Abstract
Members of the lipoxygenase multigene family, found widely in eukaryotes, have been proposed to function in nitrogen partitioning and storage in plants. Lipoxygenase gene responses to source-sink manipulations in mature soybean (Glycine max [L.] Merr.) leaves were examined using gene-specific riboprobes to the five vegetative lipoxygenases (vlxA–vlxE). Steady-state levels of all vlx mRNAs responded strongly to sink limitation, but specific transcripts exhibited differential patterns of response as well. During reproductive sink limitation, vlxA and vlxB messages accumulated to high levels, whereas vlxC and vlxD transcript levels were modest. Immunolocalization using peptide-specific antibodies demonstrated that under control conditions, VLXB was present in the cytosol of the paraveinal mesophyll and with pod removal accumulated additionally in the bundle-sheath and adjacent cells. With sink limitation VLXD accumulated to apparent high levels in the vacuoles of the same cells. Segregation of gene products at the cellular and subcellular levels may thus permit complex patterns of differential regulation within the same cell type. Specific lipoxygenase isoforms may have a role in short-term nitrogen storage (VLXC/D), whereas others may simultaneously function in assimilate partitioning as active enzymes (VLXA/B).
The lipoxygenases are a family of enzymes that are widespread in higher plants and animals. Catalyzing the hydroperoxidation of specific pentadiene moieties in long-chain fatty acids, lipoxygenase activity has been correlated with diverse processes in development, maturation, and senescence in plants (for review, see Siedow, 1991). Lipoxygenases have also been implicated in responses to wounding (Creelman et al., 1992; Farmer and Ryan, 1992; Peña-Cortés et al., 1993; Bell et al., 1995) and in plant defense and pathogen resistance (for review, see Siedow, 1991; Rosahl, 1996). The plant lipoxygenases have an enzymatic role in the biosynthesis of signaling molecules and regulatory compounds such as the jasmonates and hexenals (Vick and Zimmerman, 1987; Hildebrand, 1989; Gardner, 1991; Song and Brash, 1991).
Lipoxygenases in soybean (Glycine max [L.] Merr.) function in nitrogen and assimilate partitioning (Tranbarger et al., 1991; Grimes et al., 1993; Bunker et al., 1995), mechanisms evolved by plants to temporarily store and subsequently remobilize nutrients to meet specific needs (Dalling, 1985; Zapata et al., 1987; Peoples and Dalling, 1988). The levels of gene transcript and protein accumulation of one or more lipoxygenases in mature soybean generally increase in response to increasing levels of available nitrogen (Grimes et al., 1993). Lipoxygenase genes are regulated in response to plant nitrogen status in both tissue-specific and developmentally specific patterns (Tranbarger et al., 1991; Grimes et al., 1993). Removal of developing pods, a strong assimilate sink, causes a reallocation of nitrogen and other assimilates to lipoxygenases (Tranbarger et al., 1991; Grimes et al., 1993; Bunker et al., 1995) and to VSPA and VSPB, well-characterized as VSPs in the leaves of these “sink-regulated” plants (Franceschi et al., 1983; Wittenbach, 1983b; Staswick, 1988; Klauer et al., 1991).
Lipoxygenases (Tranbarger et al., 1991) and VSPs (Franceschi and Giaquinta, 1983a, 1983b, 1983c; Franceschi et al., 1983) accumulate transiently in the specialized cells of the PVM, a single cell layer that interconnects the veins of the leaf with the palisade parenchyma and spongy mesophyll (Fisher, 1967). The cells of the PVM are hypothesized to act as an intermediary for temporary storage and mobilization of nutrients among photosynthetic source tissues, the vasculature of the plant, and, ultimately, the reproductive sink organs, the developing pods, or regions of organogenesis during vegetative growth (Franceschi and Giaquinta, 1983a; Franceschi et al., 1983; Everard et al., 1990a, 1990b). Thus, legumes have developed structural and molecular strategies to modulate the transient supply of nitrogen to meet changing metabolic demands.
Multiple gene copies within a species have been proposed as a means for sophisticated organ-, tissue-, or cell-specific regulation to respond to specific developmental or other internal or external stimuli (Eiben and Slusarenko, 1994; Harper et al., 1994; Palmgren, 1994; Zimmer et al., 1996). Lipoxygenases in soybean are examples of such a multigene family (Bunker et al., 1995); they are very highly conserved and consist of at least eight genes. The three seed-storage lipoxygenases (L-1, L-2, and L-3) and their genes have been well characterized, but the gene products appear to be active only at the earliest stage of germination (for review, see Siedow, 1991). The cDNAs of five VLX genes, termed vlxA to vlxE, have been cloned (for derivation and terminology, see Bunker et al., 1995). Differential regulation of the several VLX isoforms may account for the diverse functions attributed to the lipoxygenase family.
The objective of the research reported in this paper was to investigate the relationship between multiple lipoxygenase genes and nitrogen partitioning in soybean, by characterizing quantitative gene response and protein subcellular localization of specific vegetative members of the lipoxygenase multigene family in mature plants subject to source-sink manipulations. To accomplish this goal, a sensitive, gene-specific RNase-protection assay was developed (Bunker et al., 1995), and antisera were raised against synthetic peptides that represent unique regions of the five VLX isoforms. The ability to quantitate relative levels of steady-state vlx mRNAs by phosphor imaging has allowed documentation of complex regulatory aspects that were previously not possible. The results of these experiments suggest that specific lipoxygenase proteins may function in short-term, inactive nitrogen storage in leaves, whereas one or more may function in assimilate partitioning as active enzymes. Specific lipoxygenase genes respond differentially within the same cell type. These experiments provide a rigorous characterization of the responses of all five vegetative members of the lipoxygenase multigene family to sink limitation in soybean, and provide a case study of integrated regulation in such a highly conserved family.
MATERIALS AND METHODS
Seeds of unnodulated soybean (Glycine max [L.] Merr. cv Wye) were planted in potting compost in 1-gallon pots and grown in controlled-environment growth chambers with a minimum light intensity of 360 to 400 μmol photons m−2 s−1, a daylength of 16 h, and a day/night temperature regime of 25/18°C. Plants were fertilized with 500 mL of Peter's Professional and, on alternate fertilization dates, Peter's Excell nutrient solutions, both prepared at 4 g L−1 concentration (Grace-Sierra Horticulture Products Co., Allentown, PA). Plants were fertilized once per week at 2 to 4 weeks of age and twice per week thereafter. At each time point from each plant in a treatment, three leaflets were selected for collection from fully expanded axial trifoliate leaves in such a way that developmental effects of leaf age were mitigated. One leaflet was randomly selected from the younger leaves, one from the older leaves, and one from the middle of the stem. Collected plant material was immediately frozen in liquid nitrogen and stored at −80°C.
RNA Analysis
For analysis of gene expression total RNA was extracted, mRNA was analyzed with RNase-protection assays, and transcript levels were quantitated with phosphor-image technology (see below). The accumulation of vspB gene transcript was analyzed for all experimental treatments to provide a standard by which to compare VLX gene response to sink limitation. Total RNA was isolated from 0.5 g of collected leaf material as described previously (Grimes et al., 1993). RNase-protection assays were performed using the RPA II kit following the manufacturer's standard protocol (Ambion, Austin, TX), using 5 μg of total RNA and 2 fmol of gel-purified antisense riboprobes. Riboprobes were 400- to 600-bp fragments (vlxA–vlxD) and a 250-bp fragment (vspB), prepared as previously described (Bunker et al., 1995), from gene-specific 5′ coding regions of the cloned cDNAs. The vlxE antisense riboprobe was generated by linearizing lox7-Ribo plasmid with BamHI and transcribing it with T7 RNA polymerase (Maxiscript kit, Ambion), incorporating [32P]CTP as the label. Sense RNA for verification of specificity of the vlxE riboprobe was prepared by linearizing the same plasmid with XhoI and transcribing it with T3 RNA polymerase. lox7-Ribo was prepared by subcloning an EcoRI- and a NcoI-cut PCR fragment (lox7-DomI) into a SmaI site in pBluescript SK(−) modified by T-addition with Taq DNA polymerase.
The 596-bp lox7-DomI fragment was isolated from a depodded soybean cDNA library by PCR amplification, using the gene-specific forward primer 5′-CACAAGCTTAAGAAGTAGCAAAGATGTTTGG-3′ (determined from the published sequence of the lox7 cDNA; Saravitz and Siedow, 1996) and the loxA/B/7-domain I reverse primer 5′-CAACTGCAGTTAGTTGGCAAAGAAAATGCGATC-3′. Gels from RNase-protection assays were exposed on x-ray film for visualization by autoradiography, then exposed on a phosphor plate for 2 h and immediately scanned for computer analysis with a phosphor imager (model 445SI, Molecular Dynamics, Sunnyvale, CA). Bands representing full-length protected riboprobes were quantitated using ImageQuant 4.1 software, following the manufacturer's protocols (Molecular Dynamics). The integrated phosphor signal volume scanned was directly proportional to radioactivity in each protected band and thus proportional to the number of gene-specific transcripts present. To allow direct comparison of results among riboprobes and experiments, the specific activity for each riboprobe was normalized to an arbitrary standard specific activity (2.0 × 105 cpm fmol−1). The ratio of standard to calculated specific activity was then used to adjust each scanned phosphor signal value. The data presented in this report are these normalized measurements.
Preparation of Peptide-Specific Antibodies
Sequences for the preparation of antibodies were chosen from vlxA to vlxD cDNA-derived amino acid sequences that maximized specificity and antigenicity, as follows: VLXA, Ac-GGIVDQGLGC-amide; VLXB, Ac-VDGIVGTGLDFC-amide; VLXC, Ac-GKGSAKDTATDFLC-amide; and VLXD, Ac-GVIDTATGILGQGC-amide.
All chosen peptides were close to the N terminus. Peptides were synthesized commercially: the VLXD-specific peptide was from Chiron Mimotopes (San Diego, CA), and the remainder were from Princeton Biomolecules (Columbus, OH). Peptides were prepared with C-terminal cysteines, and were N-terminal acetylated and C-terminal amidated.
Peptides were conjugated with Inject Maleimide activated keyhole limpet hemocyanin carrier protein (Pierce) in a 1:1 (w/w) ratio via sulfhydryl linkage. Peptide was injected into New Zealand white rabbits at 0.05 to 0.25 mg mL−1 per injection set using the monophosphoryl lipid A plus trehalose dicarynomycolate plus cell wall skeleton adjuvant system (Sigma). Rabbits were boosted at 4 and 8 weeks. Final serum collection was at 10 weeks. Serum was rimmed, coagulated, spun twice at 3500g, and stored with the addition of Tris, pH 8.0, to 0.25% and 1% thimerosal to 0.01%.
Peptide affinity-purification columns were prepared using chromatography columns (Poly-Prep, Bio-Rad) and coupling gel (Sulfo-Link, Pierce). Four milligrams of peptide was conjugated with 2 mL of coupling gel following the manufacturer's protocol. Columns were stored in 0.05% NaN3 in column buffer. Antisera were affinity purified as follows: Columns were equilibrated with column buffer (10 mm Tris/1 mm EDTA, pH 7.5). Two milliliters of serum was diluted to 20 mL and passed through the column three times (once for anti-VLXD). Columns were then washed with 20 mL of column buffer and 20 mL of 500 mm NaCl in column buffer. Antibody was eluted with 5 mL of 0.1 m Gly, pH 2.4, and 0.8-mL fractions were collected into 0.2 mL of 1 m Tris, pH 9.0. Columns were then washed with 20 mm Tris, pH 8.8 (10 mm Tris for anti-VLXD), until equilibrated at pH 8.5. Antibody was further eluted with 5 mL of 100 mm triethylamine, pH 11.5, and fractions were collected as above into 1 m Tris, pH 7.0. Columns were washed with column buffer and stored as above. Protein in the antisera fractions was quantitated using a protein assay system following the manufacturer's microassay protocol (Bio-Rad).
Specificities of these antisera have not been tested against purified proteins. Because specific, unique peptides were synthesized for antibody preparation, the probability that an antibody would cross-react with other lipoxygenase isozymes was very low. In particular, the peptide sequences recognized by anti-VLXA/B were highly dissimilar from those that anti-VLXC/D recognize, specificities strongly supported by the unique patterns of immunolocalization and differential regulation for VLXB and VLXD reported in this manuscript. However, we cannot eliminate the possibility, although remote, that anti-VLXA cross-reacts with VLXB or that anti-VLXC cross-reacts with VLXD.
Tissue Preparation, Immunolocalization, and Transmission Electron Microscopy Immunocytochemistry
Mature leaf samples were collected from 13-week-old plants with 4-week-old developing pods present (controls) and from plants of the same age that had their pods removed daily (depods). Leaf tissues were fixed, embedded, sectioned, and incubated with affinity-purified antibodies as described (Tranbarger et al., 1991). For light microscopy, anti-VLXB was diluted to 425 ng mL−1 and anti-VLXD to 1.6 mg mL−1. For epipolarization images, labeling was highlighted by reflection off of silver-enhanced immunogold particles of polarized light directed from above the section and passing through a POL cube (Leitz, Wetzlar, Germany). The tissues prepared for transmission electron microscopy were those used for light microscopy immunolocalization. The anti-VLXB antibody was diluted to 85 ng mL−1 and anti-VLXD to 145 ng mL−1. Sections were incubated overnight in the primary antibodies.
RESULTS
Response of VLX Leaf mRNAs to Reproductive Sink Removal in Soybean
To evaluate the contribution of the lipoxygenase multigene family to assimilate partitioning and storage in soybean, the responses to sink regulation by the five VLX genes in leaves were characterized and quantitated. Removal of reproductive tissue is a standard method to convert source tissues into sink-regulated ones (Geiger, 1976; Herold, 1980) and to induce VSP gene expression and protein accumulation (Wittenbach, 1982, 1983a; Staswick, 1988). Developing pods were removed daily for 6 weeks beginning 1 week after anthesis, when plants were about 9 weeks old. RNase protection assays and phosphor-image technology were used to evaluate and rigorously quantitate the steady-state levels of vlxA to vlxE and vspB mRNA in mature leaves from treated and control plants. Levels of VSP gene vspB transcript in these sink-manipulation experiments were analyzed to provide a standard by which to compare expression of the VLX genes.
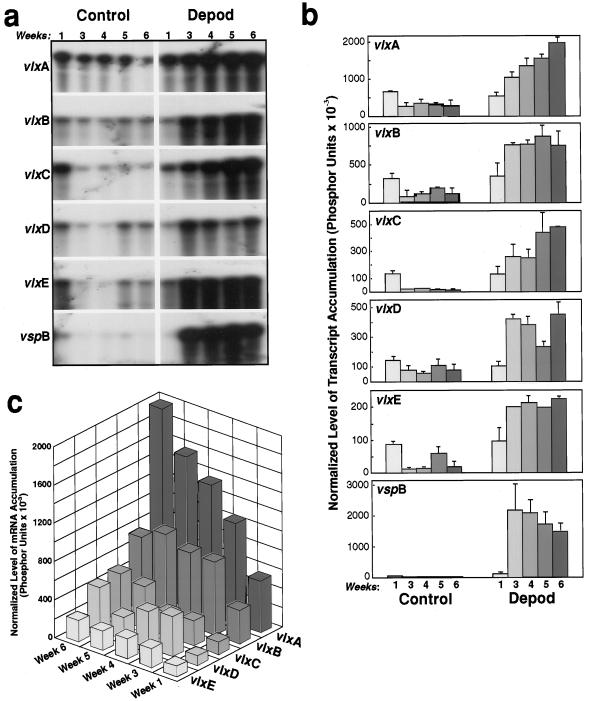
A composite autoradiograph from a representative experiment was assembled (Fig. 1a), and radiolabeled data from two independent experiments were combined and quantitated (Fig. 1, b and c). The increase of the transcript level for each VLX gene and vspB after 6 weeks of daily pod removal (above the values for the pod-bearing controls at the same age) demonstrated that transcript levels of all VLX genes in leaves responded coordinately to long-term pod removal with at least a 6-fold increase over 6 weeks (Fig. 1b). The vlx message in control plants bearing normally developing pods declined over the same time period (Fig. 1b). The vlxA transcript accumulated to the highest level during daily reproductive sink removal (Fig. 1c), although vlxC mRNA increased its message level 35-fold over that of the pod-bearing controls (Fig. 1b). However, levels of transcript for vlxA and vlxB were already at high base levels at week 1 compared with vlxC and vlxD (Fig. 1c), and these isoforms accumulated 2- to 4-fold greater levels of transcript in leaves over 6 weeks of treatment than that for vlxC/D (Fig. 1c). Response of vspB transcript level to pod removal was 99-fold above the control level (Fig. 1b), but the total transcript that accumulated was equivalent to that for vlxA.
Figure 1.
Quantitative analysis of vlx and vsp steady-state mRNA levels in sink-regulated soybean leaves from plants with pods removed daily for 6 weeks. a, Compilation of autoradiographs from a representative RNase-protection analysis. Pod removal began 1 week after anthesis with plants 9 weeks old. Selected trifoliate leaflets were harvested beginning at the time of first pod removal (Week 1) and at weekly intervals thereafter (Weeks 2–6). Total RNA was extracted and mRNA analyzed using 5 μg of total RNA and 2 fmol 32P-labeled antisense riboprobe specific to 5′ domains of vlx genes A through E and to vspB. Hybridization products were then separated on polyacrylamide gels. Only the portions of the gels representing full- length riboprobes protected by specific mRNAs are shown. b, Quantitation of vlxA through vlxE and vspB steady-state transcript from phosphor-image analysis of the 32P label in full-length protected bands from independent RNase-protection assays. The scale varies for each riboprobe. Error bars show sd (n = 2). c, Display of data described in Figure 1b, but at a common vertical scale to demonstrate comparative base levels of transcript accumulation and subsequent responses.
Response of VLX Leaf mRNAs to Removal of Shoot Tips in Soybean
The manipulation of developing shoot tips, strong vegetative sinks for nitrogen and carbon compounds in growing plants (Simpson, 1986; Turgeon, 1989), provides an alternative experimental system to characterize regulation of gene response to nitrogen and carbon assimilates. To examine responses of VLX genes in mature leaves to vegetative sink limitation, terminal and axillary shoots bearing leaves less than one-eighth expanded were removed from 30-d-old soybean plants. Shoot-tip removal continued daily for 16 d. Because shoot tips could be returned immediately to sink status by allowing regrowth, thus further testing regulation of the VLX genes by source-sink status, the shoots were allowed to resume growth for a further 2 weeks.
A composite autoradiograph from a representative experiment was assembled (Fig. 2a) and radiolabeled data from two independent experiments were combined and quantitated (Fig. 2, b and c). The increase of transcript level in leaves for each VLX gene and vspB after 16 d of shoot-tip removal (measured from the untreated controls at 16 d, data not shown) demonstrated that, as with daily pod removal, transcript levels of all VLXs in leaves responded coordinately, showing at least a 2-fold increase, whereas vlxD increased its transcript level 20-fold over that of the control plant at the same age (Fig. 2b). The vlxC transcripts also increased to nearly the same level as those of vlxD after 16 d of tip removal, both about 2- to 4-fold above the levels of vlxA and vlxB mRNAs (Fig. 2c). It is interesting that the background levels of vlxA and vlxB steady-state mRNAs in leaves from 30-d-old plants were less than one-half of those for vlxA and vlxB in leaves from 60-d-old plants (compare scales between Figs. 1b and 2b), reflecting a strong developmental difference. In control plants with tips allowed to develop normally, the transcript levels of the vlx and vsp genes remained constant or increased slightly (data not shown). The level of vspB message in treated plants was very high, responding to treatment 20-fold over that of the control plants (Fig. 1b). The responses of the vlxC and vlxD steady-state transcript levels to vegetative sink removal (Fig. 2, b and c) were therefore in contrast to those in long-term reproductive sink removal (Fig. 1, b and c), where vlxA and vlxB transcripts responded most strongly to sink limitation. Thus, it appears that vlxA/B and vlxC/D may play differing roles in nitrogen storage or partitioning in leaves, and are under developmental control as well. Cessation of vegetative sink limitation and resumption of shoot-tip growth reduced the message accumulation of all of the genes dramatically (Fig. 2, a and b). These data confirm the direct correlation between sink limitation and accumulation of VLX gene transcript in mature leaves.
Figure 2.
Quantitative analysis of vlx and vsp steady-state mRNA levels in soybean leaves from plants with shoot tips removed daily for 16 d, then with shoots allowed to regrow for 2 weeks. a, Compilation of autoradiographs from a representative RNase-protection analysis. Plants were 4 weeks old at first treatment. Terminal and axial shoots bearing leaves less than or equal to one-eighth-expanded were excised. Leaf samples were collected at the time of first tip removal (0 d), then subsequently at 4, 8, 12, and 16 d after tip removal. Beginning at 16 d, plants were allowed to regrow their shoots. Further collections were made at 1 and 2 weeks after regrowth began (23 and 30 d, respectively). Tissue collection and subsequent analysis were identical to that described for Figure 1. b, Quantitation of vlxA-E and vspB steady-state transcript levels from phosphor-image analysis of the 32P label in full-length protected bands from independent RNase-protection assays. The scale varies for each riboprobe. Error bars show sd (n = 2). c, Display of data described in Figure 2b, but at a common vertical scale to demonstrate comparative base levels of transcript accumulation and subsequent responses.
Immunocytolocalization Demonstrates That VLXB Protein Accumulates in the Cytosol and VLXD Protein Accumulates in the Vacuoles of Cells of the Leaf PVM and Bundle Sheath in Soybean Plants Subjected to Reproductive Sink Removal
Data from the examination of molecular regulation of VLX gene transcript levels described above for mature leaves (Figs. 1 and 2) demonstrate the potential for differential regulation of gene products in response to sink-limitation treatments. To understand the functional roles of specific lipoxygenase isoforms in nitrogen storage and metabolism, it is necessary to determine the cellular and subcellular sites of localization for their gene products. Because of the putative role of the PVM cells in assimilate partitioning (Franceschi and Giaquinta, 1983a; Franceschi et al., 1983; Everard et al., 1990a, 1990b), it is conceivable that VLX isoforms accumulate in the same cells. To address these questions, cellular and subcellular localization of VLXB and VLXD protein was analyzed in mature leaves from 13-week-old soybean plants from which pods had been removed daily for 4 weeks.
Generating isoform-specific antisera to VLXA through VLXE has proven difficult because of the high level of sequence conservation among these proteins. The regions of highest variability exist in the amino-terminal β-barrel domain and, consequently, regions of 12 to 16 amino acids were selected in this domain for the generation of anti-peptide antisera. Specific antisera to VLXA were obtained through VLXE using these methods, but only the antisera to VLXB, VLXD, and VLXE are of sufficient avidity for immunocytochemistry. Here we focus on VLXB and VLXD immunolocalization; the data for VLXE will be published separately.
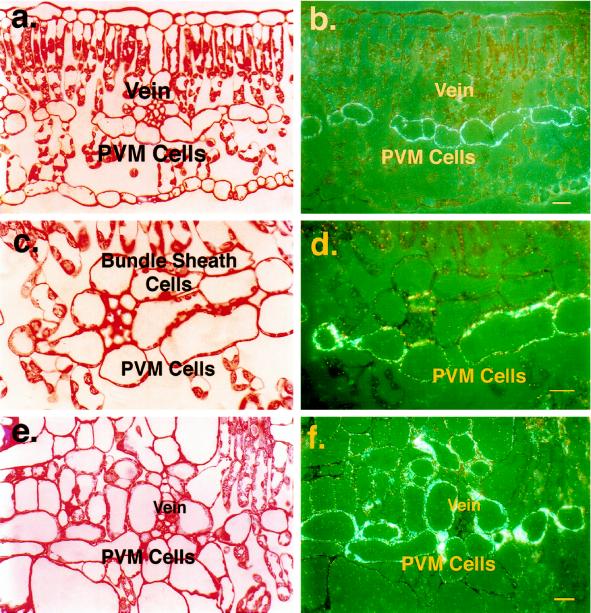
Leaf cross-sections incubated with anti-VLXB antibody were examined with bright-field (Fig. 3, a, c, and e) and epipolarization (Fig. 3, b, d, and f) light microscopy. VLXB from control plants was found localized primarily in the cytosol of cells of the PVM, the single cell layer here seen highlighted by epipolarized light reflected off of anti-VLXB-decorated silver grains (Fig. 3, b and d). In leaves of plants subjected to sink regulation by removal of developing pods, the level of VLXB accumulation in the PVM increased slightly (Fig. 3f), but this protein was found additionally in the bundle-sheath cells and to a lesser extent in the cells surrounding these tissues (Fig. 3f), suggesting a role for this enzyme in assimilate partitioning. The VLXB protein remained localized in the cytosol with sink regulation (Fig. 3f). Sections were also incubated with VLXB preimmune serum, but no staining was observed (data not shown). Transmission electron microscopy confirmed the cytosolic localization of VLXB (Fig. 4a). Anti-VLXB-decorated immunogold particles were observed primarily in the cytosol from both depodded plants (Fig. 4a) and control plants (data not shown). Density of particles within the cytosol of individual cells increased only slightly with source-sink manipulation, indicating that most of the increase in vlxB mRNA levels observed after pod removal (Fig. 1) resulted in VLXB accumulation in the bundle-sheath cells.
Figure 3.
Light microscopy immunolocalization of VLXB protein in mature soybean leaves after 4 weeks of continuous pod removal. Mature leaves were collected at 13 weeks of age, fixed, and sectioned. Sections were incubated with the peptide-specific antibody, then with protein A-conjugated gold, before silver enhancement and histochemical staining. a and b, Bright-field and epipolarization images, respectively, of leaf cross-section from untreated control plant incubated with anti-VLXB antibody. The protein under these nonsink-limited conditions can be seen localized to the cytosol of the PVM cells. Scale bar = 40 μm. c and d, Bright-field and epipolarization images, respectively, of a vascular bundle and adjacent PVM and bundle-sheath cells shown at higher magnification from untreated control plant incubated with anti-VLXB antibody. Scale bar = 25 μm. e and f, Bright-field and epipolarization images, respectively, of leaf cross-section from a plant with pods removed continuously for 4 weeks and incubated with anti-VLXB antibody. VLXB protein slightly increases its accumulation in the PVM under these sink-regulated conditions, but additional accumulation occurs in the bundle-sheath cells surrounding the vein and to a lesser extent in adjacent cells. VLXB protein remains localized in the cytosol. Scale bar = 40 μm.
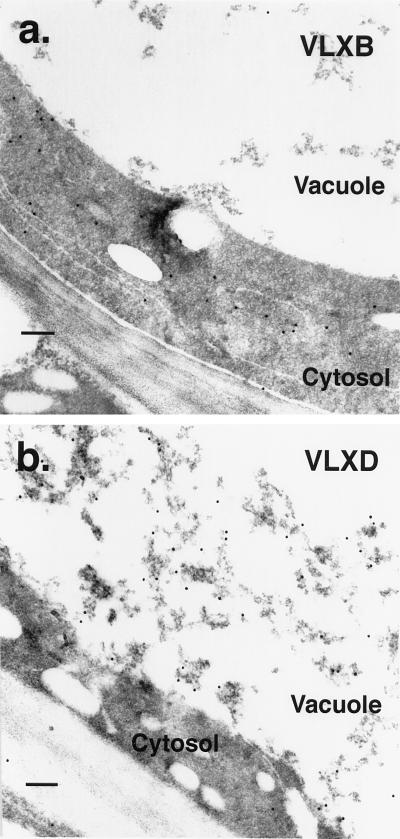
Figure 4.
Transmission electron microscopy immunocytolocalization of VLXB and VLXD protein in mature soybean leaves after 4 weeks of continuous pod removal. a, Portion of a leaf cross-section from a plant with pods removed daily for 4 weeks and incubated with anti-VLXB antibody. Tissue sections are identical to those described for Figure 3. Sections were mounted onto nickel grids, incubated with peptide-specific antibody and protein A-gold conjugate, and poststained. Immunogold particles are localized primarily in the cytosol in this cell from a sink-regulated plant. Scale bar = 200 nm. b, Portion of a leaf cross-section from a plant with pods removed daily for 4 weeks and incubated with anti-VLXD antiserum. Tissue sections are identical to those described for Figure 5 and were prepared as in a. Immunogold particles are dense in the vacuole and localize strongly to electron-dense flocculent material in this cell from a sink-regulated plant. Minimal localization is apparent elsewhere. Scale bar = 200 nm.
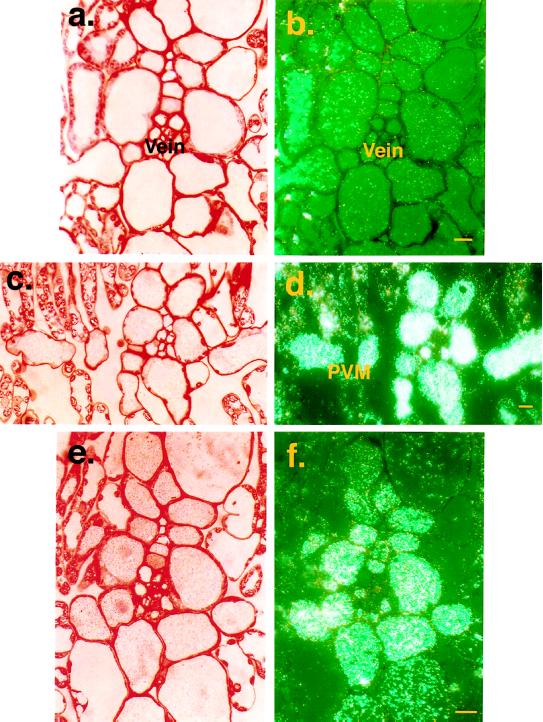
Leaf cross-sections incubated with anti-VLXD antibody were examined with bright-field (Fig. 5, a, c, and e) and epipolarization (Fig. 5, b, d, and f) light microscopy. Accumulation of VLXD protein was minimal in leaves from control plants (Fig. 5b). In contrast, VLXD protein accumulated to apparent high concentration in the vacuoles of the PVM and bundle-sheath cells in response to daily pod removal (Fig. 5, d and f). A lower level of accumulation was noted in cells adjoining these tissues. Leaf sections were also incubated with VLXD preimmune serum, but no staining was observed (data not shown). Transmission electron microscopy confirmed the vacuolar localization of VLXD (Fig. 4b). Anti-VLXD-decorated immunogold particles were readily visible in vacuoles in tissues prepared from plants with pods removed (Fig. 4b), here seen associated with electron-dense material. Little or no staining was observed in the cytosol, nor was any observed in cellular organelles such as the ER, Golgi bodies, or chloroplasts (data not shown).
Figure 5.
Light microscopy immunolocalization of VLXD protein in mature soybean leaves after 4 weeks of continuous pod removal. Sections were prepared as described for Figure 3. a and b, Bright-field and epipolarization images, respectively, of leaf cross-section from untreated control plant incubated with anti-VLXD antibodies. The protein under these nonsink-limited conditions can be seen localized at very low levels in the vacuoles of the PVM and bundle-sheath cells. Scale bar = 25 μm. c and d, Bright-field and epipolarization images, respectively, of leaf cross-section from a plant with pods removed continuously for 4 weeks and incubated with anti-VLXD antibodies. VLXD protein accumulates in the vacuoles of the PVM and bundle-sheath cells and, to a lesser extent, in adjacent cells under these sink-regulated conditions. Scale bar = 30 μm. e and f, Bright-field and epipolarization images, respectively, of a vascular bundle and surrounding bundle-sheath cells shown at higher magnification from a plant with pods removed continuously for 4 weeks and incubated with anti-VLXD antibodies. VLXD protein accumulates in the vacuoles of the PVM and bundle-sheath cells under these sink-regulated conditions. Scale bar = 25 μm.
Both cellular and subcellular immunolocalization studies of mature leaves demonstrate that VLXB and VLXD accumulate differentially in response to reproductive sink limitation in soybean. VLXB is present in the cytosol of PVM cells but, under sink-regulated conditions, accumulates additionally in the bundle sheath and surrounding cells. VLXD protein is found in the vacuoles of the PVM and bundle-sheath cells at very low levels, but accumulates to apparent high concentrations in response to sink limitation.
DISCUSSION
A quantitative analysis of molecular regulation of specific VLX mRNA levels in response to source-sink manipulations in soybean was performed and the cellular and subcellular localization of specific VLX gene products was determined. Because of the complexity of vlx mRNA accumulation patterns observed in a preliminary study (Bunker et al., 1995), and because of the cloning of a fifth VLX cDNA (vlxE/lox 7; Saravitz and Siedow, 1996), it was imperative to obtain quantitative data documenting the responses of the soybean lipoxygenase multigene family response to sink limitation. Only with quantitative data for each individual vlx mRNA is it possible to determine the potential roles of these genes and their products in nitrogen metabolism and plant function. Furthermore, while one or more lipoxygenases accumulate to very high levels in sink-regulated leaves (Tranbarger et al., 1991), there is a coordinate increase in lipoxygenase activity (Grimes et al., 1992), thus indicating a possible bifunctionality—storage and lipid peroxidation—within this multigene family. To investigate this bifunctionality and to further characterize the regulation of this multigene family, gene-specific riboprobes and a sensitive RNase-protection protocol (Bunker et al., 1995) were developed to distinguish between the highly conserved mRNA sequences. Additionally, peptide-specific antibodies were generated to visualize the cellular and subcellular localization of the specific gene products.
Steady-state levels of five VLX mRNAs were examined in leaves of mature soybean with pods removed daily, and in leaves of 4- to 6-week-old soybean with terminal and axial shoot tips removed daily. It is interesting that steady-state transcript levels of all VLX genes rose severalfold, suggesting a global regulation of the multigene family by these sink-limitation treatments. However, specific vlx isoforms demonstrated significant differential message accumulation beyond the general coordinate response. Transcripts of vlxA and vlxB accumulated to much higher levels than those for vlxC and vlxD during pod-removal treatments, whereas vlxC and vlxD transcripts accumulated to greater levels when developing shoot tips were removed. These data suggest differential functions for the specific gene products.
Cellular and subcellular localization of VLX gene products was examined in mature leaves by light and transmission electron microscopy. Previous immunolocalization studies (Vernooy-Gerritsen et al., 1983, 1984) had shown differential patterns of seed lipoxygenase accumulation during early seed germination. Here, using peptide-specific antibodies, we demonstrated that VLXB protein accumulated only in the cytosol of the PVM cells in plants with pods developing normally. The level of VLXB protein accumulation in the PVM did not appear to change significantly with sink limitation (4 weeks of pod removal), but was observed to accumulate additionally in the cytosol of bundle-sheath cells after daily pod removal. Immunolocalization studies showed that VLXD protein accumulated only in the cells of the PVM and bundle sheath and, in contrast to VLXB, only within the vacuole. VLXD protein level was very low in plants with pods developing normally, but accumulated to apparent high concentrations in plants with pods removed daily for 4 weeks. VLXD remained localized to the vacuoles under these treatment conditions.
The responses of the five VLX genes in soybean to sink manipulation provide a case study of coordinated gene regulation within a highly conserved multigene family of a single plant species. Differences of molecular regulation of specific gene transcripts, and cellular and subcellular localization of protein products, suggest that at least three lipoxygenase genes respond differentially to sink limitation and may provide a first level of regulation in cell function. The specific vacuolar localization of VLXD protein in the PVM and its accumulation in response to reproductive sink limitation suggest that this protein functions as a temporary storage protein. Biochemical studies of soybean leaf lipoxygenases demonstrate that they possess the pH optima and other characteristics of active cytosolic enzymes (Grayburn et al., 1991). Sequestration of VLXD in the typical low-pH environs of vacuoles (Boller and Wiemken, 1986) may render the VLXD isoform inactive, enabling it to function as an inert storage form (Wink, 1993; Staswick, 1994). Localization of VLXD in the cells of the PVM, a specialized cell layer involved in assimilate partitioning and storage (Franceschi and Giaquinta, 1983a; Franceschi et al., 1983; Everard et al., 1990a, 1990b), provides a further indication that VLXD is involved in the temporary storage of nitrogen in vegetative organs.
In contrast, immunolocalization data suggest that VLXB protein may function as an active enzyme. It was observed to be strictly cytosolic, and levels of protein within individual cells did not appear to vary significantly with sink- limitation treatments. However, 4 weeks of daily pod removal caused accumulation of protein in the bundle-sheath cells, in addition to the PVM, where it was found constitutively. These data are consistent with an active role for VLXB in nitrogen or assimilate partitioning in response to reproductive sink limitation. Thus, two specific VLX gene products may have completely different functions in a single cell type in response to sink limitation. Our data suggest that VLXB may function as an active enzyme in assimilate partitioning, whereas VLXD may function as a vacuolar storage protein.
In contrast to VLXB and VLXD localization to tissues specifically associated with nitrogen partitioning and storage, VLXE protein associated with thylakoid membranes of chloroplasts from diverse cell types, and the vlxE gene transcript was especially responsive to wound treatments. Because response and accumulation patterns suggest that the VLXE isozyme may be functionally and structurally distinct, and because additional studies are in progress, these data will be reported elsewhere (A.M. Fischer, L.C. Stephenson, and H.D. Grimes, unpublished data), but they suggest yet a third distinct cellular function for a soybean VLX. Bunker et al. (1995) previously demonstrated that removal of pods in sink-limitation treatments did not provoke a response from the wound-signaling pathway, suggesting that expression patterns displayed in the experiments reported in this manuscript were not due to wounding. Effects of shoot-tip removal on the wound-response pathway were not specifically tested.
How a lipoxygenase gene product might function in the PVM to mediate assimilate partitioning is unknown at this time. However, VLXB localization in the cytosol of the PVM suggests that it may have an important function in this specialized cell layer. Everard et al. (1990a) have shown that oxygen-consumption rates in the PVM are approximately 3-fold higher than in the palisade parenchyma cells, and it now becomes a distinct possibility that oxygen consumption by lipoxygenase is responsible for this. One lipoxygenase studied in mammals functions in reticulocyte maturation by remodeling membranes through peroxidation and subsequent breakdown of the membrane (Kuhn et al., 1990). Maccarrone et al. (1994) propose that soybean lipoxygenase-2, a seed lipoxygenase, can differentially oxygenate specific soybean membranes. A similar enzymatic role is conceivable for VLXB in the PVM and bundle-sheath cells, with the isozyme functioning in maintenance or dissolution of vacuolar or other membrane systems. Such an isozyme would remain cytosolic and enzymatically active in this scenario, as evidence has suggested for VLXB. Alternatively, VLXB may function in initiation of a signal transduction cascade to regulate partitioning. Several studies have implicated components of the octadecanoid pathway in the regulation of assimilate partitioning during seed development (Lopez et al., 1987; Wilen et al., 1991; Simpson and Gardner, 1995). It is clear that the biochemical activities of these lipoxygenase isoforms must now be investigated.
Molecular-regulation data from sink-limitation experiments show that levels of vlxB transcript were high in leaves of mature plants at 1 week postanthesis (“Week 1”), twice the levels observed in the 4-week-old plants, and 2-fold higher in the mature plants than the levels of vlxC and vlxD message. Because mature soybean normally undergoes translocation of assimilates from temporary storage to reproductive organs in the weeks after anthesis, the correlative high accumulation of vlxB message at this developmental stage is additional evidence that VLXB may be functioning as an active enzyme involved in assimilate partitioning. The vlxA message levels accumulate to significantly higher levels and respond to sink manipulation treatments in the same patterns as vlxB transcript. Furthermore, vlxA and vlxB nucleotide sequences are very similar, sharing 94% identity at the deduced amino acid level. Thus, we predict that VLXA will be found in the cytosol. Studies of molecular regulation demonstrate that vlxC and vlxD accumulate similar levels of transcript in both pod- and shoot-tip-removal treatments. Additionally, these two genes have closely related sequences, sharing 87% identity at the deduced amino acid level. The C-terminal coding region of the vlxC gene sits less than 1 kb upstream of the vlxD coding region, presumably the result of the duplication event that characterizes the soybean genome (Zhu et al., 1994; Shoemaker et al., 1996). It was observed that very few of these duplications in soybean are silent (Zhu et al., 1994). These data are consistent with a common role for vlxC and vlxD, encoding gene products that function as vacuolar-storage proteins. Development of antibodies to VLXA and VLXC, with sufficient avidity for immunocytochemistry, will be needed to verify subcellular localization of these isozymes.
The patterns of steady-state mRNA accumulation in leaves for vlxC and vlxD in both reproductive- and vegetative-sink limitation closely parallel each other. The fact that VLXD protein also accumulates to high levels after pod removal, whereas vlxD transcript levels accumulate only modestly, is evidence for posttranscriptional regulation. Molecular-regulation data for vlxC and vlxD transcript levels and vacuolar localization for VLXD protein thus support roles for these genes and their products in nitrogen storage and partitioning. However, molecular regulation of the lipoxygenase multigene family is complex, suggesting that additional levels of cellular regulation may exist as well. Although multiple members of the family occur within the same cell type, segregation at the cellular and subcellular level may permit complex patterns of differential regulation. These levels of developmental and spatial regulation may permit members of the lipoxygenase multigene family to have multiple roles in a complex function, e.g. processing assimilates through the PVM. Thus, by a specific function of the gene product or by multiple functions controlled by alternative regulation or localization, members of a multiple gene family such as the lipoxygenases may respond to diverse internal and external signals and yield integrated responses in plant growth and physiology.
In this report the responses to assimilate sink limitation of the five vegetative members of a highly conserved multigene family, the soybean VLXs, were quantitatively examined in mature leaves. The data strongly suggest that specific isoforms participate in temporary storage and partitioning of nitrogen and other assimilates through the specialized leaf PVM tissue. VLXD, and perhaps VLXC, may function as relatively inert vacuolar-storage proteins. VLXB, and perhaps VLXA, may function as active cytosolic enzymes in assimilate partitioning. Regulation is tightly coordinated between the gene family members and responses are specific to treatment, isoform, tissue, developmental stage, and organelle. Multiple isoforms may function simultaneously within a single cell type, and regulation of the members of this multigene family is highly coordinated.
Abbreviations:
- PVM
paraveinal mesophyll
- VLX
vegetative lipoxygenase
- VSP
vegetative storage protein
Footnotes
This research was funded in part by U.S. Department of Agriculture grant no. 95-03688 to H.D.G.
LITERATURE CITED
- Bell E, Creelman RA, Mullet JE. A chloroplast lipoxygenase is required for wound-induced jasmonic acid accumulation in Arabidopsis. Proc Natl Acad Sci USA. 1995;92:8675–8679. doi: 10.1073/pnas.92.19.8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Wiemken A (1986) Dynamics of vacuolar compartmentation. In WR Briggs, RL Jones, V Walbot, eds, Annual Review of Plant Physiology, Vol 37. Annual Reviews, Inc., Palo Alto, CA, pp 137–164
- Bunker TW, Koetje DS, Stephenson LC, Creelman RA, Mullet JE, Grimes HD. Sink limitation induces the expression of multiple soybean vegetative lipoxygenase mRNAs while the endogenous jasmonic acid level remains low. Plant Cell. 1995;7:1319–1331. doi: 10.1105/tpc.7.8.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Tierney ML, Mullet JE. Jasmonic acid/methyl jasmonate accumulate in wounded soybean hypocotyls and modulate wound gene expression. Proc Natl Acad Sci USA. 1992;89:4938–4941. doi: 10.1073/pnas.89.11.4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalling MJ (1985) The physiological basis of nitrogen redistribution during grain filling in cereals. In JE Harper, LE Schrader, RW Howell, eds, Exploitation of Physiological and Genetic Variability to Enhance Crop Productivity. American Society of Plant Physiologists, Rockville, MD, pp 55–71
- Eiben HG, Slusarenko AJ. Complex spatial and temporal expression of lipoxygenase genes during Phaseolus vulgaris (L.) development. Plant J. 1994;5:123–135. doi: 10.1046/j.1365-313x.1994.5010123.x. [DOI] [PubMed] [Google Scholar]
- Everard JD, Franceschi VR, Ku MSB. Characteristics and carbon metabolism of mesophyll and paraveinal mesophyll protoplasts from leaves of non-nodulated Glycine max. Plant Sci. 1990a;66:167–172. [Google Scholar]
- Everard JD, Ku MSB, Franceschi VR. Distribution of metabolites and enzymes of nitrogen metabolism between the mesophyll and paraveinal mesophyll of non-nodulated Glycine max. J Exp Bot. 1990b;41:855–861. [Google Scholar]
- Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DB. An unusual layer of cells in the mesophyll of the soybean leaf. Bot Gaz. 1967;128:215–221. [Google Scholar]
- Franceschi VR, Giaquinta RT. The paraveinal mesophyll of soybean leaves in relation to assimilate transfer and compartmentation. I. Ultrastructure and histochemistry during vegetative development. Planta. 1983a;157:411–421. doi: 10.1007/BF00397198. [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Giaquinta RT. The paraveinal mesophyll of soybean leaves in relation to assimilate transfer and compartmentation. II. Structural, metabolic and compartmental changes during reproductive growth. Planta. 1983b;157:422–431. doi: 10.1007/BF00397199. [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Giaquinta RT. Specialized cellular arrangements in legume leaves in relation to assimilate transfer and compartmentation: comparison of the paraveinal mesophyll. Planta. 1983c;159:415–422. doi: 10.1007/BF00392077. [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Wittenbach VA, Giaquinta RT. The paraveinal mesophyll of soybean leaves in relation to assimilate transfer and compartmentation. III. Immunohistochemical localization of specific glycopeptides in the vacuole after depodding. Plant Physiol. 1983;72:586–589. doi: 10.1104/pp.72.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner HW. Recent investigations into the lipoxygenase pathway of plants. Biochim Biophys Acta. 1991;1084:221–239. doi: 10.1016/0005-2760(91)90063-n. [DOI] [PubMed] [Google Scholar]
- Geiger DR. Effect of assimilate translocation on photosynthesis. Can J Bot. 1976;54:2337–2345. [Google Scholar]
- Grayburn WS, Schneider GR, Hamilton-Kemp TR, Bookjans G, Ali K, Hildebrand DF. Soybean leaves contain multiple lipoxygenases. Plant Physiol. 1991;95:1214–1218. doi: 10.1104/pp.95.4.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes HD, Koetje DS, Franceschi VR. Expression, activity, and cellular accumulation of methyl jasmonate-responsive lipoxygenase in soybean seedlings. Plant Physiol. 1992;100:433–443. doi: 10.1104/pp.100.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes HD, Tranbarger TJ, Franceschi VR. Expression and accumulation patterns of nitrogen-responsive lipoxygenase in soybeans. Plant Physiol. 1993;103:457–466. doi: 10.1104/pp.103.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JF, Manney L, Sussman MR. The plasma membrane H+-ATPase gene family in Arabidopsis: genomic sequence of AHA10 which is expressed primarily in developing seeds. Mol Gen Genet. 1994;244:572–587. doi: 10.1007/BF00282747. [DOI] [PubMed] [Google Scholar]
- Herold A. Regulation of photosynthesis by sink activity: the missing link. New Phytol. 1980;86:131–144. [Google Scholar]
- Hildebrand DF. Lipoxygenases. Physiol Plant. 1989;76:249–253. [Google Scholar]
- Klauer SF, Franceschi VR, Ku MSB. Protein compositions of mesophyll and paraveinal mesophyll of soybean leaves at various developmental stages. Plant Physiol. 1991;97:1306–1316. doi: 10.1104/pp.97.4.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H, Belkner J, Wiesner R, Brash AR. Oxygenation of biological membranes by the pure reticulocyte lipoxygenase. J Biol Chem. 1990;265:18351–18361. [PubMed] [Google Scholar]
- Lopez R, Dathe W, Bruckner C, Miersch O, Sembdner G. Jasmonic acid in different parts of the developing soybean fruit. Biochem Physiol Pflanzen. 1987;182:195–201. [Google Scholar]
- Maccarrone M, van Aarle PGM, Veldink GA, Vliegenthart JFG. In vitro oxygenation of soybean biomembranes by lipoxygenase-2. Biochim Biophys Acta. 1994;1190:164–169. doi: 10.1016/0005-2736(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Palmgren MG. Why isoforms of the plant plasma membrane H+-ATPase? Symp Soc Exp Biol. 1994;48:23–31. [PubMed] [Google Scholar]
- Peña-Cortés H, Albrecht T, Prat S, Weiler EW, Willmitzer L. Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta. 1993;191:123–128. [Google Scholar]
- Peoples MB, Dalling MJ. The interplay between proteolysis and amino acid metabolism during senescence and nitrogen reallocation. In: Nooden LD, Leopold AC, editors. Senescence and Aging in Plants. San Diego, CA: Academic Press; 1988. pp. 181–217. [Google Scholar]
- Rosahl S. Lipoxygenases in plants-their role in development and stress response. Z Naturforsch. 1996;51c:123–138. doi: 10.1515/znc-1996-3-401. [DOI] [PubMed] [Google Scholar]
- Saravitz DM, Siedow JN. The differential expression of wound-inducible lipoxygenase genes in soybean leaves. Plant Physiol. 1996;110:287–299. doi: 10.1104/pp.110.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker RC, Polzin K, Labate J, Specht J, Brummer EC, Olson T, Young N, Concibido V, Wilcox J, Tamulonis JP and others. Genome duplication in soybean (Glycine subgenus soja) Genetics. 1996;144:329–338. doi: 10.1093/genetics/144.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedow JN (1991) Plant lipoxygenase: structure and function. In WR Briggs, RL Jones, V Walbot, eds, Annual Review of Plant Physiology and Plant Molecular Biology, Vol 42. Annual Reviews, Inc., Palo Alto, CA, pp 145–188
- Simpson RJ (1986) Translocation and metabolism of nitrogen: whole plant aspects. In H Lambers, JJ Neeteson, I Stulen, eds, Fundamental, Ecological and Agricultural Aspects of Nitrogen Metabolism in Higher Plants. Martinus Nijhoff Publishers, Dordrecht, The Netherlands, pp 71–96
- Simpson TD, Gardner HW. Allene oxide synthase and allene oxide cyclase, enzymes of the jasmonic acid pathway, localized in Glycine max tissues. Plant Physiol. 1995;108:199–202. doi: 10.1104/pp.108.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WC, Brash AR. Purification of an allene oxide synthase and identification of the enzyme as a cytochrome P-450. Science. 1991;253:781–784. doi: 10.1126/science.1876834. [DOI] [PubMed] [Google Scholar]
- Staswick PE. Soybean vegetative storage protein structure and gene expression. Plant Physiol. 1988;87:250–254. doi: 10.1104/pp.87.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE (1994) Storage proteins of vegetative plant tissue. In RL Jones, CR Somerville, V Walbot, eds, Annual Review of Plant Physiology and Plant Molecular Biology, Vol 45. Annual Reviews, Inc., Palo Alto, CA, pp 303–322
- Tranbarger TJ, Franceschi VR, Hildebrand DF, Grimes HD. The soybean 94-kilodalton vegetative storage protein is a lipoxygenase that is localized in paraveinal mesophyll cell vacuoles. Plant Cell. 1991;3:973–987. doi: 10.1105/tpc.3.9.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R (1989) The sink-source transition in leaves. In WR Briggs, RL Jones, V Walbot, eds, Annual Review of Plant Physiology and Plant Molecular Biology, Vol 40. Annual Reviews, Inc., Palo Alto, CA, pp 119–138
- Vernooy-Gerritsen M, Bos ALM, Veldink GA, Vliegenthart JFG. Localization of lipoxygenases 1 and 2 in germinating soybean seeds by an indirect immunofluorescence technique. Plant Physiol. 1983;73:262–267. doi: 10.1104/pp.73.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernooy-Gerritsen M, Leunissen JLM, Veldink GA, Vliegenthart JFG. Intracellular localization of lipoxygenases-1 and -2 in germinating soybean seeds by indirect labeling with protein A-colloidal gold complexes. Plant Physiol. 1984;76:1070–1079. doi: 10.1104/pp.76.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick BA, Zimmerman DC (1987) Oxidative systems for modification of fatty acids: the lipoxygenase pathway. In PK Stumpf, EE Conn, eds, The Biochemistry of Plant Lipids, Vol 9. Academic Press, New York, pp 53–90
- Wilen RW, Vanrooijen GJH, Pearce DW, Pharis RP, Holbrook LA, Moloney MM. Effects of jasmonic acid on embryo-specific processes in Brassica and Linum oilseeds. Plant Physiol. 1991;95:399–405. doi: 10.1104/pp.95.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wink M. The plant vacuole: A multifunctional compartment. J Exp Bot Suppl. 1993;44:231–246. [Google Scholar]
- Wittenbach V. Effect of pod removal on leaf senescence in soybeans. Plant Physiol. 1982;70:1544–1548. doi: 10.1104/pp.70.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. Effect of pod removal on leaf photosynthesis and soluble protein composition of field-grown soybeans. Plant Physiol. 1983a;73:121–124. doi: 10.1104/pp.73.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach V. Purification and characterization of a soybean leaf storage glycoprotein. Plant Physiol. 1983b;73:125–129. doi: 10.1104/pp.73.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata F, Danso SKA, Hardarson G, Fried M. Nitrogen fixation and translocation in field-grown faba bean. Agron J. 1987;79:505–509. [Google Scholar]
- Zhu T, Schupp JM, Oliphant A, Keim P. Hypomethylated sequences: characterization of the duplicate soybean genome. Mol Gen Genet. 1994;244:638–645. doi: 10.1007/BF00282754. [DOI] [PubMed] [Google Scholar]
- Zimmer T, Ohkuma M, Ohta A, Takagi M, Schunck WH. The CYP52 multigene family of Candida maltosa encodes functionally diverse n-alkane-inducible cytochromes P-450. Biochem Biophys Res Comm. 1996;224:784–789. doi: 10.1006/bbrc.1996.1100. [DOI] [PubMed] [Google Scholar]