Summary
Relating the function of neuronal cell types to information processing and behavior is a central goal of neuroscience. In the hippocampus, pyramidal cells in CA1 and the subiculum process sensory and motor cues to form a cognitive map encoding spatial, contextual, and emotional information, which they transmit throughout the brain. Do these cells constitute a single class, or are there multiple cell types with specialized functions? Using unbiased cluster analysis, we show that there are two morphologically and electrophysiologically distinct principal cell types that carry hippocampal output. We show further that these two cell types are inversely modulated by the synergistic action of glutamate and acetylcholine acting on metabotropic receptors that are central to hippocampal function. Combined with prior connectivity studies, our results support a model of hippocampal processing in which the two pyramidal cell types are predominantly segregated into two parallel pathways that process distinct modalities of information.
Introduction
An emerging paradigm in cellular neuroscience is to understand the function of the brain in terms of individual neurons that can be grouped into distinct types based on a variety of properties. These properties of distinct cell types affect how they process information, thus enabling functional specialization within neuronal networks. A major determinant of how neurons integrate information is the shape of their dendrites (Hausser et al., 2000; Mel, 1994). For example, Purkinje cells and stellate cells have vastly different dendritic arbors that process synaptic inputs in the cerebellum differently, and in simulations of cortical pyramidal cells, even modest manipulations of dendritic architecture result in altered patterns of action potential output (Mainen and Sejnowski, 1996). Cells with different electrophysiological properties also perform distinct computations. For instance, fast-spiking interneurons and adapting interneurons respond to synaptic input in fundamentally different ways that strongly shape how these signals are processed in the cortex (Yoshimura and Callaway, 2005). The connectivity of cells within neuronal circuits also influences processing, as with the magnocellular and parvocellular pathways in the lateral geniculate nucleus of the thalamus, which form separate, parallel streams of visual information that project to segregated areas of visual cortex (Livingstone and Hubel, 1988). Neuromodulation also strongly influences the behavior of distinct cell types. For example, in the basal ganglia, two populations of medium spiny neurons that are defined by their expression of the D1 or D2 dopamine receptor form the direct and indirect pathways, which facilitate and inhibit movement, respectively (Surmeier et al., 2007). Thus, investigation of the morphology, electrophysiology, circuitry, and modulation of individual neurons can identify the different cell types within neuronal circuits and elucidate their distinct roles in processing information in the brain.
The hippocampus is the cradle of cognition—a brain structure critically involved in the formation, organization, and retrieval of new memories. The principal cell type in this region is the excitatory pyramidal neuron—one of the most-studied cells in the mammalian brain—which integrates spatial, contextual, and emotional information and transmits all hippocampal output to various targets throughout the brain. Pyramidal cells in the CA1 and subiculum regions convey this output by firing action potentials either individually or in high-frequency bursts. These distinct firing patterns are functionally important, as bursts may serve to increase the reliability of synaptic communication by increasing the probability of evoking a postsynaptic spike (Lisman, 1997; Williams and Stuart, 1999) and are involved in the induction of plasticity and the development of place fields (Epsztein et al., 2011; Golding et al., 2002). Indeed, information processing via bursts has been shown to play a key role in the formation of hippocampus-dependent memories (Xu et al., 2012). Despite the functional importance of these different firing patterns, it is not known whether the observed heterogeneity in hippocampal pyramidal cell firing patterns reflects the existence of multiple cell types or a single cell type with variable excitability (Greene and Totterdell, 1997; Jarsky et al., 2008; Staff et al., 2000; van Welie et al., 2006). A single cell type would suggest that all pyramidal cells process information similarly, whereas the existence of multiple stable cell types would allow for specialization of these principal cells in hippocampal function. Given the central goal of describing the function of the brain in terms of its different cell types and elucidating the roles of these neuronal classes in complex behavioral tasks, it is important to determine the pyramidal cell types in the hippocampus that may have different roles in information processing, learning, and memory.
Here, we show that two distinct cell types constitute hippocampal pyramidal output neurons. We show further that the two cell types are both synergistically modulated by metabotropic glutamate and acetylcholine receptors, but with opposite outcomes on long-term neuronal excitability in the two cell types. These two cell types appear to correspond to neurons that have been shown to process predominantly different modalities of information (Hargreaves et al., 2005; Knierim et al., 2006) and bias their output to different structures throughout the brain (Kim and Spruston, 2012). However, it was unknown whether these pyramidal cells differed solely in their connectivity, or rather constituted two distinct cell types with additional specialized features. Thus, our findings support a model in which the hippocampus functions through parallel processing of separate information streams by two pyramidal cell types with distinct dendritic morphology, electrophysiological properties, and different modulatory responses to neurotransmitters that are central to hippocampal function and disease (Bear et al., 2004; Disterhoft and Oh, 2006; Francis et al., 1999).
Results
Morphological and electrophysiological investigation of pyramidal neurons
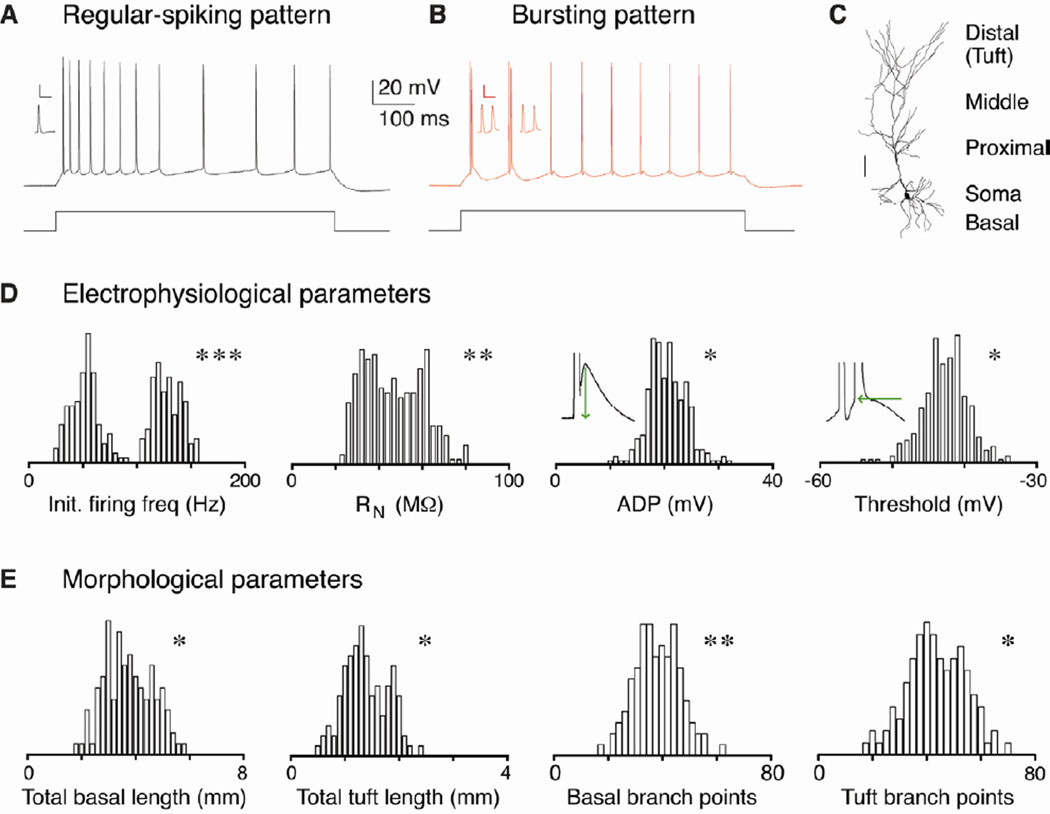
We studied the morphological and electrophysiological properties of pyramidal neurons in the CA1 and subiculum regions in acute slices of the rat hippocampus. In agreement with previous work (Greene and Mason, 1996; Jarsky et al., 2008; Staff et al., 2000; van Welie et al., 2006), suprathreshold step-current injections evoked one of two firing patterns: regular spiking or bursting (Fig. 1A-B). To determine whether these two response patterns arise from separate classes of pyramidal cells or whether they represent a single population of cells spanning a continuum of excitability, we measured electrophysiological properties using current-clamp recordings and made post-hoc anatomical reconstructions of the recorded cells (see Experimental Procedures). We examined the distribution of over 30 electrophysiological and morphological properties in a large population of pyramidal cells (n = 268, Fig. 1C-E and Table 1). If regular-spiking and bursting cells were indeed separate neuronal classes, we would expect to see multimodal distributions of some properties, versus unimodal distributions for a single class. When we examined the distribution of several electrophysiological and morphological properties (Fig. 1D-E), we found that these properties deviated significantly from a normal distribution and were poorly fit by single Gaussian functions, suggesting that there may be multiple classes of pyramidal cells throughout CA1 and the subiculum.
Figure 1. Distribution of electrophysiological and morphological properties.
A-B. Pyramidal neurons in CA1 and the subiculum respond to step current injection in vitro by firing action potentials in one of two distinct patterns: regular spiking or bursting. The former pattern consists of trains of individual action potentials, while the latter pattern begins with one or more bursts of high frequency (>100 Hz) spikes. Inset: enlarged bursts (scale bar = 50 mV and 20 ms). C. Filled pyramidal neurons with different dendritic compartments indicated (scale bar = 100 µm). D-E. All-point histograms of the distributions of several electrophysiological (n = 268 cells) and morphological properties (n = 110 cells). Illustrations of how ADP and second spike threshold were measured are inset. P values from the D’Agostino & Pearson omnibus normality test demonstrate that the properties presented are not unimodally distributed, suggesting that there are discrete groups of pyramidal cells within this population. Asterisks indicate significant differences between the groups (*p < 0.05, ** p < 0.01, *** p < 0.001).
TABLE 1.
Properties of hippocampal pyramidal cells
| Electrophysiology |
|||
|---|---|---|---|
| Reg. spiking | Bursting | p value | |
| RN (MΩ) | 55.0 ± 3.1 | 33.7 ± 2.0 | < 0.001 |
| Sag ratio | 0.84 ± 0.02 | 0.74 ± 0.02 | < 0.001 |
| Subthreshold dV/dt (mV/mS) | 2.61 ± 0.53 | 4.39 ± 0.49 | < 0.001 |
| ADP amplitude (mV) | 17.9 ± 1.3 | 22.2 ± 0.9 | < 0.001 |
| 2nd spike threshold (mV) | −40.8 ± 0.7 | −45.1 ± 0.9 | < 0.001 |
| Max 2nd falling dV/dt (mV/ms) | −56 ± 3 | −65 ± 3 | < 0.01 |
| Max 2nd rising dV/dt (mV/ms) | 240 ± 24 | 272 ± 22 | < 0.05 |
| 1st spike FWHM (ms) | 0.87 ± 0.01 | 0.82 ± 0.02 | < 0.05 |
| Resting membrane potential (mV) | −65.8 ± 0.7 | −66.2 ± 0.4 | n.s. |
| 1st spike threshold (mV) | −53.1 ± 1.0 | −52.4 ± 0.6 | n.s. |
| Max 1st spike rising dV/dt (mV/ms) | 460 ± 21 | 488 ± 21 | n.s. |
| Max 1st spike falling dV/dt (mV/ms) | −106 ± 4 | −113 ± 5 | n.s. |
| 2nd spike FWHM (ms) | 1.18 ± 0.03 | 1.14 ± 0.04 | n.s. |
| 1st spike height (mV) | 92.0 ± 1.5 | 90.3 ± 1.6 | n.s. |
| 2nd spike height (mV) | 71.3 ± 3.0 | 70.0 ± 2.3 | n.s. |
| AHP amplitude (mV) | −1.29 ± 0.25 | −1.39 ± 0.31 | n.s. |
|
Morphology |
|||
| Reg. spiking | Bursting | p value | |
| Total tuft dendrite length (mm) | 1.39 ± 0.02 | 1.49 ± 0.03 | < 0.01 |
| Total basal dendrite length (mm) | 3.89 ± 0.26 | 3.08 ± 0.17 | < 0.01 |
| Basal branching order | 4.40 ± 0.26 | 3.29 ± 0.23 | < 0.01 |
| Distance to main bifurcation (µm) | 193.8 ± 19.3 | 264.4 ± 22.8 | < 0.05 |
| Basal branch points | 50.1 ± 3.8 | 40.9 ± 3.1 | < 0.05 |
| Tuft branch points | 33.7 ± 2.8 | 41.6 ± 3.3 | < 0.05 |
| Apical branching order | 4.91 ± 0.72 | 7.93 ± 1.48 | < 0.05 |
| Proximal apical dendrite length (mm) | 1.76 ± 0.21 | 1.35 ± 0.14 | n.s. |
| Middle apical dendrite length (mm) | 1.67 ± 0.28 | 1.48 ± 0.16 | n.s. |
| Proximal apical branching order | 5.53 ± 0.47 | 4.98 ± 0.36 | n.s. |
| Middle apical branching order | 4.81 ± 0.32 | 4.16 ± 0.40 | n.s. |
| Proximal apical branch points | 25.0 ± 3.4 | 28.0 ± 2.6 | n.s. |
| Middle apical branch points | 33.7 ± 1.9 | 34.6 ± 3.2 | n.s. |
| Basal branches off soma | 3.90 ± 0.47 | 3.65 ± 0.39 | n.s. |
Mean ± S.E.M. for all values. Electrophysiology, n = 268; Morphology, n = 110
Two distinct classes of pyramidal neurons
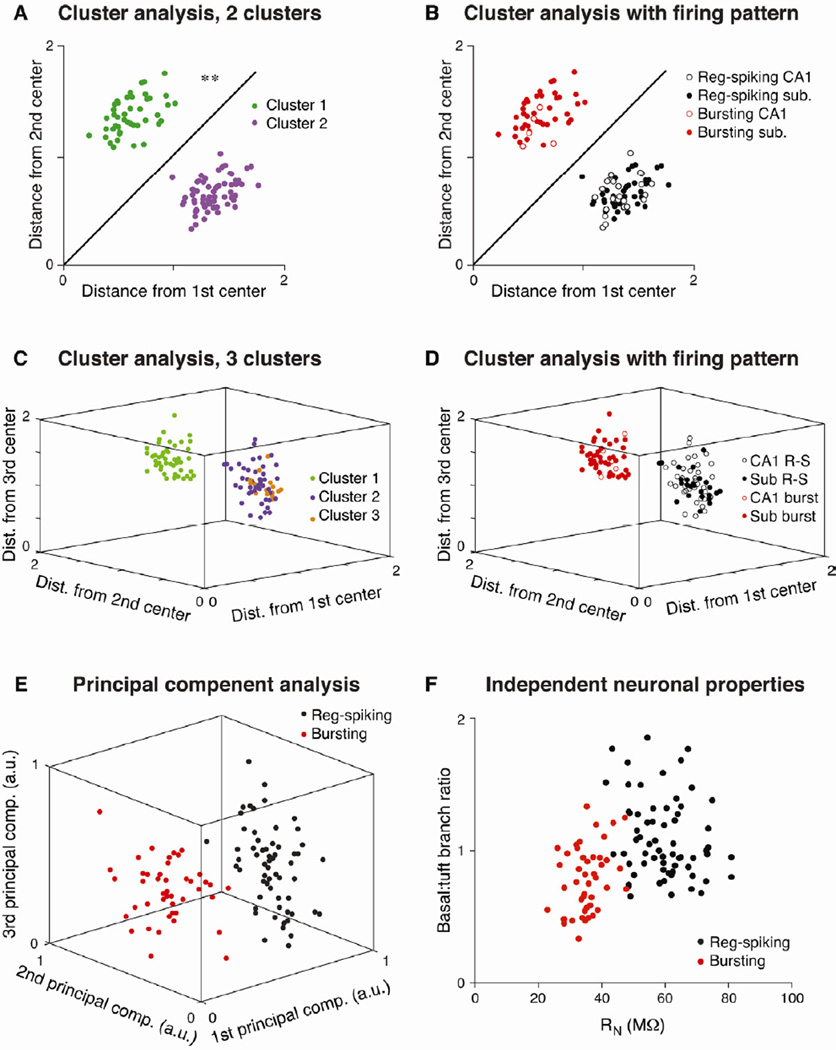
To determine the number of pyramidal cell types throughout CA1 and the subiculum, we performed an unbiased K-means cluster analysis (Efron and Tibshirani, 1986; Kong et al., 2005; Sugar and James, 2003) using 15 electrophysiological and morphological properties (see Experimental Procedures). Using all CA1 and subicular pyramidal neurons (n = 110 cells), K-means cluster analysis revealed two completely non-overlapping groups of cells (Fig. 2A), which aligned perfectly with the two step-current-induced firing patterns (Fig. 2B). We did not find evidence of more than two clusters, subgroups within the either the regular-spiking or bursting populations, or separation of CA1 and subicular neurons within either of the two clusters (Fig. 2C-D). A bootstrap analysis (see Experimental Procedures and Sugar and James, 2003) demonstrated that the data are best represented by two clusters (Supp. Fig. 1). Furthermore, a principal component analysis revealed that the first three principal components produced perfect separation of the regular-spiking and bursting cell types (Fig. 2E). Finally, a plot of only two properties (one physiological and one morphological) reveals a large degree of separation between the two cell types (Fig. 2F). Taken together, these results strongly indicate the presence of two (and not more) distinct classes of pyramidal neurons, with each cell type present in both CA1 and the subiculum.
Figure 2. Two distinct classes of hippocampal pyramidal neurons.
K-means cluster analysis assigns neurons to k groups based on the Euclidian distance of all parameters from the center of each of the k clusters (n = 110 cells). A. Plot of total distance from the center of two clusters (based on 15 electrophysiological and morphological parameters) reveals significant separation into two groups. Parameters directly related to bursting (e.g., spike frequency) were excluded from the cluster analysis. B. These two groups of cells align perfectly with the regular-spiking and bursting patterns, as every cell in the purple cluster displayed regular spiking and every cell in the green cluster displayed bursting. C. Distribution of cells into three clusters. Note the lack of separation between the orange and purple clusters (2 and 3), while the green cluster (1) is significantly separated from both. D. Similarly, when all cells are grouped into 3 clusters, separation is only apparent between regular-spiking and bursting neurons. E. A principal component analysis was performed on the same 15 electrophysiological and morphological properties, and a plot of the first three principal components shows complete separation between regular-spiking and bursting neurons. F. Qualitatively similar (but incomplete) separation between regular-spiking and bursting neurons is observed by plotting two independent parameters: input resistance and the ratio of basal to tuft dendritic branch points. Asterisks indicate significant differences between the groups (** p < 0.01).
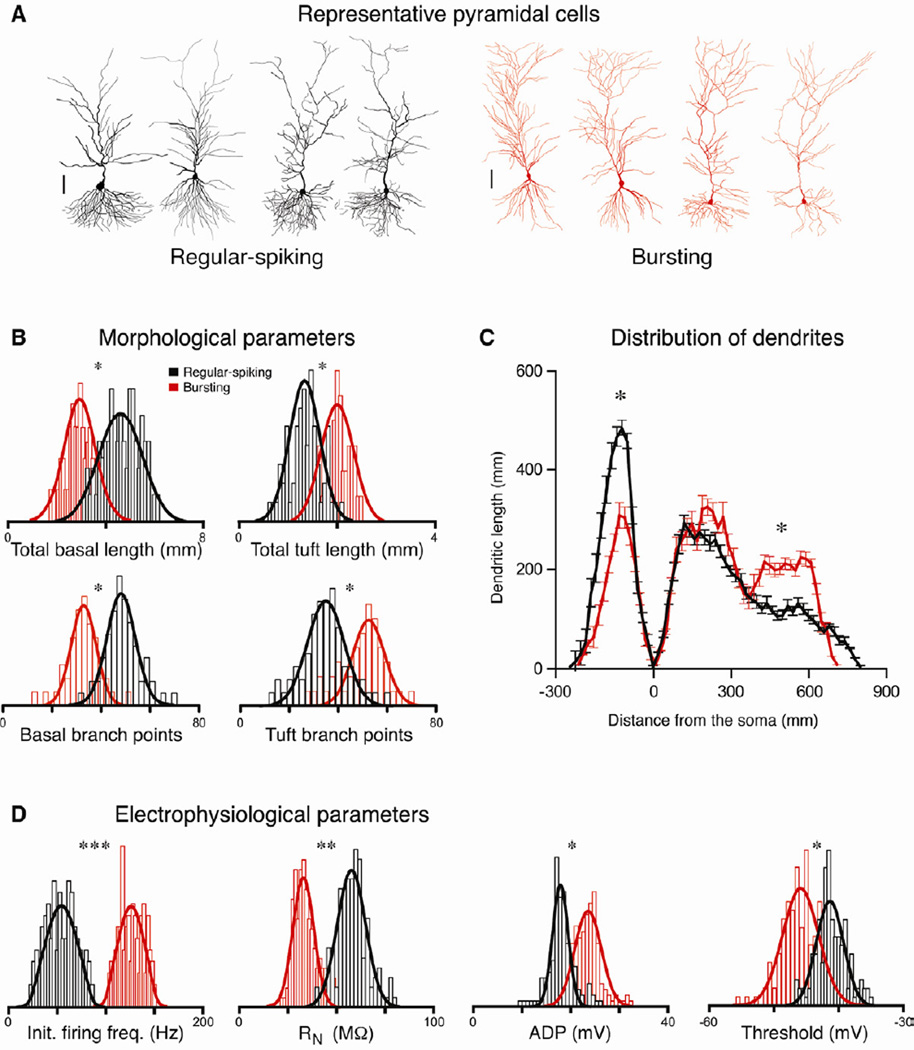
These regular-spiking and bursting cell types differ not only in their firing patterns, but also in several additional properties (Fig. 3 and Table 1). There are clear morphological differences between the two cell types: bursting cells have more extensive tuft dendrites (the distal third of the apical tree), whereas regular-spiking cells have more extensive basal dendrites (Fig. 3A-C). Regular-spiking cells also have a higher input resistance (RN), a smaller post-spike afterdepolarization (ADP), a more depolarized bursting threshold, as well as several other electrophysiological differences, relative to bursting cells (Fig. 3D and Table 1). The morphological differences in particular suggest that the regular-spiking and bursting populations do not reflect transient variance in excitability (Babadi, 2005; Beurrier et al., 1999), but rather that the two populations are discrete, stable cell types. As both of these cell types have characteristic pyramidal cell morphology and electrophysiological properties, and as we show that they are both immunopositive for a glutamatergic marker (the excitatory amino acid transporter 3, EAAT-3) and negative for a marker of GABAergic neurons (glutamic acid decarboxylase 2, GAD-2), these cell types are clearly both excitatory pyramidal neurons (Supp. Fig. 2).
Figure 3. Morphological and physiological differences between pyramidal cell types.
A. Representative reconstructions of regular-spiking (black) and bursting (red) neurons (scale bar = 100 µm). B. Histograms of the distribution of several morphological properties (n = 110 cells) and Gaussian fits of regular-spiking (black) and bursting (red) neurons. C. Distribution of dendrites by region. Plot is dendritic length as a function of distance from the soma in 20 µm segments. Negative distance denotes basal dendritic length; positive distance denotes apical length. Note that regular-spiking neurons have longer, more extensively branched basal dendrites, whereas bursting neurons have longer, more extensively branched tuft dendrites (those in the most distal third of the apical tree). There are no differences in the total dendritic length in the proximal and middle thirds of the apical dendritic tree. D. Histograms of the distribution of several electrophysiological (n = 268) properties for the two cell types. Asterisks indicate significant differences between the groups (*p < 0.05, ** p < 0.01, *** p < 0.001).
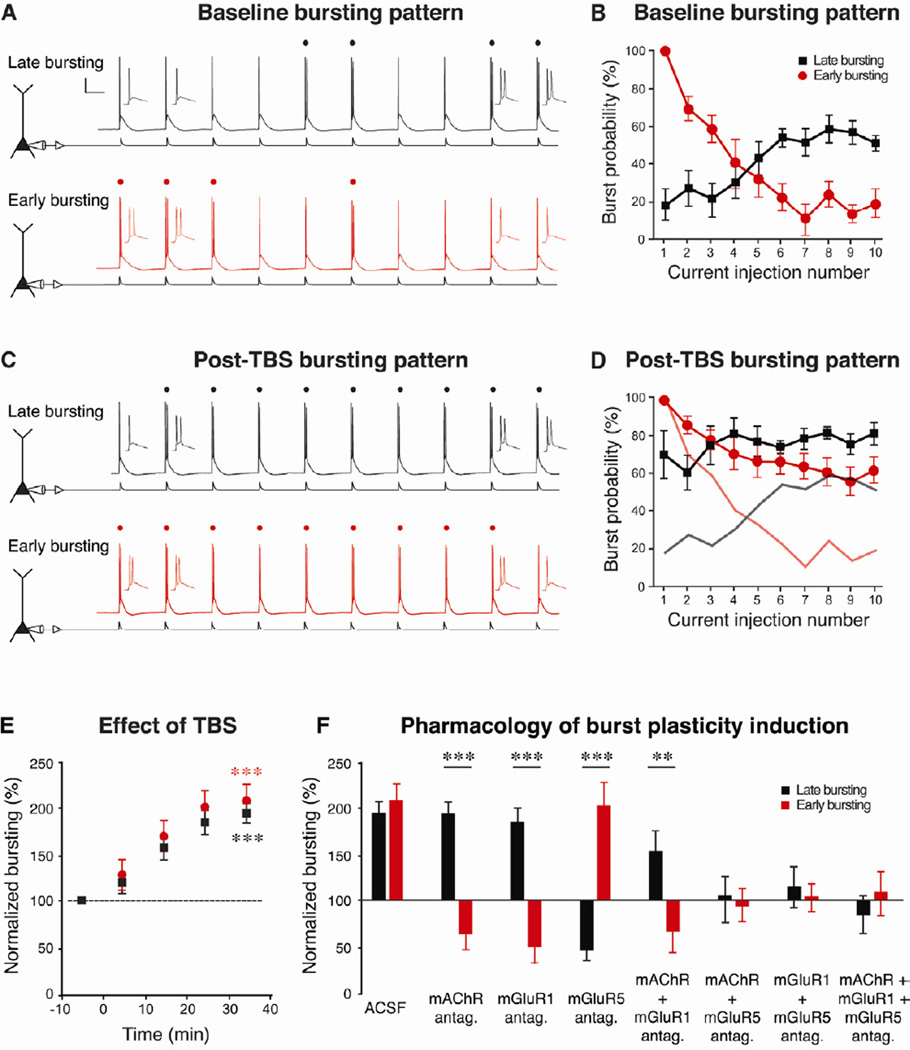
To further investigate the properties of these two types of neurons, we evoked action potential firing using trains of brief EPSC-like current injections (Moore et al., 2009). All neurons studied in this way responded with a mixture of single spikes and bursts, but the two cell types were readily distinguishable based on the temporal pattern of bursting. Regular-spiking neurons responded with single spikes early in the train and bursts later, whereas bursting neurons fired bursts early in the train and single spikes later (Fig. 4A-B). As both types of neurons can and do elicit bursts, the present nomenclature for the observed physiological heterogeneity is misleading. Therefore, we introduce a new nomenclature: late-bursting (previously “regular-spiking”) and early-bursting (previously “bursting”) pyramidal neurons. Although we chose names based on their bursting patterns in response to trains of inputs, there are many additional differences between the two cell types (summarized in Table 2).
Figure 4. Metabotropic receptors countermodulate intrinsic excitability.
A. In response to trains of repeated brief current injections, both types of neurons generated bursts (denoted by dots) and single spikes, though with different temporal patterns. Late-bursting neurons (black, previously called “regular-spiking”) displayed single spikes early in the train and bursts later, while early-bursting neurons (red, previously called “bursting”) displayed bursts early and single spikes late. B. Late-bursting neurons (black squares) showed development of bursting with repeated inputs, whereas early-bursting neurons (red circles) showed inactivation of bursting with repeated inputs. C-E. Theta-burst synaptic stimulation (TBS) increased the number of bursts evoked by the same amplitude somatic current injection (in representative traces from both cell types, 4 bursts were evoked during baseline (A) and 9 were evoked after TBS (C), indicating the induction of burst plasticity). Faded black and red lines (in D) depict the pre-TBS bursting patterns of the two types of neurons (from B). F. Normalized values for bursting (average number of bursts during 30–40 minutes after TBS, divided by the average number of bursts during the 10 minute pre-TBS baseline period) in the two cell types under a variety of pharmacological conditions (mAChRs antagonist: 10 µM atropine, mGluR1 antagonist: 25 µM LY367385, mGluR5 antagonist: 10 µM MPEP; all drugs were bath applied for the entire experiment). Asterisks indicate significant differences between the groups ** p < 0.01, *** p < 0.001). Note the opposing effects of blocking metabotropic receptors, indicating countermodulation of the two cell types.
TABLE 2.
Summary of cell-type differences
| Late-bursting cells | Early-bursting cells |
|---|---|
| Bursts late in train | Bursts early in train |
| Single spikes at threshold | Bursts at threshold |
| Small ADP | Large ADP |
| High burst threshold | Low burst threshold |
| High input resistance | Low input resistance |
| Dense basal dendrites | Sparse basal dendrites |
| Sparse tuft dendrites | Dense tuft dendrites |
| mGluR5 mediates enhanced bursting | mGluR1/mAChR mediate enhanced bursting |
| mGluR1/mAChR mediate suppressed bursting | mGluR5 mediates suppressed bursting |
Activity-dependent modulation of bursting
We studied the long-lasting modulation of pyramidal cell firing patterns using synaptic theta-burst stimulation (TBS)—a commonly used plasticity-induction protocol that mimics hippocampal activity in vivo during spatial exploration and other learning tasks. To establish a normative baseline prior to plasticity induction, we adjusted the somatic current injection amplitude to elicit on average 4 bursts out of 10 inputs per train during the baseline period, and held this amplitude constant for the duration of the experiment. After measuring neuronal output by counting the number of bursts elicited by each train during a 10-minute baseline period, we delivered TBS (see Experimental Procedures) and measured the ensuing changes in bursting. Because neuronal output in response to somatic current injection is controlled by activation of intrinsic voltage-gated or Ca2+-activated ion channels, changes in the number of burst responses were a measure of altered intrinsic postsynaptic excitability.
Expanding on previous work focusing on early-bursting cells (Moore et al., 2009), we found that both types of neurons throughout CA1 and the subiculum displayed a long-lasting increase in bursting following synaptic TBS in normal ACSF (Fig. 4C-E and Supp. Fig. 3). As shown for a representative late-bursting neuron in CA1 and an early-bursting neuron in the subiculum, 4 bursts were elicited during the baseline period (Fig. 4A) and 9 bursts were elicited by the same stimulus following TBS (Fig. 4C). This plasticity of bursting (“burst plasticity”) was activity dependent—in the absence of synaptic TBS, the level of bursting did not change over the course of 50 minutes (Supp. Fig. 3A).
Synergistic activation of metabotropic glutamate and acetylcholine receptors differentially modulates the intrinsic excitability of the two cell types
We investigated the pharmacology of burst plasticity induction in the two cell types throughout CA1 and the subiculum. We found that the induction of burst plasticity in both cell types did not require activation of ionotropic glutamate receptors or GABAA and GABAB receptors (Supp. Fig. 3B-C). Rather, plasticity induction depended on selective activation of metabotropic glutamate receptors (mGluRs) and muscarinic acetylcholine receptors (mAChRs). Interestingly, the two types of neurons differed strikingly in their response to the activation of specific subtypes of receptors (Fig. 4F and Supp. Fig. 3D-K.). Activation of mGluR5 (by delivering synaptic TBS while blocking either mAChR alone, mGluR1 alone, or both receptors together) enhanced bursting in late-bursting neurons but decreased bursting in early-bursting neurons; adding an mGluR5 antagonist blocked both of these effects. On the other hand, co-activation of mGluR1 and mAChR (by synaptic TBS while blocking mGluR5 alone) decreased bursting in late-bursting neurons but enhanced bursting in early-bursting neurons; adding antagonists of either mGluR1 or mAChR blocked both of these effects. The ability of antagonists of either mGluR1 or mAChR to completely block one direction of burst plasticity in each cell type (decreased bursting in late-bursting and enhanced bursting in early-bursting neurons) suggests that these two receptor types mediate their effects via a synergistic action (i.e., activating mGluR1 or mAChR alone has no effect). As we observed a difference between the TBS with an mGluR5 antagonist and the TBS with mGluR5 and mGluR1/mAChR antagonists, we conclude that activation of mGluR1/mAChR is necessary for these effects, but we cannot rule out a requirement for activation of additional receptors of unknown identity. Taken together, these experiments illustrate that early bursting and late-bursting cells are countermodulated: activation of mGluRs increased bursting in one class decreased it in the other, and vice versa, while mAChRs influences this plasticity further. These differences in plasticity of intrinsic excitability thus extend the differences between the two cell types (Table 2).
Burst plasticity does not interconvert cell types
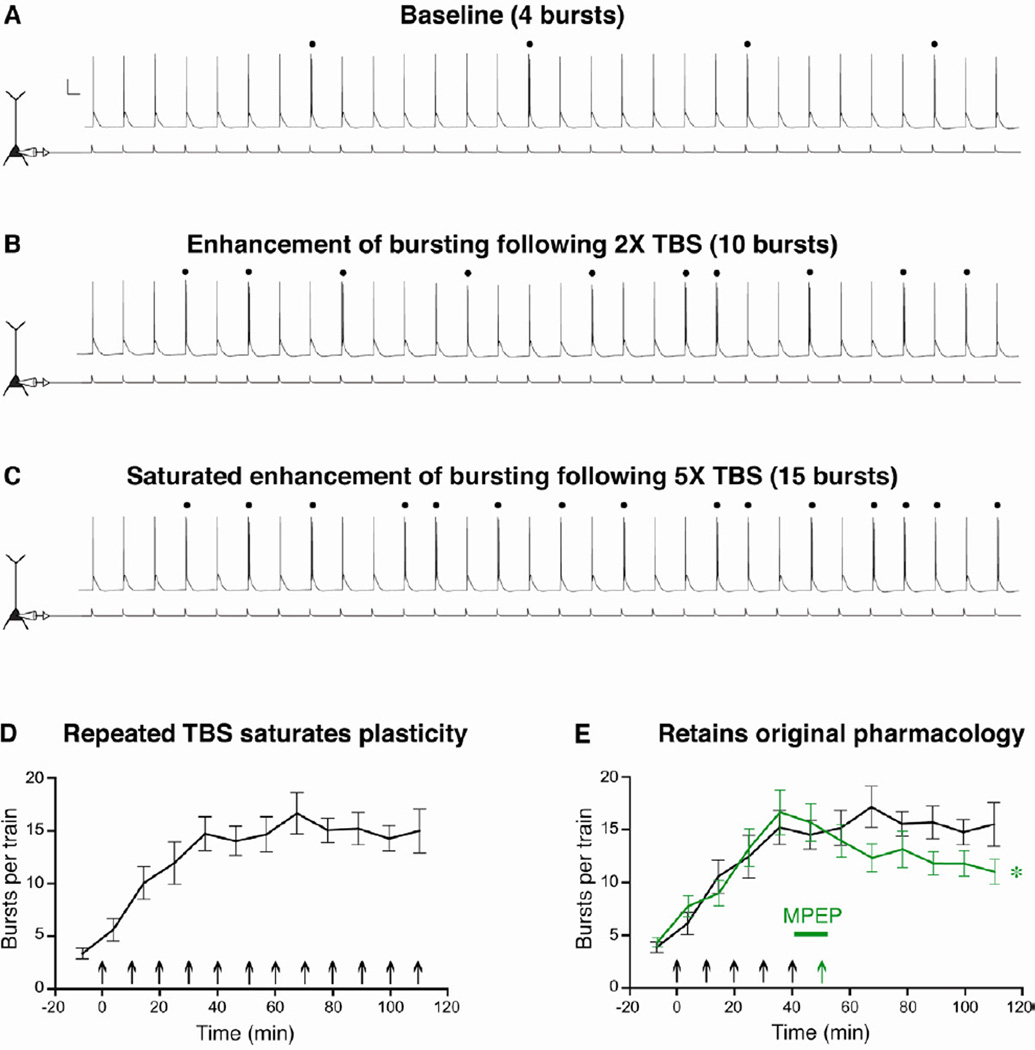
The observation that synaptic TBS differentially modulates bursting in a cell-type-dependent manner raises an intriguing question: does burst plasticity interconvert the two cell types? To test whether enhancement of bursting converts late-bursting cells to early-bursting cells, we modified the experimental paradigm in order to investigate the pharmacology of burst plasticity in a late-bursting neuron after the induction of enhanced bursting. Specifically, the enhancement was saturated by repeatedly delivering synaptic TBS every ten minutes in normal ACSF. To ensure that bursting was indeed saturated and was not due to a ceiling effect of using only 10 inputs, we used trains of 30 somatic current injections. During the baseline period, the amplitude of these injections was set to elicit approximately 4 bursts per train of 30 inputs. Repeated synaptic TBS epochs caused a much larger increase in bursting than a single TBS (Fig. 5A-D), suggesting that burst plasticity is graded. In addition, repeated induction stimuli eventually failed to enhance bursting further, suggesting that burst plasticity can be saturated.
Figure 5. Burst plasticity does not convert late-bursting cells to early-bursting.
A-C. Burst plasticity is graded and can be saturated. Representative voltage responses (top) to a train of 30, 5 Hz somatic current injections (bottom) from a late-bursting neuron at baseline (A), following 2X TBS in normal ACSF (B), and following 5X TBS (C). Bursts are denoted by dots and the scale bars represents 20 mV and 100 ms. D. In late-bursting CA1 neurons, repeated synaptic TBS epochs in normal ACSF significantly enhanced bursting to saturating levels (n = 6). E. Once enhanced bursting was saturated, MPEP (10 µm, mGluR5 antagonist) was bath applied for 10 minutes, and a final synaptic TBS epoch was delivered. Under these pharmacological conditions (green line), bursting was significantly decreased compared to experiments where MPEP was washed on but a final induction stimulus was not delivered (n = 6, *p < 0.05).
In a separate set of cells, after burst plasticity was saturated, the mGluR5-selective antagonist MPEP was applied to the bath, and a final synaptic TBS stimulus was delivered in the presence of MPEP. If saturating levels of plasticity had converted the late-bursting cell to an early-bursting cell, we would expect to see no effect of TBS in MPEP, as blocking mGluR5 did not affect enhanced bursting in early-bursting cells (see summary in Fig. 4F). However, if the late-bursting cell retained its original pharmacology (i.e., did not switch to a early-bursting cell), we would expect to see a reduction of bursting following TBS in MPEP. Indeed, the latter possibility was observed, as a single TBS in MPEP decreased bursting in late-bursting cells after the enhancement of bursting was induced (Fig. 5E). This finding suggests that burst plasticity does not serve to interconvert the two cell types, and further supports the notion that there are two stable pathways for information processing and output from the hippocampus, each dominated by a separate pyramidal cell type.
Discussion
There are two distinct classes of pyramidal cells that form separate, stable streams of hippocampal output
Previous work has shown that the firing patterns of pyramidal cells in CA1 and the subiculum can vary from regular spiking to weakly bursting to strongly bursting (Greene and Mason, 1996; Jarsky et al., 2008; Staff et al., 2000; van Welie et al., 2006), and that these firing patterns correlate with the magnitude of the calcium tail current (Jung et al., 2001). One interpretation of these observations is that regular-spiking and bursting neurons represent opposite ends of a continuous spectrum of excitability (Staff et al., 2000). The current findings, however, indicate that neurons exhibiting these different firing patterns can both in fact burst, yet they are separate, stable cell types with distinct physiological and morphological identities.
Our cluster and principal component analyses unambiguously demonstrate that there are two separate groups of cells throughout CA1 and the subiculum (see Fig. 2 and Supp. Fig. 1). The fact that we did not observe neurons with intermediate properties (i.e., between the two clusters) suggests that transitions between these groups, if they occur, must be either rapid or rare. Consistent with this, the extent of the morphological differences (see Fig. 3), the inverse induction requirements for burst plasticity (see Fig. 4), and the functional organization of output from the subiculum (see below) do not support a model of interconversion between two states (see also Fig. 5). Rather, our results strongly support the notion that these neuronal populations are stable cell types with distinct identities. Furthermore, the observed differences in spiking patterns, dendritic morphology and neuromodulation strongly suggest that these cell types process information differently. Thus, the discovery of these two discrete types of pyramidal cells that integrate hippocampal information differently, combined with our previous observation that these neurons transmit their output to different targets throughout the brain (Kim and Spruston, 2012), represents an important advancement in our understanding of how the hippocampus processes information.
While our data do not indicate the presence of more than two pyramidal cell types, there may be other subdivisions of these cells that we did not sample, either along the superficial-deep axis of the CA1/subiculum cell layer (Mizuseki et al., 2011) or along the dorsal-ventral axis of the hippocampus.
Significance of distinct cells types to information processing
There are two paths of information flow in the hippocampus: an indirect path through the well studied “trisynaptic loop” and a direct path from the entorhinal cortex (EC) to CA1 (Amaral and Witter, 1989; Witter et al., 1989). In the indirect path, information is combined into a single path, with projections from the medial and lateral EC (MEC and LEC, respectively) converging onto granule cells in the dentate gyrus (DG), and projecting in turn to CA3, CA1, and finally to the subiculum. In the direct path, information is processed in parallel, with inputs from the MEC and LEC projecting to separate areas of CA1 (Amaral and Witter, 1989), which then selectively target separate areas of the subiculum (Kim and Spruston, 2012). We have previously shown that pyramidal cells throughout the CA1 and subiculum regions are topographically organized along the proximal-to-distal axis, with cells displaying the regular-spiking pattern (i.e., late-bursting cells) predominating in CA1 and the proximal subiculum and cells showing the bursting pattern (i.e., early-bursting cells) predominating in the distal subiculum (Jarsky et al., 2008). Given this topographical organization, our data identifying late-bursting and early-bursting neurons as separate cell types suggests that these distinct neurons may contribute to functional specialization of these parallel pathways of hippocampal processing and output (Fig. 6A).
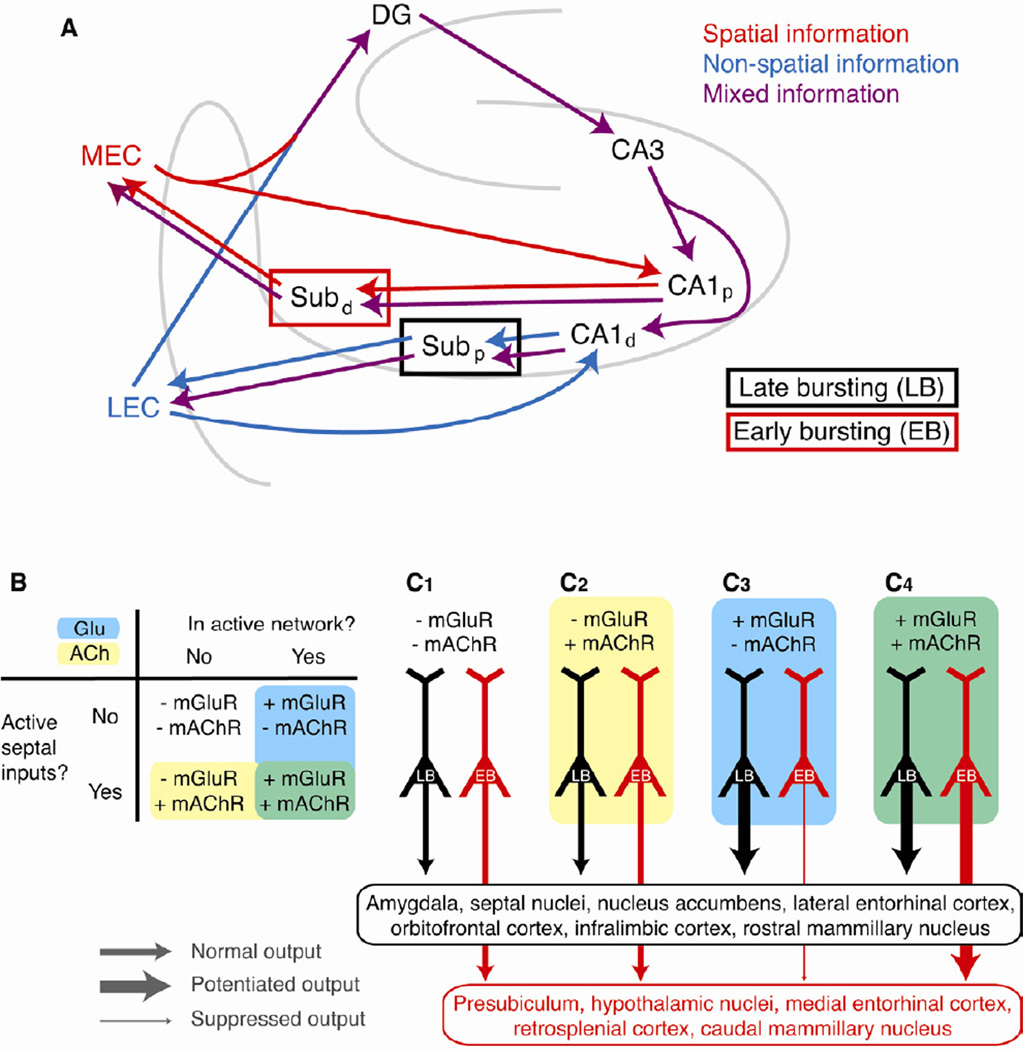
Figure 6. Countermodulation of parallel output streams from the hippocampus.
A. Separate classes of pyramidal cells preferentially connect different hippocampal inputs and outputs. Inputs from the medial entorhinal cortex (MEC) contain predominantly spatial information (red), whereas inputs from lateral entorhinal cortex (LEC) contain predominantly non-spatial information (blue). In the indirect path to CA1 through the “trisynaptic loop,” these distinct modalities of information are merged within a single information stream (purple) in the dentate gyrus. In the direct path from the EC to CA1, these biased inputs target largely separate areas of CA1, which in turn project to separate regions of subiculum that contain different proportions of the two cell types. Thus, spatial information is processed predominantly by early-bursting cells in the distal subiculum (red box) and non-spatial information is processed predominantly by late-bursting cells in the proximal subiculum (black box). Finally, late-bursting and early bursting cells in subiculum project predominantly to non-spatial (LEC) and spatial (MEC) regions of entorhinal cortex, respectively, thus forming two closed loops for processing these distinct modalities of information. Note that while projections from the two cell types are depicted as absolute, the magnitude of this preference is approximately four-fold (e.g., ~80% of the hippocampal output to LEC is carried by late-bursting cells and ~20% is carried by early-bursting cells). B-C. Neuromodulatory input from the active hippocampal network (via activation of mGluRs) and cholinergic input from the septal nuclei (via activation of mAChRs) have differential effects on the intrinsic excitability and action potential output from late-bursting and early-bursting cells. C1. In the absence of any neuromodulatory input, output from both pyramidal cell types is not modulated (medium thickness lines). C2. Similarly, cholinergic input to a cell that is not in the active hippocampal network (i.e., no mGluR activation) does not modulate excitability. C3. Glutamatergic input alone activates mGluR5, consequently enhancing output from late-bursting neurons (thick black line) and suppressing output from early-bursting neurons (thin red line). This is the countermodulation condition. C4. When mAChRs and mGluRs are activated by concurrent glutamatergic and cholinergic input, output from both types is enhanced (thick lines), with upregulation of early-bursting cells reflecting synergistic activation of mGluR1 and mAChR. Late-bursting and early-bursting cells in subiculum project predominantly to different groups of neuronal targets. Thus, depending on the specific subtypes of metabotropic receptors that are activated, hippocampal output can be bidirectionally modulated to different sets of efferent targets throughout the brain that receive preferential input from one cell type.
The primary inputs to the hippocampus from the EC contain distinct modalities of information: the MEC contains mainly spatial information and the LEC contains mainly non-spatial information (Hargreaves et al., 2005; Knierim et al., 2006). In the indirect pathway through the trisynaptic loop, these distinct modalities of information are combined into a single processing stream, because of the convergence of MEC and LEC inputs onto each dentate granule cell. In the direct temporoammonic path to CA1, however, spatial and non-spatial information remain largely segregated in parallel processing streams through anatomically separate regions of CA1. These CA1 pyramidal cells in turn project to separate areas of the subiculum that contain predominantly either late-bursting or early-bursting cells, which subsequently transmit hippocampal output to divergent brain regions (see Fig. 6). While all hippocampal targets receive projections from both early-bursting and late-bursting neurons, most regions receive approximately four times more input from one particular subtype (Kim and Spruston, 2012). Thus, pyramidal cells in the CA1 and subiculum regions form the nexus of two hippocampal circuits that process information within a single stream (the indirect pathway) and in separate, parallel streams (the direct pathway). Furthermore, our data demonstrate that these parallel pathways, carrying a different balance of non-spatial and spatial information, are composed of predominantly late-bursting or early-bursting cells in the subiculum, respectively, thus enabling functional specialization of these parallel streams of hippocampal information.
The clear morphological differences between the two cell types suggest that they process information in fundamentally different ways. Late-bursting neurons have more dense basal dendrites, suggesting that they receive more input from proximal regions of CA3 (i.e., close to the dentate gyrus) than early-bursting neurons; conversely, early-bursting neurons have more tuft dendrites, suggesting that they receive more direct temporoammonic inputs from the entorhinal cortex (Amaral and Witter, 1989; Witter et al., 1989). Thus, it is possible that these two cell types may process a different balance of direct information from cortex (from inputs selectively targeting the tuft region) and hippocampally processed information from the CA3 Schaffer collaterals (targeting the proximal apical and basal dendritic regions).
In addition to impacting information processing in the hippocampus, recent evidence suggests that distinct cell types may also form parallel streams of output from the neocortex. Pyramidal projection neurons in the frontal cortex also consist of two morphologically distinct classes that target different cortical and sub-cortical structures (Morishima and Kawaguchi, 2006). Furthermore, distinct types of layer V neurons in the medial prefrontal cortex respond differently to noradrenergic and cholinergic modulation (Dembrow et al., 2010). Finally, regular-spiking and bursting cells in layer V of barrel cortex display orthogonal forms of activity-dependent plasticity in vivo (Jacob et al., 2012). These observations, taken together with these findings, support the concept that parallel processing by distinct cell types may be a general principle of information processing across brain regions.
What mechanisms could underlie bursting differences and countermodulation of the two cell types?
The distinct firing patterns between early-bursting and late-bursting neurons (see Fig. 4A-B) indicate that these cell types must express a different complement of voltage-and/or Ca2+-gated ion channels. As a hypothetical example, early-bursting cells could express an inactivating depolarizing conductance that promotes bursting initially but not on later inputs, whereas late-bursting cells could express an inactivating hyperpolarizing conductance that limits bursting initially but not on later inputs. The distinct conductances responsible for these different firing patterns may in fact be the targets of modulation that cause the two cell types to respond differently to ACh and glutamate.
It is also possible that the observed countermodulation results from differential modulation of a common target, such as general up- or down-regulation of a conductance that influences bursting in both cell types. In this case, the molecular steps linking receptor activation to channel modulation would have to be different in the two cell types. Although mGluR1, mGluR5, and mAChR (M1/3/5 subtypes) all couple to phospholipase C (PLC) through Gq/G11, they can also activate other G proteins and transduction pathways as well (Hermans and Challiss, 2001; Niswender and Conn, 2010; Valenti et al., 2002; van Koppen and Kaiser, 2003). There are also other subtypes of mAChRs, splice variants of mGluRs, protein-protein interactions with the receptors (e.g., Homer and its associated proteins), modulators of G-proteins and their downstream targets (e.g., RGS proteins and kinases), and G-protein-independent signaling, all of which can impart cell-specific and conditional diversity on the signaling mechanisms coupled to any of these receptors (Magalhaes et al., 2012; van Koppen and Kaiser, 2003). Thus, there are numerous molecular mechanisms by which late-bursting and early-bursting hippocampal pyramidal neurons could produce divergent modulatory responses to glutamate and acetylcholine acting on similar metabotropic receptors.
Functional implications for countermodulation of hippocampal output
The pharmacological data (see Fig. 4F) reveal that specific subtypes of group I mGluRs have opposing roles in mediating enhanced and suppressed bursting. Under physiological conditions in the intact brain, however, activation of only one receptor subtype (just mGluR1 or mGluR5) is not likely to occur, but the requirement for co-activation of mAChR in order for mGluR1 to mediate its effects determines which of the two mGluRs mediates burst plasticity.
How could bidirectional burst plasticity be controlled in vivo? Our data suggest that a critical switch between enhancement and suppression of intrinsic excitability (via up- or down-regulation of bursting) is local activity. When a cell is not engaged in the active hippocampal network, there is no mGluR activation and excitability is not modulated, even when acetylcholine is present to activate mAChRs (Fig. 6B, C1–2). When a pyramidal cell is in the active network, however, glutamate release activates mGluRs. On its own, mGluR activation enhances bursting output from late-bursting cells and suppresses bursting in early-bursting cells (Fig. 6C3), in both cases via mGluR5 activation–a phenomenon that we call “countermodulation”. Given that the two cell types project predominantly to different pools of extra-hippocampal targets (Kim and Spruston, 2012), countermodulation may serve as a balance knob, dynamically and bidirectionally influencing the relative strength of hippocampal efferents from the two parallel information streams to distinct brain regions (Fig. 6B-C).
When septal cholinergic inputs are activated, bursting is enhanced in both late-bursting and early-bursting neurons, but only in neurons that are part of the active network (Fig. 6C4). In early-bursting cells this is mediated via a synergistic effect requiring co-activation of mGluR1 and mAChR, while in late-bursting cells the enhancement of bursting is mediated by mGluR5, which dominates the suppressive effect of co-activating mGluR1 and mAChR. Thus, the output from late-bursting cells is principally determined by mGluR activation, which always leads to enhancement of bursting, while the output from early-bursting cells is regulated by both mGluR, which leads to suppression of bursting on its own, but enhancement of bursting during co-activation of mAChRs. It remains possible that another condition, not yet discovered, could result in down-regulation of bursting in late-bursting cells, thus completing a suite of conditions that lead to bidirectional modulation of both cell types in the intact brain.
Our findings could promote a better understanding of the well-established dichotomy regarding the role of acetylcholine in learning and memory. Decades of work have shown that cholinergic input facilitates hippocampal activity during memory encoding and learning, but suppresses activity during memory retrieval and recall (Drever et al., 2011; Hasselmo, 1999; Micheau and Marighetto, 2011). Our results provide a potential framework for studying the mechanisms of this biphasic role of acetylcholine, as the two types of cells that process and transmit hippocampal information can be differentially modulated by mAChR activation. Furthermore, as projections to CA1 from the entorhinal cortex are more sensitive to mGluR-dependent presynaptic inhibition than mAChR-dependent inhibition (Giocomo and Hasselmo, 2007), there may be differential modulation of separate information streams flowing directly to CA1 from entorhinal cortex and indirectly through the trisynaptic circuit of the hippocampus.
Recent work in vivo has shown that cells with a higher propensity to burst are more likely to become place cells (Epsztein et al., 2010). On the surface, this would suggest that early-bursting cells are more likely to become place cells. As most of the cells in the CA1 region are late bursting (Jarsky et al., 2008), however, and as place cells are abundant in this region (Moser et al., 2008; Nakazawa et al., 2004; O'Keefe, 1976), it seems unlikely that late-bursting cells are not place cells. Rather, it is possible that both cell types can become place cells and that modulation of neuronal firing patterns with forms of plasticity similar to those described here may serve to enhance or suppress excitability, thus affecting which neurons are likely to exhibit place fields in a particular environment. Similarly, modulation of bursting could contribute to the formation of non-spatial behavioral contingencies on firing (Pastalkova et al., 2008; Wood et al., 2000).
Experimental Procedures
Solutions
Artificial cerebrospinal fluid (ACSF) consisted of (in mM): 125 NaCl, 2.5 KCl, 25 NaHCO3, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, and 25 dextrose (Fisher Scientific, Pittsburgh, PA). The pH of the ACSF was 7.3 and the osmolarity was 305–320 mOsm. ACSF was oxygenated and pH buffered by constant bubbling with a gas mixture of 95% O2/5% CO2. Internal recording solution consisted of (in mM): 115 K-gluconate, 20 KCl, 10 sodium phosphocreatine, 10 HEPES, 2 MgATP, and 0.3 NaGTP with 0.10% biocytin for morphological analysis (Sigma-Aldrich, St. Louis, MO, except KCl and HEPES, Fisher Scientific). 1 M KOH was used to pH the internal solution to 7.3–7.4. The osmolarity was 275–285 mOsm.
In a subset of experiments, one or more of the following antagonists (Sigma-Aldrich unless otherwise indicated) was also included in the perfusion ACSF and present for the entire duration of recording (unless otherwise noted): 20 µM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) to block AMPA receptors, 50 µM D-2-amino-5-phosphonopentanoate (D-AP5) and 20 µM MK-801 to block NMDA receptors, 25 µM LY367385 (Tocris) to block mGluR1, 10 µM 2-methyl-6-(phenylethynyl)-pyridine (MPEP, Tocris) to block mGluR5, and 10 µM atropine to block mAChRs.
Slice preparation and experimental setup
Male rats (p21–28; Charles River Laboratories, Wilmington, MA) were anesthetized with halothane, decapitated, and their brains rapidly removed. Transverse hippocampal slices (near-horizontal sections, 300 µm thick) were made with a Microm HM 650 V slicer (Thermo Scientific, Walldorf, Germany), transferred to an immersion storage chamber, incubated at 32–35°C for 30 minutes, and subsequently maintained at room temperature until recording.
For electrophysiological recordings, a slice was transferred to the recording chamber and maintained at 32–35°C by constant perfusion of warmed ACSF at a rate of 1 mL/s. A Zeiss Axioskop (Oberkochen, Germany) equipped with differential interference contrast optics was used in conjunction with a Hamamatsu camera system to visually identify pyramidal neurons. The subiculum was distinguished from bordering regions by the diffuse distribution of pyramidal cells compared to the tightly packed pyramidal cell layer of CA1 and the lack of distinct cortical layers seen in entorhinal cortex.
Electrophysiological recordings
Recording pipettes were fabricated (Flaming/Brown Micropipette Puller, Sutter Instruments, Novato, CA) from borosilicate capillary glass (Garner Glass Company, 4–6 MΩ open-tip resistance). To evoke synaptic responses, an extracellular stimulating pipette, fabricated from borosilicate theta glass, was filled with ACSF and placed at least 500 µm from the site of the whole-cell recording on the apical dendritic side of the soma.
Whole-cell current-clamp recordings were made using a Dagan BVC-700 amplifier (Minneapolis, MN). Only cells exhibiting a resting potential between −62 mV and −68 mV at break-in were used. Neurons were defined as either regular-spiking or bursting depending on their response to a 500 ms threshold-level current injection. With this stimulus, bursting neurons always exhibited a burst of two or more action potentials with an instantaneous frequency of greater than 100 Hz, while regular-spiking neurons always exhibited only a single spike. When the amplitude of the current injection was increased, bursting neurons typically fired additional bursts before switching to single action potentials at the end of the step, while regular-spiking neurons fired single spikes with decreasing inter-spike intervals. Regular-spiking and bursting neurons are distributed in a gradient along the proximal to distal axis from CA1 to the subiculum. Jarsky et al. (2008) reported that, in vitro, approximately 5, 30, and 80% of neurons were classified as bursting in the CA1 region near the border of CA2, at the CA1/subiculum border, and in distal subiculum, respectively. To distinguish between CA1 and subicular pyramidal neurons, all cells were located at least 100 µm from the CA1/subiculum border.
All neurons were held between −64 mV and −66 mV for the duration of the recordings. Cells that required more than 200 pA of holding current to maintain these potentials were excluded from the data set. Bridge balance and capacitance compensation were monitored and adjusted throughout the duration of each experiment; recordings in which the series resistance exceeded 40 MΩ were excluded. Recordings were generally held for at least 60 minutes, but in some cases, were maintained for more than 2 hours. At the end of each experiment, a step depolarization identical to that delivered at the beginning of the experiment was given to verify the firing properties of the neuron (i.e., regular-spiking vs. bursting).
A hyperpolarizing step current injection (−200 pA, 500 ms) was used to monitor input resistance and sag ratio, defined as the ratio of the steady state voltage (average voltage from 400–500 ms) relative to baseline, divided by the minimum voltage (usually occurring within 100 ms of the onset of the hyperpolarizing step) relative to baseline. Resting membrane potential was measured by taking the average voltage over 1 second in the absence of any current injection. The mean subthreshold voltage change (dV/dt) was calculated for each spike over a range of 20–80% of the voltage from baseline to threshold. The afterdepolarization (ADP) was calculated for each spike by finding peak voltage following the downstroke of the action potential relative to baseline. As the second spike in a burst often obscured the ADP from the first action potential, the ADP amplitude for the first spike was only calculated for inputs that did not elicit bursting. The afterhyperpolarization (AHP) was determined by calculating the difference between the minimum voltage following the spike and baseline. This value always occurred within 50 ms of the spike, corresponding to the fast AHP. The threshold for each spike was defined as the peak of the second derivative of voltage with respect to time. Maximal changes in voltage during the rising and falling phases of the action potential were calculated for each spike. Spike amplitude for each spike was defined as the difference between the peak voltage and baseline. Full width at half maximum voltage (FWHM) was calculated by determining the elapsed time between the voltage crossing half maximal amplitude (peak relative to baseline) during the rising and falling phase. To measure initial firing frequency, we measured the instantaneous frequency of the first two spikes elicited by a 500 pA depolarizing step current injection.
Neuronal output was monitored once every 20 seconds using a train of 10 somatic EPSC-like (τrise = 0.2 ms, τdecay = 6 ms) current injections at 5 Hz to evoke action potential firing. The amplitude of somatic current injections (600 - 2000 pA) was set such that, for each train, approximately 4 responses were bursts of two action potentials (while the remaining 6 responses elicited single action potentials). Once the amplitude of this current injection was set, it was maintained at this level for the duration of the experiment.
To probe long-lasting changes in intrinsic excitability and firing patterns, a theta-burst induction stimulus (TBS) consisting of theta-burst-patterned synaptic activation (5 stimuli at 100 Hz) was delivered to proximally projecting axons (Schaffer Collaterals in the case of CA1 neurons) using a bipolar theta-glass electrode, paired with a somatic current injection (2 ms square current pulse at the burst-monitoring amplitude), repeated at 5 Hz for 3 seconds. The induction stimulus was given approximately 15–20 minutes after breaking in, though burst plasticity did not depend on the elapsed time from initial break-in to when TBS was given.
Neuronal reconstruction and morphological analysis
To fill and subsequently reconstruct neurons following recording, we included biocytin in the intracellular recording pipette. Slices were fixed in paraformaldehyde (4%) and stained using an avidin-horseradish peroxidase 3,3’-diaminobenzadine reaction. Morphological reconstructions of 110 pyramidal neurons from the subiculum and CA1 region of hippocampus were made using the Neurolucida imaging system (MicroBrightField, Williston, VT) and a Leica DMLB microscope with a 63X oil-immersion lens. Morphological analyses were performed blind and measured several parameters, including soma size, total dendritic length, average dendritic width, and a Sholl-like concentric ring analysis to quantify dendritic arborization (similar to Staff et al, 2000). Briefly, we measured the total dendritic length in 20 μm diameter concentric rings emanating from the soma. By convention, basal dendrite length was represented as negative distance and apical dendrite length was represented as positive distance. We also measured the total dendritic length, average segment length, number of branch points, and branching order for apical and basal dendrites separately, as well as the distance from the soma to the bifurcation of the main apical dendrite (defined as the first bifurcation where each daughter branch has a diameter of at least 1/2 of the parent branch).
Data acquisition and statistical analysis
Voltage responses were filtered at 5 kHz, digitized at 50 kHz, and acquired using an ITC-16 analog-to-digital converter (Instrutech, Port Washington, NY). All acquisition and analysis procedures were custom programmed in IGOR Pro (Wavemetrics, Lake Oswego, OR). Statistical analyses of group data were performed with a one- or two-factor repeated measures ANOVA, where appropriate, with Prism software (GraphPad Software, Inc., San Diego, CA). When a significant main effect was detected with ANOVA tests, Bonferroni’s post-hoc correction was used to determine significance between pairwise comparisons. Normalized values are plotted as a percentage of the average value during the baseline period. Unless stated otherwise, reported values are mean ± s.e.m. For all statistical comparisons, asterisks indicate a significant effect at the following levels of significance: * = p < 0.05, ** = p < 0.01, *** = p < 0.001.
To assess the distribution of all pyramidal neurons in multi-dimensional space, we performed a K-means cluster analysis in MatLab (MathWorks, Natick, MA). First, we performed Student’s t-tests on each electrophysiological property and morphological parameter to compare bursting and regular-spiking neurons. Using only those parameters that were significantly different, we constructed a 15-dimension matrix for all 110 neurons (consisting of seven morphological properties: total basal dendritic length, total tuft dendritic length, average basal branching order, average tuft branching order, distance to main apical bifurcation, and the number of branch points in the basal and tuft regions; as well as eight electrophysiological properties: input resistance, sag ratio, subthreshold dV/dt, ADP amplitude, threshold of the second spike, maximal dV/dt during the rising and falling phases of the second spike, and the full-width at half maximum voltage (FWHM) of the first spike). Initial spike frequency was not included in the cluster analysis, though these values were significantly different between firing types. Based on these values, the K-means test selected n random cells to seed n clusters (n = 2–10). For all 15 normalized parameters, the Euclidian distance from these n seeds was calculated for all remaining cells, and each cell was then assigned to the cluster it was closest to. The cluster centers were then recalculated, and the process was repeated iteratively until the distributions ceased to change.
To determine whether the computed clusters represent a single population or arise from multiple cell types, we computed a cluster index from the 15-dimensional matrix, defined as the ratio of the sum of the square distances from each multidimensional point to its cluster center and the sum of the square distances from each point to the overall mean (Liu et al., 2008). This index varies from zero to 1, with values close to zero corresponding to very tight clusters. Assuming that the cells were defined by a single multivariate Gaussian (the null hypothesis, which we would expect if these neurons belonged to the same cell type), we calculated a million cluster index values by repeatedly drawing 110 random samples from that distribution. The p value represents the likelihood that the simulated data have a cluster index greater than the experimental data.
To determine if k clusters (2–10) were represented in the data, we applied the jump method of Sugar and James. For each integer k from 1 to a prescribed maximum, the K-means algorithm partitions the data into k clusters. For each K-means partition, the method computes the distortion, i.e., a normalized squared distance between each observation and its closest cluster center. Since the K-means partitioning may depend upon the starting points used, the K-means algorithm is repeated a number of times with different starting conditions, and a mean distortion for each prescribed value of k is obtained. A distortion curve is then generated by plotting the mean distortion as a function of k. The distortion tends to decrease as the number of clusters is increased, and this is transformed into an increase by raising the distortion to a negative power, Y. Because the distortion drops when the correct number of clusters is used, and remains roughly constant when even more clusters are employed, the transformed distortion exhibits a sudden increase, or jump, at the correct value of k. If one examines the size of the jumps in the transformed distortion, the largest jump is therefore an indication of the proper number of clusters.
We also used MatLab to perform a Principal Component analysis. We computed the principal components of the entire data set (30 properties) as well as the 15 properties were significantly different between the two populations.
Supplementary Material
Acknowledgements
The authors would like to members of the Spruston lab for helpful discussions and Adam Hantman for reagents. AG was supported by F31 NS067758, AG and SM were supported by T32 MH067564. The work was also supported by NIH RO1 NS35180 and the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
AG, SM, and NS designed the experiments. AG and SM collected the electrophysiological data, AG collected the morphological data, and AG and EB performed the immunostaining and imaging. AG analyzed the data, with input from NS and help from WK to perform the cluster and principal component analyses. AG, BM, and NS wrote the manuscript, with input from the other authors.
References
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Babadi B. Bursting as an effective relay mode in a minimal thalamic model. J Comput Neurosci. 2005;18:229–243. doi: 10.1007/s10827-005-6560-5. [DOI] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27:370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Congar P, Bioulac B, Hammond C. Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. J Neurosci. 1999;19:599–609. doi: 10.1523/JNEUROSCI.19-02-00599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembrow NC, Chitwood RA, Johnston D. Projection-specific neuromodulation of medial prefrontal cortex neurons. J Neurosci. 2010;30:16922–16937. doi: 10.1523/JNEUROSCI.3644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft JF, Oh MM. Pharmacological and molecular enhancement of learning in aging and Alzheimer's disease. J Physiol Paris. 2006;99:180–192. doi: 10.1016/j.jphysparis.2005.12.079. [DOI] [PubMed] [Google Scholar]
- Drever BD, Riedel G, Platt B. The cholinergic system and hippocampal plasticity. Behav Brain Res. 2011;221:505–514. doi: 10.1016/j.bbr.2010.11.037. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statistical Science. 1986;1:54–75. [Google Scholar]
- Epsztein J, Brecht M, Lee AK. Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron. 2011;70:109–120. doi: 10.1016/j.neuron.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epsztein J, Lee AK, Chorev E, Brecht M. Impact of spikelets on hippocampal CA1 pyramidal cell activity during spatial exploration. Science. 2010;327:474–477. doi: 10.1126/science.1182773. [DOI] [PubMed] [Google Scholar]
- Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer's disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giocomo LM, Hasselmo ME. Neuromodulation by glutamate and acetylcholine can change circuit dynamics by regulating the relative influence of afferent input and excitatory feedback. Mol Neurobiol. 2007;36:184–200. doi: 10.1007/s12035-007-0032-z. [DOI] [PubMed] [Google Scholar]
- Golding NL, Staff NP, Spruston N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature. 2002;418:326–331. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- Greene JR, Mason A. Neuronal diversity in the subiculum: correlations with the effects of somatostatin on intrinsic properties and on GABA-mediated IPSPs in vitro. J Neurophysiol. 1996;76:1657–1666. doi: 10.1152/jn.1996.76.3.1657. [DOI] [PubMed] [Google Scholar]
- Greene JR, Totterdell S. Morphology and distribution of electrophysiologically defined classes of pyramidal and nonpyramidal neurons in rat ventral subiculum in vitro. J Comp Neurol. 1997;380:395–408. doi: 10.1002/(sici)1096-9861(19970414)380:3<395::aid-cne8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Hargreaves EL, Rao G, Lee I, Knierim JJ. Major dissociation between medial and lateral entorhinal input to dorsal hippocampus. Science. 2005;308:1792–1794. doi: 10.1126/science.1110449. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends Cogn Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- Hausser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science. 2000;290:739–744. doi: 10.1126/science.290.5492.739. [DOI] [PubMed] [Google Scholar]
- Hermans E, Challiss RA. Structural, signalling and regulatory properties of the group I metabotropic glutamate receptors: prototypic family C G-protein-coupled receptors. The Biochemical journal. 2001;359:465–484. doi: 10.1042/0264-6021:3590465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob V, Petreanu L, Wright N, Svoboda K, Fox K. Regular spiking and intrinsic bursting pyramidal cells show orthogonal forms of experience-dependent plasticity in layer V of barrel cortex. Neuron. 2012;73:391–404. doi: 10.1016/j.neuron.2011.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Mady R, Kennedy B, Spruston N. Distribution of bursting neurons in the CA1 region and the subiculum of the rat hippocampus. J Comp Neurol. 2008;506:535–547. doi: 10.1002/cne.21564. [DOI] [PubMed] [Google Scholar]
- Jung HY, Staff NP, Spruston N. Action potential bursting in subicular pyramidal neurons is driven by a calcium tail current. J Neurosci. 2001;21:3312–3321. doi: 10.1523/JNEUROSCI.21-10-03312.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Spruston N. Target-specific output patterns are predicted by the distribution of regular-spiking and bursting pyramidal neurons in the subiculum. Hippocampus. 2012;22:693–706. doi: 10.1002/hipo.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knierim JJ, Lee I, Hargreaves EL. Hippocampal place cells: parallel input streams, subregional processing, and implications for episodic memory. Hippocampus. 2006;16:755–764. doi: 10.1002/hipo.20203. [DOI] [PubMed] [Google Scholar]
- Kong JH, Fish DR, Rockhill RL, Masland RH. Diversity of ganglion cells in the mouse retina: unsupervised morphological classification and its limits. J Comp Neurol. 2005;489:293–310. doi: 10.1002/cne.20631. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci. 1997;20:38–43. doi: 10.1016/S0166-2236(96)10070-9. [DOI] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Magalhaes AC, Dunn H, Ferguson SS. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. British journal of pharmacology. 2012;165:1717–1736. doi: 10.1111/j.1476-5381.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Mel B. Information Processing in Dendritic Trees. Neural Comput. 1994;6:1031–1085. [Google Scholar]
- Micheau J, Marighetto A. Acetylcholine and memory: a long, complex and chaotic but still living relationship. Behav Brain Res. 2011;221:424–429. doi: 10.1016/j.bbr.2010.11.052. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Diba K, Pastalkova E, Buzsaki G. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat Neurosci. 2011;14:1174–1181. doi: 10.1038/nn.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SJ, Cooper DC, Spruston N. Plasticity of burst firing induced by synergistic activation of metabotropic glutamate and acetylcholine receptors. Neuron. 2009;61:287–300. doi: 10.1016/j.neuron.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima M, Kawaguchi Y. Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J Neurosci. 2006;26:4394–4405. doi: 10.1523/JNEUROSCI.0252-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB. Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci. 2008;31:69–89. doi: 10.1146/annurev.neuro.31.061307.090723. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, McHugh TJ, Wilson MA, Tonegawa S. NMDA receptors, place cells and hippocampal spatial memory. Nat Rev Neurosci. 2004;5:361–372. doi: 10.1038/nrn1385. [DOI] [PubMed] [Google Scholar]
- Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J. Place units in the hippocampus of the freely moving rat. Experimental neurology. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Itskov V, Amarasingham A, Buzsaki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321:1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staff NP, Jung HY, Thiagarajan T, Yao M, Spruston N. Resting and active properties of pyramidal neurons in subiculum and CA1 of rat hippocampus. J Neurophysiol. 2000;84:2398–2408. doi: 10.1152/jn.2000.84.5.2398. [DOI] [PubMed] [Google Scholar]
- Sugar CA, James GM. Finding the number of clusters in a dataset: an information-theoretic approach. Journal of the American Statistical Association. 2003;98:750–763. [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Valenti O, Conn PJ, Marino MJ. Distinct physiological roles of the Gq-coupled metabotropic glutamate receptors Co-expressed in the same neuronal populations. J Cell Physiol. 2002;191:125–137. doi: 10.1002/jcp.10081. [DOI] [PubMed] [Google Scholar]
- van Koppen CJ, Kaiser B. Regulation of muscarinic acetylcholine receptor signaling. Pharmacology & therapeutics. 2003;98:197–220. doi: 10.1016/s0163-7258(03)00032-9. [DOI] [PubMed] [Google Scholar]
- van Welie I, Remme MW, van Hooft JA, Wadman WJ. Different levels of Ih determine distinct temporal integration in bursting and regular-spiking neurons in rat subiculum. J Physiol. 2006;576:203–214. doi: 10.1113/jphysiol.2006.113944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Mechanisms and consequences of action potential burst firing in rat neocortical pyramidal neurons. J Physiol. 1999;521(Pt 2):467–482. doi: 10.1111/j.1469-7793.1999.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter MP, Van Hoesen GW, Amaral DG. Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci. 1989;9:216–228. doi: 10.1523/JNEUROSCI.09-01-00216.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 2000;27:623–633. doi: 10.1016/s0896-6273(00)00071-4. [DOI] [PubMed] [Google Scholar]
- Xu W, Morishita W, Buckmaster PS, Pang ZP, Malenka RC, Sudhof TC. Distinct neuronal coding schemes in memory revealed by selective erasure of fast synchronous synaptic transmission. Neuron. 2012;73:990–1001. doi: 10.1016/j.neuron.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci. 2005;8:1552–1559. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.