Summary
Hutchinson-Gilford progeria syndrome (HGPS, OMIM 176670) is a rare disorder characterized by segmental accelerated aging and early death from coronary artery disease or stroke. Nearly 90% of HGPS sufferers carry a G608G mutation within exon 11 of LMNA, producing a truncated form of prelamin A, referred to as “progerin”. Here, we report the isolation of naïve multipotent skin-derived precursor (SKP) cells from dermal fibroblast cultures from HGPS donors. These cells form spheres and express the neural crest marker, nestin, in addition to the multipotent markers, OCT4, Sox2, Nanog and TG30; these cells can self-renew and differentiate into smooth muscle cells (SMCs) and fibroblasts. The SMCs derived from the HGPS-SKPs accumulate nuclear progerin with increasing passages. A subset of the HGPS-naïve SKPs express progerin in vitro and in situ in HGPS skin sections. This is the first in vivo evidence that progerin is produced in adult stem cells, and implies that this protein could induce stem cells exhaustion as a mechanism contributing to aging. Our study provides a basis on which to explore therapeutic applications for HGPS stem cells and opens avenues for investigating the pathogenesis of other genetic diseases.
Keywords: Lamin A, Progerin, HGPS, Progeria, Adult stem cells
Introduction
Hutchinson-Gilford progeria syndrome (HGPS, OMIM 176670) is a rare disorder characterized by segmental accelerated aging and early death from coronary artery disease or stroke (Brown et al., 1985). Nearly 90% of HGPS sufferers carry a G608G mutation within exon 11 of LMNA, producing a truncated form of prelamin A, referred to as “progerin” (Cao and Hegele, 2003; De Sandre-Giovannoli et al., 2003; Eriksson et al., 2003).
A-type and B-type lamins represent critical building blocks of the nuclear lamina (Aebi et al., 1986; Fuchs and Weber, 1994; Steinert and Roop, 1988). The LMNA G608G mutation in HGPS induces severe abnormalities in nuclear morphology, heterochromatin organization, mitosis and DNA replication and DNA repair (Cao and Hegele, 2003; Cao et al., 2011; Cao et al., 2007; De Sandre-Giovannoli et al., 2003; Dechat et al., 2007; Dreesen and Stewart, 2011; Eriksson et al., 2003; Goldman et al., 2004; Lutz et al., 1992; McClintock et al., 2006; Shumaker et al., 2006; Worman, 2012). So far it is unknown whether progerin is present in stem cells.
Recently, rare fibroblasts from elderly individuals were found to exhibit nuclear phenotypes identical to those of HGPS cells (Cao et al., 2007; Scaffidi and Misteli, 2006). Skin sections from a subject with HGPS showed that progerin was localized primarily in vascular and dermal cells in vivo (McClintock et al., 2006). Similarly, skin sections from healthy individuals revealed the presence of progerin in a subset of dermal fibroblasts (McClintock et al., 2007). Recently, progerin was detected in HGPS coronary arteries, and remarkably, it was also present in non-HGPS individuals (Olive et al., 2010). Collectively, these findings demonstrate that progerin may be implicated in normal aging.
Two HGPS-induced pluripotent stem (iPS) cell models have been generated; the HGPS-iPS cells were able to differentiate into different cellular lineages (Ho et al., 2011; Liu et al., 2011; Zhang et al., 2011). The HGPS-iPS cells that differentiated into vascular SMCs accumulated progerin and displayed the nuclear envelope alteration and premature senescence previously observed in HGPS fibroblasts (Cao et al., 2011; Capell and Collins, 2006).
Recently, several adult stem cell populations have been identified in human skin (Blanpain et al., 2007; Hunt et al., 2009; Jahoda et al., 2003; Watt et al., 2006). One particular population, skin-derived precursor (SKP) stem cells, was isolated from mouse and human dermis and was expanded with methods normally used to culture central nervous system (CNS) stem cells and exhibit multipotent properties (Fernandes et al., 2006; Toma et al., 2001). These SKP cells similarly to CNS neurospheres express nestin, an intermediate filament protein expressed in neural precursors (Toma et al., 2001; Toma et al., 2005).
Here, we report the isolation of naïve multipotent skin-derived precursor (SKP) cells from dermal fibroblast cultures from HGPS donors. The SMCs derived from the HGPS-SKPs accumulate nuclear progerin with increasing passages. A subset of the HGPS-naïve SKPs express progerin in vitro and in situ in HGPS skin sections. This is the first in vivo evidence that progerin is produced in adult stem cells, and implies that this protein could induce stem cells exhaustion as a mechanism contributing to aging.
Results
Skin-derived precursor (SKPs) cells are present in primary human dermal fibroblast cultures
Given that both SKPs and primary fibroblast cultures are derived from the dermis, we speculated that a small number of SKP cells would remain in primary dermal fibroblast cultures. In preliminary observations, we found that a few viable cells were present in frozen aliquots of primary fibroblast cultures that experienced stress as the result of extreme temperature variations during storage, or shipment. These rare cells were able to expand and could be passaged several times. This suggested that a small number of cells with proliferation potency and resistance to temperature stress were present in human fibroblast cultures.
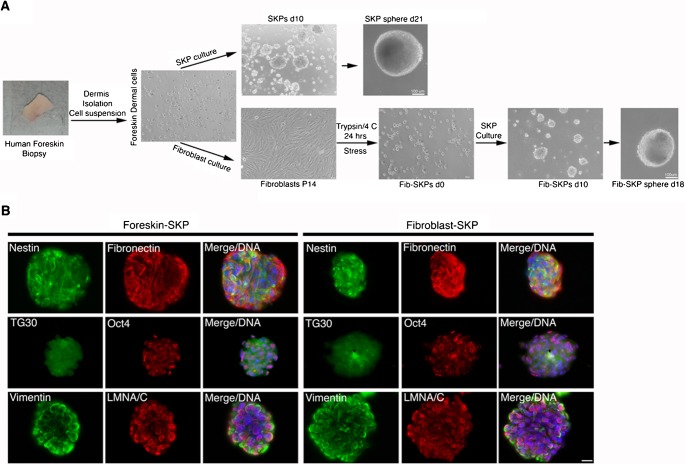
To test this hypothesis, we developed a method to isolate and compare SKP cells from foreskin tissues with the ones isolated from foreskin dermal fibroblast cultures. Human foreskin biopsies of normal individuals (age 0 to 12 years) were obtained post-surgically from the dermatology clinic at TU-Munich in accordance with the guidelines of the Ethics Committee executive board. The foreskin samples were cut in half: one half was used to establish fibroblast cultures, and the other half was used to establish SKP cultures, as specified in Materials and Methods (Toma et al., 2005).
Using the SKP culture method (Toma et al., 2005), we reliably generated cultures containing floating spheres that shared the growth characteristics of those described previously (Biernaskie et al., 2007) (Fig. 1). The spheres, with diameters between 50 and 100 µm, became visible by day 8 to 14 and grew to over 200 µm in diameter by week 3 (Fig. 1A). Furthermore, the SKP spheres expressed nestin and displayed antigenic properties similar to those described previously (Fig. 1B) (Toma et al., 2001), and were also positive for fibronectin and vimentin, as reported previously (Toma et al., 2001). Cells within these spheres exhibited stem cell-like properties, as they expressed the human embryonic stem (ES) cell markers, Oct4 and TG30 (Fig. 1B). The nestin-positive cells exhibited a positive signal for lamin A/C. These findings demonstrate that the SKP spheres must derive from an adult stem cell population within the human foreskin dermis.
Fig. 1. Generation of SKP spheres from human foreskin biopsy and from respective foreskin primary fibroblast culture.
(A) The method for SKP sphere cultures is outlined starting from a typical skin biopsy (skin image), followed by the dermal cell suspension which was either used directly for SKP cultures or for the establishment of primary fibroblast cultures as described in Materials and Methods. Typical 3D SKP spheres at day 10, 18 and 21 in culture are shown. SKP denotes skin-derived precursor and Fib-SKPs denotes SKPs derived from primary fibroblast cultures. Experiments repeated four times. (B) Immunofluorescence analysis performed on SKPs derived from foreskin biopsies (left) and SKPs derived from foreskin fibroblast cultures (right) taken between day 16 to day 18 in culture and stained with the indicated antibodies (n = 3). Nuclei were counterstained with a DNA stain dapi (blue). Scale bar: 20 µm.
We then investigated whether this population of dermal cells that selectively expands to generate the SKP spheres under controlled growth conditions consisting of epidermal growth factor (EGF) and fibroblast growth factor 2 (FGF2) could also be present in dermal fibroblast cultures derived from the same skin biopsy. To establish the fibroblast culture, the foreskin dermal-cellular suspension was directly grown in DMEM medium containing 15% FBS (Materials and Methods). We used the fibroblast cultures from population doublings (PPDs) 20 and above that typically correspond to fibroblasts available from cell banks.
Foreskin fibroblast cultures from PPDs 20 to 35 were processed for SKPs isolation (Materials and Methods). Briefly, cells detached by trypsin were incubated at 4°C for 24 hours (Fig. 1). After this treatment the majority of the cells died (85±3%) as observed by trypan blue staining. Cells were resuspended in SKP growth medium containing EGF and FGF2 growth factors and monitored for growth daily. While the first day only single cells could be observed, by day 3 to 4 a few clusters of 2 to 6 cells were observed. By day 8 to 10, floating spheres from different sizes ranging from 50 to 100 µm in diameter were visible (Fig. 1A), and by day 15 to 18 typical 3D spheres of an average of 200 µm were present (Fig. 1A) and after 3 to 4 weeks some spheres became larger than 300 µm in diameter.
To investigate the expression of stem cell markers in the SKP spheres derived from foreskin fibroblast cultures (Fib-SKP), spheres from day 16 to18 were screened for the neural crest stem cell marker nestin, and the ES marker, Oct4, both previously reported to be expressed in SKPs (Toma et al., 2005). We found that these markers were consistently expressed in the fibroblast-derived spheres (Fig. 1B): the majority of cells stained strongly for nestin, and Oct4 showed characteristic punctuate nuclear staining in these spheres (Fig. 1B). Collectively, these findings indicate that a subpopulation of cells present in primary fibroblast cultures can be isolated using the method described above (Materials and Methods).
Naïve stem cell precursor isolation from HGPS primary fibroblast cultures
Encouraged by the findings described above, we investigated whether similar stem cell precursors could be isolated from primary fibroblast cultures derived from subjects with HGPS. Dermal fibroblasts (HGADFN003, HGADFN164, HGADFN169, HGADFN178 and HGADFN188) from subjects with HGPS were obtained from the Progeria Research Foundation (www.progeriaresearch.org) and were age-matched with normal dermal fibroblasts (GMO3348E, GMO3349C, GMO8998A, GMO1652C and GMO2036A) obtained from the Coriell Institute for Medical Research (Camden, NJ). The Institutional Review Board at TU Munich approved the use of the previously established primary fibroblast cultures from human skin biopsies derived from healthy donors and HGPS patients.
Prior to the isolation of the naïve stem cells from the HGPS fibroblasts, we first characterized the status of A-type lamins in the fibroblast cultures. All of the experiments were performed with fibroblast cultures with PPDs ranging 20 to 35. Lamin A, lamin B1 and progerin were screened by immunocytochemistry and western blot analysis (Fig. 2; supplementary material Fig. S1). Lamins A and B1 were detected in all nuclei: however, lamin B1 was variable in its expression, indicating a decreased level in some HGPS nuclei as reported previously (Liu et al., 2011). In contrast, no variation in lamin B1 levels was observed in control counterparts (Fig. 2A).
Fig. 2. Typical SKP spheres were derived from HGPS primary dermal fibroblast cultures.
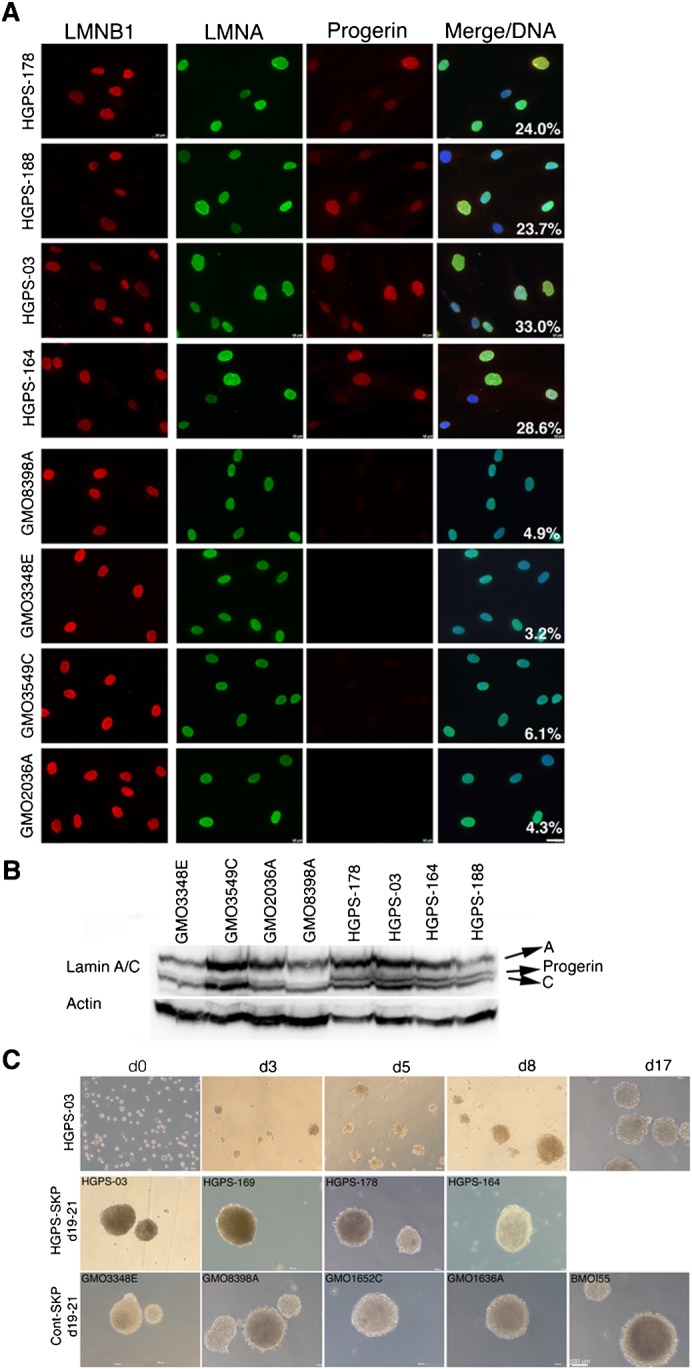
(A) Immunofluorescence detection of lamin B1, lamin A and progerin in HGPS and normal fibroblast cultures from PPD 20 to 35 are shown. The percentage of cells showing dysmorphic nuclei is indicated (n = 3). Scale bar: 20 µm. (B) Western Blot analysis of total HGPS and normal fibroblast lysates were probed as indicated (n = 3). (C) In vitro formation of SKP spheres derived from HGPS-003 (HGADFN003) from day 0 to 17 in culture. Typical 3D spheres derived from four HGPS and four normal fibroblast strains and a human mesenchymal stem cell line (BMOI.55) from day 19 to 21 in culture are shown. At least five SKP cultures have been established for each fibroblast strains used in this study. Scale bar: 100 µm.
Progerin signal intensity was variable in the HGPS nuclei, indicating different amounts of progerin, as previously reported (Goldman et al., 2004; McClintock et al., 2006) (Fig. 2A,B). In contrast, progerin was not detected in normal cells (Fig. 2A,B). As alterations in the nuclear size and shape characterize the HGPS nuclear phenotype, the percent of abnormal nuclei was determined for all eight strains used at PPDs 20 to 35 and as previously described (McClintock et al., 2006; Paradisi et al., 2005). An average of 24% to 33% of the HGPS nuclei exhibited nuclear abnormalities; in contrast, only 3% to 6% of the control cells exhibited dysmorphic nuclei (Fig. 2A).
We next determined whether naïve stem cells were present in the HGPS fibroblast cultures. Four HGPS and four control fibroblast strains were used to establish the SKP sphere cultures (Materials and Methods). In parallel, the human mesenchymal line, BMOI.55 (Okamoto et al., 2002), was used for the comparison of the 3D sphere morphologies (Fig. 2C).
Strikingly, all of these above cultures behaved in a manner similar to that described herein for HGPS-03. The cells that survived the initial stresses formed small cell clusters (4 to 10 cells) between day 3 and 4 (Fig. 2C). By day 5 to 8, we observed the formation of floating spheres of an average of 50 µm and larger (Fig. 2C). By day 17, typical 3D spheres ranged in size from 100 to 200 µm. After 3 to 4 weeks in culture, the HGPS-spheres became larger in diameter (200 to 400 µm) and resembled those observed in the SKP cultures derived from foreskin biopsies (Fig. 1). The spheres generated from the control fibroblasts and BMOI.55 mesenchymal stem cells exhibited identical formation kinetics and reached the typical 3D sphere diameter of ∼200 µm at about the same time (i.e., by day 18) (Fig. 2C).
The SKP spheres developed in a manner that was similar to all of the fibroblast cultures tested in this study, and were morphologically similar to the SKP spheres derived from human foreskin biopsies. We established five SKP cultures for each eight-fibroblast strains used in this study. Between 90 to 180 spheres formed from a starting population of 2×106 fibroblasts, independently of whether the cells were derived from HGPS or normal-fibroblast cultures. Consequently, the average frequency of SKP sphere formation per SKP culture ranged from 0.0045% to 0.009%. The variability in sphere formation likely reflects the asynchronous and heterogeneous properties of the primary fibroblast strains. Nevertheless, the fibroblasts cultures at PPDs 20 to 35 invariably generated the characteristic floating spheres when cultured in SKP growth medium as described in Materials and Methods. Together, these data indicate that this method produces reproducible results and allows the generation of a sufficient number of naïve HGPS precursor cells to screen their stem cell properties.
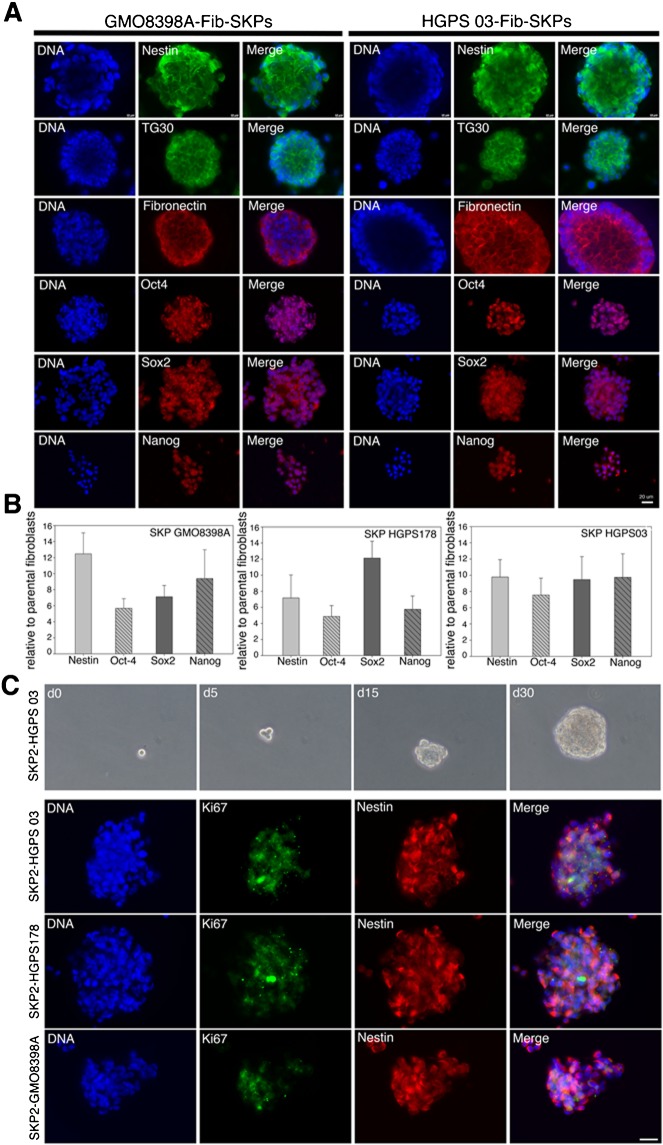
HGPS-SKP-spheres express neural crest stem cell and embryonic stem (ES) cell markers
Nestin was consistently expressed in the HGPS-spheres (Fig. 3A). Oct4 showed its characteristic nuclear staining both in the HGPS and normal spheres (Fig. 3A). Additionally, the HGPS-SKP and normal SKP spheres expressed the pluripotent markers Nanog, Sox2, and TG30 (Fig. 3A).
Fig. 3. SKP spheres derived from HGPS fibroblasts express stem cell markers.
(A) SKP-spheres derived from normal fibroblasts (left) and HGPS fibroblasts (right) from day 18 to 21 in culture were immunostained for indicated stem cell proteins (n = 3; similar stainings were obtained in SKP-spheres derived from the other fibroblast strains). (B) Real time PCR analysis of stem cell markers (Nestin, Oct4, Sox2 and Nanog) on HGPS and normal SKP sphere cultures from day 21 to 24 relative to the parental fibroblast cultures. All values are presented as mean ± S.D. (P<0.05; n = 4). (C) HGPS-SKPs were capable of self-renewal and proliferation. Limiting dilution assays showed that a single cell derived from an HGPS003-SKP sphere taken at day 18 re-formed a sphere denoted SKP2-HGPS003 (phase contrast microscopy). A typical SKP2 sphere appeared after approximately 30 days. SKP2 spheres from HGPS and normal cells at day 25 to 30 were double stained with anti-Ki67 and anti-Nestin antibodies. Nuclei are stained blue with DAPI. Scale bars: 20 µm.
Gene expression profiling with real-time PCR confirmed that the cells in the SKP spheres express higher levels of nestin, Oct4, Sox2 and Nanog mRNA relative to the parental fibroblast cultures (Fig. 3B). These findings indicate that the fibroblast cultures derived from HGPS subjects contain naïve stem cell precursors that can be isolated by utilizing the SKP culture conditions described above.
HGPS-SKP spheres contain cells capable of self-renewal
To examine whether the cells within the SKP spheres could undergo self-renewal by serial cloning in vitro, eight HGPS-SKP spheres were cloned to test their capacity for self-renewal. By day 15, the single cells derived from the spheres had formed small clusters, and a typical sphere appeared after ∼30 days (Fig. 3C); approximately 0.2% of the cells from a single dissociated sphere were capable of forming new spheres. Proliferating cells were detected by Ki67 staining, indicating that the cells in the re-formed spheres were able to divide (Fig. 3C).
Naïve HGPS-SKPs differentiate into smooth muscle cells and fibroblasts
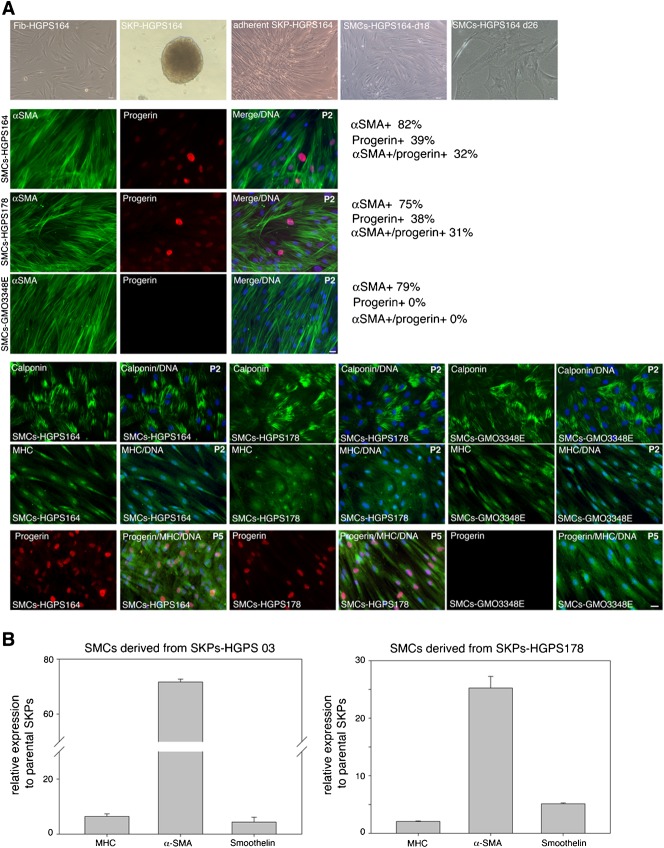
Previously, different populations of progenitor cells located in the skin were shown to differentiate into neuroectodermal and mesodermal cell lineages in vitro (Amoh et al., 2005a; Amoh et al., 2005b; Fernandes et al., 2006; Gingras et al., 2007; Hoogduijn et al., 2006; Jahoda et al., 2003; Yu et al., 2006). Smooth muscle and fibroblast cells are principal cellular targets of progerin nuclear accumulation in vivo, in both HGPS and elderly populations (McClintock et al., 2006; McClintock et al., 2007; Olive et al., 2010). Therefore, we investigated the potential of these HGPS-SKPs to differentiate into smooth muscle cells (SMCs) and fibroblasts.
The HGPS and normal SKP spheres were collected at day 21, and induced to differentiate into SMCs or into fibroblasts (Materials and Methods; supplementary material Fig. S2). Gene expression analysis confirmed that, upon induction to differentiate into SMCs, mRNA encoding SMC differentiation markers (α-smooth muscle actin [αSMA], smooth muscle myosin heavy chain [MHC] and Smoothelin) were increased in the HGPS and normal SMC preparations, relative to the SKP-sphere cultures (Fig. 4B). Immunocytochemistry confirmed the expression of SMC markers, αSMA, calponin 1, and MHC, in the SMCs derived from the HGPS and normal SKPs (Fig. 4A). The percent of αSMA-positive cells in HGPS and normal SMC cultures indicated that an average of 75% to 85% of the cells underwent differentiation (Fig. 4A).
Fig. 4. Characterization of smooth muscle cells-derived from HGPS-SKPs.
(A) SKP-spheres derived from HGPS and normal fibroblasts were directed to differentiate into smooth muscle cells (SMCs). Four SMCs directed differentiation experiments were performed using two normal (GMO3348E, GMO8398A) and three HGPS-SKP strains (HGADFN03, HGADFN164, HGADFN178). Phase contrast imaging recapitulating the different culture steps from the fibroblast culture to the SKP sphere formation and to the SMCs differentiation (upper panel). SMCs from passage 2 (P2) and 5 (P5) were immunostained for indicated SMC markers and progerin. At passage 2 the percent of αSMA-positive and progerin-positive cells are indicated (n = 3). Scale bar: 20 µm. (B) Real-time PCR analysis of the SMC markers, myosin heavy chain (MHC), α-smooth muscle actin (αSMA) and smoothelin as indicated relative to the parental HGPS SKP-sphere cultures. All values are presented as mean ± S.D. (P<0.05; n = 3).
At early passage 2, progerin was detected in an average of 38 to 39% of the HGPS-SMCs, of which 31 to 32% were also αSMA positive (Fig. 3C). At later passage 5, the number of progerin-positive cells in the HGPS-SMCs cultures increased dramatically ranging 80 to 90% of the cells (Fig. 3D). These results indicate that naïve HGPS stem cells are capable of differentiating into SMCs in vitro and inducing the accumulation of progerin in HGPS-SMC nuclei with increasing cellular age in vitro; as reported in recent HGPS-iPSCs studies (Liu et al., 2011; Zhang et al., 2011), and in our previous in situ studies (McClintock et al., 2006; Olive et al., 2010). Collectively, these studies suggest that progerin could be a contributor to the molecular events that lead to the vascular disease in HGPS.
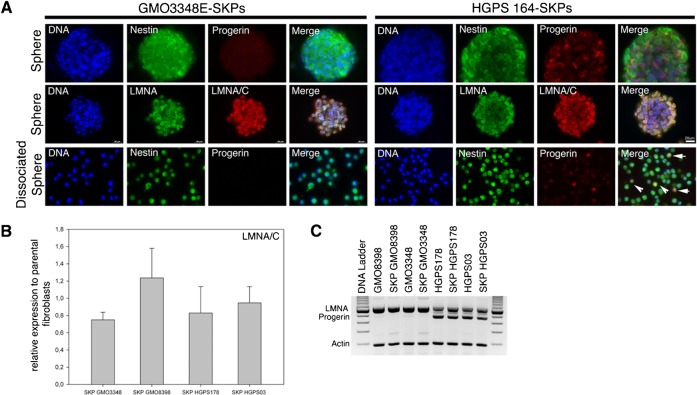
Naïve HGPS- SKPs express progerin in vitro and in situ in HGPS
We next investigated whether progerin was already present in naïve HGPS stem cells. Immunohistochemistry of HGPS and normal SKP spheres indicated that lamin A/C was present in all nuclei and progerin was detectable at varying levels in a subset of the nuclei in HGPS-SKP spheres (Fig. 5A). Screening of the SKP cells from dissociated HGPS spheres indicated that an average of 14% to 26% of the cells exhibited a detectable progerin-positive signal, whereas the normal SKPs showed no progerin signal (Fig. 5A). Moreover, quantitative real-time PCR, semi-quantitative PCR and Western blot analyses indicated that the levels of lamin A/C and progerin in HGPS-SKPs were comparable to the parental fibroblast cultures (Fig. 5B,C; supplementary material Fig. S3).
Fig. 5. Progerin is expressed in naïve HGPS-SKPs in vitro.
(A) HGPS-SKP and Normal-SKP spheres immunostained with anti-progerin and anti-nestin antibodies or anti-lamin A (LMNA) and anti-lamin A/C (LMNA/C) (lower panels), and counterstained with a DNA stain (dapi). Lower panel, immunofluorescence analysis of dissociated SKP spheres derived from normal (GMO3348E) and HGPS (HGPS164) cultures at day 18 were stained with anti-progerin and nestin antibodies as indicated (n = 4). Arrows in Merge image indicate progerin-positive cells. Scale bar: 20 µm. (B) Real-time PCR analysis of lamin A/C in HGPS-SKP spheres and normal fibroblast-SKP spheres collected at day 21 in relation to respective parental fibroblast cultures. All values are presented as mean ± S.D. (P<0.05; n = 4). (C) Semi-quantitative PCR analysis of lamin A and progerin transcripts in fibroblasts and corresponding SKP sphere cultures as indicated (n = 3).
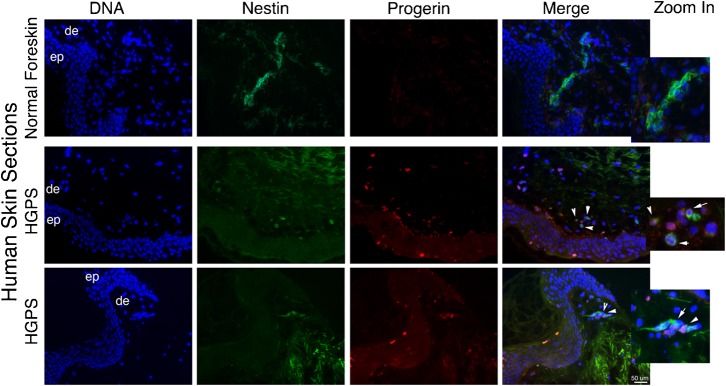
These in vitro data suggest that adult stem cells in HGPS patients might already express progerin in vivo. To answer this question, immunohistochemistry was performed on frozen sections from a skin biopsy of a 9-year-old HGPS patient (HGADFN143) characterized previously as containing progerin-positive cells in the vascular system and the dermis (McClintock et al., 2006; McClintock et al., 2007). Because the neuroepithelial stem cell marker, nestin, is expressed in SKPs derived from human skin (Toma et al., 2005), we compared the distribution of nestin-positive cells with the progerin signal in the rare HGPS skin sections available (Fig. 6). Nestin-positive cells were distributed throughout the dermis in the human foreskin derived from a young unaffected individual (Fig. 6), and some cells were found as linear patches. However, we detected no progerin-positive cells in these foreskin samples. In the HGPS skin sections, the progerin-positive cells were detected within the dermis and the upper layer of the epidermis (Fig. 6), as reported previously (McClintock et al., 2006; McClintock et al., 2007), and very few nestin-positive cells were detected in HGPS skin (Fig. 6). Examination of these rare nestin-positive cells revealed the presence of low levels of progerin (Fig. 6). Collectively, these findings indicated that progerin is present in a subset of nestin-positive cells in HGPS skin.
Fig. 6. Progerin is expressed in nestin-positive cells in vivo in HGPS skin sections.
In situ localization of progerin and nestin on normal human foreskin sections and on skin sections derived from a subject with HGPS (HGADFN143). DNA was stained with dapi. Morphologic entities are indicated: epidermis (ep), dermis (de), Arrows indicate progerin- and nestin-positive cells on Merge images. Scale bar: 50 µm. Zoom in by a factor of 4.
Discussion
In the present study, we developed a new method allowing the isolation of naïve multipotent skin-derived precursor (SKP) cells from human primary fibroblast cultures. We show that the SKPs derived from foreskin fibroblast cultures share similar stem cell properties with the cells isolated directly from human foreskin biopsies. These SKPs express the neural crest marker nestin and the multipotency markers OCT4, Sox2, Nanog and TG30. These cells also display the capacity for self-renewal and differentiation. For a proof of principle using this approach, we isolated naïve SKPs from primary fibroblast cultures established from skin biopsies taken from children with HGPS.
The existence of SKPs in mouse and human dermis has been demonstrated, and these cells have been expanded by methodologies normally used to culture central nervous system (CNS) stem cells (Toma et al., 2001; Toma et al., 2005). SKPs exhibit some similarities to CNS neurosphere cultures (Toma et al., 2001; Toma et al., 2005), and the clonal analysis of early and late passages of dermal spheres has demonstrated that SKPs are multipotent and can differentiate into cells with neuronal, glial and mesenchymal phenotypes (Fernandes et al., 2006; Toma et al., 2001). Based on these studies and the fact that primary fibroblasts were also derived from the dermis, we hypothesized that a subpopulation of SKPs might exist in primary fibroblast cultures. Moreover, preliminary observations indicated that a subset of cells in fibroblast cultures were resistant to stress (i.e., extreme temperature variations) and could be expanded in vitro. Combining these two independent observations, we developed the method described herein to isolate naïve SKPs from HGPS fibroblasts.
Because skin biopsy samples from patient with HGPS are not readily available, the approach described herein offers a remarkable alternative to characterize naïve adult stem cells from pre-existing fibroblast cultures. Furthermore, to assess the potential role of adult stem cells in the development of HGPS disease, it was important to determine whether progerin was present in naïve HGPS stem cells, as shown in this study.
Recently, two models of induced pluripotent stem cells from HGPS patient fibroblasts have been established (Liu et al., 2011; Zhang et al., 2011). The lamin A/C gene encodes lamins A and C, which are involved in maintaining the function of the transcription factors required for the differentiation of adult stem cells and embryonic stem cells (Constantinescu et al., 2006; Hutchison and Worman, 2004). A-type lamins are expressed in most differentiated somatic cells, but the expression of these lamins is reduced or absent in cells that have a low degree of differentiation or are highly proliferative (Agrelo et al., 2005). The degree of maturation between embryonic versus adult stem cells might be partly linked to the expression of A-type lamins.
In contrast with HGPS-SKPs, which express a detectable amount of A-type lamins, including progerin, HGPS iPS cells resemble embryonic stem cells because they lack both lamin A/C and progerin expression in the undifferentiated state following the reprogramming process (Liu et al., 2011; Zhang et al., 2011). In our study, a significant number of cells in HGPS-SKP spheres exhibited a positive signal for progerin expression. Because A-type lamins are expressed in adult stem cells, the detection of progerin in HGPS-SKPs may not be surprising. However, this observation represents the first evidence that progerin is present in HGPS adult stem cells, further supporting a role for progerin in adult stem cell dysfunction in HGPS.
Previously, the screening of skin sections derived from a subject with HGPS showed that progerin was present in dermal nuclei, blood vessels, arrector pili muscle, and keratinocyte nuclei in the uppermost layers of the epidermis (McClintock et al., 2006). In the present study, using skin sections from the same HGPS patient, we demonstrated the presence of rare nestin-positive cells in the dermal compartment, a subset of which exhibited a progerin-positive nuclear signal. This in situ observation reinforces the hypothesis that the accelerated aging exhibited by HGPS patients might partly be the result of adult stem cell depletion and the progressive deterioration of tissue functions, as suggested previously (Scaffidi and Misteli, 2008). HGPS iPS cells could not have provided this potential link because lamin A/C and progerin are remarkably repressed in these reprogrammed iPS cells (Liu et al., 2011; Zhang et al., 2011).
HGPS-iPS cells when directed to differentiate, showed phenotypic alterations similar to those previously reported in HGPS fibroblasts (Cao et al., 2011; Cao et al., 2007; De Sandre-Giovannoli et al., 2003; Dechat et al., 2007; Eriksson et al., 2003; Goldman et al., 2004; Lutz et al., 1992; McClintock et al., 2006; Shumaker et al., 2006). These alterations included nuclear abnormalities, progerin expression and accumulation, altered DNA repair mechanisms and premature senescence (Liu et al., 2011; Zhang et al., 2011).
Lamin A/C is present in most somatic cells; however, specific cell types (e.g., vascular smooth muscle cells and fibroblasts) appear more susceptible to cellular dysfunction due to progerin expression in vivo in HGPS patients. The reason that the HGPS disease appears to develop and affect only certain tissues (skin, bone, vasculature and adipocyte) remains unclear (Gordon et al., 2007).
Interestingly, in the HGPS-iPS models in which cells were directed to differentiate into neural progenitors, endothelial cells, fibroblasts, VSMCs, and mesenchymal stem cells (MSCs), progerin levels were the highest in MSCs, VSMCs, and fibroblasts, and these cells displayed increased DNA damage and nuclear abnormalities similar to these previously observed in primary HGPS fibroblast cultures (Zhang et al., 2011). In a second HGPS iPS study, iPS cells were able to differentiate into specialized mesoderm-derivatives such as smooth muscle cells (SMCs) and endothelial cells (Liu et al., 2011). Again, similar nuclear abnormalities and premature senescence were observed upon progerin accumulation in the differentiated SMCs (Liu et al., 2011; Zhang et al., 2011).
HGPS-SKPs were also able to differentiate into fibroblasts and SMCs with a markedly similar efficiency as that reported for HGPS-iPS cells (Liu et al., 2011; Zhang et al., 2011). In all of the studies SMCs were characterized by the expression of the SMC markers α-smooth muscle actin, calponin 1, and smooth muscle myosin heavy chain. In addition, progerin was found to be accumulated in differentiated cells in vitro (Liu et al., 2011; Zhang et al., 2011). Interestingly, HGPS iPS cells and HGPS-SKPs directed to differentiate into SMCs recapitulate the same SMC phenotypic changes that were previously observed in situ in HGPS biopsies (McClintock et al., 2006; Olive et al., 2010; Stehbens et al., 2001). Altogether, these studies provide new cellular models to further dissect the molecular mechanisms triggered by progerin expression in SMCs, and in the future, these models will aid in the understanding of how vascular disease develops in HGPS patients.
Except for fibroblasts and lymphoblasts, no patient-derived cell lineages are available for study. Now, with the development of HGPS iPS cell lines and the naïve HGPS SKPs reported in this study, multiple HGPS cell lineages with endogenous levels of progerin and other lamins can be generated. The new cellular lineages will allow the study of the pathogenesis of HGPS in the cell types that are affected in vivo by HGPS. HGPS disease modeling in the near future will allow the elucidation of how progerin impacts bone, skin, vascular tissue and adipose tissue regeneration and homeostasis. These future studies will provide new possibilities for the development of therapeutic avenues to restore or prevent the cellular damage triggered by progerin expression in these specific cell types. Moreover, the use of HGPS-iPS cells and HGPS-SKPs will undoubtedly provide major insights into how progerin in adult stem cells might limit HGPS tissue regeneration in vivo.
In the present study, we provide evidence that a subset of nestin-positive cells express progerin in vivo. These nestin-positive cells must correspond to the multipotent progenitor cells known as SKPs (Fernandes et al., 2004). This result suggests that, in vitro, the progerin-positive HGPS-SKP cells recapitulate the same molecular events that lead to progerin accumulation in adult stem cells in vivo. Collectively, our data demonstrate the expression of progerin in dermal adult stem cells from an HGPS patient, providing the first direct evidence that HGPS stem cells might age prematurely as previously suggested (Scaffidi and Misteli, 2008). Furthermore, because progerin is detectable in the normal tissues of healthy individuals, progerin is relevant to both HGPS disease and normal aging (Bökenkamp et al., 2011; McClintock et al., 2007; Olive et al., 2010; Scaffidi and Misteli, 2006).
In conclusion, our work provides an incentive to explore new therapies for the prevention of progerin accumulation in naïve HGPS adult stem cells. Moreover, the methods described in this study permit the isolation of adult stem cells from primary fibroblast cultures in cell banks around the world, providing a valuable tool with which to better understand the pathogenesis of HGPS and other genetic diseases.
Materials and Methods
Preparation of skin-derived precursor (SKP) cells and primary dermal fibroblast cultures from identical foreskin samples
Human skin samples were obtained from post-surgery materials in accordance with the Institutional Review Ethic Committee at TU-Munich. Patients signed informed consent form. Human skin tissue samples were transported to laboratory in ice-cold PBS containing 2% antibiotic mix (penicillin/streptomycin) and fungizone 2 µg/ml. The specimens were washed with cold PBS buffer, cut into 4–6 mm2 pieces and incubated in dispase 1 U/ml overnight at 4°C followed by 15 minutes at 37°C. The epidermis was manually removed from tissue pieces after dispase incubation and the dermis was minced into smaller pieces following enzymatic digestion with collagenase XI (0.05 mg/ml) for 2 hr at 37°C. Afterwards, tissue homogenates were dissociated and passed through a 70 µm cell strainer (BD Falcon, USA), and centrifuged at 1,500 rpm for 7 min. The cell pellet was resuspended in PBS and split into two equal aliquots.
Both samples were centrifuged and one pellet was resuspended in 4 ml SKP proliferation medium (DMEM-F12, 3:1 and 40 ng/ml FGF2, 20 ng/ml EGF (both from BD Biosciences), B27 (Invitrogen), and 1 µg/ml fungizone (Invitrogen)) and then transferred to a 25 cm2 tissue culture flask (BD Biosciences) as described previously (Toma et al., 2005). The cells were grown at 37°C for 3 to 4 weeks with addition of growth medium every three days keeping the final concentration of the growth factors as above. Foreskin-SKP spheres could be passaged at least three times using trypsin solution (Invitrogen).
The second pellet from the foreskin cell samples was resuspended in fibroblast growth medium DMEM containing 15% fetal calf serum, 2 mM glutamine, 100 IU/ml penicillin, and 100 mg/ml streptomycin. Cells were passaged when they reached 80% confluency. After three passages, frozen aliquots of passage three and above were stored for further experiments.
To generate SKP sphere cultures from human primary fibroblast cultures, fibroblast cultures from PPDs 20 to 35 were used. Fibroblasts were grown in 10 cm tissue culture plates in DMEM containing 15% FBS as mentioned above and processed for SKP sphere formation when they reached 80% confluency. One plate corresponded in average to 2×106 cells and was washed with PBS and incubated with 2 ml trypsin (0.25%, Invitrogen) solution for 1 hr at 37°C. Detached cells were directly collected with 10 ml of PBS containing 100 IU/ml penicillin, and 100 mg/ml streptomycin. The cell suspension, in a tightly closed 15 ml falcon tube, was then further incubated in a fridge at 4°C for 24 hours. After this treatment, the cell suspension was centrifuged. The final cell pellet was resuspended in SKP growth medium containing EGF and FGF as described above, and allowed to grow in 25 cm2 flask with low adherence properties at 37°C with 5% CO2 incubator. SKP growth medium supplemented with the growth factors was added to the flask every three days. The cultures were monitored for growth every day. For each fibroblast strains used in this study, three 10 cm dishes were used in parallel to generate enough SKP spheres for the different analyses. Moreover, SKP derived from fibroblast cultures of the different strains haven been repeated at least five times.
Human foreskin biopsy sections
Foreskin biopsies from unaffected individual from 0 to 12 years old were obtained from post-surgery materials at the Dermatology Clinic at TU-Munich and in accordance with the guidelines of the Ethics Committee executive board at TU-Munich. Skin biopsies were embedded in Optimum Cooling Temperature medium (O.C.T.) and cryopreserved for tissue sectioning. Serial 6 µm frozen skin sections were prepared and stored at −80°C.
HGPS skin biopsy and primary dermal fibroblast cells
Dermal fibroblasts from subjects with HGPS were obtained from the Progeria Research Foundation Cell and Tissue Bank (www.progeriaresearch.org). The following fibroblasts were used: HGADFN003 (M, age 2), HGADFN178 (F, age 7), HGADFN164 (F, age 4) and HGADFN188 (F, age 2). Age-matched control dermal fibroblasts were obtained from the Coriell Institute for Medical Research (Camden, NJ). The following cell lines were used: GM01652C (F, age 11), GM02036A (F, age 11), GM03348E (M, age 10), GM08398A (M, age 8). We also used the human mesenchymal line, BMOI.55 (kindly provided by Toguchida T. and Aoyama T. at Kyoto University) (Okamoto et al., 2002).
The Institutional Review Board at TU-Munich approved the use of human cells previously established from skin biopsies from HGPS patients and unaffected individuals.
Cells were cultured in DMEM containing 15% fetal bovine serum, 1% glutamine and 1% penicillin/streptomycin. Cells were subcultured at 80% confluency to keep cultures in growth phase and collected at population doublings between 20 and 35.
The Progeria Research Foundation Cell and Tissue Bank kindly provided frozen skin sections derived from a skin biopsy of a 9-year-old donor with HGPS carrying LMNA G608G mutation (HGADFN143). Sections of the same skin biopsy were used previously to determine the in vivo pattern of expression of progerin in the HGPS skin (McClintock et al., 2006).
Directed differentiation into smooth muscle cells
Single spheres from day 21 to 26 were plated into 6 well culture dishes and cells were allowed to adhere and outgrow from the spheres in SKP medium for 48 hours. To obtain smooth muscle cells (SMCs), the medium was replaced with SMC differentiation medium consisting of high-glucose Dulbecco's modified Eagle medium (Invitrogen) containing 5% FBS, 5 ng/mL PDGF-BB (Invitrogen), and 2.5 ng/mL TGF-β1 (Invitrogen) as previously described (Hill et al., 2010). Medium was changed every 3 days. Screening for SMC markers was performed after 3 to 4 weeks in cultures in SMC differentiation medium. These experiments have been repeated four times.
Directed differentiation into fibroblasts
SKP spheres from day 21 to 26 were plated into 6 well culture dishes and immediately grown in fibroblast supporting medium consisting of low-glucose DMEM containing 15% fetal calf serum, 2 mM glutamine, 100 IU/ml penicillin, and 100 mg/ml streptomycin. Cells were passaged when they reached 80% confluency and underwent repeated passaging and cryopreservation. Screening for the fibroblast marker: prolyl-4-hydroxylase beta (P4HB) an enzyme involved in collagen synthesis was performed on passage 8 and above using anti-P4HD Ab (AF0910-1, Acrys).
Protein extraction and western blotting
To obtain fibroblast protein extracts, cells were scraped from culture dishes in chilled PBS, centrifuged at 450 × g for 5 min at 4°C, washed in PBS, and the final cell pellet was resuspended in Laemmli sample buffer (BioRad) and boiled 5 minutes at 95°C. For SKP-sphere cultures, the cultures were collected in a 15 ml falcon tube, directly centrifuged, and the SKP pellets were processed as described above.
Equal amounts of extracts were loaded in parallel on a 10% polyacrylamide gel. After separation by electrophoresis, proteins were transferred to nitrocellulose membranes and incubated with blocking buffer as described previously (McClintock et al., 2006). Membranes were incubated with primary antibodies: anti-progerin antibody (McClintock et al., 2007), anti-lamin A/C kindly provided by Dr. N. Chaudhary, anti-actin Ab (Sigma), anti-lamin B1 (sc-6216, Santa Cruz), washed, and then incubated with the corresponding secondary antibody coupled to horseradish peroxidase (Jackson ImmunoResearch Laboratories). Proteins were visualized using the enhanced chemiluminescence detection system (Amersham Pharmacia Biotech). Signals were analyzed by densitometry using Quantity One 1-D analysis software (Bio-Rad) on the scanned images. Protein quantification was normalized to the internal control actin.
Immunocytochemistry
Fibroblasts were directly grown on coverslips and SKP spheres were cytospun on glass coverslips. Cells were either fixed in 4% paraformaldehyde for 10 minutes at room temperature or fixed in 100% methanol at −20°C for 10 minutes. Cells were then permeabilized with 0.3% Triton X-100, followed by blocking with 5% FBS in PBS. Cells or spheres were incubated in primary antibody and secondary antibodies (Alexa Fluors, Invitrogen) respectively for 1 hour. Primary antibodies used in this study were anti-progerin Ab (McClintock et al., 2007), anti-lamin A/C Ab (Chaudhary and Courvalin, 1993) (kindly provided by Dr. Nilabh Chaudhary), anti-nestin Ab (MAB5326, Chemicon, 1:100), anti-vimentin Ab (Chemicon 1:200), anti-fibronectin (F3648, Sigma, 1:600), anti-Nanog Ab (ab21624, abcam, 1:400), anti-Oct4 Ab (ab19857, abcam, 1:400), Sox2 (ab97959, abcam, 1:400), anti-TG30 Ab (Millipore, 1:400), anti-lamin A Ab (133A2, abcam, 1:200) and prolyl4-hydroxylase (Acris 1:200).
The secondary antibodies were affinity purified Alexa Fluor 488 goat or donkey IgG antibodies (Molecular Probes) and Cy3-conjugated IgG antibodies (Jackson ImmunoResearch laboratories). All samples were also counterstained with dapi Vectashield mounting medium (Vector Inc.). Images were acquired on an Axioplan fluorescence microscope (Carl Zeiss, Germany).
Immunostaining of SMC markers was carried out on cells fixed with 4% paraformaldehyde solution for 10 minutes and permeabilized with PBS containing 0.3% Triton X-100 for 30 minutes. Antibodies against αSMA (MO851, Dako, 1:100), calponin (M3556, Dako, 1:100), and smooth muscle heavy chain (SM-MHC) (IS066, Dako, 1:25) were used.
For Ki67 staining spheres were fixed in 4% paraformaldehyde for 10 minutes at room temperature followed by 30 minutes incubation in 2N HCL/PBS solution. Coverslips were then washed in PBS at least 5 times and incubated in blocking buffer for 30 minutes. Spheres were then processed with anti-Ki67 Ab (610968, BD Bioscences, 1/50).
Nuclear dismorphology was calculated as percentage of abnormal nuclear by a direct count of 300 cells per coverslip in triplicate and from at least three independent experiments.
The percentages of αSMA- and progerin-positive cells were determined by a direct count of 300 cells per coverslip in triplicate and from three independent experiments.
Statistical analysis
Results are presented as mean ± S.D. Comparisons were performed with student's t-test. P<0.05 was defined as statistically significant.
Immunohistochemistry
Stainings were performed on 6 µm frozen sections fixed by methanol/acetone (1 V/1 V) at −20°C for 10 minutes and washed in PBS, then blocked in PBS buffer containing 3% BSA, 10% normal goat serum and 0.3% Triton X-100 for 30 minutes and 1 hour in the same buffer without Triton X-100. Slides were incubated with the anti-progerin Ab 972S9 (McClintock et al., 2007) together with anti-nestin Ab (MAB5326, Chemison) for 1 hour. After 6 washes in blocking buffer, slides were incubated with secondary antibodies. Slides were washed in blocking buffer and in PBS, then mounted with Vectashield mounting medium (Vector Inc.)
Real-time RT-PCR analysis
We synthesized cDNA using Omniscript Reverse Transcriptase (Qiagen) using total cellular RNA as template. RNA from fibroblasts, SKP-spheres cultures and SMC cultures were used. Primers were designed using Primer3 (http://frodo.wi.mit.edu). The list of genes that were validated by RT-PCR and their corresponding primers are shown in supplementary material Table S1.
Real-time RT-PCR reactions contained Power SYBR Green PCR mastermix (Applied Biosystems), 300 nM of each primer, and 50 ng of template in a 20 µl reaction volume. Amplification was carried out using the Mx3000P Real-Time PCR Detection System (Stratagene) with an initial denaturation at 95°C for two minutes followed by 40 cycles at 95°C for 35 seconds and 60°C for 20 seconds. Three experiments were performed for each assay, in which the samples were run in triplicate. GAPDH was used as an endogenous control and quantification was performed using the relative quantification method where the real-time PCR signal of the experimental RNA was measured in relation to the signal of the control. The 2(ΔΔCT) method was used to calculate relative changes in gene expression (Livak and Schmittgen, 2001).
Supplementary Material
Acknowledgments
We would like to thank Dr Toguchida T. and Dr Aoyama T. at Kyoto University for providing the mesenchymal stem cells BMOI.55, Dr Chaudhary N. for anti-lamin A/C antibody. We thank the Progeria Research Foundation, Lorraine Fast and the patient families for providing HGPS fibroblasts. This work was supported by the Alexander von Humboldt Foundation (5090371), the Christine Kühne Center for Allergy Research and Education (CK-CARE), and the Bayerischen Staatsministerium (to K.D.). Conceived and designed the experiments: K.D. Performed the experiments and analyzed the data: V.W., D.R., D.G. and K.D. Analyzed the data and contributed reagents and analysis tools: L.B.G., M.H., R.S. and J.R. Wrote the manuscript: K.D.
Footnotes
Competing interests: The authors declare that there are no competing interests.
References
- Aebi U., Cohn J., Buhle L., Gerace L. (1986). The nuclear lamina is a meshwork of intermediate-type filaments. Nature 323, 560–564 10.1038/323560a0 [DOI] [PubMed] [Google Scholar]
- Agrelo R., Setien F., Espada J., Artiga M. J., Rodriguez M., Pérez-Rosado A., Sanchez-Aguilera A., Fraga M. F., Piris M. A., Esteller M. (2005). Inactivation of the lamin A/C gene by CpG island promoter hypermethylation in hematologic malignancies, and its association with poor survival in nodal diffuse large B-cell lymphoma. J. Clin. Oncol. 23, 3940–3947 10.1200/JCO.2005.11.650 [DOI] [PubMed] [Google Scholar]
- Amoh Y., Li L., Campillo R., Kawahara K., Katsuoka K., Penman S., Hoffman R. M. (2005a). Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc. Natl. Acad. Sci. USA 102, 17734–17738 10.1073/pnas.0508440102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoh Y., Li L., Katsuoka K., Penman S., Hoffman R. M. (2005b). Multipotent nestin-positive, keratin-negative hair-follicle bulge stem cells can form neurons. Proc. Natl. Acad. Sci. USA 102, 5530–5534 10.1073/pnas.0501263102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J. A., McKenzie I. A., Toma J. G., Miller F. D. (2007). Isolation of skin-derived precursors (SKPs) and differentiation and enrichment of their Schwann cell progeny. Nat. Protoc. 1, 2803–2812 10.1038/nprot.2006.422 [DOI] [PubMed] [Google Scholar]
- Blanpain C., Horsley V., Fuchs E. (2007). Epithelial stem cells: turning over new leaves. Cell 128, 445–458 10.1016/j.cell.2007.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bökenkamp R., Raz V., Venema A., DeRuiter M. C., van Munsteren C., Olive M., Nabel E. G., Gittenberger-de Groot A. C. (2011). Differential temporal and spatial progerin expression during closure of the ductus arteriosus in neonates. PLoS ONE 6, e23975 10.1371/journal.pone.0023975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. T., Kieras F. J., Houck G. E., Jr, Dutkowski R., Jenkins E. C. (1985). A comparison of adult and childhood progerias: Werner syndrome and Hutchinson-Gilford progeria syndrome. Adv. Exp. Med. Biol. 190, 229–244. [DOI] [PubMed] [Google Scholar]
- Cao H., Hegele R. A. (2003). LMNA is mutated in Hutchinson-Gilford progeria (MIM 176670) but not in Wiedemann-Rautenstrauch progeroid syndrome (MIM 264090). J. Hum. Genet. 48, 271–274 10.1007/s10038-003-0025-3 [DOI] [PubMed] [Google Scholar]
- Cao K., Capell B. C., Erdos M. R., Djabali K., Collins F. S. (2007). A lamin A protein isoform overexpressed in Hutchinson-Gilford progeria syndrome interferes with mitosis in progeria and normal cells. Proc. Natl. Acad. Sci. USA 104, 4949–4954 10.1073/pnas.0611640104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao K., Blair C. D., Faddah D. A., Kieckhaefer J. E., Olive M., Erdos M. R., Nabel E. G., Collins F. S. (2011). Progerin and telomere dysfunction collaborate to trigger cellular senescence in normal human fibroblasts. J. Clin. Invest. 121, 2833–2844 10.1172/JCI43578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capell B. C., Collins F. S. (2006). Human laminopathies: nuclei gone genetically awry. Nat. Rev. Genet. 7, 940–952 10.1038/nrg1906 [DOI] [PubMed] [Google Scholar]
- Chaudhary N., Courvalin J. C. (1993). Stepwise reassembly of the nuclear envelope at the end of mitosis. J. Cell Biol. 122, 295–306 10.1083/jcb.122.2.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinescu D., Gray H. L., Sammak P. J., Schatten G. P., Csoka A. B. (2006). Lamin A/C expression is a marker of mouse and human embryonic stem cell differentiation. Stem Cells 24, 177–185 10.1634/stemcells.2004-0159 [DOI] [PubMed] [Google Scholar]
- De Sandre-Giovannoli A., Bernard R., Cau P., Navarro C., Amiel J., Boccaccio I., Lyonnet S., Stewart C. L., Munnich A., Le Merrer M. et al. (2003). Lamin a truncation in Hutchinson-Gilford progeria. Science 300, 2055 10.1126/science.1084125 [DOI] [PubMed] [Google Scholar]
- Dechat T., Shimi T., Adam S. A., Rusinol A. E., Andres D. A., Spielmann H. P., Sinensky M. S., Goldman R. D. (2007). Alterations in mitosis and cell cycle progression caused by a mutant lamin A known to accelerate human aging. Proc. Natl. Acad. Sci. USA 104, 4955–4960 10.1073/pnas.0700854104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesen O., Stewart C. L. (2011). Accelerated aging syndromes, are they relevant to normal human aging? Aging (Albany NY) 3, 889–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M., Brown W. T., Gordon L. B., Glynn M. W., Singer J., Scott L., Erdos M. R., Robbins C. M., Moses T. Y., Berglund P. et al. (2003). Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature 423, 293–298 10.1038/nature01629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes K. J. L., McKenzie I. A., Mill P., Smith K. M., Akhavan M., Barnabé-Heider F., Biernaskie J., Junek A., Kobayashi N. R., Toma J. G. et al. (2004). A dermal niche for multipotent adult skin-derived precursor cells. Nat. Cell Biol. 6, 1082–1093 10.1038/ncb1181 [DOI] [PubMed] [Google Scholar]
- Fernandes K. J., Kobayashi N. R., Gallagher C. J., Barnabé-Heider F., Aumont A., Kaplan D. R., Miller F. D. (2006). Analysis of the neurogenic potential of multipotent skin-derived precursors. Exp. Neurol. 201, 32–48 10.1016/j.expneurol.2006.03.018 [DOI] [PubMed] [Google Scholar]
- Fuchs E., Weber K. (1994). Intermediate filaments: structure, dynamics, function, and disease. Annu. Rev. Biochem. 63, 345–382 10.1146/annurev.bi.63.070194.002021 [DOI] [PubMed] [Google Scholar]
- Gingras M., Champigny M. F., Berthod F. (2007). Differentiation of human adult skin-derived neuronal precursors into mature neurons. J. Cell. Physiol. 210, 498–506 10.1002/jcp.20889 [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Shumaker D. K., Erdos M. R., Eriksson M., Goldman A. E., Gordon L. B., Gruenbaum Y., Khuon S., Mendez M., Varga R. et al. (2004). Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA 101, 8963–8968 10.1073/pnas.0402943101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon L. B., McCarten K. M., Giobbie-Hurder A., Machan J. T., Campbell S. E., Berns S. D., Kieran M. W. (2007). Disease progression in Hutchinson-Gilford progeria syndrome: impact on growth and development. Pediatrics 120, 824–833 10.1542/peds.2007-1357 [DOI] [PubMed] [Google Scholar]
- Hill K. L., Obrtlikova P., Alvarez D. F., King J. A., Keirstead S. A., Allred J. R., Kaufman D. S. (2010). Human embryonic stem cell-derived vascular progenitor cells capable of endothelial and smooth muscle cell function. Exp. Hematol. 38, 246–257 10.1016/j.exphem.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J. C., Zhou T., Lai W. H., Huang Y., Chan Y. C., Li X., Wong N. L., Li Y., Au K. W., Guo D. et al. (2011). Generation of induced pluripotent stem cell lines from 3 distinct laminopathies bearing heterogeneous mutations in lamin A/C. Aging (Albany NY) 3, 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogduijn M. J., Gorjup E., Genever P. G. (2006). Comparative characterization of hair follicle dermal stem cells and bone marrow mesenchymal stem cells. Stem Cells Dev. 15, 49–60 10.1089/scd.2006.15.49 [DOI] [PubMed] [Google Scholar]
- Hunt D. P. J., Jahoda C., Chandran S. (2009). Multipotent skin-derived precursors: from biology to clinical translation. Curr. Opin. Biotechnol. 20, 522–530 10.1016/j.copbio.2009.10.004 [DOI] [PubMed] [Google Scholar]
- Hutchison C. J., Worman H. J. (2004). A-type lamins: guardians of the soma? Nat. Cell Biol. 6, 1062–1067 10.1038/ncb1104-1062 [DOI] [PubMed] [Google Scholar]
- Jahoda C. A. B., Whitehouse C. J., Reynolds A. J., Hole N. (2003). Hair follicle dermal cells differentiate into adipogenic and osteogenic lineages. Exp. Dermatol. 12, 849–859 10.1111/j.0906-6705.2003.00161.x [DOI] [PubMed] [Google Scholar]
- Liu G. H., Barkho B. Z., Ruiz S., Diep D., Qu J., Yang S. L., Panopoulos A. D., Suzuki K., Kurian L., Walsh C. et al. (2011). Recapitulation of premature ageing with iPSCs from Hutchinson-Gilford progeria syndrome. Nature 472, 221–225 10.1038/nature09879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Lutz R. J., Trujillo M. A., Denham K. S., Wenger L., Sinensky M. (1992). Nucleoplasmic localization of prelamin A: implications for prenylation-dependent lamin A assembly into the nuclear lamina. Proc. Natl. Acad. Sci. USA 89, 3000–3004 10.1073/pnas.89.7.3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock D., Gordon L. B., Djabali K. (2006). Hutchinson-Gilford progeria mutant lamin A primarily targets human vascular cells as detected by an anti-Lamin A G608G antibody. Proc. Natl. Acad. Sci. USA 103, 2154–2159 10.1073/pnas.0511133103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintock D., Ratner D., Lokuge M., Owens D. M., Gordon L. B., Collins F. S., Djabali K. (2007). The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS ONE 2, e1269 10.1371/journal.pone.0001269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T., Aoyama T., Nakayama T., Nakamata T., Hosaka T., Nishijo K., Nakamura T., Kiyono T., Toguchida J. (2002). Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 295, 354–361 10.1016/S0006-291X(02)00661-7 [DOI] [PubMed] [Google Scholar]
- Olive M., Harten I., Mitchell R., Beers J. K., Djabali K., Cao K., Erdos M. R., Blair C., Funke B., Smoot L. et al. (2010). Cardiovascular pathology in Hutchinson-Gilford progeria: correlation with the vascular pathology of aging. Arterioscler. Thromb. Vasc. Biol. 30, 2301–2309 10.1161/ATVBAHA.110.209460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradisi M., McClintock D., Boguslavsky R. L., Pedicelli C., Worman H. J., Djabali K. (2005). Dermal fibroblasts in Hutchinson-Gilford progeria syndrome with the lamin A G608G mutation have dysmorphic nuclei and are hypersensitive to heat stress. BMC Cell Biol. 6, 27 10.1186/1471-2121-6-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P., Misteli T. (2006). Lamin A-dependent nuclear defects in human aging. Science 312, 1059–1063 10.1126/science.1127168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaffidi P., Misteli T. (2008). Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing. Nat. Cell Biol. 10, 452–459 10.1038/ncb1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker D. K., Dechat T., Kohlmaier A., Adam S. A., Bozovsky M. R., Erdos M. R., Eriksson M., Goldman A. E., Khuon S., Collins F. S. et al. (2006). Mutant nuclear lamin A leads to progressive alterations of epigenetic control in premature aging. Proc. Natl. Acad. Sci. USA 103, 8703–8708 10.1073/pnas.0602569103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehbens W. E., Delahunt B., Shozawa T., Gilbert-Barness E. (2001). Smooth muscle cell depletion and collagen types in progeric arteries. Cardiovasc. Pathol. 10, 133–136 10.1016/S1054-8807(01)00069-2 [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Roop D. R. (1988). Molecular and cellular biology of intermediate filaments. Annu. Rev. Biochem. 57, 593–625 10.1146/annurev.bi.57.070188.003113 [DOI] [PubMed] [Google Scholar]
- Toma J. G., Akhavan M., Fernandes K. J., Barnabé-Heider F., Sadikot A., Kaplan D. R., Miller F. D. (2001). Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 3, 778–784 10.1038/ncb0901-778 [DOI] [PubMed] [Google Scholar]
- Toma J. G., McKenzie I. A., Bagli D., Miller F. D. (2005). Isolation and characterization of multipotent skin-derived precursors from human skin. Stem Cells 23, 727–737 10.1634/stemcells.2004-0134 [DOI] [PubMed] [Google Scholar]
- Watt F. M., Lo Celso C., Silva-Vargas V. (2006). Epidermal stem cells: an update. Curr. Opin. Genet. Dev. 16, 518–524 10.1016/j.gde.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Worman H. J. (2012). Nuclear lamins and laminopathies. J. Pathol. 226, 316–325 10.1002/path.2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Fang D., Kumar S. M., Li L., Nguyen T. K., Acs G., Herlyn M., Xu X. (2006). Isolation of a novel population of multipotent adult stem cells from human hair follicles. Am. J. Pathol. 168, 1879–1888 10.2353/ajpath.2006.051170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Lian Q., Zhu G., Zhou F., Sui L., Tan C., Mutalif R. A., Navasankari R., Zhang Y., Tse H. F. et al. (2011). A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell 8, 31–45 10.1016/j.stem.2010.12.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.