Abstract
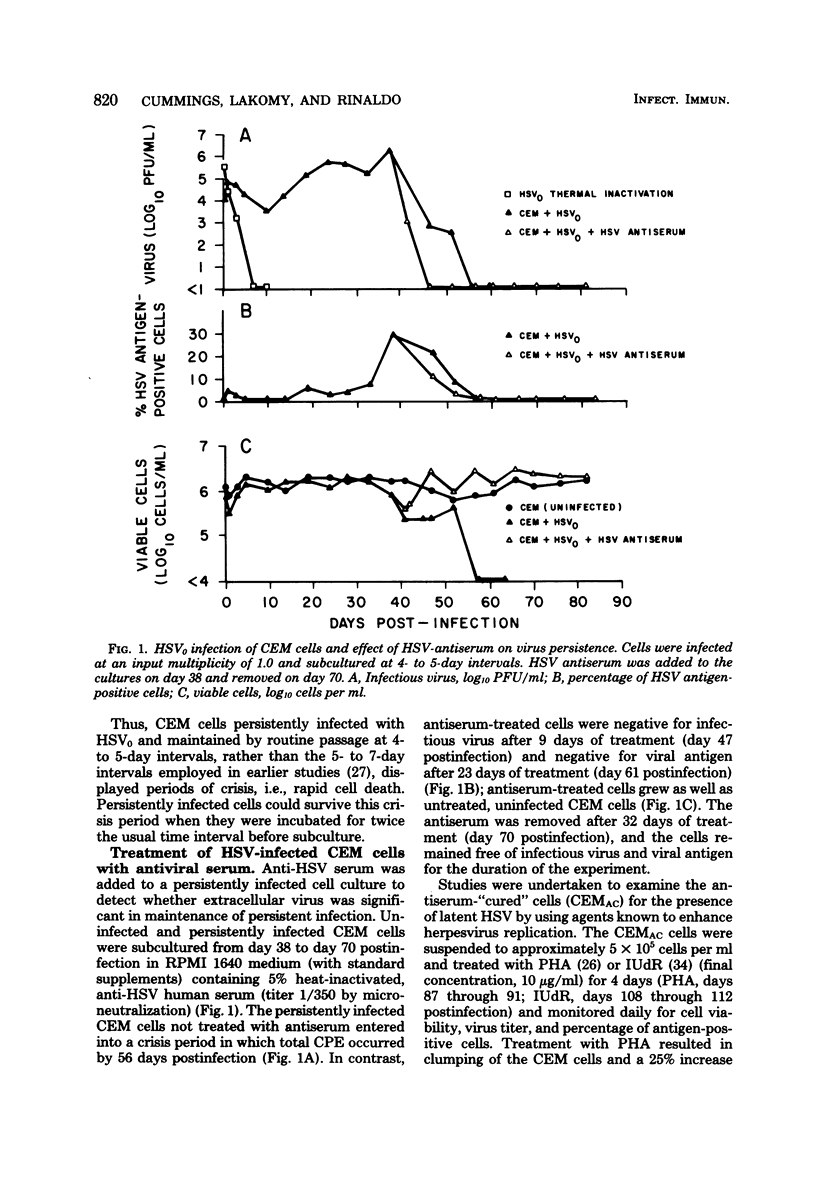
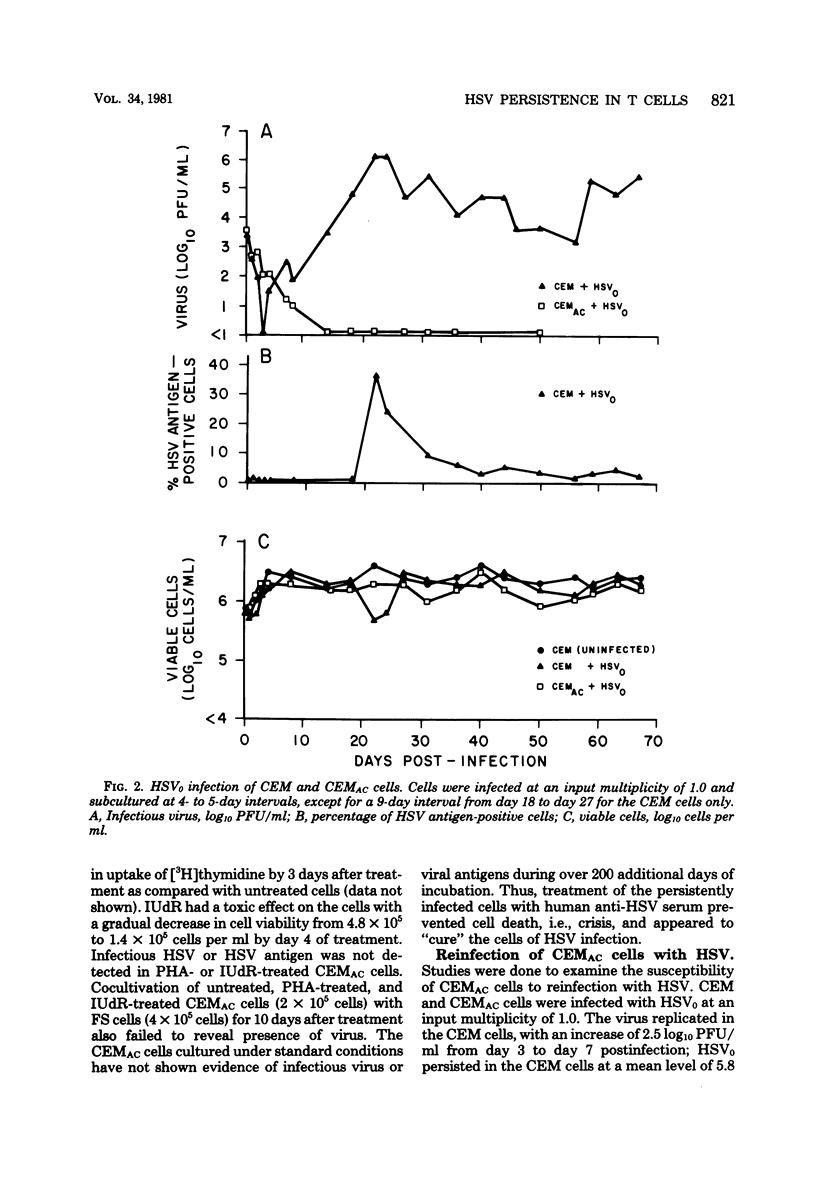
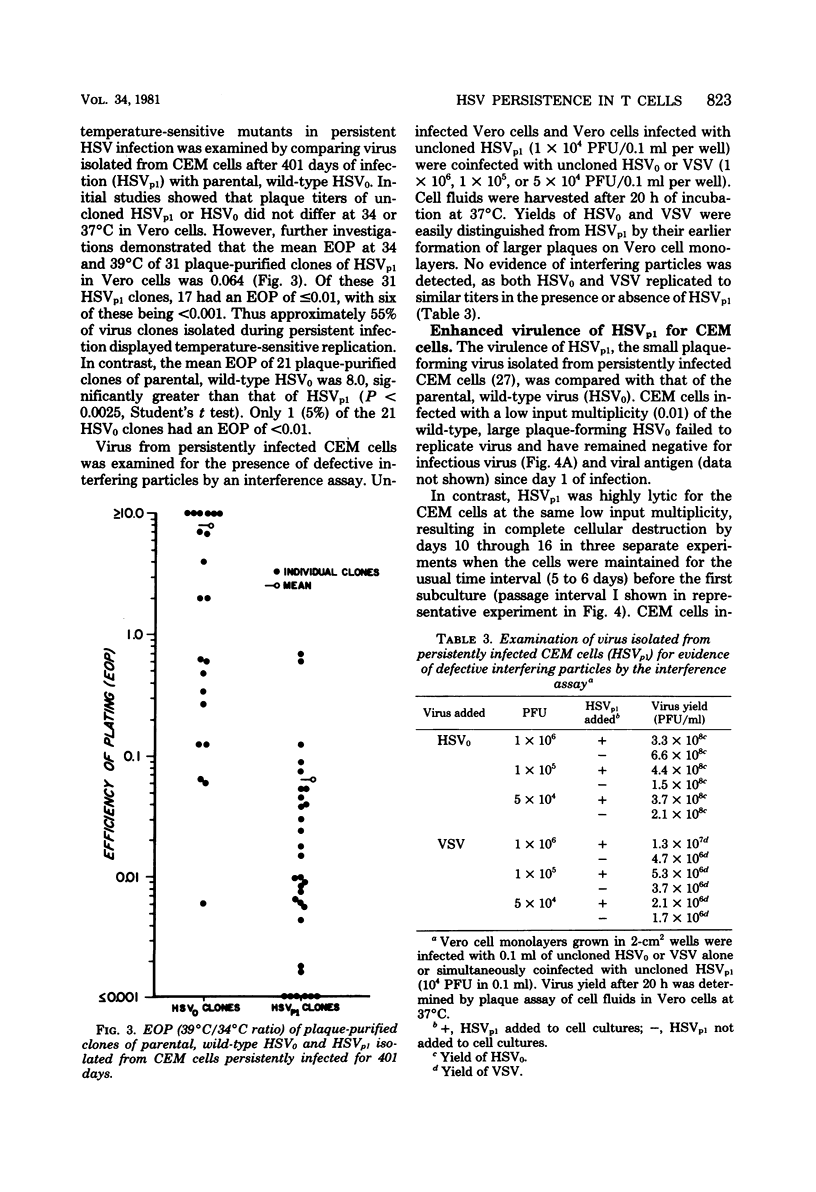
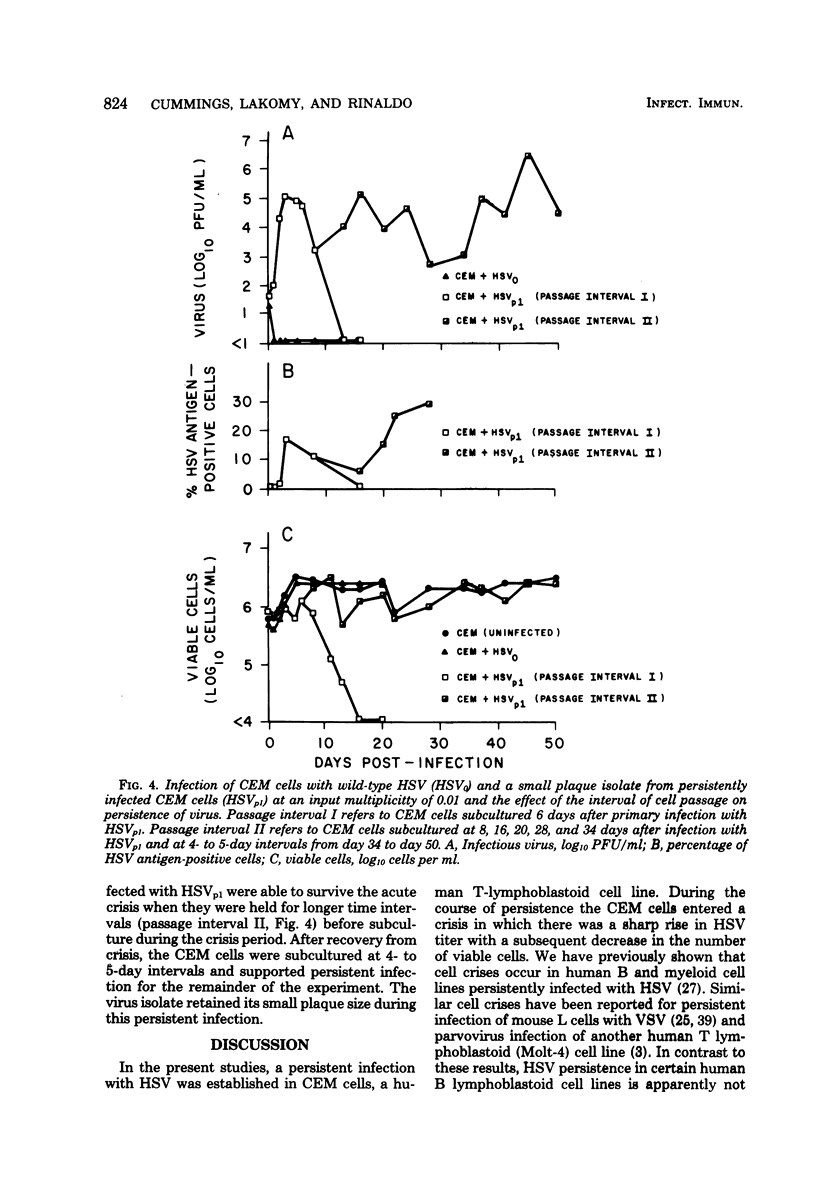
Persistent, dynamic-state infection with herpes simplex virus (HSV) type 1 has been maintained in human T lymphoblastoid (CEM) cells for many months after initial infection with the wild-type virus (HSV0) (input virus/cell multiplicity of 1.0). Persistently infected cells grew as well as uninfected cells, except during occasional periods of crisis (increased viral replication and cytopathic effect). Cells could survive the crisis when they were maintained for twice the usual time interval (8 to 10 rather than 4 to 5 days) before subculture. Interferon was not detectable in the cultures. HSV0 was compared with HSVp1, a small plaque-forming isolate from persistently infected CEM cells. Primary infection of CEM cells with HSV0 at a low input multiplicity (0.01) led to abortive replication, whereas infection with HSVp1 at the same multiplicity resulted in either rapidly lytic or persistent infection depending upon the time interval of subculture. Approximately 55% of plaque-purified clones of HSVp1, as compared with only 5% of HSV0 clones, displayed temperature-sensitive growth in Vero cells. Defective interfering virus was not detectable in uncloned HSVp1 by interference assay. Persistently infected cultures "cured" by treatment with HSV antiserum or incubation at 39 degrees C were resistant to reinfection with HSV but permissive for vesicular stomatitis virus replication, suggesting that these treatments modulated a shift from the dynamic-state of the static-state, latent infection. These studies provide a model for characterization of HSV persistence and latency in a highly differentiated human cell line.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. A., Flowers A., Sundeen R., Merk L. P. Chemotherapy and immunotherapy of three human lymphomas serially transplantable in the neonatal Syrian hamster. Cancer. 1972 Feb;29(2):524–533. doi: 10.1002/1097-0142(197202)29:2<524::aid-cncr2820290244>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass L. R., Hetrick F. M. Persistent infection of a human lymphocyte cell line (Molt-4) with the kilham rat virus. J Infect Dis. 1978 Feb;137(2):210–212. doi: 10.1093/infdis/137.2.210. [DOI] [PubMed] [Google Scholar]
- Black P. H. The oncogenic DNA viruses: a review of in vitro transformation studies. Annu Rev Microbiol. 1968;22:391–426. doi: 10.1146/annurev.mi.22.100168.002135. [DOI] [PubMed] [Google Scholar]
- Blackmore R. V., Morgan H. R. Self-limiting infection with herpes simplex virus in cell culture. Acta Virol. 1967 Jan;11(1):1–12. [PubMed] [Google Scholar]
- Doller E., Aucker J., Weissbach A. Persistence of herpes simplex virus type 1 in rat neurotumor cells. J Virol. 1979 Jan;29(1):43–50. doi: 10.1128/jvi.29.1.43-50.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doller E., Duff R., Rapp F. Resistance of hamster cells transformed by herpes simplex virus type 2 to superinfection by herpes simplex viruses. Intervirology. 1973;1(3):154–167. doi: 10.1159/000148842. [DOI] [PubMed] [Google Scholar]
- Dunn J. E., Meinke W. J., Spizizen J. Further characterization of herpes virus persistence. J Gen Virol. 1979 May;43(2):467–471. doi: 10.1099/0022-1317-43-2-467. [DOI] [PubMed] [Google Scholar]
- Eron L., Kosinski K., Hirsch M. S. Hepatitis in an adult caused by Herpes simplex virus type I. Gastroenterology. 1976 Sep;71(3):500–504. [PubMed] [Google Scholar]
- Floyd R., Glasser R., Vonka V., Benyesh-Melnick M. Studies on the growth of herpes simplex virus in lymphoblastoid cells. Acta Virol. 1971 Mar;15(2):133–142. [PubMed] [Google Scholar]
- Furukawa T. Persistent infection with human cytomegalovirus in a lymphoblastoid cell line. Virology. 1979 Apr 15;94(1):214–218. doi: 10.1016/0042-6822(79)90452-5. [DOI] [PubMed] [Google Scholar]
- Hampar B., Burroughs M. A. Mechanism of persistent herpes simplex virus infection in vitro. J Natl Cancer Inst. 1969 Sep;43(3):621–634. [PubMed] [Google Scholar]
- Hampar B., Copeland M. L. Persistent Herpes Simplex Virus Infection In Vitro with Cycles of Cell Destruction and Regrowth. J Bacteriol. 1965 Jul;90(1):205–212. doi: 10.1128/jb.90.1.205-212.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland J. J., Villarreal L. P., Welsh R. M., Oldstone M. B., Kohne D., Lazzarini R., Scolnick E. Long-term persistent vesicular stomatitis virus and rabies virus infection of cells in vitro. J Gen Virol. 1976 Nov;33(2):193–211. doi: 10.1099/0022-1317-33-2-193. [DOI] [PubMed] [Google Scholar]
- Inglot A. D., Albin M., Chudzio T. Persistent infection of mouse cells with Sindbis virus: role of virulence of strains, auto-interfering particles and interferon. J Gen Virol. 1973 Jul;20(1):105–110. doi: 10.1099/0022-1317-20-1-105. [DOI] [PubMed] [Google Scholar]
- Ju G., Udem S., Rager-Zisman B., Bloom B. R. Isolation of a heterogeneous population of temperature-sensitive mutants of measles virus from persistently infected human lymphoblastoid cell lines. J Exp Med. 1978 Jun 1;147(6):1637–1652. doi: 10.1084/jem.147.6.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J., Shope T. C., Peterson W. D., Jr Epstein-barr virus-negative human malignant T-cell lines. J Exp Med. 1974 May 1;139(5):1070–1076. doi: 10.1084/jem.139.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai A., Matsumoto S. Interfering and noninterfering defective particles generated by a rabies small plaque variant virus. Virology. 1977 Jan;76(1):60–71. doi: 10.1016/0042-6822(77)90282-3. [DOI] [PubMed] [Google Scholar]
- Leinbach S. S., Summers W. C. Herpes simplex virus type 1 infection of isogenic Epstein-Barr virus genome-negative and -positive Burkitt's lymphoma-derived cell lines. J Virol. 1979 Apr;30(1):248–254. doi: 10.1128/jvi.30.1.248-254.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Goldin A. L., Glorioso J. C. Persistence of herpes simplex virus genes in cells of neuronal origin. J Virol. 1980 Jul;35(1):203–210. doi: 10.1128/jvi.35.1.203-210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y. Studies of L cells persistently infected with VSV: factors involved in the regulation of persistent infection. J Gen Virol. 1977 May;35(2):265–279. doi: 10.1099/0022-1317-35-2-265. [DOI] [PubMed] [Google Scholar]
- Preble O. T., Youngner J. S. Temperature-sensitive viruses and the etiology of chronic and inapparent infections. J Infect Dis. 1975 Apr;131(4):467–473. doi: 10.1093/infdis/131.4.467. [DOI] [PubMed] [Google Scholar]
- Ramseur J. M., Friedman R. M. Prolonged infection of L cells with vesicular stomatitis virus. Defective interfering forms and temperature-sensitive mutants as factors in the infection. Virology. 1978 Mar;85(1):253–261. doi: 10.1016/0042-6822(78)90429-4. [DOI] [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Richter B. S., Black P. H., Callery R., Chess L., Hirsch M. S. Replication of herpes simplex virus and cytomegalovirus in human leukocytes. J Immunol. 1978 Jan;120(1):130–136. [PubMed] [Google Scholar]
- Rinaldo C. R., Jr, Richter B. S., Black P. H., Hirsch M. S. Persistent infection of human lymphoid and myeloid cell lines with herpes simplex virus. Infect Immun. 1979 Aug;25(2):521–525. doi: 10.1128/iai.25.2.521-525.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robey W. G., Graham B. J., Harris C. L., Madden M. J., Pearson G. R., Vande Woude G. F. Persistent herpes simplex virus infections established in two Burkitt lymphoma derived cell lines. J Gen Virol. 1976 Jul;32(1):51–62. doi: 10.1099/0022-1317-32-1-51. [DOI] [PubMed] [Google Scholar]
- Roumillat L. F., Feorino P. M., Caplan D. D., Lukert P. D. Analysis and characterization of herpes simplex virus after its persistence in a lymphoblastoid cell line for 15 months. Infect Immun. 1980 Aug;29(2):671–677. doi: 10.1128/iai.29.2.671-677.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneurin J. M., Desgranges C., Lavoue M. F., de-The G. Herpes simplex-RAJI(A44), a new cell line for serologic testing by immunofluorescence. J Immunol. 1976 Sep;117(3):950–952. [PubMed] [Google Scholar]
- Sekellick M. J., Marcus P. I. Persistent infection. II. Interferon-inducing temperature-sensitive mutants as mediators of cell sparing: possible role in persistent infection by vesicular stomatitis virus. Virology. 1979 May;95(1):36–47. doi: 10.1016/0042-6822(79)90399-4. [DOI] [PubMed] [Google Scholar]
- St Jeor S., Rapp F. Cytomegalovirus replication in cells pretreated with 5-iodo-2'-deoxyuridine. J Virol. 1973 Jun;11(6):986–990. doi: 10.1128/jvi.11.6.986-990.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Thacore H., Youngner J. S. Cells persistently infected with newcastle disease virus: I. Properties of mutants isolated from persistently infected L cells. J Virol. 1969 Sep;4(3):244–251. doi: 10.1128/jvi.4.3.244-251.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahlne A., Lycke E. Herpes simplex virus infection of mouse neuroblastoma cells (39880). Proc Soc Exp Biol Med. 1977 Oct;156(1):82–87. doi: 10.3181/00379727-156-39880. [DOI] [PubMed] [Google Scholar]
- WALKER D. L. THE VIRAL CARRIER STATE IN ANIMAL CELL CULTURES. Prog Med Virol. 1964;6:111–148. [PubMed] [Google Scholar]
- Youngner J. S., Dubovi E. J., Quagliana D. O., Kelly M., Preble O. T. Role of temperature-sensitive mutants in persistent infections initiated with vesicular stomatitis virus. J Virol. 1976 Jul;19(1):90–101. doi: 10.1128/jvi.19.1.90-101.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Preble O. T., Jones E. V. Persistent infection of L cells with vesicular stomatitis virus: evolution of virus populations. J Virol. 1978 Oct;28(1):6–12. doi: 10.1128/jvi.28.1.6-13.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


