Abstract
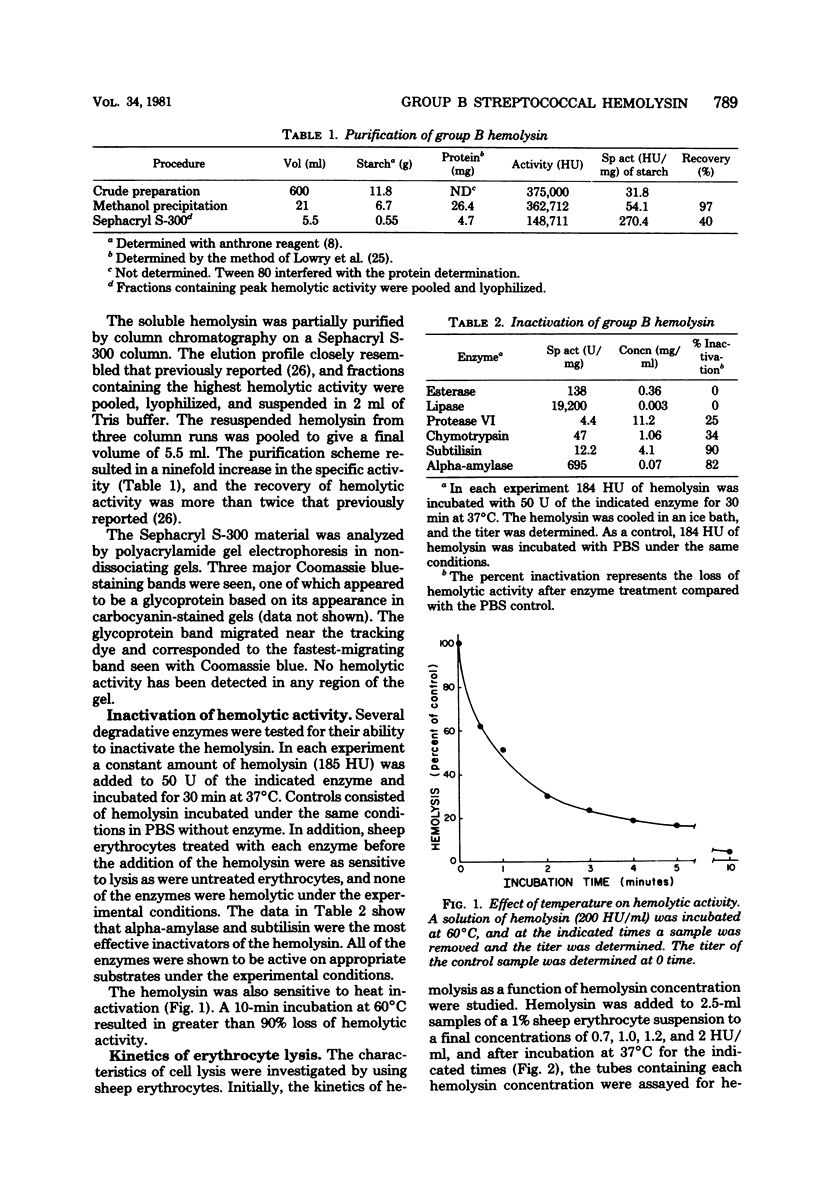
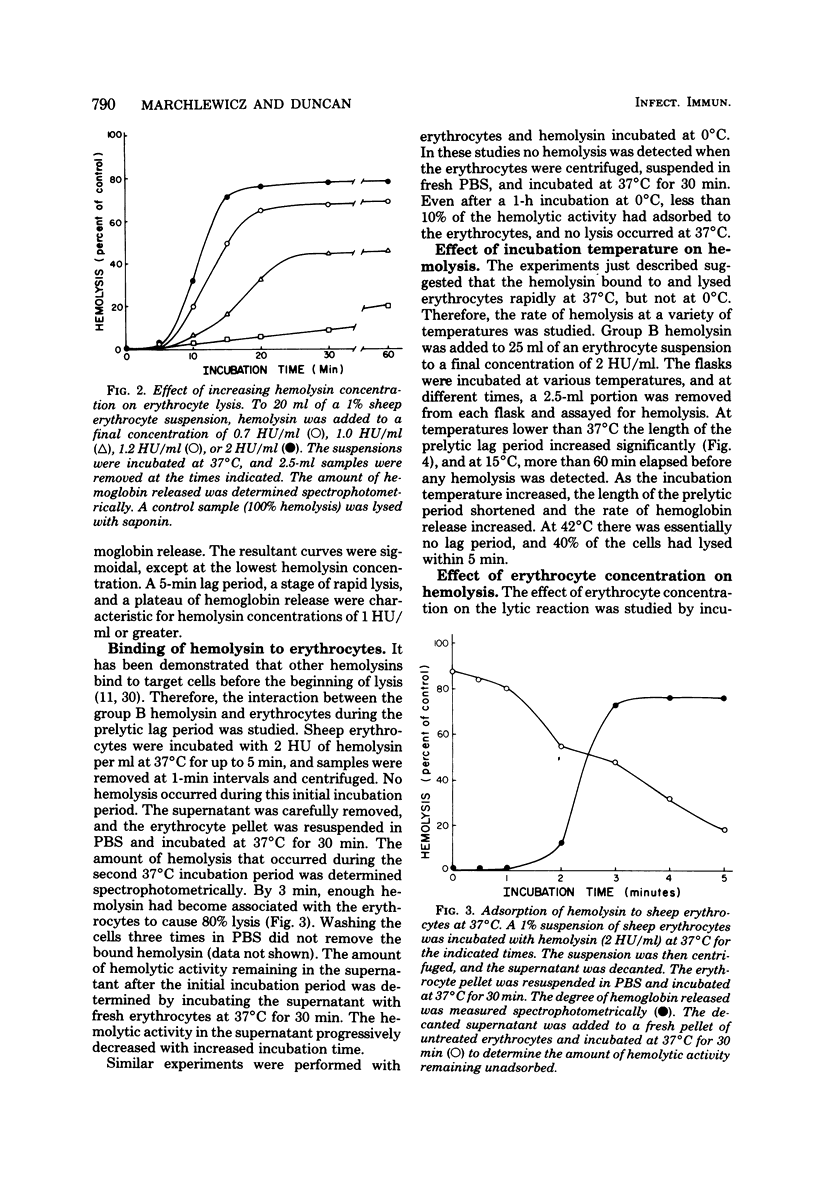
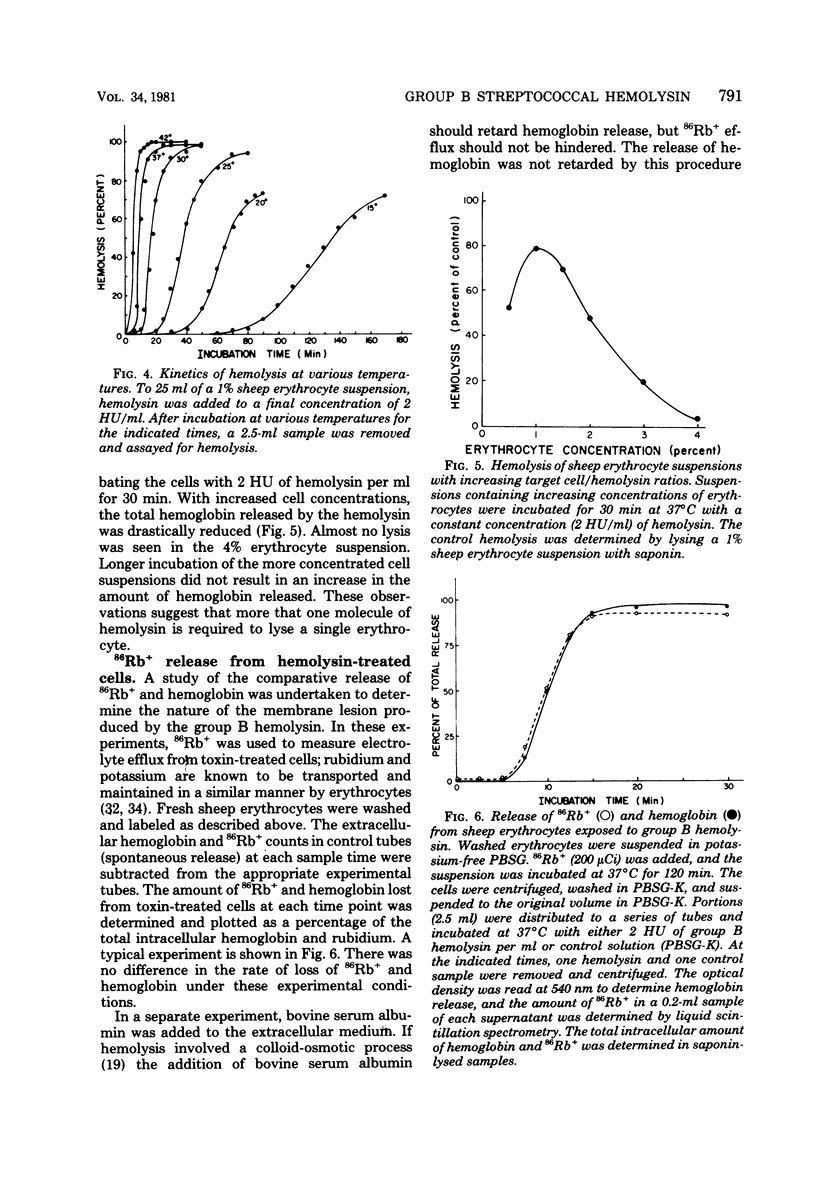
An improved procedure for the isolation and purification of the hemolysin produced by a group B streptococcus was developed, and the inactivation of partially purified hemolysin by several enzymes was studied. Hemolysin obtained in buffer containing starch and Tween 80 was inactivated by subtilisin and alpha-amylase, suggesting that the hemolysin may consist of a protein hemolytic moiety complexed to starch which acts as a carrier or stabilizer. Properties of the hemolytic reaction were studied by using sheep erythrocytes as target cells. Experiments to examine the kinetics of hemolysis at different hemolysin concentrations resulted in a family of sigmoidal curves characterized by a short prelytic lag phase followed by a period of rapid release of hemoglobin. The binding of the group B hemolysin at 37 degrees C was rapid; within 3 min, most of the cells had bound sufficient hemolysin to produce lysis. In contrast, the hemolysin did not bind to erythrocytes at 0 degrees C. The length of the prelytic lag period and the rate of hemolysis were also temperature dependent. A decrease in total hemolysis was observed when the target cell/hemolysin ratio was increased, suggesting that a multihit response is required for lysis. Intracellular 86Rb and hemoglobin were released at the same rate from hemolysin-treated cells, indicating that a colloid-osmotic process is not involved in the lytic mechanism.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E., Raynaud M. Action de la streptolysine O sur les membranes cellulaires. I. Fixation sur la membrane érythrocytaire. Ann Inst Pasteur (Paris) 1968 Jun;114(6):812–827. [PubMed] [Google Scholar]
- Anthony B. F., Okada D. M. The emergence of group B streptococci in infections of the newborn infant. Annu Rev Med. 1977;28:355–369. doi: 10.1146/annurev.me.28.020177.002035. [DOI] [PubMed] [Google Scholar]
- Atwood K. C., Norman A. On the Interpretation of Multi-Hit Survival Curves. Proc Natl Acad Sci U S A. 1949 Dec;35(12):696–709. doi: 10.1073/pnas.35.12.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNHEIMER A. W., RODBART M. The effect of nucleic acids and of carbohydrates on the formation of streptolysin. J Exp Med. 1948 Aug;88(2):149–168. doi: 10.1084/jem.88.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. J., Barrett F. F. Group B streptococcal infections in infants. The importance of the various serotypes. JAMA. 1974 Nov 25;230(8):1158–1160. [PubMed] [Google Scholar]
- Bernheimer A. W. COMPARATIVE KINETICS OF HEMOLYSIS INDUCED BY BACTERIAL AND OTHER HEMOLYSINS. J Gen Physiol. 1947 Mar 20;30(4):337–353. doi: 10.1085/jgp.30.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra G. B. Effect of detergents on streptolysin S precursor. Infect Immun. 1980 Aug;29(2):306–310. doi: 10.1128/iai.29.2.306-310.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie K. E., Haukenes G. Measles virus specific precipitins in sera from patients with chronic active hepatitis. Scand J Infect Dis. 1979;11(2):99–106. doi: 10.3109/inf.1979.11.issue-2.01. [DOI] [PubMed] [Google Scholar]
- Duncan J. L., Mason L. Characteristics of streptolysin S hemolysis. Infect Immun. 1976 Jul;14(1):77–82. doi: 10.1128/iai.14.1.77-82.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias N., Heller M., Ginsburg I. Binding of streptolysin S to red blood cell ghosts and ghost lipids. Isr J Med Sci. 1966 May-Jun;2(3):302–309. [PubMed] [Google Scholar]
- Ferrieri P., Gray E. D., Wannamaker L. W. Biochemical and immunological characterization of the extracellular nucleases of group B streptococci. J Exp Med. 1980 Jan 1;151(1):56–68. doi: 10.1084/jem.151.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M., Koshimura S. Isolation and properties of a streptococcal hemolysin formed in the presence of colistin. Jpn J Exp Med. 1975 Dec;45(6):457–466. [PubMed] [Google Scholar]
- GINSBURG I., GROSSOWICZ N. A cell-bound hemolysin of group A streptococci. Bull Res Counc Isr Sect E Exp Med. 1958 Dec;7E:237–246. [PubMed] [Google Scholar]
- GINSBURG I., HARRIS T. N., GROSSOWICZ N. OXYGEN-STABLE HEMOLYSINS OF GROUP A STREPTOCOCCI. I. THE ROLE OF VARIOUS AGENTS IN THE PRODUCTION OF THE HEMOLYSINS. J Exp Med. 1963 Dec 1;118:905–917. doi: 10.1084/jem.118.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Pastewka J. V., Peacock A. C. Differential staining of phosphoproteins on polyacrylamide gels with a cationic carbocyanine dye. Anal Biochem. 1973 Nov;56(1):43–51. doi: 10.1016/0003-2697(73)90167-x. [DOI] [PubMed] [Google Scholar]
- Hingson D. J., Massengill R. K., Mayer M. M. The kinetics of release of 86rubidium and hemoglobin from erythrocytes damaged by antibody and complement. Immunochemistry. 1969 Mar;6(2):295–307. doi: 10.1016/0019-2791(69)90166-9. [DOI] [PubMed] [Google Scholar]
- Inoue K., Akiyama Y., Kinoshita T., Higashi Y., Amano T. Evidence for a one-hit theory in the immune bactericidal reaction and demonstration of a multi-hit response for hemolysis by streptolysin O and Clostridium perfringens theta-toxin. Infect Immun. 1976 Feb;13(2):337–344. doi: 10.1128/iai.13.2.337-344.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjems E., Perch B., Henrichsen J. Serotypes of group B streptococci and their relation to hyaluronidase production and hydrolysis of salicin. J Clin Microbiol. 1980 Feb;11(2):111–113. doi: 10.1128/jcm.11.2.111-113.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. Y., Wang M. T., de Faria J. B., Akao T. Streptolysin S: improved purification and characterization. Arch Biochem Biophys. 1978 Dec;191(2):804–812. doi: 10.1016/0003-9861(78)90423-x. [DOI] [PubMed] [Google Scholar]
- Marchlewicz B. A., Duncan J. L. Properties of a hemolysin produced by group B streptococci. Infect Immun. 1980 Dec;30(3):805–813. doi: 10.1128/iai.30.3.805-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan T. W., Baker C. J., Straus D. C., Mattingly S. J. Association of elevated levels of extracellular neuraminidase with clinical isolates of type III group B streptococci. Infect Immun. 1978 Sep;21(3):738–746. doi: 10.1128/iai.21.3.738-746.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley T. D., Duncan J. L. Characteristics of streptolysin O action. Infect Immun. 1971 Dec;4(6):683–687. doi: 10.1128/iai.4.6.683-687.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOLOMON A. K. The permeability of the human erythrocyte to sodium and potassium. J Gen Physiol. 1952 May;36(1):57–110. doi: 10.1085/jgp.36.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus D. C., Mattingly S. J., Milligan T. W., Doran T. I., Nealon T. J. Protease production by clinical isolates of type III group B streptococci. J Clin Microbiol. 1980 Sep;12(3):421–423. doi: 10.1128/jcm.12.3.421-423.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summary of the workshop on perinatal infections due to group B Streptococcus. J Infect Dis. 1977 Jul;136(1):137–152. doi: 10.1093/infdis/136.1.137. [DOI] [PubMed] [Google Scholar]
- Whittam R., Ager M. E. Vectorial aspects of adenosine-triphosphatase activity in erythrocyte membranes. Biochem J. 1964 Nov;93(2):337–348. doi: 10.1042/bj0930337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson H. W. Analysis of group B streptococcal types associated with disease in human infants and adults. J Clin Microbiol. 1978 Feb;7(2):176–179. doi: 10.1128/jcm.7.2.176-179.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson H. W., Facklam R. R., Wortham E. C. Distribution by serological type of group B streptococci isolated from a variety of clinical material over a five-year period (with special reference to neonatal sepsis and meningitis). Infect Immun. 1973 Aug;8(2):228–235. doi: 10.1128/iai.8.2.228-235.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


