Summary
Parasitic filarial nematodes that belong to the Onchocercidae family live in mutualism with Wolbachia endosymbionts. We developed whole-mount techniques to follow the segregation patterns of Wolbachia through the somatic and germline lineages of four filarial species. These studies reveal multiple evolutionarily conserved mechanisms that are required for Wolbachia localization to the germline. During the initial embryonic divisions, Wolbachia segregate asymmetrically such that they concentrate in the posteriorly localized P2 blastomere, a precursor to the adult germline and hypodermal lineages. Surprisingly, in the next division they are excluded from the germline precursor lineage. Rather, they preferentially segregate to the C blastomere, a source of posterior hypodermal cells. Localization to the germline is accomplished by a distinct mechanism in which Wolbachia invade first the somatic gonadal cells close to the ovarian distal tip cell, the nematode stem cell niche, from the hypodermis. This tropism is associated with a cortical F-actin disruption, suggesting an active engulfment. Significantly, germline invasion occurs only in females, explaining the lack of Wolbachia in the male germline. Once in the syncytial environment of the ovaries, Wolbachia rely on the rachis to multiply and disperse into the germ cells. The utilization of cell-to-cell invasion for germline colonization may indicate an ancestral mode of horizontal transfer that preceded the acquisition of the mutualism.
Keywords: Wolbachia, Bacteria, Filarial nematodes, Mutualism, Parasite, Symbiosis
Introduction
Filarial parasites cause debilitating diseases such as lymphatic filariasis (caused by Brugia malayi, Brugia timori, and Wuchereria bancrofti) and onchocerciasis (River Blindness, caused by Onchocerca volvulus), with a billion people at risk, and over 120 million infected individuals in tropical areas (Taylor et al., 2005). With the exception of Loa loa, these human parasites harbor the Wolbachia endosymbionts. These alpha-proteobacteria are widely distributed among arthropods, but restricted to the family of Onchocerchidae in the nematodes (Werren et al., 1995; Ferri et al., 2011). In arthropods, the Wolbachia are maternally inherited and have developed an array of strategies based on the manipulation of their host reproduction to spread through populations (Serbus et al., 2008). Wolbachia have been classified in different supergroups, defined by multilocus sequence typing (MLST) based on five conserved and rapidly evolving genes (Baldo and Werren, 2007). Some supergroups are specific to arthropods (A,B,E,H,I,K), others to nematodes (C,D,J), and one supergroup encompasses both phyla (F) (Lo et al., 2002; Casiraghi et al., 2005).
In filarial nematodes, Wolbachia are mutualistic and required for fertility and survival (Hoerauf et al., 2000; Fenn and Blaxter, 2004). Their removal by antibiotic treatments from Brugia malayi or from Onchocerca volvulus leads to extensive apoptosis (Landmann et al., 2011). In addition, the Wolbachia released from the nematodes into the human body trigger an inflammatory reaction that underlies the lymphedema and corneal occlusion associated with these neglected diseases (Taylor et al., 2000; Turner et al., 2006; Debrah et al., 2009; Turner et al., 2009). For these reasons, Wolbachia have become major drug targets, since the current treatments do not kill adult worms (Slatko et al., 2010).
Experimental studies and genome analysis suggest Wolbachia contribute to the biosynthesis of diverse metabolic pathways, among them nucleotides, riboflavin, FAD and heme biosynthesis (Foster et al., 2005; Wu et al., 2009; Strübing et al., 2010). Wolbachia also help the worms to escape the host's immune system, and therefore confer longevity (Hansen et al., 2011). In most adult filarial species harboring Wolbachia, the endosymbionts are present in two sites, in the female gonad, from which they are vertically transmitted to the progeny, and in the hypodermis forming the lateral chords. Hypodermal chords are a site of intense metabolism, providing nutrients to developing germ cells and growing embryos in the female gonads. Chords harbor the highest Wolbachia titer in the worm, and the strong evolutionary conserved tropism of Wolbachia for this tissue suggests participation to the metabolism. Wolbachia removal leads indeed to cytoskeleton defects in the chords, and apoptosis occurs in the gonads in a non-cell autonomous manner (Landmann et al., 2011). It has been recently suggested that the Wolbachia present in the lateral chords initially show a tropism for both the female and the male gonads, but remain only in the adult female reproductive apparatus (Fischer et al., 2011). A thorough charcaterization of the cellular and molecular basis of the Wolbachia transmission to the chords and subsequently to the germline will lead to the identification of potential drug targets.
Here we take advantage of newly developed whole-mount immunofluorescent techniques for localizing Wolbachia in filarial nematodes, and a recent cocladogenesis study of Wolbachia in a wide range of filarial species to identify conserved mechanisms of Wolbachia soma and germline transmission during nematode development (Ferri et al., 2011). We examine Wolbachia transmission in four filarial species, all of which belong to the secernentean class of nematodes. An important feature of this class of nematode is a fixed and almost identical embryonic lineages (Skiba and Schierenberg, 1992; Malakhov, 1994; Dolinski et al., 2001; Lahl et al., 2003). Significantly Caenorhabditis elegans with its defined lineage is a member of this class and provides a framework for the studies described here.
We previously reported the asymmetric transmission of Wolbachia in the B. malayi embryo, whose embryonic development resembles to that of C. elegans (Landmann et al., 2010). In secernentean nematodes, embryogenesis consists of mitotic divisions following a fixed lineage, encompassing gastrulation. Divisions then stop (i.e. at about 550 cells in C. elegans) and morphogenesis is achieved by a dramatic remodeling of the hypodermal epithelium, transforming a ball of cells into a worm-shaped embryo (Sulston et al., 1983). Specifically, we demonstrate that Wolbachia supergroups C and D follow the same evolutionary-conserved pattern of asymmetric segregation during embryogenesis to reach a subset of hypodermal cells prior to morphogenesis. They rely on cell fusion to colonize the lateral chords, before invading the posterior pseudocoelom in both male and female, but enter only the female distal ovary. Wolbachia may rely on engulfment to enter into the somatic gonad, as suggested by the associated cortical F-actin disruption. We then highlight the importance of the ovarian rachis for Wolbachia multiplication and spread into the germline, identifying therefore new cell mechanisms crucial for sustaining the mutualistic association between Wolbachia and its nematode host.
Materials and Methods
Specimens
Brugia malayi specimens were supplied alive and Dirofilaria immitis adults were supplied frozen, by TRS Laboratories (Athens, Georgia), and by the Filariasis Research Reagent Resource Center at the University of Georgia and funded by the National Institutes of Health. Litomosoides sigmondontis specimens were raised in jirds, Meriones unguiculatus, at the Muséum National d'Histoire Naturelle, Paris, France (C.M.). Onchocerca dewittei japonica adults were recovered from a Japanese wild boar in Feb. 2011 in Ôita prefecture, Japan (S.U.). Dirofilaria immitis adult living worms were kindly provided by Prof. Wieslaw Kozek, Univeristy of Puerto Rico. Worms obtained alive were fixed approximately 1 to 3 days after their removal from the host, while worms fixed prior to shipping were typically used 1 to 2 weeks after reception.
Stainings and immunostainings
Procedures for propidium iodide (PI) and phalloidin stainings, as well as immunostainings in embryos, larval and adult stages were performed as described in detail in (Landmann et al., 2010). Briefly, worms were either cut (for immunostainings) or not (for PI and phalloidin stainings), placed in PBST with 3.2% paraformaldehyde for fixation, with an equal volume of heptane, under rotation for 10 minutes. After a brief centrifugation, they were rinsed in PBST for 5 minutes, and treated in RNAse (1 mg/mL) in PBST overnight, before proceeding to immunostainings. Small larval stages were freeze cracked after fixation and before RNAse treatment.
Propidium iodide stains both host and bacterial DNA. We favored propidium iodide since it preferentially stains the bacterial DNA, which appears as bright foci compared to the much larger host nuclei. In addition PI does not have the issue of penetration as do antibodies, allowing whole-mount stainings. The specificity has been established (Landmann et al., 2010), and we show again colocalization of PI foci with an anti-Wolbachia ftsZ antibody and anti-WSP (supplementary material Fig. S4).
Antibodies
A chicken polyclonal anti-WSP antibody was kindly provided by J. Foster, New England Biolabs, and used at a 1:100 dilution to confirm that propidium iodide-stained DNA foci were corresponding to Wolbachia (supplementary material Figs S3, S4).
A rabbit polyclonal anti-Wolbachia ftsZ antibody used at a 1:500 dilution was raised against the following conserved peptide sequence (accession number of Wolbachia ftsZ: CAT19364) and affinity-purified: eelqkyvdtlivipnqnlfrvanekttfsdafkladnvlhigirgvtdlmvmpglinldfadietvmsemgkamigtgeaegedraisaaeaaisnplld.
Microtubule stainings were performed using the monoclonal DM1á antibody (Cell Signaling Technology) at a dilution of 1:100.
Microscopy and Imaging:
Confocal microscope images were captured on an inverted photoscope (DMIRB; Leitz) equipped with a laser confocal imaging system (TCS SP2; Leica) using an HCX PL APO 1.4 NA 63 oil objective (Leica) at room temperature. 3-D tissue reconstructions were performed using the Volocity5 software.
Results
Investigating the transmission of different Wolbachia supergroups at the tissue and cell levels required the use of a reliable technique we could apply in several filarial nematode species. We opted mainly for a combination of fluorescent phalloidin and propidium iodide (PI) throughout this study, revealing the cortical actin, therefore the cell membranes, as well as the total DNA respectively. The identity of the Wolbachia, appearing as DNA foci, was confirmed by specific immunostainings (cf. Material and Methods).
Wolbachia mitotic asymmetric segregation pattern during embryogenesis is conserved across species of Onchocercidae harboring the supergroups C and D
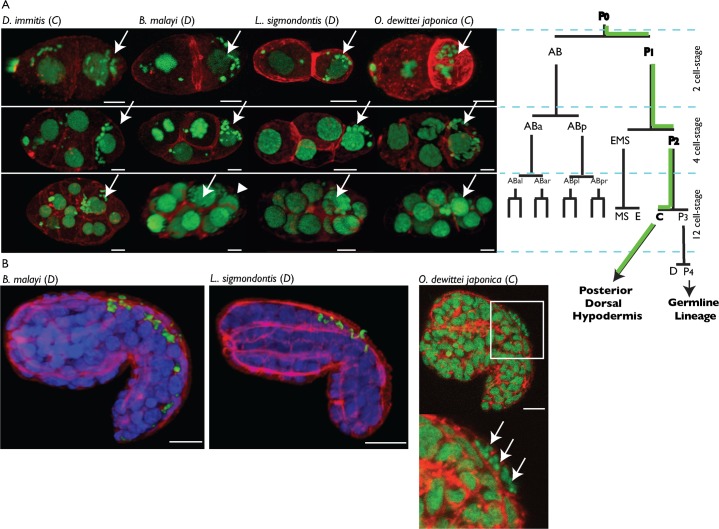
We have previously described the segregation of Wolbachia supergroup D during the embryogenesis of Brugia malayi (Landmann et al., 2010). In the B. malayi zygote, the Wolbachia concentrate to the posterior pole and become asymmetrically inherited by the germline precursor lineage, successively in P1 and P2 (Fig. 1A). In the 12-cell stage B. malayi embryo however, the highest Wolbachia titer is always observed in the C blastomere, with a minor concentration in its sister, the germline precursor P3 (Fig. 1A, arrow and arrowhead respectively; supplementary material Movie 1). This raises the question of the evolutionary conservation of this transmission mechanism based on asymmetric segregation. To address these issues, we decided to look at the behavior of two Wolbachia supergroups during early embryogenesis in four species of Onchocercidae. Brugia malayi and Litomosoides sigmondontis, two species raised in laboratory rodent hosts carrying the Wolbachia supergroup D, while Dirofilaria immitis and Onchocerca dewittei japonica, harboring the supergroup C, were collected from their natural hosts, Canis lupus and Sus scrofa leucomystax (Japanese wild boar) respectively. We observed that in all four species, Wolbachia follow the same segregation pattern, leading to a highest titer in the C blastomere at the 12-cell stage (Fig. 1A). Unlike in B. malayi, the presence of Wolbachia was not always detected in the germline precursor P3 in D. immitis and L. sigmondontis, but sometimes in the C blastomere only. More strikingly, in the O. dewittei japonica 12-cell stage embryo, the Wolbachia segregated exclusively into the C blastomere (Fig. 1A, n>10 embryos; supplementary material Movie 2). In this species the Wolbachia asymmetric segregation during early embryogenesis is perfect, unlike in B. malayi, where we showed the variable presence of Wolbachia in other early blastomeres (Landmann et al., 2010) (supplementary material Movie 1).
Fig. 1. Wolbachia supergroups C and D segregate into the C blastomere and its hypodermal progeny during filarial embryogenesis.
(A) Propidium iodide -green- and phalloidin -red- stainings of 2, 4 and 12-cell stage embryos. For sake of simplicity, the C. elegans lineage nomenclature was used to name the early blastomeres, based on the division pattern. The green path on the lineage tree shows the Wolbachia asymmetric segregation. On confocal images, arrows point to the blastomeres indicated in bold fonts on the lineage tree, and the arrowhead points to the P3 blastomere in the B. malayi 12-cell stage. In all species the AB lineage divides ahead of the posterior lineage. Anterior to the left. (B) Immunostainings of B. malayi and L. sigmondontis embryos in early elongation with an anti-WSP (green), PI -blue- and phalloidin -red-, cf. supplementary material Movies 3, 4. O. dewittei japonica is stained as in (A), arrows point to Wolbachia. Anterior to the right, dorsal part to the top. Scale bar = 5 µm.
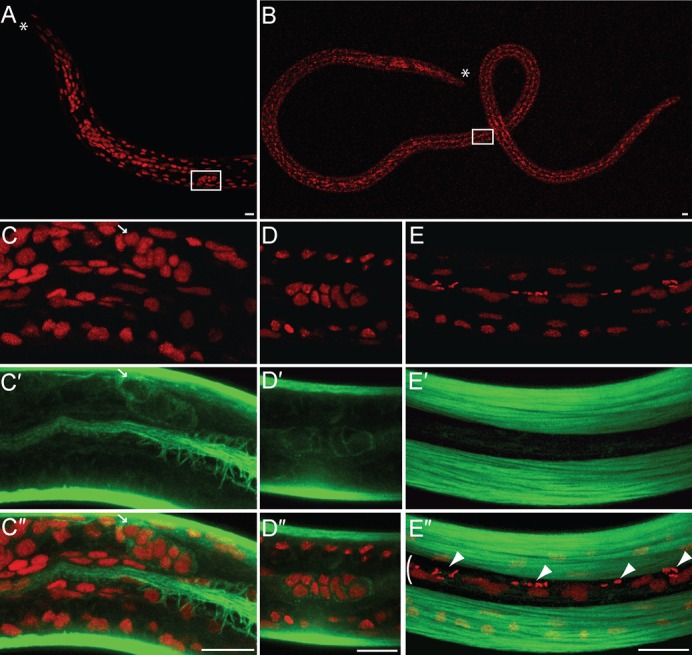
To confirm the identity of the single infected blastomere in the O. dewittei japonica 12-cell stage embryo, we compared the Wolbachia localization after morphogenesis had begun in B. malayi, L. sigmondontis, and O. dewittei japonica (Fig. 1B). When elongation begins, the C hypodermal progeny is located at the posterior dorsal surface of the embryo (Sulston et al., 1983) (Fig. 2A,B). During early embryogenesis in B. malayi, the Wolbachia in blastomeres other than C almost always disappear, in contrast to the hypodermal progeny of C. As a consequence, almost only the posterior dorsal surface contains Wolbachia (supplementary material Movies 3, 4). In O. dewittei japonica, strictly only these cells harbor the endosymbionts. (Fig. 1B).
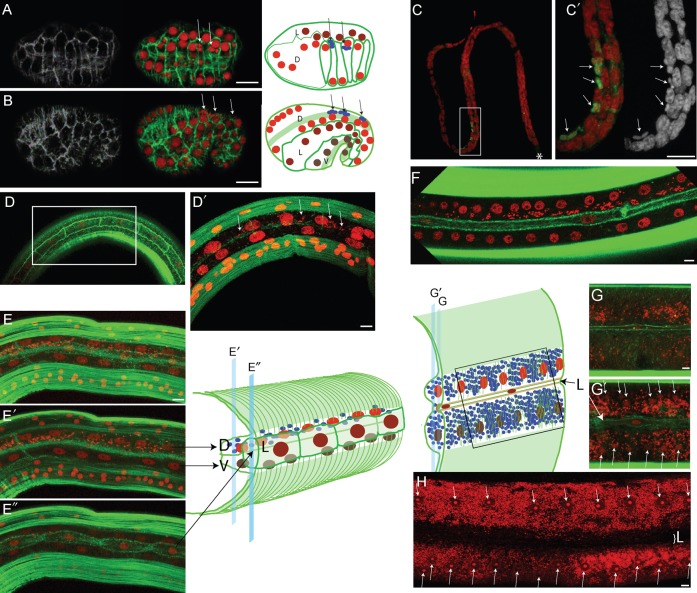
Fig. 2. Hypodermal cell fusion correlates with Wolbachia dispersion in B. malayi lateral chords.
All stages were stained with propidium iodide -red- and phalloidin -green-, except for (C,C′) where the anti-ftsZ is in green, and phalloidin was not used in (C,C′). (A) top view of the embryonic dorsal intercalation. (B) Lateral view of early elongation. In (A,B) arrows point to Wolbachia. In addition to cell membranes, the schematic drawing in (B) shows the underlying muscle precursors -green stripes-, and the Wolbachia -arrows pointing to blue foci-. D = dorsal hypodermis -red nuclei-; L = lateral hypodermis -burgundy nuclei-; V = ventral hypodermis -brown nuclei-. (C) Microfilaria stained with ftsZ, the star indicating the anterior. (C′) is a magnification of (C). (D,D′) Posterior lateral chord of a L3 at 3 dpi showing the hypodermal circumferential actin bundles reaching the unfused lateral hypodermal cells in (D), and (D′) the dorsal and ventral hypodermal syncytia underneath the lateral cells, with few Wolbachia in the dorsal syncytium (arrows). (E) Confocal merge of a posterior lateral chord in a L3 at 7 dpi, separating muscle quadrants. (E′) deep view showing the dorsal and ventral syncytia, with Wolbachia populating the dorsal hypodermis. (E″) surface view showing the yet unfused lateral hypodermis. (F) Merge of surface sections of a 30 dpi immature worm, showing the fused lateral syncytium, while the Wolbachia are still confined in the dorsal syncytium. (G,G′) section of an adult female lateral chord, showing the Wolbachia in the basal compartment (G), and a deeper view showing the ventral/dorsal syncytium chord with nuclei (arrows) surrounded by numerous Wolbachia (G′). The thin lateral hypodermal syncytium (“L”) does not fuse with the ventral/dorsal syncytium. Positions and depth of the G and G′ confocal images are indicated on the schematic drawing. (H) Adult female lateral chord, arrows point to the chord nuclei, “L” indicates the position of the lateral hypodermal syncytium. Note that in adult, the Wolbachia can occupy most of the lateral chord cytoplasm. Scale bar = 5 µm.
The comparison of Wolbachia distribution during early embryogenesis in several filarial species shows that: i. Wolbachia supergroups C and D rely on similar evolutionary-conserved mechanisms to segregate into embryonic hypodermal cells derived from the C blastomere, and ii. Wolbachia presence in B. malayi P3 and P4 germline precursors is due to imperfect asymmetric segregation. Taken together, these data show that in both supergroups, Wolbachia are not maintained in the embryonic germline precursors, but are inherited by blastomeres destined for a hypodermal fate.
The hypodermal cells derived from C are the source of all Wolbachia in larval and adult syncytial chords
We focused on the laboratory strain B. malayi to follow the Wolbachia invasion of hypodermal tissues in embryonic, larval and adult stages. In secernentean nematodes, such as B. malayi and C. elegans, embryonic morphogenesis is driven through contractions of hypodermal circumferential actin bundles leading to epithelial cell shape changes (Zhang et al., 2010). Prior to embryonic morphogenesis, the major hypodermis is composed of two rows of dorsal cells, separated from ventral hypodermal cells on either side of the embryo by lateral hypodermal cells. The posterior left and right dorsal hypodermal cells derived from C, that contain Wolbachia, intercalate in a process similar to that in C. elegans (Fig. 2A) (Chisholm and Hardin, 2005). When morphogenesis occurs soon after, the Wolbachia remain exclusively located in the apical compartment of the posterior dorsal hypodermis, above the nuclei and under the actin bundles (Fig. 2B, arrows, n>100).
Once morphogenesis is completed, a motile prelarval stage called microfilaria (mf) is released from the worm's uterus into the vertebrate host. In mf, nuclei density is very high (Fig. 2C), and because nuclei are tightly surrounded by actin-rich muscle quadrants, phalloidin staining does not allow a good visualization of hypodermal cortical actin (data not shown). We were therefore unable to follow the Wolbachia in the microfilaria hypodermal cells. We therefore chose to stain the Wolbachia in mf with an anti-ftsZ (Fig. 2C,C′). FtsZ concentrates at the septum when the bacterium divides, but is otherwise localized around the bacterial DNA. In mf, the bacteria are always located from the anterior ½ to the ¾ of the worm's length (n>200), where hypodermal cells derived from the C embryonic blastomere are expected to be found, based on C. elegans development (Sulston et al., 1983). We detected at most a few dozen of Wolbachia, and in addition Wolbachia localize identically in mf of the two other species we observed, L. sigmondontis and D. immitis (data not shown).
Multiplication of Wolbachia follows hypodermal cell fusion
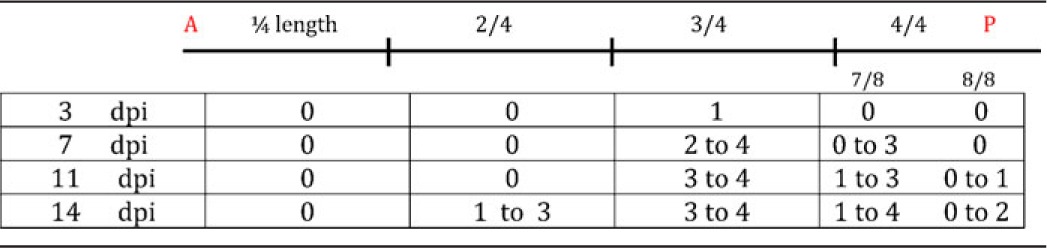
To establish the onset of Wolbachia proliferation and population of the hypodermal tissues, we selected different larval developmental time points. After blood-meal infection of the mosquitos, the mf develop to second-stage (L2) and then third-stage larvae (L3). We did not observe a significant increase in Wolbachia number or distribution during this phase (data not shown). Upon mosquito biting, the L3 larvae penetrate in the vertebrate host. We next scored the Wolbachia density at different days post-vertebrate host infection (dpi), along the larvae antero-posterior axis (Table 1). A massive Wolbachia multiplication takes place between 3 and 7 dpi, and correlates with a spread along the A-P axis, in the hypodermis (n>10 for each dpi).
Table 1. Qualitative scoring of Wolbachia density in L3 and L4 B. malayi larvae.
L3 (at 3, 7 and 11 days post-infection -dpi-) and L4 (at 14 dpi) were stained with propidium iodide and phalloidin, the Wolbachia localization along the A-P axis was established by confocal microscopy, and their density, established as propidium iodide foci, was scored into arbitrary categories ranging from 0 to 4 as follows: 0 = no Wolbachia; 1 = few, scattered Wolbachia; 2 = numerous Wolbachia; 3 = very numerous (i.e. ∼100(s) around each nucleus), 4 = the cytoplasm is filled with Wolbachia.
To better understand the relationship between the Wolbachia multiplication and the lateral chords morphogenesis, we followed the major changes during the worm hypodermis development. When embryonic morphogenesis begins, three rows of hypodermal cells are present on either side of the embryo, such as in C. elegans (Fig. 2B) (Zhang et al., 2010). One peculiarity of filarial nematodes is that the hypodermal dorsal and ventral nuclei migrate towards and below the lateral hypodermis (Fig. 2D,D′ at 3dpi, Fig. 2E–E″ at 7 dpi), to create in larval stages thick lateral chords isolating two wide submedian muscle quadrants on each side. As in C. elegans, cell fusion regulates the formation of mature hypodermis (Alper and Podbilewicz, 2008). Dorsal cells on one part and ventral cells on the other part fuse to create two syncytia, located under the lateral hypodermis in B. malayi. We observed that during larval development, the lateral hypodermal cells divide and provide additional nuclei to the dorsal syncytium by fusion as in C. elegans (data not shown). These two syncytia are clearly visible in L3 at 3 dpi before the Wolbachia density increases (Fig. 2D′, compare with the dorsal syncytium at 7 dpi in Fig. 2E′). Wolbachia colonize first the dorsal syncytium of the lateral chords (Fig. 2E′). In 30 dpi young adults, the very elongated and thin lateral cells have fused to create their own syncytium above the secretory-excretory canal, along the equator (Fig. 2F). In mature adults, the apical surface of the dorsal and ventral rows increase, creating larger lateral chords. The lateral syncytium is embedded in the fused “dorsoventral” syncytium, again in a manner similar to C. elegans (compare Fig. 2E to Fig. 2E″, with Fig. 2G,G′, and with (Alper and Podbilewicz, 2008)). We previously described that in some adults, half of a chord is Wolbachia-infected, very likely to be the dorsal part (Landmann et al., 2010). The thinness of the hypodermis under the lateral syncytium (similar to what is observed above the muscle quadrants) may act as a barrier to Wolbachia dispersion, which accumulate basally around the dorsal and ventral nuclei and are excluded from the equatorial line (Fig. 2H). Collectively these findings indicate that all the Wolbachia originate from the embryonic C blastomere's hypodermal progeny, and that hypodermal cell fusion precedes Wolbachia multiplication and dispersion in the lateral chords, first in the dorsal rows in the posterior of the larva, and second along the entire chords.
There is no Wolbachia in the B. malayi gonadal primordium in L3 larvae
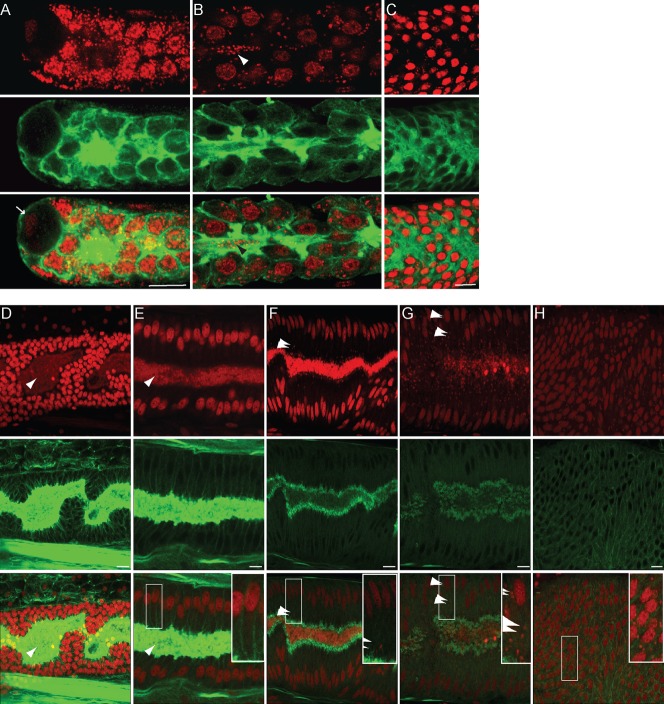
Wolbachia are mutualistic in filarial nematodes, and their presence in the female germline is mandatory to ensure their transmission and the survival of the progeny (Landmann et al., 2011). In contrast, the adult male germline is devoid of Wolbachia (Kozek, 1977; Landmann et al., 2010). To understand how and when the endosymbionts colonize the female, but not the male, germline, we followed the development of the gonad in B. malayi larval stages and young adults, and confirmed the critical observations in L. sigmondontis.
While it is very difficult to locate the gonadal primordium (GP) in microfilariae without a specific germline marker due to the nuclei density, it is possible to identify the GP in L3 larval stages taken from mosquitoes about 14 days after their mf-infected blood meal. Although the precise nature of these cells is not yet established, the GP must contain primordial germ cells, surrounding somatic gonad precursors, and a leading tip cell similar to the C. elegans distal tip cell, known to be critical for proliferation and migration of the GP (Kimble and Crittenden, 2005; Byrd and Kimble, 2009). The developing germ cells are flanked by somatic gonad precursors and their basal membrane, and in both sexes the GP appears as a bag of 10 cells in L3 larvae from mosquitoes. In these larvae the GP has already migrated toward the anterior in the female, where it reaches the junction between the developing oesophagus and intestine (Fig. 3A,C–C″), while it is located in the male mid-body (Fig. 3B,D–D″) (Bain, 1972). The GP contacts the body wall only in female, where the gonad anchor cell will induce the vulva differentiation from a group of competent hypodermal cells during the remaining larval development (supplementary material Fig. S1). Based on DNA and actin staining to localize the GPs, none of the observed L3 GPs ever contained any Wolbachia (n>100, Fig. 3C–C″,D–D″; supplementary material Movie 5). In L3 larvae, the apical part of the lateral chords embedded between wide muscle quadrants is still narrow, while these chords run deep toward the central axis of the worm. Most if not all of the Wolbachia are located in the posterior half of the larvae, strictly in the dorsal row of lateral hypodermal chords (Fig. 3E–E″).
Fig. 3. The Wolbachia are absent from the gonadal primordium in B. malayi male and female L3 larvae.
B. malayi L3 larvae stained with PI -red- and phalloidin -green-. (A) anterior part of a female L3 larva and (B) male L3 larva, all obtained from mosquitoes 14 days after their mf-infected blood meal. The gonadal primordium (GP, white box) is anterior in the female and in the mid-body in the male. The anterior is marked by an asterisk. (C–C″) female GP (arrow pointing toward the anchor cell) located at the junction between the developing oesophagus and intestine, and apposed to the hypodermis. (D–D″) male GP. (E–E″) surface detail in the posterior half of a L3 larva, where the Wolbachia (arrowheads) localize in a lateral hypodermal chord (bracket). All the images are merges of confocal sections, the anterior of the worms being oriented to the left. Scale bar = 10 µm.
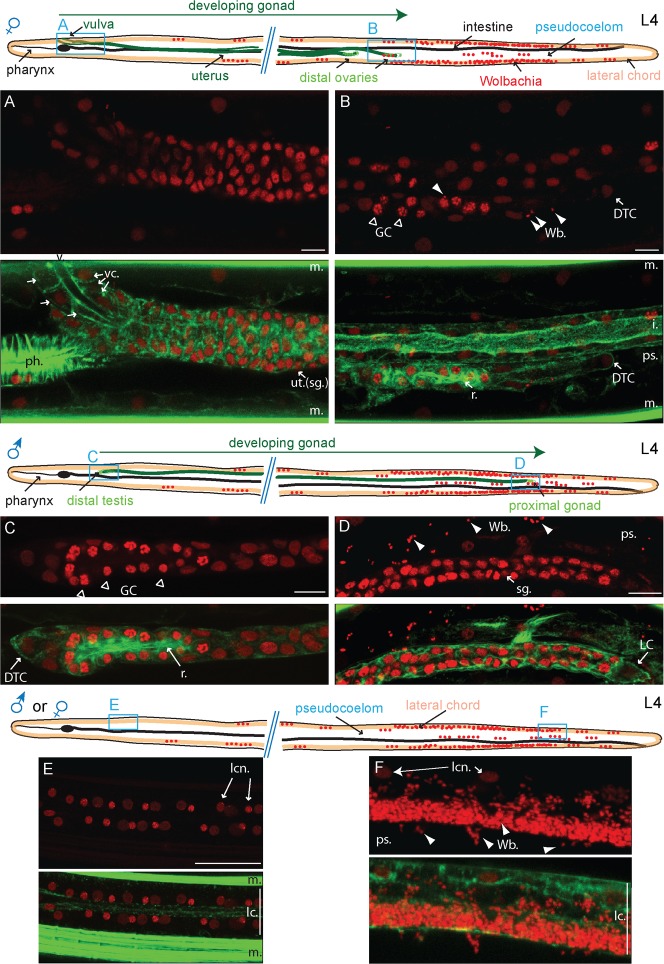
Wolbachia invade only the female distal gonad from lateral chords, in L4 larval stages
The female GP is anchored to the developing vulva in the anterior (Fig. 4A). The distal part has split into two ovaries migrating towards the posterior, led by a distal tip cell capping each ovary (Fig. 4B). The few germ cells located in the distal ovaries are all connected by a central actin-rich structure called the rachis, forming a syncytium (Fig. 4B). At this 8 to 10 dpi stage begins the germ cell mitotic proliferation. In contrast to the female where the distal gonad migrates toward the posterior, the distal testis is already anterior (Fig. 4C), and in the male the proximal somatic gonad elongates toward the posterior, led by a “linker cell” (Fig. 4D). We never observed any Wolbachia in the B. malayi male gonad, at any level, from the distal to the proximal end (Fig. 4C,D, n>50 males). In the B. malayi female, very few Wolbachia are present in the migrating distal ovaries. More specifically, they are observed in the distal tip cell or in the closely associated somatic cells forming the gonad (Fig. 4B, n>50 females). This suggests that the L4 larval stage (at 8 to 10 dpi) is the earliest stage during which the Wolbachia appear in the female gonad. We observed an identical behavior in L. sigmondontis L4 larvae (data not shown).
Fig. 4. The Wolbachia start invading the female ovary distal tips from the adjacent posterior lateral chords in L4 larvae, but are absent from the L4 male gonad.
B. malayi L4 larvae (8 to 10 dpi) stained with propidium iodide -red- and phalloidin -green-. The localization of the images taken is indicated on the schematic drawings. (A,B) anterior -proximal- part, and posterior -distal- part of the female gonad respectively. (C,D) anterior -distal- part and posterior -proximal- part of the male gonad respectively. (E) Top down view of a typical anterior lateral chord. (F) Longitudinal view of a typical posterior lateral chord. DTC, distal tip cell; GC, germ cells; i., intestine; sg., somatic gonad; lc., lateral chord; lcn, lateral chord nuclei; LC, linker cell; m., muscle; ps., pseudocoelom; r., rachis; ut., uterus; v., vulva; vc, vulval cells; Wb, Wolbachia; All images are oriented with the L4 larvae anterior part to the left. Scale bar = 20 µm.
In 8 to 10 dpi L4 larvae, the Wolbachia are found in the posterior ½ of the worm's length in the lateral chords, and are almost or completely absent from the anterior of the worm (Fig. 4E). Using whole mount larvae, we were able to observe the pseudocoelomic fluid in which the organs develop, and found free Wolbachia almost exclusively in the posterior (Fig. 4D). There, the Wolbachia concentrate in the basal compartment of the lateral chords, and cross the chords through the basal lamina to access the pseudocoelom in both male and female (Fig. 4F).
Taken together, these data show that the endosymbionts cross the lateral chords basal membrane to enter the pseudocoelom in the posterior of male and female L4 larvae, but the gonad tropism is female-specific.
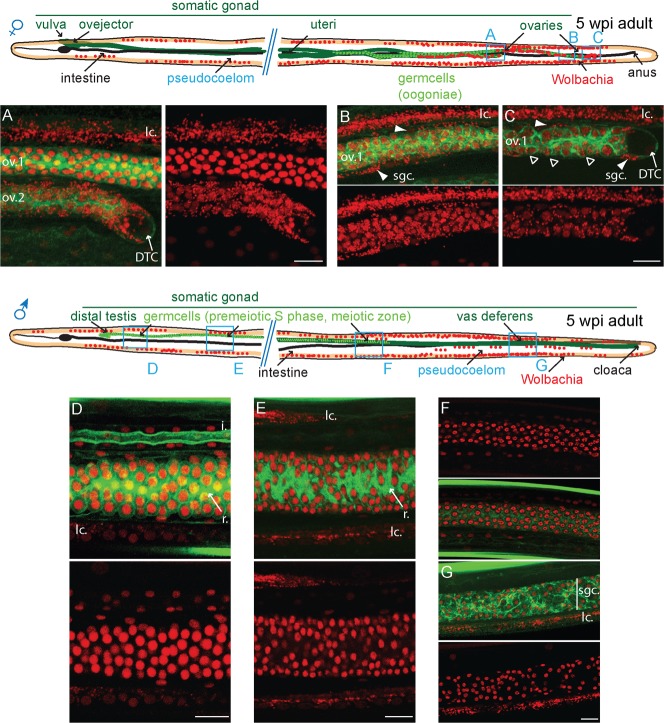
Only the distal somatic ovary is favorable to Wolbachia multiplication in young adult gonads
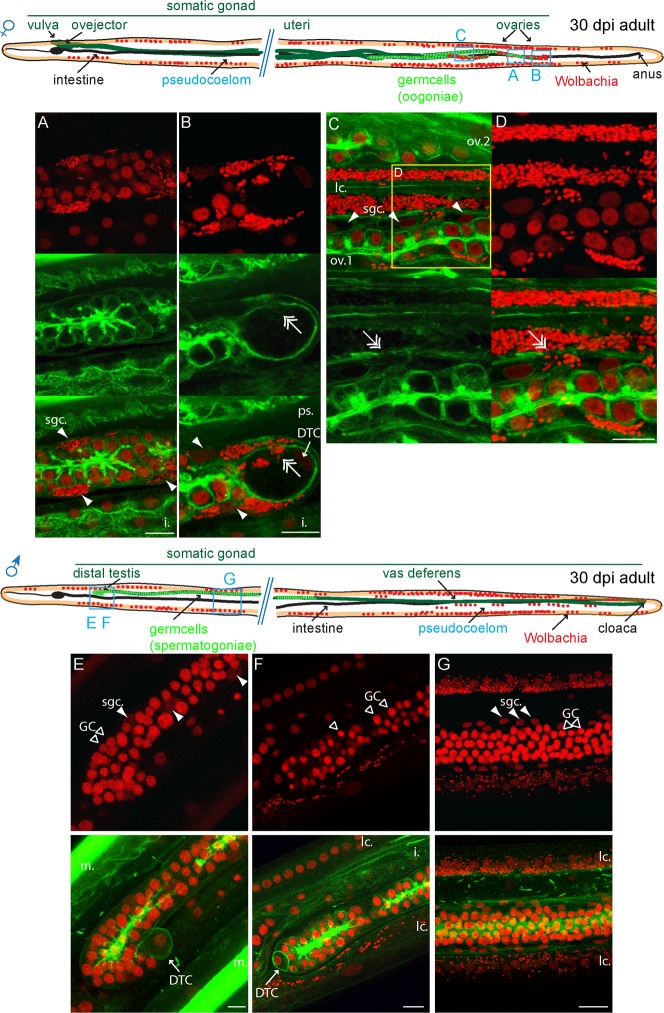
We then followed how Wolbachia colonize the germline in young adult (Fig. 5). At 30 dpi, the ovaries are not yet coiled, but the distal tips are in the posterior of the female. The Wolbachia are present in large amount in the somatic gonad, the epithelium surrounding the germ cells (Fig. 5A,B; supplementary material Movie 6). This indicates that they first replicate in these cells before infecting the germ line. We observed only their presence in the vicinity of the distal tip, at no more than 300 µm from it (n>30, Fig. 5C). In addition, the entry into somatic cells, for example in the somatic gonad epithelium (Fig. 5D) or into the distal tip cell from the somatic gonad epithelium (Fig. 5B) is associated with a local cortical actin weakening (Fig. 5B,D, double arrows; supplementary material Fig. S2). At this stage, the vast majority of germ cells and the connecting rachis are still devoid of bacteria. In 30 dpi males, the distal testis located in the anterior has often reached its adult morphology. The male distal tip cell makes a U-shaped turn (Fig. 5E). Wolbachia are often present in the adjacent anterior lateral chords, but never in the distal testis (n>30, Fig. 5F). We could not detect any bacteria in the male gonad, even in the proximal gonad, in spite of numerous Wolbachia in the surrounding lateral chords (Fig. 5G; supplementary material Movie 7).
Fig. 5. Cortical actin disruption upon Wolbachia entry into the female somatic gonad.
B. malayi 30 dpi young adults stained with propidium iodide -red- and phalloidin -green-. The localization of the confocal images is indicated on the schematic drawings. (A–D) female. (A,B) tip of the most distal ovary. (C) Confocal section at the level of the first ovary, at about 300 µm from the distal ovary tip, and at the surface of the second ovary. The two ovaries are separated by the basal part of a lateral chord. Arrowheads point to the sgc, the somatic gonad cells , double arrows to Wolbachia-associated cortical actin disruption. The split and merged D panels are an enlargement from, and indicated on (C). DTC, distal tip cell; i, intestine; lc., lateral chord; ov.1, most distal ovary; ov.2, most proximal ovary. (E–G) male. Open arrowheads point to the GC, germ cells. m, muscles. Scale bar = 10 µm.
Wolbachia presence has been previously reported to be transient in the B. malayi male gonad, by in situ hybridization techniques, at 5 weeks post infection (Fischer et al., 2011). We therefore extended our observation to this stage in both female and male (n>50, Fig. 6). In 5wpi female, the Wolbachia are no longer restricted to the somatic cells of the distal gonad, but are found in all the distal germ cells (Fig. 6A–C). In contrast, we never found any Wolbachia within the male gonad by using a direct propidium iodide detection at any level observed, neither in the germ cells, before or during meiosis starting at this stage (Fig. 6D–F) nor in the proximal somatic gonad, the vas deferens (Fig. 6G; supplementary material Movies 8–10).
Fig. 6. Wolbachia tropism for the somatic distal gonad is female-specific.
B. malayi 5wpi adults stained with propidium iodide -red- and phalloidin -green-. The localization of the confocal images is indicated on the schematic drawings. (A–C) female. (A) distal tip of the second ovary “ov.2”, located at 700 µm of the most posterior ovary “ov.1”. (B,C) posterior distal ovary. Arrowheads point to infected somatic gonad epithelial cells “sgc”. In (C) open arrowheads point to infected germline. (D–G) male. (D) zone of mitotic proliferation, (E) premeiotic S phase zone, (F) meiotic zone, (G) vas deferens. I, intestine; lc., lateral chords; r, rachis. Scale bar = 10 µm.
These observations support a Wolbachia tropism for the gonad that is specific to the female. In the 5wpi female, the bacteria are almost never detected beyond ∼700 µm from the distal tip of the ovary (Fig. 6A, “ovary NO. 1”; supplementary material Movie 11). Unlike in the distal tip where they first multiply in the surrounding somatic gonad, the few Wolbachia leading the invasion of the germ cells toward the proximal end of the ovary are detected in the center of the gonad, often in actin-rich areas devoid of nuclei, the rachis (supplementary material Movie 11). This raised the question of the Wolbachia colonization of the germ cells in the mature ovary.
The rachis, Wolbachia route to the proximal germline
In secernentean nematodes, proliferating and maturing germline nuclei are organized as a syncytium. Nuclei are surrounded by a membrane forming a basal peduncle, connected to the rachis by a pore. Until cellularization takes place, all the germline nuclei share the same cytoplasm. To optimize Wolbachia transmission to all germ cells, it is tempting to think that they populate the oogoniae before cellularization. We observed ovaries of several species of adult filarial nematodes, and found indeed Wolbachia present in the syncytial gonad (Fig. 7). We found however two modes of transmission. In B. malayi (Fig. 7A), as well as in D. immitis and O. dewittei japonica (supplementary material Fig. S3), Wolbachia are present around all the germline nuclei in the syncytial ovary. We also found in these species numerous amount of Wolbachia in the rachis, suggesting that the rachis either favors their multiplication, and/or serve as a route to infect the proximal syncytium in young adults and newly born germline nuclei in older adults (Fig. 7B, arrowhead). While the rachis is always enriched in filamentous actin, the peduncles are enriched in microtubules that surround the nuclei, as observed in B. malayi (supplementary material Fig. S4).
Fig. 7. Two modes of population in the syncytial ovary.
Longitudinal confoncal sections of B. malayi and L. sigmondontis adult ovaries stained with propidium iodide -red- and phalloidin -green-. (A–C) B. malayi female ovary. (A) distal tip, the arrow points to the DTC, (B) more proximal ovary, the arrowheads point to the Wolbachia in the rachis, (C) cellularization zone. (D–H) L. sigmondontis ovary, from distal to proximal ends. Arrowheads point to the Wolbachia. (D–G) syncytial ovary, (H) cellularization. Scale bar = 5 µm.
Instead of being sinuous (i.e. like in B. malayi), and extremely branched (i.e. in D. immitis), L. sigmondontis rachis is a simple tube around which peduncles emanate as a rosette (Fig. 7D–G). In contrast to the previous species, all the Wolbachia of the distal syncytial ovary are found in the rachis, as revealed with the propidium iodide staining (Fig. 7D,E). We confirmed that the propidium iodide staining, marking the entire distal rachis, corresponds to a massive Wolbachia population, using a specific anti-WSP antibody (supplementary material Fig. S5). Prior to cellularization, the cytoplasmic peduncles containing the germline nuclei elongate. Progressively, Wolbachia leave the rachis and disperse into the rosette (Fig. 7F,G). After cellularization, all the germcells contain Wolbachia. These observations stress the crucial role of the rachis in the Wolbachia population of the oocytes, therefore for the transmission to the progeny.
Discussion
To define transmission mechanisms of Wolbachia during filarial nematode development, we took advantage of newly developed whole-mount immunofluorescent techniques as a complementary approach to the traditional histological methods commonly used in filarial nematode research (Landmann et al., 2010). An accurate robust transmission of Wolbachia to the female germline is critical in filarial nematodes because they rely on the presence of the endosymbionts for their growth, development, fertility and long term survival (Slatko et al., 2010; Landmann et al., 2011; Tamarozzi et al., 2011). We previously described the asymmetric segregation of Wolbachia during Brugia malayi early embryogenesis to the posterior blastomeres, which produce posterior hypodermal cells as well as the germline lineage (Landmann et al., 2010). However a lack of markers prevented lineage tracing of Wolbachia through late embryogenesis and into the microfilaria. The fixed secernentean embryonic lineage and the presence of Wolbachia in the germline precursors led us to postulate that localization of Wolbachia to the adult ovary may occur through segregation with the germline lineage during nematode development. However recent work in B. malayi has produced convincing data that Wolbachia do not fate map to the germline through their mitotic segregation pattern, and germline localization requires invasion from neighboring somatic cells (Fischer et al., 2011).
To explore this issue further we examined the transmission of different Wolbachia supergroups in multiple nematode species. We find that Wolbachia, regardless of their supergroup or the filarial host, rely on multiple evolutionarily conserved mechanisms to reach the ovary. During embryogenesis through the motile pre-larval microfilarial stage, Wolbachia distribution in the nematode is determined entirely through asymmetric segregation patterns during host mitosis. In all nematode species examined, this leads to an asymmetric concentration of Wolbachia in the C founder blastomere. As development progresses, Wolbachia concentrate to the C hypodermal progeny. Segregation with the C lineage results in Wolbachia populating the posterior lateral chords in L3 and L4 larval stages. Once the Wolbachia titer becomes high in the posterior lateral chords, the bacteria cross the basal membrane of the chords, diffuse in the posterior pseudocoelom in both males and females, but invade only the distal gonad of female worms, by crossing the cell membrane of somatic gonad epithelium, as previously observed using electron microscopy (Taylor and Hoerauf, 1999).
In the embryo, Wolbachia rely on mitotic division to asymmetrically segregate to hypodermal blastomeres
The transmission to hypodermal blastomeres by mitotic division is stereotypical in the four species we studied. Based on lessons from C. elegans, another secernentean nematode with an identical early lineage, it is likely that the Wolbachia integrate, directly or indirectly, posterior polarity cues or their downstream effectors to segregate into the P1 and P2 blastomeres, and that these cues are conserved across the Onchocercidae family of filarial parasites (for a review on embryonic polarity establishment in C. elegans, cf. (Munro and Bowerman, 2009)). Surprisingly when P2 divides, they no longer segregate in the posterior P3 germline precursor, but rather concentrate into the more dorsal-anterior C founder cell, suggesting that Wolbachia localization is likely to not depend on germline determinants.
Hypodermal cell fusion precedes the spread of Wolbachia in lateral chords
We find a major increase of the bacterial titer in larval chords between 3 and 7 dpi (cf. Table 1), consistent with previous quantitative PCR data (McGarry et al., 2004). In the larval lateral chords, Wolbachia first spread along the anterior-posterior axis. It is only at later stages that the bacteria multiply to fill most of the hypodermal syncytium of the lateral chords. Cell fusion therefore precedes Wolbachia dispersion and multiplication in the hypodermis. Mechanisms leading to formation of hypodermal syncytia have been characterized in C. elegans (Alper and Podbilewicz, 2008). Disturbing cell fusion may prevent Wolbachia multiplication in human filarial parasites.
In the L4 larva, Wolbachia invade the ovary from the lateral chords, crossing cell membranes
We found that Wolbachia colonize the distal tip of the gonad from adjacent chords. This was recently reported (Fischer et al., 2011), but in contrast to the conclusions of this study based on in situ hybridized sections, we never detected Wolbachia in the male germline at any developmental stages. Our data, based on direct detection of bacterial DNA strongly argue for a strict ovarian tropism. An anatomical peculiarity of filarial nematodes starting in the fourth larval stage is the different localization of the distal gonad in male and female worms. In the male, the distal testis resides in the anterior, while in the female the two ovaries have extended in the posterior. The highest density of Wolbachia occurs in the posterior hypodermis adjacent to the point where Wolbachia populate the female gonad. This posterior high density may be a factor linked to their release into the posterior pseudocoelom only, occuring in both males and females. In the female gonad, the Wolbachia enter only the distal tip. We find that the gonad invasion coincides with the beginning of germline mitotic proliferation around 8 dpi, a process under the control of the distal tip cell (DTC) in nematodes. This cell acts as a stem cell niche, signaling a germline stem cell-like pool to divide via a notch pathway (Hubbard, 2007). It is possible that the surrounding somatic gonad epithelium may also be influenced by the DTC, and express in response specific cell surface receptors, absent from the male gonad and from the proximal female ovary, and used by the Wolbachia for docking. It was demonstrated in Drosophila that newly introduced Wolbachia show a tropism for the somatic stem cell niche of the ovary, before invading the germline (Frydman et al., 2006). Similarly, we found in B. malayi a bacterial proliferation in the distal somatic gonad, suggesting again evolutionarily conserved mechanism for the gonad tropism.
Two mechanisms of cell-to-cell transmission entry can be envisaged. Local membrane lysis, by secretion of a serine protease as previously suggested, could be used by Wolbachia to cross membranes (Fischer et al., 2011). Alternatively, manipulation of the host cytoskeleton and trafficking could be envisaged. For instance, internalization after engulfment has been described for many intracellular pathogens. After engulfment, bacteria transiently disassemble the actin network to pass this otherwise impermeable physical barrier. This step is crucial for cell invasion (Carabeo, 2011). We indeed observe a local cortical actin disruption associated with internalized Wolbachia, suggesting that they manipulate the actin dynamics at the cell cortex. Future studies will be needed to clarify whether they secrete actin depolymerization factor(s), or if they increase the activity of endogenous depolymerization factors, as Salmonella do (Dai et al., 2004).
A mode of transmission unique to filarial nematodes
Despite the obligate mutualism between Wolbachia and their filarial hosts, the vertical transmission to the female to its progeny follows a circuitous route. First segregating with and then away from the germline lineage, requiring cell invasion mechanisms to become established in the germline. In insects such as Drosophila, Wolbachia germline localization requires a direct concentration at the embryonic pole cells, the germline precursors. However recent studies demonstrate that Wolbachia are also capable of reaching the germline through a distinct somatic route requiring cell-cell invasion. The somatic stem-cell niche tropism in flies could reflect an ancient and conserved mechanism of germline invasion by horizontal transfer that may reinforce the direct transmission through the germline precursors (Frydman et al., 2006). Similarly, the presence of Wolbachia in hypodermal cells of filarial nematodes may reflect an ancestral somatic tissue-preference after horizontal transfer in the arthropod vector or the vertebrate host.
In a second time, the successful transfer to the progeny could have allowed the development of diverse mutualisms across Onchocercidae carrying the Wolbachia supergroups C and D. Support for a common ancestor's transmission pattern in different Onchocercidae, rather than a similar parallel evolution of infection mechanisms, comes from the absence of recombination in Wolbachia supergroups C and D, carried by different species of nematodes, unlike in arthropod specimens, hosting sometimes several supergroups (Casiraghi et al., 2001; Casiraghi et al., 2003; Bordenstein et al., 2009).
In addition to the hypodermal fate and the distal somatic gonad cells, we also demonstrate that the rachis is a favorable environment for bacterial replication. In insects, Wolbachia are present in Golgi-related host vesicles (Cho et al., 2011).
Wolbachia-enriched cells must support their high membrane demand. While the C. elegans rachis has been described to be enriched in Golgi apparatus (Abi-Rached and Brun, 1975), D. immitis rachis seems devoid of such organelles, rather located around the germline nuclei (Lee, 1975). Differences among nematodes species may account for the different localizations of Wolbachia we observed in distal ovaries. We noticed microtubules running between the rachis and the peduncles, as described in Ascaris (Foor, 1967), and the Wolbachia may use a microtubule cytoskeleton to reach the oogoniae, as they do in Drosophila (Ferree et al., 2005).
This study suggests a number of new cellular processes, which could deliver potential drug-targets against filarial diseases. Future efforts will focus on the mechanisms of recognition of the distal somatic gonad cell surface leading to the ovarian population, and on the identification of factors required for Wolbachia replication.
Supplementary Material
Acknowledgments
This work was supported by the Sinsheimer Laboratories Insurance fund (433317-09577), and by the Bill and Melinda Gates Foundation for financial support of the AWOL consortium through a grant awarded to the Liverpool School Of Tropical Medicine.
Footnotes
Competing interests: The authors declare that there are no competing interests. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Abi-Rached M., Brun J. (1975). Etude ultrastructurale des relations entre ovocytes et rachis au cours de l’ovogenese du nematode Caenorhabditis elegans. Nematologica 21, 151–162 10.1163/187529275X00518 [DOI] [Google Scholar]
- Alper S., Podbilewicz B. (2008). Cell fusion in Caenorhabditis elegans. Methods Mol. Biol. 475, 53–74 10.1007/978-1-59745-250-2_4 [DOI] [PubMed] [Google Scholar]
- Bain O. (1972). Recherches sur la morphogenèse des Filaires chez l’hôte intermédiaire. Ann. Parasitol. Hum. Comp. 47, 251–303. [Google Scholar]
- Baldo L., Werren J. H. (2007). Revisiting Wolbachia supergroup typing based on WSP: spurious lineages and discordance with MLST. Curr. Microbiol. 55, 81–87 10.1007/s00284-007-0055-8 [DOI] [PubMed] [Google Scholar]
- Bordenstein S. R., Paraskevopoulos C., Dunning Hotopp J. C., Sapountzis P., Lo N., Bandi C., Tettelin H., Werren J. H., Bourtzis K. (2009). Parasitism and mutualism in Wolbachia: what the phylogenomic trees can and cannot say. Mol. Biol. Evol. 26, 231–241 10.1093/molbev/msn243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd D. T., Kimble J. (2009). Scratching the niche that controls Caenorhabditis elegans germline stem cells. Semin. Cell Dev. Biol. 20, 1107–1113 10.1016/j.semcdb.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carabeo R. (2011). Bacterial subversion of host actin dynamics at the plasma membrane. Cell. Microbiol. 13, 1460–1469 10.1111/j.1462-5822.2011.01651.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casiraghi M., Anderson T. J., Bandi C., Bazzocchi C., Genchi C. (2001). A phylogenetic analysis of filarial nematodes: comparison with the phylogeny of Wolbachia endosymbionts. Parasitology 122, 93–103 10.1017/S0031182000007149 [DOI] [PubMed] [Google Scholar]
- Casiraghi M., Werren J. H., Bazzocchi C., Biserni A., Bandi C. (2003). dnaA gene sequences from Wolbachia pipientis support subdivision into supergroups and provide no evidence for recombination in the lineages infecting nematodes. Parassitologia 45, 13–18. [PubMed] [Google Scholar]
- Casiraghi M., Bordenstein S. R., Baldo L., Lo N., Beninati T., Wernegreen J. J., Werren J. H., Bandi C. (2005). Phylogeny of Wolbachia pipientis based on gltA, groEL and ftsZ gene sequences: clustering of arthropod and nematode symbionts in the F supergroup, and evidence for further diversity in the Wolbachia tree. Microbiology 151, 4015–4022 10.1099/mic.0.28313-0 [DOI] [PubMed] [Google Scholar]
- Chisholm A. D., Hardin J. (2005). Epidermal morphogenesis. WormBook 1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. O., Kim G. W., Lee O. K. (2011). Wolbachia bacteria reside in host Golgi-related vesicles whose position is regulated by polarity proteins. PLoS ONE 6, e22703 10.1371/journal.pone.0022703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai S., Sarmiere P. D., Wiggan O., Bamburg J. R., Zhou D. (2004). Efficient Salmonella entry requires activity cycles of host ADF and cofilin. Cell. Microbiol. 6, 459–471 10.1111/j.1462-5822.2004.00375.x [DOI] [PubMed] [Google Scholar]
- Debrah A. Y., Mand S., Marfo-Debrekyei Y., Batsa L., Pfarr K., Lawson B., Taylor M., Adjei O., Hoerauf A. (2009). Reduction in levels of plasma vascular endothelial growth factor-A and improvement in hydrocele patients by targeting endosymbiotic Wolbachia sp. in Wuchereria bancrofti with doxycycline. Am. J. Trop. Med. Hyg. 80, 956–963. [PubMed] [Google Scholar]
- Dolinski C., Baldwin J. G., Thomas W. K. (2001). Comparative survey of early embryogenesis of Secernetea (Nematoda), with phylogenetic implications. Can. J. Zool. 79, 82–94 10.1139/z00-179 [DOI] [Google Scholar]
- Fenn K., Blaxter M. (2004). Are filarial nematode Wolbachia obligate mutualist symbionts? Trends Ecol. Evol. (Amst.) 19, 163–166 10.1016/j.tree.2004.01.002 [DOI] [PubMed] [Google Scholar]
- Ferree P. M., Frydman H. M., Li J. M., Cao J., Wieschaus E., Sullivan W. (2005). Wolbachia utilizes host microtubules and Dynein for anterior localization in the Drosophila oocyte. PLoS Pathog. 1, e14 10.1371/journal.ppat.0010014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri E., Bain O., Barbuto M., Martin C., Lo N., Uni S., Landmann F., Baccei S. G., Guerrero R., de Souza Lima S. et al. (2011). New insights into the evolution of Wolbachia infections in filarial nematodes inferred from a large range of screened species. PLoS ONE 6, e20843 10.1371/journal.pone.0020843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer K., Beatty W. L., Jiang D., Weil G. J., Fischer P. U. (2011). Tissue and stage-specific distribution of Wolbachia in Brugia malayi. PLoS Negl. Trop. Dis. 5, e1174 10.1371/journal.pntd.0001174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foor W. E. (1967). Ultrastructural aspects of oocyte development and shell formation in Ascaris lumbricoides. J. Parasitol. 53, 1245–1261 10.2307/3276689 [DOI] [PubMed] [Google Scholar]
- Foster J., Ganatra M., Kamal I., Ware J., Makarova K., Ivanova N., Bhattacharyya A., Kapatral V., Kumar S., Posfai J. et al. (2005). The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 3, e121 10.1371/journal.pbio.0030121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frydman H. M., Li J. M., Robson D. N., Wieschaus E. (2006). Somatic stem cell niche tropism in Wolbachia. Nature 441, 509–512 10.1038/nature04756 [DOI] [PubMed] [Google Scholar]
- Hansen R. D., Trees A. J., Bah G. S., Hetzel U., Martin C., Bain O., Tanya V. N., Makepeace B. L. (2011). A worm’s best friend: recruitment of neutrophils by Wolbachia confounds eosinophil degranulation against the filarial nematode Onchocerca ochengi. Proc. Biol. Sci. 278, 2293–2302 10.1098/rspb.2010.2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerauf A., Volkmann L., Nissen-Paehle K., Schmetz C., Autenrieth I., Büttner D. W., Fleischer B. (2000). Targeting of Wolbachia endobacteria in Litomosoides sigmodontis: comparison of tetracyclines with chloramphenicol, macrolides and ciprofloxacin. Trop. Med. Int. Health 5, 275–279 10.1046/j.1365-3156.2000.00544.x [DOI] [PubMed] [Google Scholar]
- Hubbard E. J. A. (2007). Caenorhabditis elegans germ line: a model for stem cell biology. Dev. Dyn 236, 3343–3357 10.1002/dvdy.21335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J., Crittenden S. L. (2005). Germline proliferation and its control. WormBook 1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozek W. J. (1977). Transovarially-transmitted intracellular microorganisms in adult and larval stages of Brugia malayi. J. Parasitol. 63, 992–1000 10.2307/3279832 [DOI] [PubMed] [Google Scholar]
- Lahl V., Halama C., Schierenberg E. (2003). Comparative and experimental embryogenesis of Plectidae (Nematoda). Dev. Genes Evol. 213, 18–27 10.1007/s00427-002-0289-1 [DOI] [PubMed] [Google Scholar]
- Landmann F., Foster J. M., Slatko B., Sullivan W. (2010). Asymmetric Wolbachia segregation during early Brugia malayi embryogenesis determines its distribution in adult host tissues. PLoS Negl. Trop. Dis. 4, e758 10.1371/journal.pntd.0000758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmann F., Voronin D., Sullivan W., Taylor M. J. (2011). Anti-filarial activity of antibiotic therapy is due to extensive apoptosis after Wolbachia depletion from filarial nematodes. PLoS Pathog. 7, e1002351 10.1371/journal.ppat.1002351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. C. (1975). Dirofilaria immitis: ultrastructural aspects of oocyte development and zygote formation. Exp. Parasitol. 37, 449–468 10.1016/0014-4894(75)90015-6 [DOI] [PubMed] [Google Scholar]
- Lo N., Casiraghi M., Salati E., Bazzocchi C., Bandi C. (2002). How many wolbachia supergroups exist? Mol. Biol. Evol. 19, 341–346 10.1093/oxfordjournals.molbev.a004087 [DOI] [PubMed] [Google Scholar]
- Malakhov V. V. (1994). Nematodes: Structure, Development, Classification, And Phylogeny. Washington; London: Smithsonian Institution Press. [Google Scholar]
- McGarry H. F., Egerton G. L., Taylor M. J. (2004). Population dynamics of Wolbachia bacterial endosymbionts in Brugia malayi. Mol. Biochem. Parasitol. 135, 57–67 10.1016/j.molbiopara.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Munro E., Bowerman B. (2009). Cellular symmetry breaking during Caenorhabditis elegans development. Cold Spring Harb. Perspect. Biol. 1, a003400 10.1101/cshperspect.a003400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbus L. R., Casper-Lindley C., Landmann F., Sullivan W. (2008). The genetics and cell biology of Wolbachia-host interactions. Annu. Rev. Genet. 42, 683–707 10.1146/annurev.genet.41.110306.130354 [DOI] [PubMed] [Google Scholar]
- Skiba F., Schierenberg E. (1992). Cell lineages, developmental timing, and spatial pattern formation in embryos of free-living soil nematodes. Dev. Biol. 151, 597–610 10.1016/0012-1606(92)90197-O [DOI] [PubMed] [Google Scholar]
- Slatko B. E., Taylor M. J., Foster J. M. (2010). The Wolbachia endosymbiont as an anti-filarial nematode target. Symbiosis 51, 55–65 10.1007/s13199-010-0067-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strübing U., Lucius R., Hoerauf A., Pfarr K. M. (2010). Mitochondrial genes for heme-dependent respiratory chain complexes are up-regulated after depletion of Wolbachia from filarial nematodes. Int. J. Parasitol. 40, 1193–1202 10.1016/j.ijpara.2010.03.004 [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N. (1983). The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev. Biol. 100, 64–119 10.1016/0012-1606(83)90201-4 [DOI] [PubMed] [Google Scholar]
- Tamarozzi F., Halliday A., Gentil K., Hoerauf A., Pearlman E., Taylor M. J. (2011). Onchocerciasis: the role of Wolbachia bacterial endosymbionts in parasite biology, disease pathogenesis, and treatment. Clin. Microbiol. Rev. 24, 459–468 10.1128/CMR.00057-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. J., Hoerauf A. (1999). Wolbachia bacteria of filarial nematodes. Parasitol. Today 15, 437–442 10.1016/S0169-4758(99)01533-1 [DOI] [PubMed] [Google Scholar]
- Taylor M. J., Cross H. F., Bilo K. (2000). Inflammatory responses induced by the filarial nematode Brugia malayi are mediated by lipopolysaccharide-like activity from endosymbiotic Wolbachia bacteria. J. Exp. Med. 191, 1429–1436 10.1084/jem.191.8.1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. J., Bandi C., Hoerauf A. (2005). Wolbachia bacterial endosymbionts of filarial nematodes. Adv. Parasitol. 60, 245–284 10.1016/S0065-308X(05)60004-8 [DOI] [PubMed] [Google Scholar]
- Turner J. D., Langley R. S., Johnston K. L., Egerton G., Wanji S., Taylor M. J. (2006). Wolbachia endosymbiotic bacteria of Brugia malayi mediate macrophage tolerance to TLR- and CD40-specific stimuli in a MyD88/TLR2-dependent manner. J. Immunol. 177, 1240–1249. [DOI] [PubMed] [Google Scholar]
- Turner J. D., Langley R. S., Johnston K. L., Gentil K., Ford L., Wu B., Graham M., Sharpley F., Slatko B., Pearlman E. et al. (2009). Wolbachia lipoprotein stimulates innate and adaptive immunity through Toll-like receptors 2 and 6 to induce disease manifestations of filariasis. J. Biol. Chem. 284, 22364–22378 10.1074/jbc.M901528200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren J. H., Zhang W., Guo L. R. (1995). Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proc. Biol. Sci. 261, 55–63 10.1098/rspb.1995.0117 [DOI] [PubMed] [Google Scholar]
- Wu B., Novelli J., Foster J., Vaisvila R., Conway L., Ingram J., Ganatra M., Rao A. U., Hamza I., Slatko B. (2009). The heme biosynthetic pathway of the obligate Wolbachia endosymbiont of Brugia malayi as a potential anti-filarial drug target. PLoS Negl. Trop. Dis. 3, e475 10.1371/journal.pntd.0000475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Gally C., Labouesse M. (2010). Tissue morphogenesis: how multiple cells cooperate to generate a tissue. Curr. Opin. Cell Biol. 22, 575–582 10.1016/j.ceb.2010.08.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.