlor (28%), bioallethrin (26%), malathion (15%), diazinon (12%) and transfluthrin (11%). This study was approved by the Human Investigation Committee at Wayne State University, the University of the Philippines and the Bulacan Provincial Hospital. Informed consent was obtained from the mothers for themselves and their infants. Maternal blood and hair were obtained at midgestation and at birth cord blood, meconium, and infant hair were collected after birth to analyze for the above pesticides, including lindane and DDT. Pesticide analysis was by gas chromatography/mass spectrometry (GC/MS) using previously published procedures (Bielawski et al., 2005; Corrion et al., 2005; Ostrea et al., 2006, Posecion et al 2006).
Neurodevelopmental Outcome
The Griffiths test assesses five developmental scales: motor, social, hearing/language, eye/hand coordination and performance (Griffiths 1976; Huntley 1996). Based on the literature (Diamond A, 2007, Rendeli et al 2002, Burns et al 2004, Hart et al 2004, Bloom et al 2001) we choose three factors to represent neurodevelopment at 24 months. All three factors were from the Griffith Mental Development Scales (Griffiths 1976; Huntley 1996) 1) Locomotor subscale (gross motor development including balance and coordination of movements), 2) social development (Personal-Social [daily activities and child interaction] and Language subscales [receptive and expressive language]), and 3) Performance subscale (visuospatial skills and reaction time).
Neurobehavioral development of the children was assessed by the Griffiths Mental Developmental Scale (Griffiths 1976; Huntley 1996) by one of three Griffiths certified, developmental pediatricians at 24 months. Their inter-rater agreement by Cohen’s kappa was 0.8525 (p<0.0001).
Covariates
Several potential confounders to the child’s neurodevelopmental performance were also evaluated including infant’s gender, maternal intelligence (assessed by a modified WAIS-III performance subscale; Wechsler 1981), status of the home stimulation of the child (Home Observation for Measurement of the Environment [HOME]; Caldwell & Bradley, 1984) and socioeconomic status ([SES] Roberto Scale; Roberto, 2002). The Roberto scale is widely used in the Philippines and is based on home structure and appearance. The scale ranges from A (highest) to E (lowest).
Prior to study inclusion, the validity of the WAIS-III for use this the study population was performed to determine subtests that were suitable for the study population. A group of 30 females that were not among the current subjects but from the same population were administered the performance subtest of the WAIS-III. Based on their performance scores, the following subtests were selected to comprise the short form for computing the performance IQ: Picture completion, matrix reasoning, picture arrangement and object assembly. For the Griffiths test, covariates included postnatal exposure to propoxur by hair analysis of the children, and serum lead at 24 months.
Statistical analysis
Mean (standard deviation) and frequency distribution were calculated to describe the demographic and socioenvironmental characteristics of the study population. Comparisons were made between pesticide exposed and non-exposed infants on multiple demographic/ socioenvironmental characteristics as well as the infant’s gestation, birth weight, length, and head circumference by the Student t-test or Mann-Whitney test for continuous variables and the Pearson’s chi-square analysis or Fisher’s Exact Test for categorical variables.
Structural Equation Modeling using LISERL 8.72 was performed to determine the effects of fetal exposure to pesticides (as determined by meconium analysis) on the child’s neurodevelopment at 24 months as assessed by the Griffiths test while controlling for the following confounders: infant gender, socioeconomic status, maternal intelligence and HOME. Chlorpyrifos, pretilachlor, malathion, diazinon, lindane and DDT were excluded in the outcome analysis since only a few matrices were positive (<1.0 %) for these pesticides. Similarly, due to the very low prevalence of pyrethroids (2.5%), we preliminarily assessed the relationship of these pesticides with 24 month neurodevelopment before the structural equation model was tested. No significant bivariate relationships between pyrethroids and neurodevelopment at 24 months were found. As a result of the low prevalence and non-significant results, these pesticides were also excluded from the path analysis. Model fit was assessed using the comparative fit index (CFI), root mean square error of approximation (RMSEA) and 90% confidence interval for RMSEA. CFI> 0.95 and RMSEA <0.06 indicate a good fitting model (Hu & Bentler, 1999). Due to non-normality in the data the Satorra-Bentler scaled chi-square was used (Satorra & Bentler, 1994). Paths that were not significant at p≤0.05 were removed from the model unless there were strong theoretical reasons for including them. Modifications to the model were based on both substantive clinical and statistical grounds. For the final model, results are reported as standardized regression coefficients. Prior to performing the path analysis, SPSS version 19 was used to assess the assumptions of linearity and multivariate normality. To identify multivariate outliers Mahalanobis distance, which is a measure of the multivariate distance of a case from the centroid of all other cases, was used (Tabachnick & Fidell, 2007). Mahalanobis distance can be evaluated using a χ2 statistic. The probability of the χ2 statistic is an indication of the probability that the values for that case come from the same multivariate distribution of the other cases. A criteria of p < 0.001 was used to indicate multivariate outliers. Cases with p < 0.001 have a very small probability of coming from the same multivariate distribution as the other cases. Using this criterion multivariate outliers were removed from the data set. An iterative process was used to clean the data. To reduce the influence of outlying cases, univariate outliers were winsorized and extremely skewed variables transformed.
Results
A total of 793 mother/infant dyads were initially enrolled at birth and 754 dyads (95.1%) were successfully followed up to 2 years of age. Among those not assessed at follow up (N=39), six children were deceased, 29 could not be located, and four mothers voluntarily withdrew from the study. The only significant difference between the 754 mother/infant pairs assessed at 2 years and those not assessed was marital status. Those not assessed were less likely to be married (43.6% vs 73.6%, p<0.03).
Of the 754 mother/infant pairs, only singletons were included in the analysis (N=741). Nine cases were identified as multivariate outliers among the set of predictors and outcomes using Mahalanobis distance, p < 0.001. The multivariate outliers were removed from the dataset leaving 732 cases. Overall only 1.3% of all data were missing. Complete data were available for 590 cases. Cases missing data on more than 25% of the variables of interest were dropped from further analyses (N = 9). Of the remaining data, 133 cases were missing data on at least one variable. Missing data were then imputed using PRELIS 2.52. Data could not be imputed for 27 cases leaving 697 cases with complete data after imputation. The data were then imported back into SPSS and a second evaluation for multivariate outliers was performed. One additional case was identified as a multivariate outlier and removed leaving a final data set of 696 cases with complete data for the path analysis.
From an initial survey of the study site, the predominant pesticides that were used in the homes included propoxur, cyfluthrin, bioallethrin, transfluthrin and chlorpyrifos. The principal farm pesticides were chlopyrifos, cypermethrin, pretilachlor, malathion and diazinon. The prevalence of pesticide exposure based on the analysis of maternal hair, maternal blood, cord blood, infant hair or meconium obtained at birth, is shown in Table 1. Although most pesticides were detected in the various matrices, the prevalence of chlorpyrifos, pretilachlor, malathion, diazinon, lindane and DDT was <1.0% (Ostrea el al, 2009) and are excluded from the table and outcome analysis. Cyfluthrin, bioallethrin, transfluthrin and cypermethrin belong to the family of pyrethoids and are presented as a group because of similarity in their pharmacologic action. The number of samples was comparatively less for cord blood and meconium because cord blood was not obtained in those infants born at home or in an outside hospital. For meconium, some samples were insufficient in amount for analysis. The highest rate of pesticide exposure (Table 1) was to propoxur (21.3% in meconium and 21.1% in maternal hair) and to pyrethroids (2.5% in meconium and 13.9% in maternal hair), especially bioallethrin. In a previous publication, we compared pesticide exposure in subjects who had all five matrices available for analysis and the highest rate of pesticide exposure was detected in meconium followed by maternal hair (Ostrea et al., 2009). Thus, meconium and maternal hair are ideal matrices to analyze for prenatal exposure to pesticide.
Table 1.
Prevalence of prenatal exposure to propoxur and grouped pyrethroids (bioallethrin, cyfluthrin, transfluthrin and cypermethrin) by the analysis of meconium, cord blood, infant hair, maternal hair and maternal blood at birth.
| Meconium (N=717) | Cord blood (N=630) | Infant hair (N=730) | Maternal hair (N=740) | Maternal blood (N=740) | |
|---|---|---|---|---|---|
| Propoxur | 153 (21.3%) | 12 (1.9%) | 1 (0.1%) | 156 (21.1%) | 22 (3.0%) |
| Grouped pyrethroids | 18 (2.5%) | 0 (0.0%) | 0 (0.0%) | 103 (13.9%) | 0 (0.0%) |
Relation between exposure and neurodevelopment
To determine the relationship between fetal exposure to pesticide and the child’s neurodevelopment at 2 years of age, fetal exposure to pesticide was based on meconium analysis, because meconium is a sensitive matrix to analyze for prenatal exposure to pesticide and, because, unlike maternal hair, meconium is produced by fetal tissue and therefore representative of fetal exposure to the pesticides. The demographic characteristics of the mother/infant dyads of this subgroup (N=680) are shown in Table 2. There were no significant differences in maternal and infant demographic characteristics or pregnancy complications in the pesticide-exposed versus non-exposed infants. Birth weight, head circumference, length and gestation were not significantly different between groups. The characteristics of the home and environment are shown in Table 3 and were not significantly different in the exposed versus non exposed groups except for a higher use of water seal toilet in the exposed group (87.4% versus 80.1%, p<0.04) and a higher use of well water in the unexposed group (44.9% versus 34.8%, p=0.02). Overall, there was an high (>90%) prevalence of flies, roaches and mosquitoes in the homes of both groups the subjects and the overall use of home pesticide was reported from 16.8% of the homes.
Table 2.
Demographics of the study population
| Total | Not exposed | Exposed | p* | |
|---|---|---|---|---|
| Maternal characteristics | (N=696) | (N=537) | (N=159) | |
| Maternal age (yr) ** | 25.8 (5.9) | 25.7 (5.8) | 26.2 (6.0) | 0.38 |
| Gravida ** | 2.4 (1.7) | 2.4 (1.7) | 2.3 (1.5) | 0.52‡ |
| Parity ** | 1.2 (1.5) | 1.3 (1.6) | 1.1 (1.4) | 0.50‡ |
| Married % | 74.0 | 73.3 | 76.1 | 0.48 |
| Oligohydramnios % | 1.8 | 1.5 | 2.7 | 0.30† |
| Meconium stained % | 11.6 | 11.4 | 12.2 | 0.78 |
| C-section % | 16.6 | 15.9 | 18.9 | 0.38 |
| Vertex presentation % | 97.2 | 97.4 | 96.9 | 0.78† |
| PROM > 24 % | 0.9 | 1.0 | 0.6 | 1.00† |
| Hypertension/PIH % | 5.2 | 6.0 | 2.5 | 0.08 |
| Vaginal bleeding % | 0.4 | 0.6 | 0 | 1.00† |
| Active smoking % | 2.1 | 2.1 | 2.0 | 1.00† |
| Cocaine use % | 0 | 0 | 0 | |
| Opiate use % | 0.3 | 0.4 | 0 | 1.00† |
| Marijuana use % | 0.4 | 0.6 | 0 | 1.00† |
| Alcohol use % | 0.7 | 0.7 | 0.6 | 1.00† |
| Methamphetamine use % | 0.9 | 1.1 | 0 | 0.37† |
| Infant characteristics | ||||
| Male % | 54.5 | 55.8 | 50.3 | 0.22 |
| Gestation (wk) ** | 38.7 (1.2) | 38.6 (1.2) | 38.6 (1.4) | 0.38 |
| Weight (gm) ** | 2900.6 (424.4) | 2911.9 (425.9) | 2862.0 (418.3) | 0.20 |
| Length (cm) ** | 48.8 (2.4) | 48.9 (2.4) | 48.5 (2.6) | 0.10 |
| Head circumference (cm) ** | 33.1 (1.5) | 33.1 (1.5) | 33.0 (1.6) | 0.27 |
| Oxygen use after birth % | 5.7 | 5.1 | 7.7 | 0.21 |
| Resp distress % | 1.0 | 0.7 | 1.9 | 0.20† |
| Transient tachypnea % | 1.2 | 1.1 | 1.3 | 1.00† |
| Meconium aspiration syndrome % | 0.4 | 0.4 | 0.6 | 0.54† |
| Asphyxia % | 1.0 | 0.7 | 1.9 | 0.20† |
| Jaundice % | 3.2 | 3.0 | 3.8 | 0.61 |
| Sepsis % | 11.1 | 10.1 | 14.6 | 0.12 |
Based on Student t-test or Mann-Whitney test ‡ for continuous variables and Pearson’s chi-square analysis or Fisher’s Exact Test† for categorical variables
Mean ± standard deviation
Table 3.
Characteristics of the home and environment of the study population
| Total | Non-Exposed | Exposed | p* | |
|---|---|---|---|---|
| Home/Environment | (N=696) | (N=537) | (N=159) | |
| Roberto scale (A – C) % | 38.9 | 37.6 | 43.4 | 0.19 |
| Makeshift homes % | 6.8 | 6.3 | 8.2 | 0.42 |
| Water seal toilet % | 81.8 | 80.1 | 87.4 | 0.04 |
| Source of water (well) % | 42.6 | 44.9 | 34.8 | 0.02 |
| Waste water in canals/river % | 62.4 | 61.7 | 64.8 | 0.48 |
| Flies % | 91.8 | 90.3 | 96.9 | 0.008 |
| Cockroach % | 90.7 | 90.5 | 91.2 | 0.79 |
| Mosquitoes % | 98.0 | 97.6 | 99.4 | 0.21† |
| Cellphone % | 45.1 | 44.5 | 47.2 | 0.55 |
| Use of home pesticides % | 16.8 | 16.9 | 16.4 | 0.86 |
| Lead recycling plant % | 6.5 | 5.6 | 9.5 | 0.08 |
Pearson’s chi square analysis
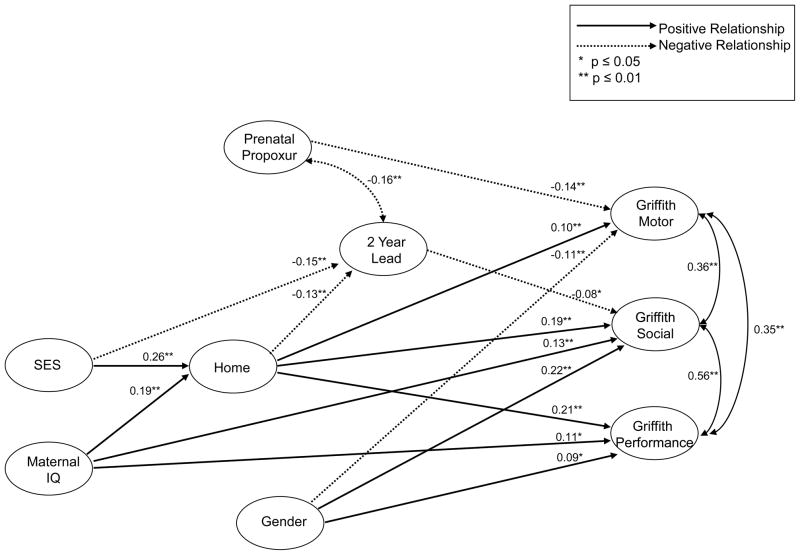
The final path model showing the effects of fetal exposure to environmental pesticides on child’s neurodevelopment is shown in Figure 1. Only variables that were significantly related to neurodevelopmental outcome were included in the figure. Standardized coefficients are presented. Overall, model fit was excellent χ2 (27, n=696) = 52.35, p=0.0006, RMSEA=0.037 (90%CI, 0.021–0.052), and CFI=0.98.
Figure 1.
Path analysis model showing the relationship between prenatal exposure to propoxur and confounders on child neurobehavioral development as assessed by the Griffiths test at 24 months of age
Direct effects on neurodevelopment
Exposure to propoxur was negatively related to motor development (β= −0.14, p<0.001), but was unrelated to social and performance development. Other variables were related to neurobehavioral development at 24 months. The HOME score (β=0.10, p=0.006), and infant gender with boys scoring higher than girls (β= −0.11, p=0.004) were related to motor development. Along with exposure to propoxur, the HOME score and gender accounted for 4% of the total motor development variance. Social development was predicted by 2-year lead exposure (β=−0.08, p=0.03), the HOME score (β=0.19, p<0.001), infant gender (girls scored higher than boys) (β=0.22, p<0.001), and maternal IQ (β=0.13, p<0.001). These variables accounted for 12% of social development variance. For performance development the related variables were HOME (β=0.20, p<0.001), infant gender, with girls scoring higher than boys (β=0.09, p=0.046) and maternal IQ (β=0.11, p=0.01). These variables accounted for 7% of performance development variance.
Indirect and Total Effects
In addition to the direct effects listed above, the following variables had indirect effects on 24 month neurobehavioral development that were mediated by other study variables. SES had significant indirect effects on motor (β=0.02, p=0.02), social (β=0.06, p<0.001) and performance development (β=0.05, p<0.001). The effects of SES on development were mediated through lead exposure and HOME environment. In addition to its direct effects on development, maternal IQ also had significant indirect effects on motor (β=0.02, p=0.02), social (β=0.04, p<0.001) and performance development (β=0.04, p=0.002). The indirect effects of maternal IQ on development were mediated through the HOME environment.
Discussion
This prospective, cohort study of fetal exposure to environmental pesticides, specifically propoxur and pyrethroids, and their potential adverse effects on the neurodevelopment of the children at 2 years of age is the first study to examine five matrices (maternal hair, maternal blood, cord blood, infant hair and meconium) to determine antenatal pesticide exposure: As previously reported (Ostrea et al., 2009), meconium was found to have the highest positive rate to pesticides. This is consistent with the high detection rate of many xenobiotics in this matrix including licit and illicit drugs, nicotine metabolites and alcohol metabolites (Ostrea et al., 1999). The repository nature of meconium provides a wide window of xenobiotic exposure. Meconium is first formed at around the third or fourth week of gestation, thus, most xenobiotics that the fetus is exposed to during gestation are deposited in meconium through fetal swallowing and/or bile secretion (Ostrea et al., 1989). Since meconium, unlike fetal urine, is not normally excreted in utero, compounds that deposit in meconium accumulate and increase in concentration, thus enhancing their detection after birth.
The high exposure rate to home pesticides, even in an agricultural area such as Bulacan, is likely due to the high prevalence of flies, roaches and mosquitoes in the area. This observation parallels reports of high exposure rate to home pesticides among pregnant women and their infants residing in urban areas (Whyatt et al., 2003; Whyatt et al., 2004; Ostrea et al., 2002). Thus, whether in urban or rural areas, pesticide use is widespread and constitutes a serious health risk in pregnant women. In our study, spray pesticides were commonly used, principally Baygon™ (91.5%) which contains propoxur and cyfluthrin. Organophosphates were of low prevalence because they were not the common pesticides used at home. Improper use of these home pesticides was evident in a subgroup study showing that 39.9% of the spraying was done by the pregnant woman, themselves and reentry time to the sprayed area was ≤ 60 minutes in 73.2% of the cases. Poor education and inadequate labeling regarding the safe use of the pesticide were major reasons for their improper use.
The effects of prenatal exposure to pesticides on the neurodevelopmental outcome in the children was assessed at 24 months of age by the Griffiths Mental Development Scales since several investigators have suggested that the effects of prenatal exposures may not be obvious in early life (Waddington et al., 1957; Scarr-Salapetek, 1976; McCall 1981). Neuronal plasticity, which is the ability of the nervous system to continue to remodel and change throughout the developmental period in response to environmental insults (Rice & Barone, 2000; Barone et al., 2000; Bourgeois et al., 1993; Rakic et al., 1986; King et al., 1991) may act as a temporary compensating mechanism to interference in brain development and anatomical and functional effects of developmental insults may be subtle at early life but become apparent at later ages (Rice 1996; Qiao et al., 2002; Qiao et al., 2003). We also chose to not examine the global overall Griffith’s score (general quotient) but instead, to examine subfactors to see if there was a differential influence of pesticide exposure on specific neurodevelopmental outcomes. Since motor ability may influence performance in the cognitive, social, and performance domains, especially true in risk populations, (Rendeli et al., 2002; Burns et al., 2004) we chose to assess motor individually. As the literature has suggested there is a relation between sociability and language, (Diamond, 2007; Hart et al., 2004; Bloom et al., 2001; Carpenter, et al., 1998;), we chose to examine these scales as a single social/language factor and since both the Griffiths hand/eye coordination subscale and the performance subscales are performance measures we examined them as a single factor labeled performance.
Our results show that propoxur has a significant adverse effect on the Griffiths motor subscale at 24 months. This is important since motor development determines the sequence in which certain perceptual, language and cognitive abilities unfold. (Bushnell et al., 1993; Webster R et al., 2005; Diamond A, 2007). In a study of 378 children at age 5–6 years, quantitative and qualitative aspects of motor performance were related to several aspects of cognition, after controlling for the influence of attention (Wassenberg at al., 2005). Motor development is one of the earliest functions to develop in children. A study of Griffiths test among Filipino children at 24 months (Reyes et al., 2010), showed a relatively higher mean subquotient scores in motor tasks, self-help and social which coincide with developmental expectations during infancy, which is basically to develop ambulation and an awareness of their surroundings (First & Palfrey 1994). Mean subquotient in hearing and language have been found to be among the lowest, as emergence of speech is not generally expected until just before the toddler years (Capute & Accardo 1996). The early development of motor function in children may be a reason why adverse effect of prenatal exposure to propoxur was evident in motor compared to other functions (social and performance). However, because of delayed neurotoxicity, adverse effects on other neurobehavioral functions may occur later. Thus, it is important that follow up of the children is made which is what we are currently doing.
The influence of other factors on motor development were also observed in this study, such as HOME and infant gender. For social development the related variables were two year lead exposure, the HOME score, infant gender, and maternal IQ. For performance development the related variables were the HOME score, infant gender, and maternal IQ.
This is the first study to report on the adverse effect of propoxur, a carbamate, on the child’s neurodevelopment. The strengths of this study are its large sample size (N=754 mother/infant dyads) that was prospectively studied with a high (95.1%) follow-up rate and the use of fetal tissue (meconium) as a sensitive biomarker of fetal exposure to pesticides. Propoxur, inhibits acetylcholinesterase and elicit cholinergic hyperstimulation similar to the action of organophosphates. However, unlike the organophosphates, the anticholinesterase action of propoxur is transient and may last only from minutes to a few hours (Bjorling et al., 2007). Yet, as shown in this study, prenatal exposure to propoxur is associated with neurodevelopmental delays in children similar to what have been observed with organophosphates (Eskenazi et al. 2007; Handal et al. 2008; Rauh et al. 2006, Bouchard et al. 2010; Grandjean et al. 2006; Ruckart et al. 2004; Eskenazi 2007), which indicates the high vulnerability of the fetal brain even to transient prolongation of acetylcholine action secondary to pesticide effect. On the other hand, other pharmacologic action of anticholinesterase pesticides may also play a role that is not mediated by a neurotransmitter (acetylcholine) effect. In undifferentiated PC12 cells which is a standard in vitro model for neuronal development, exposure of the cells to organophosphates and carbamate have shown inhibition of DNA synthesis, signs of oxidative stress as evidenced by measurements of lipid peroxidation, transcriptional effects on neurotrophic factors, their receptors and modulators and a shift of the transmitter fate of the cells away from the cholinergic phenotype and toward the catecholaminergic phenotype. (Slotkin TA et al., 2007, Slotkin et al., 2008).
With regards to the pyrethroids, there was very little prenatal exposure as measured by meconium and there was no relationship between pyrethroid exposure and neurodevelopment at 24 months. However, the smaller sample size of infants positive for grouped pyrethroids (2.5%) compared to propoxur (21.3%) may have biased our results towards the null, resulting in our failure to observe an adverse effect. The absence of identifiable, significant adverse direct effects of pyrethroid at age 24 months should not be interpreted as a benign exposure. In animal studies following exposure to pyrethroids, delayed neurologic effects were observed. Early postnatal exposure to bioallethrin in rats showed that, as adults, the rats exhibited hyperactivity and a decrease in muscarinic ACh receptors, as well as decreased learning as measured by a Morris swim maze (Eriksson 1997; Talts, et al., 1998). Neonatal rats have been shown to be 4–17 times more vulnerable to the acute toxicity of pyrethroids (including permethrin (type I), deltamethrin (type II), cypermethrin (type II)) than adult rats (Sheets at al., 2000; Cantalamessa F, 1993). The higher toxicity in neonatal rats is affected by the lower capacity for metabolic detoxification doses (Sheets et al, 1994; Sheets LP 2000).
In the outcome analysis of pesticides as predictors of the Griffiths test, we did not include pesticides with very low prevalence (<1%) e.g., chlorpyrifos, pretilachlor, malathion, diazinon, and DDT (Ostrea el al, 2009) because of insufficient power to demonstrate any significant relationship. Interactions between pesticides with regards to the outcome measures were also not analyzed, except for propoxur and pyrethroids, since interactions between pesticides of very low prevalence would have given extremely low power. For propoxur and grouped pyrethroids, a post hoc simple regression analysis to determine interaction between the two groups of pesticide did not add anything to the prediction of Griffiths outcome.
In the follow up study of the children up to 2 years of age, we determined ongoing exposure to propoxur and pyrethroids in children’s hair since these pesticides constituted the highest pesticide exposure antenatally. The ongoing exposure at 2 years of age to propoxur was 12.4% and 0.6% to bioallethrin. Unlike prenatal exposure, we did not find any significant association between postnatal exposure to propoxur and bioallethrin to the Griffiths test. This indicates that prenatal pesticide exposure has the highest risk to subsequent adverse neurodevelopment in children since it is during fetal life that brain growth and development are its maximum rate and vulnerability. (Bruckner, 2000; Eriksson, 1997; Barone et al., 2000).
In summary, a longitudinal study of a large group of children in the Philippines who were prenatally exposed to propoxur showed subsequent adverse effect of the pesticide on motor development at 24 months of age. This is an important observation since propoxur is widely used in the United States and globally, as well (USEPA 2009). The co-morbid association of motor performance and subsequent cognitive and language development is a compelling reason for the long term, longitudinal study of these prenatally, pesticide exposed children.
Acknowledgments
This study was supported by grants from NIH/NICHD (R01HD039428), US Environmental Protection Agency (RFA 2001-STAR-H1. No. R829395) and EHS Center Grant P30 ES06639 from NIH/NIEHS, Wayne State University, Detroit, Michigan
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barone S, Jr, Das KP, Lassiter TL, White LD. Vulnerable processes of nervous system development: a review of markers and methods. Neurotoxicology. 2000;21:15–36. [PubMed] [Google Scholar]
- 2.Bielawski D, Ostrea E, Posecion N, Corrion M, Seagraves J. Detection of several classes of pesticides and metabolites in meconium by gas chromatography/mass spectrometry. Chromatographia. 2005;62:623–29. doi: 10.1365/s10337-005-0668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjørling-Poulsen M, Andersen HR, Grandjean P. Potential developmental neurotoxicity of pesticides used in Europe. Environ Health. 2008;7:50. doi: 10.1186/1476-069X-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloom L, Tinker E, Kofsky E. The Intentionality Model and Language Acquisition: Engagement, Effort, and the Essential Tension in Development Author(s) Monographs of the Society for Research in Child Development. 2001;66(4) [PubMed] [Google Scholar]
- 5.Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the Macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle CA, Decoufle P, Yeargin-Allsopp M. Prevalence and health impact of developmental disabilities in US children. Pediatrics. 1994;93:399–403. [PubMed] [Google Scholar]
- 7.Bruckner JV. Differences in sensitivity of children and adults to chemical toxicity: The NAS Panel Report. Regulatory Toxicol Pharmacol. 2000;31:280–85. doi: 10.1006/rtph.2000.1393. [DOI] [PubMed] [Google Scholar]
- 8.Burns Y, O’Callaghan M, McDonell B, Rogers Y. Movement and motor development in the ELBW infant at 1 year is related to cognitive and motor abilities at 4 years. Early Human Dev. 2004;80:19–29. doi: 10.1016/j.earlhumdev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Bushnell E, Boudreau J. Motor development and the mind: The potential role of motor abilities as a determinant of aspects of perceptual development. Child Dev. 1993;64:1005–1021. [PubMed] [Google Scholar]
- 10.Caldwell BM, Bradley RH. Administration manual, revised edition: Home observation for measurement of the environment. Little Rock: University of Arkansas at Little Rock; 1984. [Google Scholar]; California Health and Human Services Agency. A Report to the Legislature. CHHS: Department of Developmental Services; 1999. Changesin the Population of Persons with Autism and Pervasive Developmental Disorders in Cali through 1998. [Google Scholar]
- 11.Cantalamessa F. Acute toxicity of two pyrethroids, permethrin, and cypermethrin in neonatal and adult rats. Arch Toxicol. 1993;67:510–513. doi: 10.1007/BF01969923. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter M, Nagell K, Tomasello M, Butterworth G, Moore C. Social cognition, joint attention, and communicative competence from 9 to 15 months of age. Monographs of the Society for Research in Child Development. 1998;63:1–174. Blackwell Publishing on behalf of the Society for Research in Child Development Stable URL: http://www.jstor.org/stable/1166214. [PubMed] [Google Scholar]
- 13.Corrion ML, Ostrea EM, Jr, Bielawski DM, Posecion NC, Jr, Seagraves JJ. Detection of prenatal exposure to several classes of environmental toxicants and their metabolites by gas chromatography-mass spectrometry in maternal and umbilical cord blood. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;882:221–29. doi: 10.1016/j.jchromb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Crump KS, Kjellstrom T, Shipp AM, Silvers A, Stewart A. Influence of prenatal mercury exposure upon scholastic and psychological test performance: benchmark analysis of a New Zealand cohort. Risk Anal. 1998;18(6):701–13. doi: 10.1023/b:rian.0000005917.52151.e6. [DOI] [PubMed] [Google Scholar]
- 15.Diamond A. Interrelated and interdependent. Dev Science. 2007;10:152–158. doi: 10.1111/j.1467-7687.2007.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engel SM, Berkowitz GS, Barr DB, et al. Prenatal Organophosphate Metabolite and Organochlorine Levels and Performance on the Brazelton Neonatal Behavioral Assessment Scale in a Multiethnic Pregnancy Cohort. Am J Epidemiol. 2007;165:1397–404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- 17.Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ Health Perspect. 1999;107:409–19. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksson P. Developmental neurotoxicity of environmental agents in the neonate. Neurotoxicology. 1997;18:719–26. [PubMed] [Google Scholar]
- 20.Grandjean P, Harari R, Barr D, Debes F. Pesticide exposure and stunting as independent predictors of neurobehavioral deficits in Ecuadorian school children. Pediatrics. 2006;117:546–56. doi: 10.1542/peds.2005-1781. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths R. The Abilities of Babies: A Study in Mental Measurement. University of London Press; London, UK: 1976. [Google Scholar]
- 22.Handal AJ, Lozoff B, Breilh J, Harlow SD. Neurobehavioral development in children with potential exposure to pesticides. Epidemiology. 2007;18(3):312–320. doi: 10.1097/01.ede.0000259983.55716.bb. [DOI] [PubMed] [Google Scholar]
- 23.Hart K, Fujiki K, Brinton B, Hart C. The relationship between social behavior and severity of language impairment. J Speech, Language Hearing Res. 2004;47:647–662. doi: 10.1044/1092-4388(2004/050). [DOI] [PubMed] [Google Scholar]
- 24.Holene E, Nafstad I, Skaare JU, Sagvolden T. Behavioural hyperactivity in rats following postnatal exposure to sub-toxic doses of polychlorinated biphenyl congeners 153 and 126. Behav Brain Res. 1998;94:213–24. doi: 10.1016/s0166-4328(97)00181-2. [DOI] [PubMed] [Google Scholar]
- 25.Holland N, Furlong C, Bastaki M, et al. Paraoxonase polymorphisms, haplotypes, and enzyme activity in Latino mothers and newborns. Environ Health Perspect. 2006;114:985–91. doi: 10.1289/ehp.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hollingshead A. Four factor index of social status. Department of Sociology, Yale University; New Have, CT: 1975. [Google Scholar]
- 27.Hu L, Bentler P. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 28.Huntley M. The Griffiths Mental Developmental Scales Manual from birth to two years. Assoc Res Infant Child Dev. 1996 [Google Scholar]
- 29.Jacobson JL, Jacobson SW, Humphrey HE. Effects of in utero exposure to PCBs and related contaminants on cognitive functioning in young children. J Pediatr. 1990;116:38–45. doi: 10.1016/s0022-3476(05)81642-7. [DOI] [PubMed] [Google Scholar]
- 30.King AJ, Moore DR. Plasticity of auditory maps in the brain. Trends Neurosci. 1991;14:31–37. doi: 10.1016/0166-2236(91)90181-s. [DOI] [PubMed] [Google Scholar]
- 31.McCall RB. Nature-nurture amd the two realms of development: A proposed integration with respect to mental development. Child Dev. 1981;52:1–12. [Google Scholar]
- 32.National Scientific Council on the Developing Child. The Timing and Quality of Early Experiences Combine to Shape Brain Architecture: Working Paper #5. 2007 http://www.developingchild.net.
- 33.Ostrea EM, Jr, Morales V, Ngoumgna E, Prescilla R, Tan E, Hernandez E, Ramirez GB, Cifra HL, Manlapaz ML. Prevalence of fetal exposure to environmental toxins as determined by meconium analysis. Neurotoxicology. 2002;23:329–39. doi: 10.1016/s0161-813x(02)00077-3. [DOI] [PubMed] [Google Scholar]
- 34.Ostrea EM, Jr, Brady MJ, Parks PM, Asensio DC, Naluz A. Drug screening of meconium in infants of drug-dependent mothers: An alternative to urine testing. J Pediatr. 1989;115:474–77. doi: 10.1016/s0022-3476(89)80860-1. [DOI] [PubMed] [Google Scholar]
- 35.Ostrea EM, Jr, Villanueva-Uy E, Bielawski DM, Posecion NC, Jr, Corrion ML, Jin Y, Janisse JJ, Ager JW. Maternal hair--an appropriate matrix for detecting maternal exposure to pesticides during pregnancy. Environ Res. 2006;101:312–22. doi: 10.1016/j.envres.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Ostrea EM., Jr . Testing for illicit drugs and other agents in the neonate: A review of laboratory methods and the role of meconium analysis. In: Moyer VA, editor. Current Problems in Pediatrics. Vol. 29. Mosby; St Louis, Mo: 1999. pp. 41–56. [PubMed] [Google Scholar]
- 37.Ostrea EM, Jr, Bielawski DM, Posecion NC, Jr, Corrion M, Villanueva-Uy E, Bernardo RC, Jin Y, Janisse JJ, Ager JW. Combined analysis of prenatal (maternal hair and blood) and neonatal (infant hair, cord blood and meconium) matrices to detect fetal exposure to environmental pesticides. Environmental Research. 2009;109(1):116–122. doi: 10.1016/j.envres.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Posecion N, Ostrea E, Bielawski D, Corrion M, Seagraves J, Jan Y. Detection of exposure to environmental pesticides during pregnancy by the analysis of maternal hair using GC-MS. Chromatographia. 2006;64:681–87. doi: 10.1365/s10337-006-0092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qiao D, Seidler FJ, Padilla S, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: what is the vulnerable period? Environ Health Perspect. 2002;110:1097–1103. doi: 10.1289/ehp.021101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiao D, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environ Health Perspect. 2003;111:536–544. doi: 10.1289/ehp.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rauh VA, Garfinkel R, Perera FP, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:e1845–59. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- 43.Rendeli C, Salvaggio E, Sciascia Cannizzaro G, Bianchi E, Calderelli M, Guzzetta F. Child’s Nervous System. 2002;18:231–234. doi: 10.1007/s00381-002-0557-4. [DOI] [PubMed] [Google Scholar]
- 44.Reyes A, Pacifico R, Benitez B, Villanueva-Uy E, Lam H, Ostrea E. Use of the Griffiths Mental Development in an Agro-Industrial Province in the Philippines. Child Care, Health Dev. 2009;36:354–360. doi: 10.1111/j.1365-2214.2010.01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rice D, Barone S., Jr Critical periods of vulnerability for the developing nervous system:evidence from humans and animal models. Environ Health Perspect. 2000;108 (Suppl 3):511–533. doi: 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rice D. Evidence of delayed neurotoxicity produced by methylmercury. Neurotoxicology. 1996;17:583–596. [PubMed] [Google Scholar]
- 47.Roberto N. The marketer’s guide to socioeconomic classification of consumers: Insights and challenges of target marketing in the Philippines using SEC indicators. Sage Publications; Thousand Oaks, CA: 2002. [Google Scholar]
- 48.Rosas LG, Eskenazi B. Pesticides and child neurodevelopment. Curr Opin Pediatr. 2008;20:191–7. doi: 10.1097/MOP.0b013e3282f60a7d. [DOI] [PubMed] [Google Scholar]
- 49.Rosenstein L, Chernoff N. Spontaneous and evoked EEG changes in perinatal rats following in utero exposure to Baygon: a preliminary investigation. Bull Environ Contamin Toxicol. 1978;20:624–32. doi: 10.1007/BF01683575. [DOI] [PubMed] [Google Scholar]
- 50.Rotenberg M, Shefi M, Dany S, Dore I, Tirosh M, Almog S. Differentiation between organophosphate and carbamate poisoning. Clin Chim Acta. 1995;234:11–21. doi: 10.1016/0009-8981(94)05969-y. [DOI] [PubMed] [Google Scholar]
- 51.Ruckart PZ, Kakolewski K, Bove FJ, Kaye WE. Long-term neurobehavioral health effects of methyl parathion exposure in children in Mississippi and Ohio. Environ Health Perspect. 2004;112:46–51. doi: 10.1289/ehp.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scarr-Salapatek S. An evolutionary perspective on infant intelligence: Species patterns and individual variations. In: Lewis M, editor. Origins of intelligence. New York: Plenum; 1976. [Google Scholar]
- 53.Satorra A, Bentler PM. Corrections to test statistics and standard errors in covariance structure analysis. In: von Eye A, Clogg CC, editors. Latent variables analysis: Applications for developmental research. Thousand Oaks, CA: Sage; 1994. pp. 399–419. [Google Scholar]
- 54.Schantz S, Bowman RE. Learning in monkeys exposed perinatally to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Neurotoxicol Teratol. 1989;11(1):13–19. doi: 10.1016/0892-0362(89)90080-9. [DOI] [PubMed] [Google Scholar]
- 55.Schettler T, Stein J, Reich F, Valenti M, Walinga D. A report by Greater Boston Physicians for Social Responsibility. Cambridge; MA: 2000. In Harm’s Way: Toxic Threats to Child Development; pp. 9–22. [Google Scholar]
- 56.Sheets LP, Doherty JD, Law MW, Reiter LW, Crofton KM. Age-dependent differences in the susceptibility of rats to deltamethrin. Toxicol Appl Pharmacol. 1994;126:186–190. doi: 10.1006/taap.1994.1106. [DOI] [PubMed] [Google Scholar]
- 57.Sheets LP. A consideration of age-dependent differences in susceptibility to organophosphorus and pyrethroid insecticides. Neurotoxicology. 2000;21:57–63. [PubMed] [Google Scholar]
- 58.Tabachnick BG, Fidell LS. Using Multivariate Statistics. 5. Allyn & Bacon; [Google Scholar]
- 59.Talts U, Talts JF, Eriksson P. Differential expression of muscarinic subtype mRNAs after exposure to neurotoxic pesticides. Neurobiol Aging. 1998;19:553–559. doi: 10.1016/s0197-4580(98)00095-5. [DOI] [PubMed] [Google Scholar]
- 60.U.S. Environmental Protection Agency. Integrated Risk Information System (IRIS) on Baygon. National Center for Environmental Assessment, US Environmental Protection Agency. Baygon (Propoxur) Health Advisory. Office of Drinking Water; Washington, DC: 1989. [Google Scholar]
- 61.U.S. Environmental Protection Agency. Integrated Risk Information System (IRIS) on Baygon. National Center for Environmental Assessment, Office of Research and Development; Washington, DC: 1999. [Google Scholar]
- 62.U.S. Environmental Protection Agency. [Accessed August 2009];Pesticide Industry Sales and Usage Report: 2000–2001 Market Estimates. http://www.epa.gov//oppbead1/pestsales.
- 63.Waddington CH. Canalization of development and genetic assimilation of acquired characters. Nature. 1959;183:1654–5. doi: 10.1038/1831654a0. [DOI] [PubMed] [Google Scholar]
- 64.Waliszewski SM, Aguirre AA, Infanzon RM, Rivera J, Infanzon R. Time trend of organochlorine pesticide residues in human adipose tissue in Veracruz, Mexico: 1988–1997 survey. Sci Total Environ. 1998;221:201–4. doi: 10.1016/s0048-9697(98)00279-4. [DOI] [PubMed] [Google Scholar]
- 65.Wassenberg R, Feron F, Kessels A, Hendriksen J, Kalff A, Kroes M, Hurks P, Beeren M, Jolles J, Vles J. Relation Between Cognitive and Motor Performance in 5- to 6-Year-Old Children: Results From a Large-Scale Cross-Sectional Study. Child Dev. 2005;76:1092–1103. doi: 10.1111/j.1467-8624.2005.00899.x. [DOI] [PubMed] [Google Scholar]
- 66.Webster R, Majnemer A, Platt R, Shevell M. Motor function at school age in children with a preschool diagnosis of developmental language impairment. J Pediatr. 2005;146:80–85. doi: 10.1016/j.jpeds.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 67.Weiss B. Vulnerability of children and the developing brain to neurotoxic hazards. Environ Health Perspect. 2000 Jun;108(Suppl 3):375–81. doi: 10.1289/ehp.00108s3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weschler D. Manual for the Wechsler Adult Intelligence Scale Revised. San Antonio, Texas: The Psychological Corporation; 1981. [Google Scholar]
- 69.Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, Hoepner LA, Garfinkel R, Hazi Y, Reyes A, Ramirez J, Cosme Y, Perera FP. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111:749–56. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, Hoepner LA, Diaz D, Dietrich J, Reyes A, Tang D, Kinney PL, Perera FP. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect. 2004;112:1125–32. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Young JG, Eskenazi B, Gladstone EA, et al. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26:199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]