Summary
The relationship between integrin expression and function in pathologies is often contentious as comparisons between human pathological expression and expression in cell lines is difficult. In addition, the expression of even integrins αvβ6 and αvβ8 in tumor cell lines is not comprehensively documented. Here, we describe rabbit monoclonal antibodies (RabMabs) against the extracellular domains of αv integrins that react with both native integrins and formalin fixed, paraffin embedded (FFPE) human tissues. These RabMabs, against αvβ3 (EM22703), αvβ5 (EM09902), αvβ6 (EM05201), αvβ8 (EM13309), and pan-αv (EM01309), recognize individual integrin chains in Western blots and in flow cytometry. EM22703 detected a ligand-induced binding site (LIBS), reporting an epitope enhanced by the binding of an RGD-peptide to αvβ3. αvβ8 was rarely expressed in human tumor specimens, and weakly expressed in non-small-cell lung carcinoma (NSCLC). However, ovarian carcinoma cell lines expressed αvβ8, as did some melanoma cells, whereas U87MG glioma lacked αvβ8 expression. We observed an unexpected strong expression of αvβ6 in tumor samples of invasive ductal breast adenoma, colorectal carcinoma (CRC), and NSCLC. αvβ3 was strongly expressed in some invasive NSCLC cohorts. Interestingly, PC3 prostate cell and human prostate tumors did not express αvβ3. The RabMabs stained plasma membranes in FFPE-immunohistochemistry (IHC) samples of tumor cell lines from lung, ovary, colon, prostate, squamous cell carcinoma of head and neck (SCCHN), breast, and pancreas carcinomas. The RabMabs are unique tools for probing αv integrin biology, and suggest that especially αvβ6 and αvβ8 biologies still have much to reveal.
Keywords: Integrin, Alphav, Paraffin embedded, Rabbit-monoclonal, Immunohistology
Introduction
Five integrins share the αv chain: αvβ3, αvβ5, αvβ6, αvβ8, and αvβ1. αvβ3 and αvβ5 are well characterized, αvβ6 less so, and αvβ8 and αvβ1 the least, which may reflect the availability of specific antibodies. For example, in the case of murine monoclonal antibodies (MuMabs), LM609 binds to and inhibits αvβ3 (Cheresh and Spiro, 1987; Lin et al., 1998), PIF6 binds to αvβ5 (Weinacker et al., 1994), and 5C4 binds to β6 (Sipos et al., 2004). However, specific antibodies to β5, β6, and β8 chains are rare. This is unfortunate as the αv biologies are complex and interesting. For example, αvβ3 and αvβ5 are involved in tumor growth and angiogenesis (Desgrosellier and Cheresh, 2010), whereas αvβ6 and αvβ8 enhance the activation of latent TGFbeta (Lacy-Hulbert et al., 2007; Sheppard, 2005), and αvβ8 expression has been connected to the inhibition of tumor cell growth (Cambier et al., 2000; Fang et al., 2011). However, limitations in detecting antibodies make it hard to identify integrin distributions or activation states that could be relevant to human pathologies.
Integrins are dimeric cell surface proteins which control cell attachment. 14 alpha chains associate with 8 beta chains to form 24 receptors, each an obligate heterodimer. Integrins are differentially glycosylated, spliced, and activated in response to their cellular and extracellular environments (Bellis, 2004; Campbell and Humphries, 2011; Fornaro and Languino, 1997). This subtle regulation of cell attachment to extracellular matrix and to other cells, coordinates intracellular signaling responses to growth factors, and drives diverse cell behaviors (Hynes, 2002; Schwartz and Ginsberg, 2002). Occasionally, valuable antibodies have been described which detect those LIBS that alter following ligation or activation of integrins (Honda et al., 1995; Mould et al., 1995). The structural basis of such LIBS is unknown, with few exceptions (Honda et al., 1995).
αv integrins are being targeted in the clinics, notably in cancer therapies, so the characterization of their distribution in human tissue is important (Cox et al., 2010). αv expression in tumors may reveal significant information for diagnosis, prognosis, and therapeutic outcome. Yet despite 25 years of research, and hundreds of specific antibodies that can stain fresh frozen tissues, monoclonal antibodies that specifically stain integrins in FFPE material remain rare. Sampling, storage, and logistics for frozen tissue are challenging, involving strict maintenance of cool-chains. Furthermore, histomorphology is not optimally maintained in cryostat sections, compared with FFPE biopsies, so information on target distribution may be lost. In addition, some tissues are difficult to obtain, except as FFPE biopsies.
The FFPE process involves cross-linking, dehydration, hydrophobic environments, and heat, all of which can destroy or conceal epitopes. Integrins are large, conformationally active transmembrane proteins and have many epitopes that might be lost. Nevertheless, some antibodies do bind to αv integrins in FFPE material; often these are rabbit polyclonal antibodies, targeting the short conserved integrin cytoplasmic domain. However, polyclonal antibodies have numerous disadvantages, not least mortality of their hosts. In addition, antibodies against integrins' cytoplasmic domains may complicate the interpretation of the results as staining can report ambiguous distribution, while the location of functional integrin heterodimers is unambiguously at the cell surface. Optimal antibodies should recognize the active heterodimeric extracellular domains. Monoclonal antibodies are the reagents of choice, being uniform and available in essentially unlimited amounts. Thus, there are gaps in our knowledge of integrin pathology due to the lack of monoclonal antibodies that can identify integrins in FFPE material.
As rabbit polyclonal antibodies can bind epitopes in FFPE material (Pytela et al., 2008), we thought that RabMabs might also recognize integrin extracellular domains there. Lagomorphs have longer complementarity-determining regions (CDRs) than rodents, and thus potentially have high binding affinity; also, as they are out-bred, their MHC loci are more diversified than the in-bred rodent populations often used for monoclonal production. Here we describe RabMabs against extracellular domains of αv integrins, one of which detects a LIBS on αvβ3. These RabMabs have helped to better understand the relationships between expression of native heterodimers and cell proliferation on viable cells, in archival FFPE material, and with biochemistry.
Results
Rabbit monoclonal antibody generation
Primary bleeds of approximately one third of the immunized animals reacted with the DTM-integrins by ELISA. IHC using primary bleeds gave strong membrane staining of cells known to express the target integrins (Table 1) being stained on FFPE cell-line microarray (CMA). Twenty to thirty percent of the resulting fused spleenocytes multiclones had the desired specificity by ELISA – and 1–2% were reactive in IHC. Multiclones showing the lowest background staining on Raji and Sf9, and the strongest specific membrane staining were recloned to monoclonality and then banked. cDNA from the heavy and light chains was cloned, sequenced, and expressed in a 293-EBNA cell line, and the recombinant RabMabs were purified and studied further.
Table 1. Flow cytometry of viable cells.
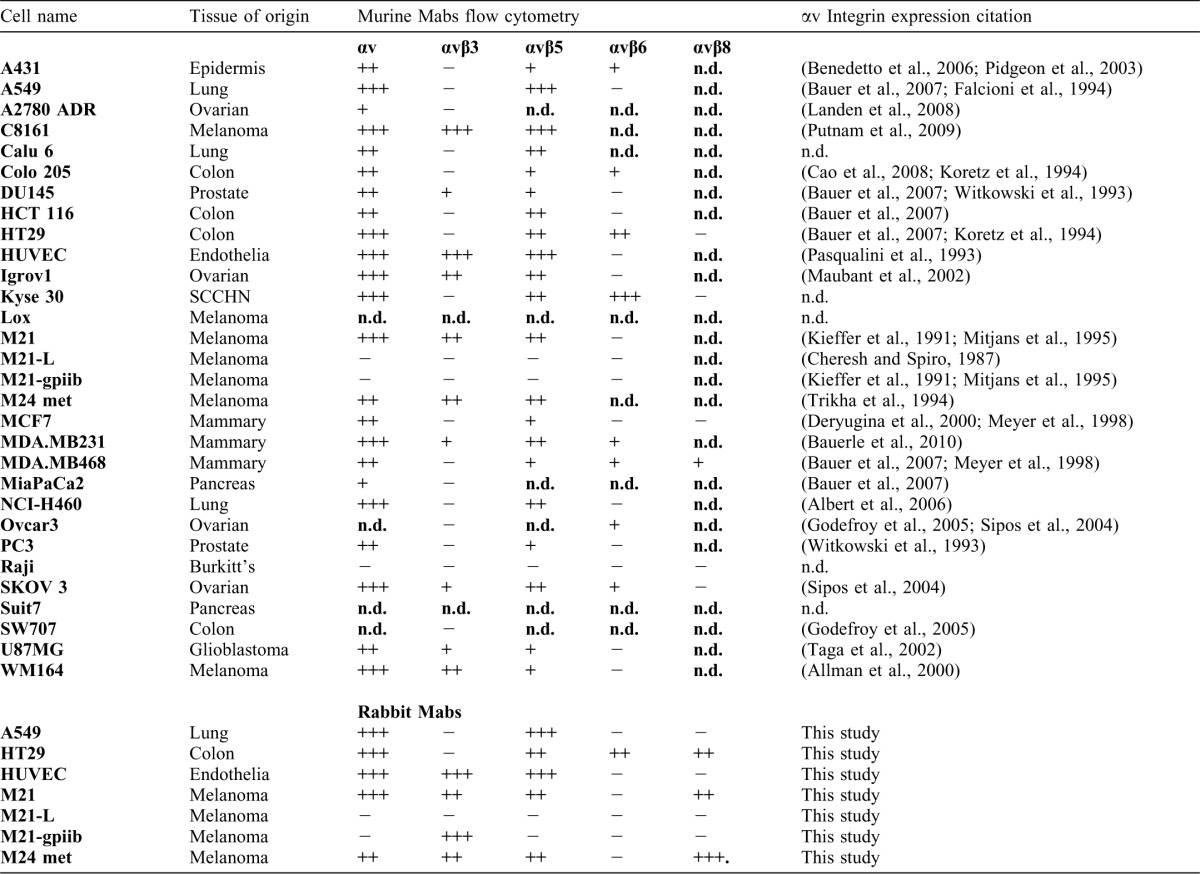
Murine monoclonal antibodies against: αv (17E6); αvβ3 (LM609); αvβ5 (P1F6); and β6 (10C5). Rabbit monoclonals against: αvβ3 (EM22703), αvβ5 (EM09902), αvβ6 (EM05201), αvβ8 (EM13309), and αv (EM01309). Intensity is scored as median intensity of fluorescence (MIF) normalized to second layer (i.e. 1 is background MIF: 2 is twice background). − = MIF<2; + = MIF 2–4; ++ = MIF 4–9; and +++ = MIF>10. Literature citation to (sometimes partial) integrin profile is provided. Literature data on αvβ8 and αvβ6 expression on tumor cell lines are very limited. n.d. = not determined, or no literature available. Lox, Suit7, and SW707 were not investigated by cytometry.
Integrin expression profile on human tumor cells
To generate integrin target profiles, we used MuMabs in flow cytometry of a panel of human cells (Table 1). In general, our results are in agreement with what has been reported in literature regarding integrin expression on these cells. For example, the M21 cell series M21 (αvβ3 +; αvβ5 +; αvβ6 −) and M21-L (αvβ3 +/−; αvβ5 −; αvβ6 −), the colon carcinoma line HT29 (αvβ3−; αvβ5+; αvβ6+), the NSCLC line A549 (αvβ3−; αvβ5+; αvβ6−), and HUVECs (αvβ3+; αvβ5+; αvβ6−) had the integrin expression profiles reported in the literature (Table 1).
Some integrin expression patterns are controversial. Expression of αvβ3 integrin on the prostate cell line PC-3 has been reported as being high (Zheng et al., 2000), low (Witkowski et al., 1993), or not occurring (Haywood-Reid et al., 1997), and similar results have been found on MDA-MB231 breast carcinoma. We confirmed that the PC-3 cell line does not express αvβ3 and that low levels of this integrin are detected on MDA-MB-231. Expression of αvβ6 is not extensively documented. We confirmed αvβ6 expression on HT29 cells (Kemperman et al., 1997), and found high levels of this integrin on the SCCHN line Kyse30, and low levels on MDA-MB231 and -468 breast carcinoma lines. αvβ6 expression on clinical SCCHN has been associated with invasive tumor behavior (Janes and Watt, 2004; Sipos et al., 2004; Xue et al., 2001). αvβ8 integrin expression has not been reported for the tumor cell lines we examined in this study, and no αvβ8-specific monoclonal reagent for flow cytometry appears to be commercially available. As our results otherwise agreed with the literature descriptions, we arrayed the cell lines as paraffin CMAs for the primary FFPE screening of the RabMabs.
The RabMabs bind purified intact integrins via individual chains of the complex
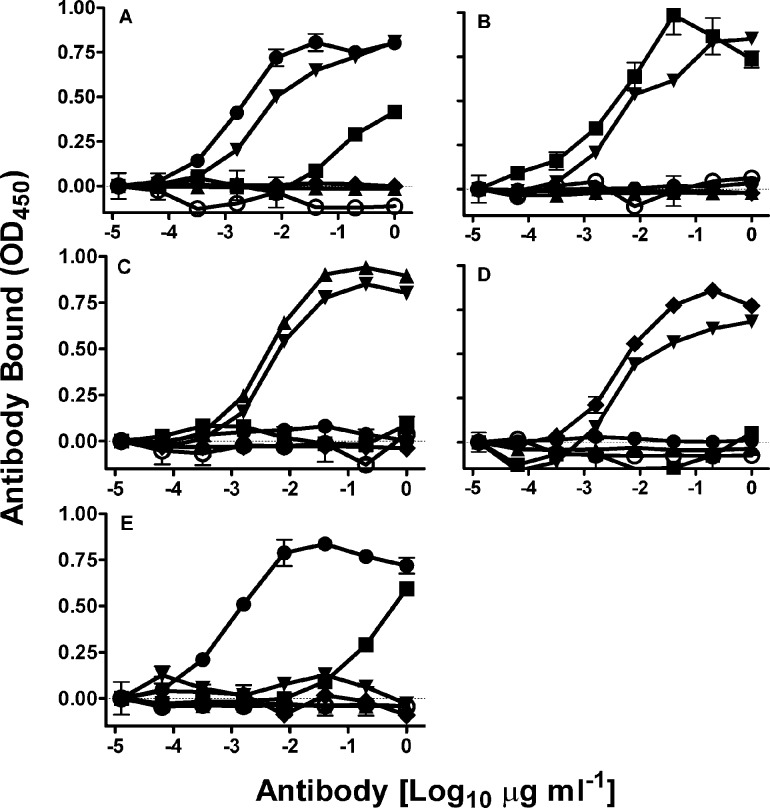
The binding specificities of the RabMabs were characterized on recombinant DTM-integrins (i.e. the immunogens) and native gpiibβ3 using ELISA in physiological divalent cations (Fig. 1). EM22703 bound to αvβ3 and gpiibβ3 (EC50 2 ng ml−1) (Fig. 1A,E). EM09902 bound to αvβ5 (EC50 5 ng ml−1) and also weakly bound to αvβ3 and gpiibβ3 (EC50 ∼7 µg ml−1) (Fig. 1A,B,E). EM05201 bound to αvβ6 (EC50 ∼5 ng ml-1), EM13309 bound to αvβ8 (EC50 ∼10 ng ml−1), and EM01309 bound to all αv integrins (EC50 ∼10 ng ml−1) but not gpiibβ3. These data suggested that the RabMabs bound to the extracellular domains of native integrin complexes. Unexpectedly, EM00212 did not bind even to native gpiibβ3 (EC50>>10 µg ml−1). EM22703 did not bind to the β3 cytoplasmic domain (it binds to DTM-αvβ3 that lacks this domain), but it did react with gpiibβ3. The EC50s suggest the antibodies have picomolecular binding affinities.
Fig. 1. ELISA profile of EBNA-recombinant rabbit anti-integrin monoclonal antibodies.
Plates coated with soluble recombinant integrins (A) αvβ3; (B) αvβ5; (C) αvβ6; (D) αvβ8; or (E) native platelet gpiibβ3 (1 µg/ml) were incubated with recombinant antibodies from clones EM22703 (circles, closed); EM09902 (squares); EM05201 (triangles, up); EM13309 (diamonds); EM01309 (triangles, down); EM00212 (circles, open).
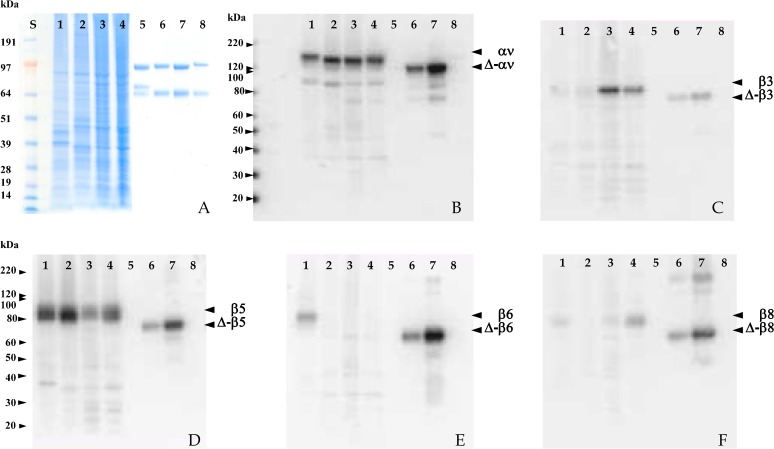
We next studied the antibodies on Western blots of whole cell lysates. Preliminary experiments showed that the RabMabs blotted the DTM-integrins, and that the signal from the non-reduced proteins was stronger than from the reduced proteins, suggesting some conformational specificity; detection limits were below 5 ng integrin per lane. We calculated that this sensitivity might detect integrins in cell lysates (Fig. 2). In octylglucoside lysates of M21, HT29, M24, and A549 cells (Fig. 2A, lanes 1–4), the pan-αv antibody EM01309 bound to a band of ∼150 kDa (Fig. 2B, lanes 1–4), and to the α-chain of recombinant αvβ3 (Fig. 2B, lanes 6, 7) but not to gpiibβ3 (Fig. 2B, lane 8). There was weak reactivity with a doublet component and with a protein of approximately 95 kDa. Faster migrating proteins were also weakly detected in the positive control lanes, and may be partially degraded αv. Both αv and β chains of DTM-integrins are transmembrane truncated and so migrate faster than the major staining bands in the cell lysate that, therefore, likely identify the cellular integrin αv chain (Kraft et al., 1999; Mehta et al., 1998).
Fig. 2. Characterization of EBNA-recombinant rabbit anti-integrin antibodies on Western blots of whole cell lysates.
Detergent lysates of tumor cell lines (10 µg protein) and purified integrins were resolved on SDS-PAGE gels. HT29 (lane 1), A549 (lane 2); M21 (lane 3); and M24 (lane 4). (A) Lanes 1–4 stained with Coomassie brilliant blue. Lanes 5–8 recombinant DTM-integrins αvβ3, αvβ5, αvβ6, and αvβ8 (750 ng). (B–F) Western blots probed with (B) EM01309; (C) EM22703; (D) EM09902; (E) EM05201; or (F) EM13309; and bound antibody detected using ECL. Molecular markers were run in parallel as indicated on the left gel margins. In (B–F), positive control integrins were loaded in lanes 6 (7.5 ng) and 7 (25 ng) vs. negative control integins (100 ng) in lane 8 as follows: (B) αvβ5 vs. gpiibβ3; (C) αvβ3 vs. αvβ6; (D) αvβ5 vs. αvβ6; (E) αvβ6 vs. αvβ3; (F) αvβ8 vs. αvβ6. Note that in each blot the integrin negative control is not stained.
The αvβ3-antibody (EM22703) bound to the β3 chain in Western blots (Fig. 2C), strongly stained a protein in M21 and M24 melanoma cells running at ∼85 kDa (lanes 3, 4), that can be seen only very faintly in HT29 and A549 cells (lanes 1, 2). In DTM-αvβ3 (Fig. 2C, lanes 6, 7), a band running at the same position as the recombinant DTM-β3 chain was observed. Melanoma cells (e.g. M21) expressed αvβ3, whereas HT29 and A549 did not. Similar results have been previously reported for both melanoma cells (Clark et al., 1994; Felding-Habermann et al., 1992; Kieffer et al., 1991) and HT29 and A549 lines (Bauer et al., 2007; Kemperman et al., 1997).
The αvβ5-specific antibody EM09902 bound to a diffuse band of 80–95 kDa in all tumor cells examined (Fig. 2D), and to a protein with the mobility of recombinant β5 on DTM-αvβ5 (Fig. 2D, lanes 6, 7), but no binding to DTM-αvβ6 controls was observed (lane 8). Flow cytometry showed that HT29 and A549 expressed αvβ5 strongly, whereas M21 expressed this integrin weakly and expression in the M24 line was moderate. These cell lines have previously been reported to express αvβ5 (Burvenich et al., 2008; Felding-Habermann et al., 1992; Kemperman et al., 1997).
The αvβ6-specific antibody EM05201 bound to a compact protein band at ∼90 kDa in HT29 cells (Fig. 2E, lane 1). It also stained a protein migrating in the same position as the DTM-β6 chain (Fig. 2E, lanes 6, 7), but did not stain DTM-αvβ3 (Fig. 2E, lane 8). To our knowledge, the αvβ6 expression pattern for M21, M24, or A549 cells has not been reported. However, HT29 cells have been shown to express αvβ6 (Kemperman et al., 1997), an epithelial integrin, not yet reported on melanoma (Sheppard, 1996).
The αvβ8-specific antibody EM13309 bound weakly to a protein band migrating at ∼90 kDa in HT29 cells and M21 cells; binding was moderate in M24 cells (Fig. 2F, lanes 1–4). EM13309 also stained a protein migrating in the same position as the recombinant DTM-β8 chain, but did not stain DTM-αvβ6 (Fig. 2F, lanes 6–8). We were unable to find an αvβ8 expression profile for HT29, M21, M24, or A549 cells in the literature; however, αvβ8 is expressed in cells that, like melanocytes, are in the neural crest lineage (Nishimura et al., 1998).
The RabMabs recognize the integrin heterodimers by flow cytometry on viable cells
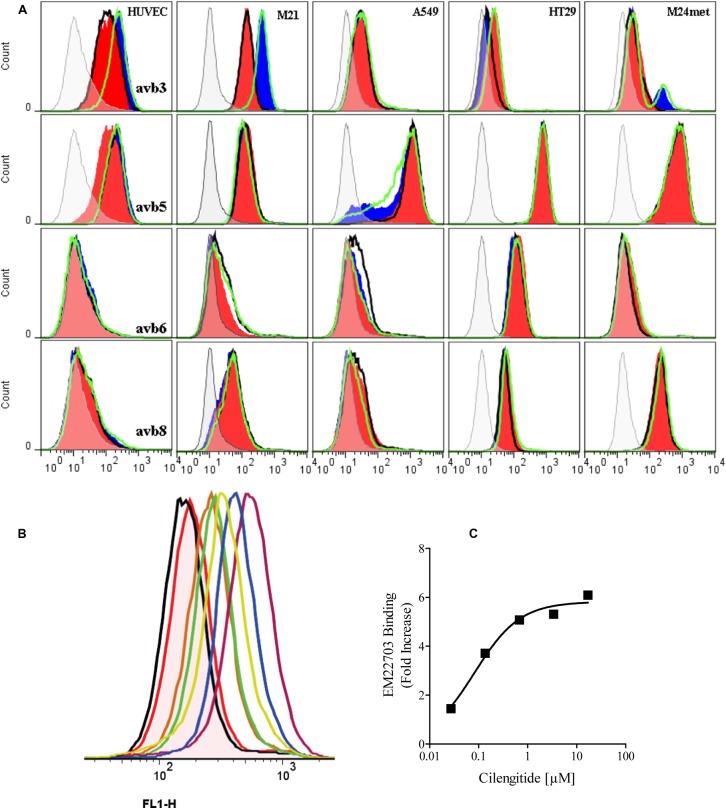
We next investigated whether the RabMabs could bind their targets on viable cells. The antibodies bound strongly and specifically to a similar set of cells stained in FFPE material (Table 1; Fig. 3A), but with interesting variations. The patterns of expression in flow cytometry with EM22703 (anti-αvβ3) and EM09902 (anti-αvβ5) matched literature and our in-house data obtained using MuMabs LM609 (anti-αvβ3) and P1F6 (anti-αvβ5). M21 cells and HUVECs expressed αvβ3, HT29 and A549 cells displayed expression levels approaching background, and M21-L cells did not express any of the integrins. However, EM22703, which in ELISA also recognized gpiibβ3, bound to M21-gpiib but not to M21-L cells. Thus, EM22703 binds to an epitope on the β3 chain, in a complex with either αv or gpiib. EM09902 (anti-αvβ5) stained most cells with the exception of M21-L and M21-gpiib, which do not express αv, and so lack cell surface αvβ5. M21, HUVEC, A549, and HT29 cells express αvβ5 (Table 1). EM05201 (anti-αvβ6) detected a strong signal on HT29 and on no other cell line, a pattern similar to the αvβ6 specific murine antibody 10C5 (Table 1). EM13309 (anti-αvβ8) detected a signal on HT29 cells and on two melanoma lines (M21 and M24-met), but not on A549, HUVECs, M21-L, nor M21-gpiib lines. The EM01309 antibody unambiguously detected αv chains in ELISA of the intact recombinant proteins (Fig. 1) and on Western blots (Fig. 2). However, in viable cell flow cytometry, it recognized the αv chain only on HUVECs and weakly stained M24-met cells. Nevertheless, M21, A549, and HT-29 cells express αv (Table 1; Fig. 4). Control murine anti-αv Mab, 17E6 (Mitjans et al., 1995), bound strongly in flow cytometry to all cells with the exception of M21-L and M21-gpiib. EM00212 (anti- β3A-cytoplasmic domain) gave the same signal as the second layer antibody, and it acted as a control for cellular integrity.
Fig. 3. Viable cell flow cytometry with the RabMabs shows strong LIBS signals from anti-αvβ3 antibody EM22703, and αvβ8 signals.
(A) Flow cytometry in the presence of physiological divalent cations (black open); 1 mM Mn2+ (red closed); 10 µM cilengitide in physiological cations (blue closed); 10 µM cilengitide in 1 mM Mn2+ (green open). Horizontal panels show staining with EM22703 (αvβ3); EM09902 (αvβ5); EM05201 (αvβ6); and EM13309 (αvβ8). Vertical panels show staining on HUVECs, M21, A549, HT29, and M24Met. Gray shading shows binding of EM00212 and the second layer controls, which superimpose. (B,C) Variation of EM22703 flow cytometry signal on M21 cells with cilengitide concentration. (B) red = 0 µM; black = 4 nM; brown = 40 nM; green = 100 nM; yellow = 400 nM; grape = 4 µM; blue = 100 µM.
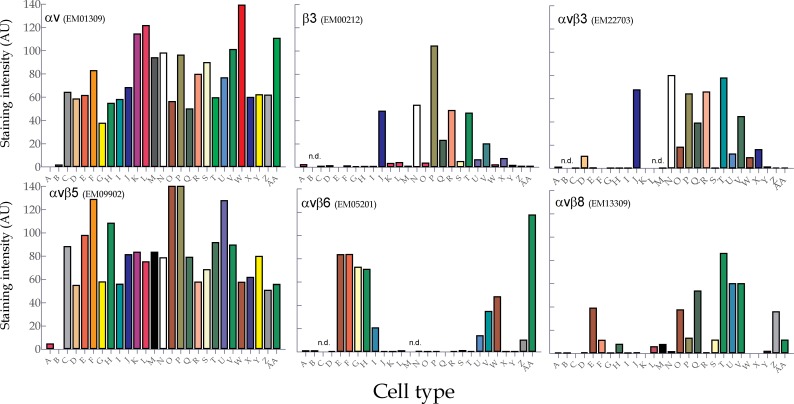
Fig. 4. Automated image analysis of FFPE human tumor cell line staining using anti-integrin αv RabMabs.
Image analysis of human tumor cell lines in TMAs stained with RabMabs. The cells are grouped by tumor-of-origin: (A) T-lymphoma: Raji: and (B) insect production cell line: Sf9, serve as negative controls. (C–E) Mammary carcinomas: MCF7; MDA-MB231; MDA-MB468. (F) Carcinoid: A431. (G–I) Colorectal carcinomas: Colo 205; HT-29; SW707. (J) Glioma: U87MG. (K–M) Lung carcinomas: A549; Calu-6; H460. (N–R) Melanomas: C8161; Lox; M21; M24-met; WM164. (S–V) Ovarian carcinomas: A2780ADR; Igrov1; OVCAR-3; SKOV3. (W) Pancreatic carcinoma: Suit7. (X–Z) Prostate carcinomas: DU145; MiaPaCa2; PC-3. (AA) SCCHN: Kyse30. AU = adsorption units. n.d. = not determined. αvβ5 histograms for Lox and M24-met are truncated for comparability from original values of 146 AU (Lox) and 178 AU (M21).
Thus the RabMabs recognized the dissociated preferably non-reduced integrin chains in blots. They also bound to them specifically in the context of intact, native integrin heterodimers on the cell surface, and in the functionally active heterodimeric DTM-immunogens.
EM22703 recognizes a LIBS epitope
As integrins undergo conformational changes on binding ligands (to produce LIBS) whose gain or loss can be reported by antibodies (Du et al., 1993; Luo et al., 2005), we investigated whether the RabMabs reported such LIBS. αv-integrins bind the Arg-Gly-Asp sequence in their ligands (Xiong et al., 2002). The RabMabs showed little change in binding to cells co-incubated with an RGD peptide, cilengitide, during antibody staining. The only exception was EM22703, in which the signal increased by 3–10 fold following incubation with RGD-peptide on M21, M24-Met and HUVEC cells. In M24-Met line, only a sub-population of ∼15% of the cells developed the LIBS epitope on exposure to cilengitide, whereas for both M21 and HUVECs the entire detected population expressed the novel epitope (Fig. 3A). The signal increase was concentration dependent (Fig. 3B), with an EC50 of ∼100 nM on M21 cells (Fig. 3C). The change in LIBS expression was not a result of activation, as high concentrations of Mn2+, a known activator and conformational modulator of αv integrins, had no effect on the antibody signals. Also, the signal that developed in physiological divalent cations with cilengitide was not affected by Mn2+ (Fig. 3A).
The RabMabs recognize specific groups of human cell lines in FFPE preparations
To develop robust staining protocols, the RabMabs were screened using a widely used automated clinical processing machine. The RabMabs were screened on sectioned FFPE CMAs and tumor-xenografts, where they stained strongly and specifically. Mild protease treatment was optimal for the antibodies against αvβ3, αvβ5, αvβ6, and αvβ8. Tris-EDTA pre-treatment was optimal for the antibodies against β3 cytoplasmic domain and αv. The antibodies strongly stained cell plasma membranes, with some punctuate intracellular staining. For αvβ8 pronounced staining was also frequently observed in cytoplasm. Quantitative image analysis of the CMA stainings is shown in Fig. 4.
EM01309: This pan-αv-specific antibody stained all adherent human cell lines, but did not stain Raji B-cell lymphoma or Sf9 insect cells. It labeled membranes, with some punctuate intracellular staining (Figs 4, 5). It also stained cell lines when these were cultivated as subcutaneous xenografts in immune-suppressed mice, with pronounced membrane staining.
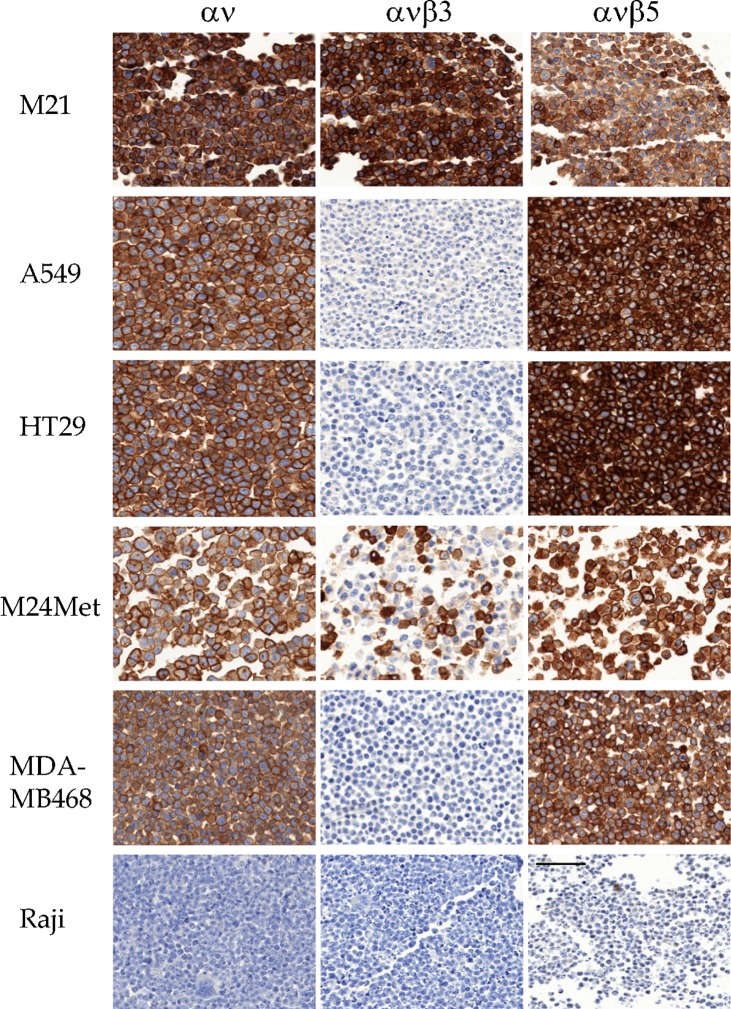
Fig. 5. Human tumor cell lines stained in FFPE microtissue array using RabMabs.
M21 and M24-met melanoma, A549 NSCLC, HT29 CRC, MDA-MB468 mammary carcinoma, and Raji B-cell lymphoma are shown stained with EM01309 (αv), EM22703 (αvβ3), and EM09902 (αvβ5). Scale bar = 50 µm.
EM22703: This αvβ3-specific antibody stained a subset of cell lines, including melanomas (3/4) (WM164; M24met; M21; but not Lox), glioma (U87MG), and most ovarian carcinomas (Igrov1; SKOV3 weakly Ovcar3; but not A2780ADR), whereas CRC (Colo205; HT29; SW707), NSCLC (Calu6; NHI-H460; A549), SCCHN (Kyse30), mammary (MCF7; MDA-MB468; MDA-MB231), and prostate lines (PC3; DU145; MiaPaCa2) were unstained (Figs 4, 5). EM22703 strongly labeled plasma membranes.
EM09902: This αvβ5-specific antibody stained all adherent tumor cells on the CMA (Figs 4, 5). Some stainings were intense (e.g. M21, M24met, HT29, and A549). EM09902 labeled plasma membranes. It also stained sectioned subcutaneous HT29 tumor xenografts from mice. Although EM09902 staining was intense, it was specific: Raji lymphoma and Sf9 insect cells were unstained.
EM05201: This αvβ6-specific antibody stained plasma membranes of a subset of cell lines, including CRC (3/3) (HT29; Colo205; SW707), SCCHN (Kyse30), mammary (1/3) (MDA-MB468), and carcinoid (A431) lines, but not melanomas (M21) glioma (U87MG), or ovarian carcinomas (3/4) (Igrov1; A2780ADR; Ovcar3). Prostate lines (DU145; MiaPaCa2) were unstained or faintly positive (PC3) (Figs 4, 6).
Fig. 6. Human tumor cell lines stained on FFPE microtissue array using RabMabs.
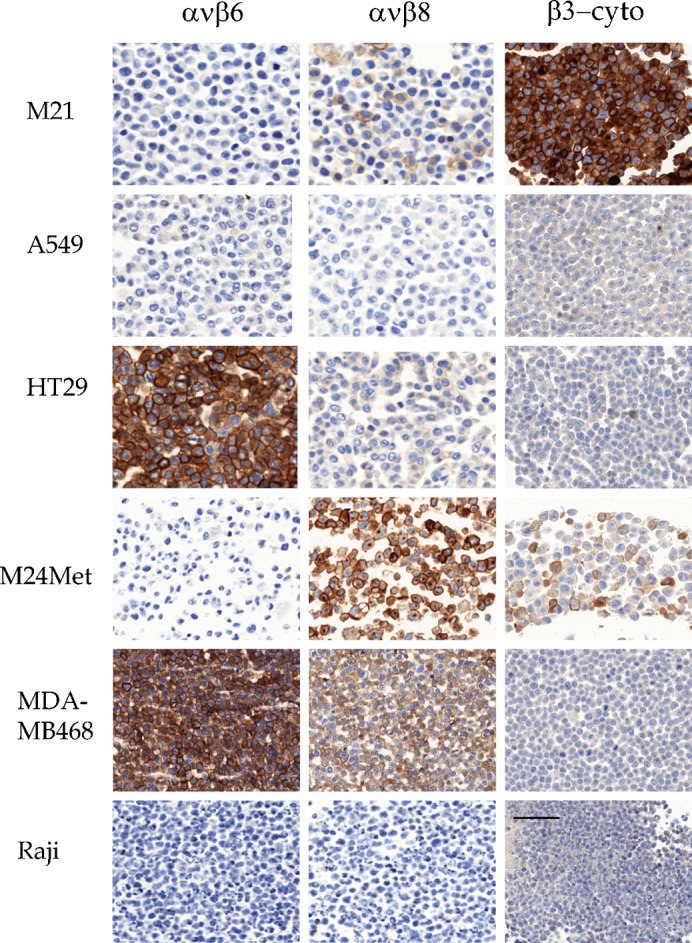
EM05201, EM13309, and EM00212, M21 and M24-met melanoma, A549 NSCLC, HT29 CRC, MDA-MB468 mammary carcinoma, and Raji B-cell lymphoma stained with EM05201 (αvβ6), EM13309 (αvβ8), and EM00212 (cyto-β3). Scale bar = 50 µm.
EM13309: This αvβ8-specific antibody stained a subset of tumor cell lines, including ovarian carcinomas (3/3) (Ovcar3; Igrov1; SKOV3), some melanomas (2/4) (Lox; M24-met; and M21, but only weakly), and a breast carcinoma (1/3) (MDA-MB-468), and weakly stained a CRC line (1/4: HT29). A prostate line (1/2) (PC3) stained weakly. It stained no lung (0/4) or glioma lines (Figs 4, 6). Staining defined the plasma membrane; but, in contrast to the other antibodies, EM13309 also often stained the cytoplasm that sometimes dominated the membrane staining. In flow cytometry, viable cells showed a clear staining. It was notable that the cell lines that stained well for αvβ8 were strongly proliferative ovarian carcinomas, and did not include the glioblastoma derived line U87MG (Fang et al., 2011).
EM00212: As the staining patterns with the αvβ6 and αvβ8 antibodies were unexpected, we studied the antibody EM00212, an anti-β3 cytoplasmic domain reagent, to verify specificity. The low staining intensity of Sf9 and Raji, known to not express αvβ3, was considered as background. EM00212 stained the same cell lines as EM22703, with some variations in intensity of staining; for example, the faint signal on Lox with EM22703 was not seen. EM00212 strongly marked plasma membranes (Figs 4, 6). Thus the staining from an anti-cytoplasmic β3 RabMab closely matched the results from the extracellular domain-specific reagent EM22703, and was distinct from the staining with EM13309 and EM05201. Cells that stained for αvβ3 tended not to stain for αvβ6, with the exception of the SKOV3 and OVCAR3 ovarian carcinoma lines.
The RabMabs recognize their targets in FFPE archival human tumor samples
Human TMAs and normal archival paraffin tissue specimens were stained with the RabMabs. Strong staining without unspecific background was observed in a subset of samples. Six to nine samples of each tumor were examined, and representative galleries of data are shown (Figs 7, 8). Archival FFPE samples of solid tumors, shown for melanoma, NSCLC, CRC, mammary, and prostate carcinomas, strongly expressed membranous αv integrins.
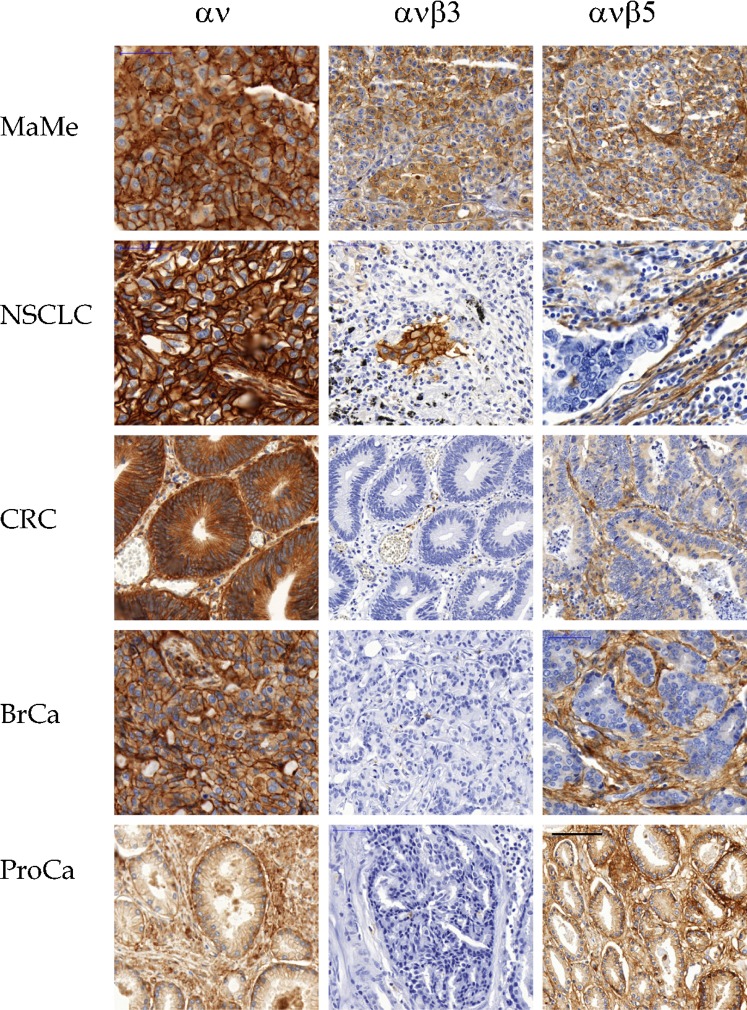
Fig. 7. IHC of archival human tumors.
Malignant melanoma (MaMe) and non-small-cell lung carcinoma (NSCLC), colorectal carcinoma (CRC), invasive ductal breast carcinoma (BrCa), and prostate carcinoma (PrCa) are shown stained with EM01309 (αv), EM22703 (αvβ3), and EM09902 (αvβ5). Scale bar = 50 µm.
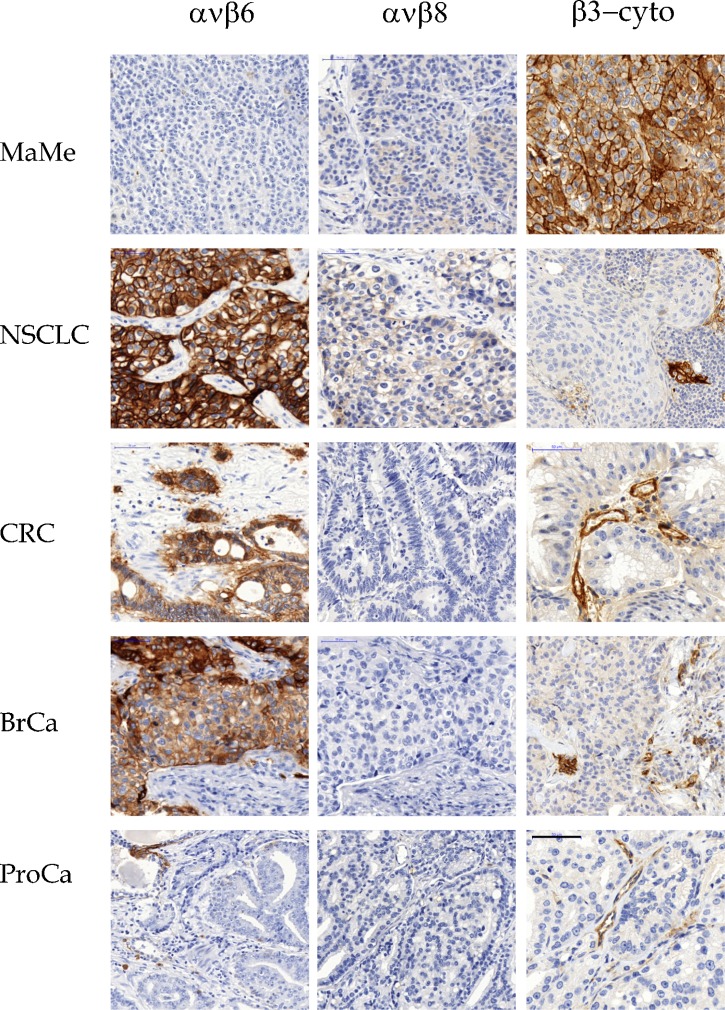
Fig. 8. IHC of archival human tumors.
Malignant melanoma (MaMe) and non-small-cell lung carcinoma (NSCLC), colorectal carcinoma (CRC), invasive ductal breast carcinoma (BrCa), and prostate carcinoma (PrCa) are shown stained with EM05201 (αvβ6), EM13309 (αvβ8), and EM00212 (cyto-β3). Scale bar = 50 µm.
Melanomas stained for αvβ3, as previously reported (Albelda et al., 1990; Hart et al., 1991), as well as for αvβ5, particularly in the connective tissues and vasculature (Fig. 7). αvβ6 expression was absent, and αvβ8 was weakly expressed (Fig. 8). In NSCLC, αvβ3 strongly labeled complexes of tumor cells at the invasion front, and tumor vasculature (Fig. 7). αvβ5 was prevalent in cells within the tumor stroma and occasionally on tumor cells. αvβ6 was intensely expressed on tumor cells, but absent from interstices and vasculature (Fig. 8). A weak membrane staining for αvβ8 was frequently observed in NSCLC (Fig. 8). In CRC, only vasculature stained for αvβ3, whereas αvβ5 was heavily expressed throughout the tumor and its interstices (Fig. 7). αvβ6 expression was confined to apparently invasive cohorts, and αvβ8 expression was absent (Fig. 8). A different pattern was seen in mammary carcinomas, where αvβ3 expression was absent except on vasculature, αvβ5 was confined to interstices and vasculature (Fig. 7), and αvβ6 was strongly expressed on the tumor cells (Fig. 8). There was no αvβ8 expression in mammary carcinomas (Fig. 8). Prostate cancer samples expressed essentially only αvβ5 in the tumor environment, with vasculature expressing some αvβ3 (Figs 7, 8).
Discussion
Effective and specific antibodies have provided foundations for progress and understanding in the field of integrin research. Here we describe a matched set of RabMabs that can stain integrins in archived FFPE tissue. The RabMabs also function in flow cytometry of viable cells, where EM22703 acts as a LIBS reagent, reporting binding of nanomolar concentrations of an antagonistic αvβ3 inhibitor cilengitide, and in Western blotting, on DTM-integrins. The antibodies revealed unexpected distributions of the αv integrins in human archival paraffin embedded materials, notably αvβ3 in some invasive NSCLC, and αvβ6 in mammary carcinoma.
Integrins are large complex molecules, where ligand binding in the extracellular head domains depends on divalent cations which can act to stimulate (usually Mg2+) or inhibit (usually Ca2+) ligand binding. Mn2+ is often a strong-activator. EM22703 is a LIBS antibody. It binds to unligated αvβ3 on viable cells and in FFPE tissues. However, in flow cytometry, the EM22703 signal is increased 3–10 fold in the presence of nanomolar amounts (≥50 nM) of cilengitide (Dechantsreiter et al., 1999), and is not inhibited by micromolar levels of this RGD peptide, nor it is affected by Mn2+. Thus it is not a ligand-mimetic antibody like WOW-1 and PAC-1 (Pampori et al., 1999) or an ion-sensitive reporter. Cilengitide induces large conformational changes in αvβ3 upon binding (Arnaout et al., 2007; Xiong et al., 2002). The precise molecular basis for the EM22703 LIBS signal is not clear, but it is not induced by manganese ions, which both activate and cause conformational changes (Hynes, 2002). LIBS antibodies that report β3 activation (Honda et al., 1995) may be sensitive to divalent cations or to the presence of ligands, or may modulate ligand binding (Frelinger et al., 1990; Frelinger et al., 1991). However, EM22703 does not report this type of epitope. It still needs to be determined whether the EM22703 LIBS signal might be detectable in clinical specimens during RGD-based therapy.
Currently, standard antibodies recognizing αv integrin (e.g. LM609; P1F6; 10C5; 17E6) can only detect their epitopes in cryopreserved tissues and, with few exceptions, do not show specific staining in paraffin tissue blocks. This has locked integrin researchers out of the FFPE archives that bank many important and rare clinical samples. We used commercial methodology to generate FFPE-competent anti-integrin RabMabs, five of which we describe here: it seems the techniques may be generally applicable for producing FFPE-reactive antibodies against integrin extracellular domains. In summary, the antibodies stains in FFPE material support published integrin distributions seen in cryostat materials. However, this now enables the greater spatial resolution of FFPE immunohistology. We also show new characterizations of αvβ8 for which no FFPE-capable monoclonal antibodies have been described, and find that ovarian carcinoma cell lines express αvβ8.
Western blotting showed the epitopes of the RabMabs localized to individual integrin chains, though they bind the intact heterodimers on viable cells. The signals in Western blots were decreased under reducing conditions, implying a conformational aspect to the epitopes. The immunogens were made in insect cells with divergent glycosylation from mammalian forms. The above mentioned cross reactivity and conformational aspects suggest that sugars are not involved in the RabMab epitopes. Integrins are obligate heterodimers, so antibodies binding only one chain may define the complex in IHC, providing (a) they recognize external domain of the intact complex, (b) they recognize a chain with a limited number of partners, and, not least, (c) they function in IHC. Three integrin beta chains are believed monogamous for αv: β5, β6 and β8. β3 also binds gpiib, which is expressed only in the megakaryocyte lineage, so IHC staining for β3 can often be unequivocally assigned to αvβ3 (Fig. 7: NSCLC). As such, binding of the RabMabs to external domains of integrin chains may be used for the tissue localization of the αvβx complexes. Furthermore, it is known that the epitope of antibody LM609 maps to the β3 chain but it detects only αvβ3, not gpiibβ3 (Cheresh and Spiro, 1987; Lin et al., 1998). Consequently, experimental context may affect whether a single chain or a complex-dependent epitope is detected. The RabMabs described here recognize conformational epitopes on individual chains, but clearly they do so in the context of the intact integrin heterodimers on vital cells. In this study, this was highlighted by the EM22703 LIBS antibody against αvβ3, and by EM01309 against αv.
For the RabMab EM01309, biochemistry and FFPE techniques reported an identical pan-αv profile, which was consistent with results reported with 17E6 (Mitjans et al., 1995) and LM142 (Lawler and Hynes, 1989). Interestingly, however, these profiles did not match the flow-cytometry patterns of EM01309. It strongly bound to HUVECs, moderately bound M24 cells, but did not bind to M21. The molecular basis for this unusual recognition profile is under investigation, but it does not seem to be modulated by Mn2+ or RGD peptides.
In summary, data found by FFPE IHC supported the distributions of αvβ3, αvβ5, and αvβ6 found using biochemistry and cell biological tools. For example, flow cytometry profiles with LM609 and EM22703 (binding αvβ3) are similar. However, unlike LM609, EM22703 also recognizes β3 on Western blots, on FFPE material, and is a LIBS antibody. P1F6 and EM09902 (anti-αvβ5) stained all adherent tumor cells, but not M21-L or Raji lymphoma, both known to not express αvβ5 (Nagel et al., 2003). For EM05201 (anti-αvβ6), cells with an SCC origin and breast carcinoma, NSCLC, and CRC tissues were strongly stained. For EM13309 (anti-αvβ8), we had no appropriate comparator antibody available, but the biochemical, cytometric, and IHC patterns were unequivocal. These surprisingly revealed αvβ8 expression in ovarian tumor cell lines. The distribution predicted by staining of tumor cell lines is largely reflected in clinical archival material.
The integrin distributions we observed on FFPE material were unexpected. αvβ8 is implicated in activation of TGFb (Lacy-Hulbert et al., 2007) and, as confirmed here, is present on astrocytes (Milner et al., 1999; Nishimura et al., 1998). αvβ8 inhibits angiogenesis and growth of transformed epithelial and glioblastoma multiform (GBM) (Tchaicha et al., 2011), and promotes invasion in GBM cell lines (Cambier et al., 2000; Fang et al., 2011). A microRNA miR-93 that targets β8 in U87MG cells promotes cell growth, but here we found that U87MG expresses little αvβ8, confirming recent reports by others (Tchaicha et al., 2011). This difference may reflect the antibodies used. αvβ8 was also unexpectedly expressed in several ovarian carcinoma cell lines, in some melanoma lines (e.g. M24-met), and in a breast carcinoma MDA-MB468 line. Nishimura and colleagues (Nishimura et al.,1994) reported β8 mRNA expression in a normal ovary, but we could find no report of its expression in ovarian or mammary carcinomas. αvβ8 was not strongly expressed in the restricted number of breast carcinoma or melanoma tissue samples that were examined in this study. We note that M24-met and MDA-MB468 are derived from metastases: if αvβ8-dependent TGFb activation mediated immune suppression at metastatic sites, one could speculate that this would be an appropriate expression pattern. However, this speculation clearly awaits detailed study.
αvβ6 is also implicated in TGFb activation (Margadant and Sonnenberg, 2010; Sheppard, 2005), and is associated with SCCHN (Xue et al., 2001), with a poor prognosis in CRC (Bates et al., 2005) and with inflammation of the lung (Horan et al., 2008). We confirmed αvβ6 expression in NSCLC and CRC, and found it strongly expressed in human mammary carcinomas and on the mammary line MDA-MB468. αvβ6 expression in mammary carcinoma has been previously noted (Arihiro et al., 2000). In the present study, we confirmed and extended previous results, confirming observations on mRNA that αvβ6 was expressed in the basal layers of normal colonic epithelium and in a set of kidney tubuli. As with αvβ8, expression of αvβ6 in invasive tumors of the breast and lung supports the concept of an immunosuppressive role for αvβ6 via local activation of latent TGFb at tumor margins (Thomas et al., 2006; Xue et al., 2001).
We found that αvβ5 was ubiquitous on attached tumor cells and in tumors. The expression of αvβ5 and αvβ6 was often complementary: where both were expressed, αvβ5 was frequently in stromal compartments, with αvβ6 in the tumor, for example in the breast and, to a lesser extent, in the lung and colon. In prostate carcinoma (which generally lacked αvβ6 and αvβ8 and, except the vasculature, αvβ3), αvβ5 was expressed both in the tumor cells and in tumor stroma. A switch between αvβ5 and αvβ6 which supports survival of squamous cell carcinomas has been noted (Janes and Watt, 2004), while stratified carcinomas maintain αvβ5 expression.
αvβ3 is well characterized, and we confirmed its expression in melanoma cell lines, in malignant melanoma and its vasculature, and in ovarian cancer cell lines. We also confirmed αvβ3 expression on glioma, but found it was not expressed on PC-3 prostate carcinoma cells. Unexpectedly, in clinical specimens, αvβ3 was occasionally expressed on invasive cohorts in NSCLC.
Why do RabMabs function so well in FFPE material, where murine antibodies largely fail to recognize FFPE-embedded integrins? The immunogen integrin was made in insect cells, where glycoprotein sugar processing differs from mammalian forms, and this can enhance immunogenicity (Gu et al., 2009; Tomiya et al., 2004; Wilson et al., 1998). Rabbits are out-bred, so the diversity of type I MHC loci and antibody diversity is greater than the in-bred rodent populations often used for monoclonal antibody production. Finally, lapidomorphs express CDR domains that are longer than those in rodentia (Liu and Wolf, 1998), which permits more precise spatial imaging of structures. The resulting antibody response detects integrin epitopes in FFPE material that are apparently invisible to rodent immune systems. It is very fitting that Dr. Robert Pytela, a discoverer of the integrins, invented rabbit monoclonal antibody technologies.
We have struggled to identify integrin antibodies that react reproducibly in FFPE material with integrin extracellular domains, though occasional monoclonals may do so (e.g. GoH3). FFPE tissue banks are a resource of great importance, from which integrin researchers have been essentially locked out. Of course, some polyclonal antibodies directed against the cytoplasmic domains do recognize integrins in FFPE material, but such antibodies cannot be used in viable cell flow cytometry. In addition, polyclonal antibodies are “mortal” – producer animals die. When discussing the use of antibodies in clinical and especially in diagnostic applications, monoclonals are the gold standard. The RabMabs show that, although hard, the situation is not hopeless.
In conclusion, we have routinely generated rabbit monoclonal antibodies against extracellular domains of human αv integrins. They function in biochemistry, cell biology, and FFPE immunohistology, and can report receptor RGD-occupancy of αvβ3 at nanomolar concentrations in viable cells. These RabMabs reveal unexpected integrin expression patterns, notably of αvβ8 in ovarian and mammary carcinomas, and αvβ6 in mammary carcinomas, and are proving to be invaluable tools for investigating the links between cell biology and pathologies driven by integrins.
Materials and Methods
Immunogens
Human recombinant transmembrane-truncated (DTM) extracellular domains of integrins αvβ3, αvβ5, αvβ6, and αvβ8 were generated using the baculovirus system, and purified from High Five insect cell lines (Kraft et al., 1999; Mehta et al., 1998; Xiong et al., 2007). Integrin chains were truncated at the juxta-membrane residues C-termini (αv chain: IQP987; β3: GPD718; β5: TPN719; β6: PPN706; and β8: YLR684) (Mehta et al., 1998; Ulmer, 2010). A β3A cytoplasmic domain (H722DR…RGT762) was produced as C-terminal fusion on glutathione S-transferase.
Cells and mouse monoclonal antibodies
MuMabs against integrins αvβ3 (LM609), αvβ5 (P1F6), αvβ6 (10C5), αv (17E6), and β1 (P4C10) were obtained from Millipore (Schwalbach, Germany), anti-mouse-FITC was obtained from Becton Dickinson (Heidelberg, Germany), and anti-rabbit-Alexa-488 was obtained from Invitrogen (Darmstadt, Germany). Cells from ATCC (Wessel, Germany) were cultured as recommended, in DMEM (A431; A549; Calu-6; Colo205; HT29; M21 series; M24met; MCF7; MDA-MB-231; MiaPaCa2; PC3; SKOV3; SW707; Suit-S2; U87MG; WM164), RPMI (A2780 ADR; Igrov1; Lox; MDA-MB 468; NCI-H460; Ovcar3; Raji), RPMI/Ham's F12 (1:1) (Kyse30), DMEM/Ham's F12 (C8161), or MEM alpha+ (DU145) supplemented with 10% FCS in a 5% CO2–95% air atmosphere. Human umbilical vein endothelial cells (HUVECs) were produced in-house and maintained as previously described in complete EGM MV medium (EBM: Promo cell, Heidelberg, Germany): 2% (v/v) endothelial cell growth supplement, 10 ng/ml EGF, 1 µg/ml hydrocortisone (Mikkelsen et al., 2008).
Cells with previously well characterized integrin profiles were used to investigate RabMab staining and LIBS activities. M21 cells express αvβ3 and αvβ5 (Wayner et al., 1991), and M21-L is a sub-clone with low αv expression (Cheresh and Spiro, 1987; Mitjans et al., 1995). M21-gpiib is M21-L transfected with the integrin gpIIb and expresses gpiibβ3 (Kieffer et al., 1991; Mitjans et al., 1995). HT29 cells express αvβ5 and αvβ6 (Kemperman et al., 1997). A549 cells express αvβ5, but no αvβ3 or αvβ6 (Burvenich et al., 2008; Falcioni et al., 1994).
Immunization and primary screening
RabMabs against DTM-αvβ3, DTM-αvβ5, DTM-αvβ6, DTM-αvβ8, and cytoplasmic domain of β3 were generated under proprietary protocols by Epitomics (Burlingame, CA, USA) (Epitomics inc, 2010; Pytela et al., 2008; Spieker-Polet et al., 1995), and screened by ELISA to identify antibodies specifically binding DTM-integrins (Mehta et al., 1998). Reactive hybridomas were subsequently screened by immunohistology on FFPE-sectioned human tumor cells pre-screened by flow cytometry (Table 1). Hybridomas were recloned by serial dilution and expanded. The monoclonal RabMab cells were harvested and banked. Immunoglobulin genes were amplified by PCR from the monoclonals and the heavy and light chain cDNA cloned into an EBNA expression system for recombinant antibody production. The IgGs were purified and stored under aseptic conditions at 4°C, or for long storage at −80°C. The binding characteristics of the final EBNA recombinant antibodies described here were identical with the hybridoma-derived antibodies.
Immunohistochemistry
Cultured human tumor-derived cell lines were harvested, fixed in buffered formaldehyde (4%; pH 7.0; 16–24 h, 20°C), embedded in paraffin, and set en bloc for cell-line microarrays (CMAs). Sections 3 µm thick were mounted on positively charged slides (SuperFrost Plus; Menzel-Gläser, Braunschweig, Germany) and stored at −80°C under desiccant. An automated tissue immunohistology processing machine (Discovery XT; Ventana Medical Systems Inc., Tuscon, AZ, USA [VMSI]) was used for tissue staining. Optimal procedures involved heating the de-paraffinized sections in Tris-EDTA buffer pH 8 or incubating them with protease 1 (0.5 U/ml, 8 min; 37°C: VMSI) or protease 2 (0.1 U/ml, 12 min; 37°C: VMSI). Endogenous peroxidase was blocked by incubation in 3% hydrogen peroxide. Sections were incubated with RabMabs diluted in PBS, followed by incubation with HRP conjugated secondary anti-rabbit antibody (16 min; 37°C: Ventana OmniMap or UltraMap Kits), and development with 3,3′-diaminobenzidine tetrahydrochloride (DAB)/H2O2. Counterstaining was done with haematoxylin and samples were washed, dehydrated, and mounted under Entellan® Neu (VWR, Darmstadt, Germany). The milder protease treatment (0.1 U/ml) was optimal for antibodies against αvβ3, αvβ5, αvβ6, and αvβ8. The Tris-EDTA treatment was optimal for the antibodies against β3 cytoplasmic domain and αv. Commercial FFPE human normal tissue (provitro GmbH, Berlin, Germany) and human tumor tissue (Asterand plc, Detroit, MI, USA) microarrays (TMAs) were processed in parallel to the cell line microarrays as described above.
The stained CMAs were digitized with the MiraxScan device (Zeiss, Oberkochen, Germany) to a resolution of 1 pixel = 0.23×0.23 µm2. The MiraxScan calibrated brightness for each slide prior to scanning. The scans were analyzed with the Visiopharm Integrator System image analysis software (Visiopharm A/S, Hoersholm, Denmark). Cells were detected by their darker blue nuclear and cytoplasmic staining. The positive (brown) stained area was calculated as percent area of the viable tissue area. Antibody staining (arbitrary unit) was calculated as
Antibody staining = Area fraction * (255-Intensity) of the brown color
Each RabMab was cloned into an identical IgG backbone, so their relative staining intensities reported by a labeled second layer could be directly compared.
Western blotting
Representative cell lines, shown by IHC or flow cytometry to express the target integrins, were grown to semiconfluency, harvested, and processed for Western blotting (Mitjans et al., 1995; Sipos et al., 2004). The solubilized detergent extracts were resolved by PAGE under reducing and non-reducing conditions (4–12% SDS-PAGE Bis-Tris gels; MOPS buffer system) (Invitrogen; NuPAGE-MOPS system). Molecular weight standards for enhanced chemoluminescence (ECL; Magic Marker XP, Invitrogen, Karlsruhe, Germany), Coomassie blue staining (SeeBlue2; Invitrogen, Karlsruhe, Germany), and DTM-recombinant integrins were run in parallel as mass and blotting controls. The gels were blotted onto nitrocellulose papers in a semi-dry apparatus (Trans-Blot: Biorad, Munich, Germany), blocked (PBS; 5% w/v BSA; 0.1% Tween-20), and the transferred proteins probed with the RabMabs (0.02–10 µg/ml) diluted in T-PBS (PBS; 0.1% Tween-20). After washing in T-PBS, bound RabMabs were detected using HRP-conjugated goat-anti-rabbit antibodies (1:100,000 in T-PBS: Biorad, Munich, Germany), and visualized using enhanced chemoluminescence (Lumi-Light plus; Roche, Mannheim, Germany). The images were captured in digital format (Versadoc; Biorad, Munich, Germany).
Viable cell flow cytometry
Viable cell flow cytometry on human tumor cell lines was performed essentially as detailed elsewhere (Mitjans et al., 1995). Cells were harvested from culture using trypsin (0.5 µg/ml)/EDTA (0.2 µg/ml), which did not affect expression of the integrins, and washed in FACs saline buffer (PBS; 0.9 mM CaCl2; 0.5 mM MgCl2; 0.5% w/v BSA). They were then incubated with antibody diluted in FACs buffer (1 µg/ml; 60 min; 4°C), washed and stained using Alexa488 labeled goat-anti-rabbit IgG (Invitrogen, Karlsruhe, Germany) (30 min; 4°C). Finally, cells were re-washed and subjected to flow-cytometry collecting 20000 events. Murine anti-integrin antibodies were used under identical conditions (but at 10 µg/ml). The EM00212 antibody against the β3 cytoplasmic domain does not recognize its epitope on viable cells, and was used as the isotype matched RabMab control. The mean intensity of fluorescence (MIF) was expressed as the ratio to the MIF of the negative control (cells stained with PI, with an isotype matched control, and secondary labeled antibody).
For detection of LIBS epitopes, cells were washed and suspended in FACS saline buffer and then incubated for 15 min with various concentrations of cyclic RGD peptide, reactive with integrins αvβ3 and αvβ5 (Goodman et al., 2002). Cells were then incubated with RabMabs in the presence of the peptide; the washing, staining, and flow-cytometry procedure was done as described above.
Acknowledgments
Immunization, fusion, ELISA primary screening, antibody molecular cloning procedures and recombinant antibody production were performed under a service agreement with Epitomics. We thank the Epitomics experimental teams in Burlingame, CA, USA under Dr. Li Li and the team in Vienna, under Dr. Franz Leichfried and Lis Knogler, for their expert work. All screening protocols, IHC, biochemistry, and cell biology experimental studies were devised and performed in the authors' laboratories. We thank the molecular biology and protein technology teams at Merck KGaA under Dr. Detlev Güssow and Dirk Mueller-Pompalla for the cDNA cloning and production of the immunogens, and Kerstin Leidinger, Ina Seibel, Catherine Eichhorn, and Jutta Welge for expert technical support, and Dr. Francesc Mitjans who supported the early stages of this project. Dr. Sandra Mendes kindly commented on the manuscript.
Footnotes
Competing interests: The authors declare no competing interests apart from their corporate affiliation.
References
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. (1990). Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 50, 6757–6764. [PubMed] [Google Scholar]
- Albert J. M., Cao C., Geng L., Leavitt L., Hallahan D. E., Lu B. (2006). Integrin alpha v beta 3 antagonist Cilengitide enhances efficacy of radiotherapy in endothelial cell and non-small-cell lung cancer models. Int. J. Radiat. Oncol. Biol. Phys. 65, 1536–1543 10.1016/j.ijrobp.2006.04.036 [DOI] [PubMed] [Google Scholar]
- Allman R., Cowburn P., Mason M. (2000). In vitro and in vivo effects of a cyclic peptide with affinity for the alpha(nu)beta3 integrin in human melanoma cells. Eur. J. Cancer 36, 410–422 10.1016/S0959-8049(99)00279-8 [DOI] [PubMed] [Google Scholar]
- Arihiro K., Kaneko M., Fujii S., Inai K., Yokosaki Y. (2000). Significance of alpha 9 beta 1 and alpha v beta 6 integrin expression in breast carcinoma. Breast Cancer 7, 19–26 10.1007/BF02967183 [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Goodman S. L., Xiong J. P. (2007). Structure and mechanics of integrin-based cell adhesion. Curr. Opin. Cell Biol. 19, 495–507 10.1016/j.ceb.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates R. C., Bellovin D. I., Brown C., Maynard E., Wu B., Kawakatsu H., Sheppard D., Oettgen P., Mercurio A. M. (2005). Transcriptional activation of integrin ß6 during the epithelial-mesenchymal transition defines a novel prognostic indicator of aggressive colon carcinoma. J. Clin. Invest. 115, 339–347 10.1172/JCI200523183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer K., Mierke C., Behrens J. (2007). Expression profiling reveals genes associated with transendothelial migration of tumor cells: a functional role for alphavbeta3 integrin. Int. J. Cancer 121, 1910–1918 10.1002/ijc.22879 [DOI] [PubMed] [Google Scholar]
- Bauerle T., Komljenovic D., Merz M., Berger M. R., Goodman S. L., Semmler W. (2011). Cilengitide inhibits progression of experimental breast cancer bone metastases as imaged noninvasively using VCT, MRI and DCE-MRI in a longitudinal in vivo study. Int. J. Cancer 128, 2453–2462 10.1002/ijc.25563 [DOI] [PubMed] [Google Scholar]
- Bellis S. L. (2004). Variant glycosylation: an underappreciated regulatory mechanism for [beta]1 integrins. Biochim. Biophys. Acta 1663, 52–60 10.1016/j.bbamem.2004.03.012 [DOI] [PubMed] [Google Scholar]
- Benedetto S., Pulito R., Crich S. G., Tarone G., Aime S., Silengo L., Hamm J. (2006). Quantification of the expression level of integrin receptor alpha(v)beta3 in cell lines and MR imaging with antibody-coated iron oxide particles. Magn. Reson. Med. 56, 711–716 10.1002/mrm.21023 [DOI] [PubMed] [Google Scholar]
- Burvenich I., Schoonooghe S., Vervoort L., Dumolyn C., Coene E., Vanwalleghem L., Van Huysse J., Praet M., Cuvelier C., Mertens N. et al. (2008). Monoclonal antibody 14C5 targets integrin αvβ5. Mol. Cancer Ther. 7, 3771–3779 10.1158/1535-7163.MCT-08-0600 [DOI] [PubMed] [Google Scholar]
- Cambier S., Mu D. Z., O'Connell D., Boylen K., Travis W., Liu W. H., Broaddus V. C., Nishimura S. L. (2000). A role for the integrin alphavbeta8 in the negative regulation of epithelial cell growth. Cancer Res. 60, 7084–7093. [PubMed] [Google Scholar]
- Campbell I. D., Humphries M. J. (2011). Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 3, a004994 10.1101/cshperspect.a004994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Du P., Jiang S. H., Jin G. H., Huang Q. L., Hua Z. C. (2008). Enhancement of antitumor properties of TRAIL by targeted delivery to the tumor neovasculature. Mol. Cancer Ther. 7, 851–861 10.1158/1535-7163.MCT-07-0533 [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Spiro R. C. (1987). Biosynthetic and functional properties of an Arg-Gly-Asp- directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J. Biol. Chem. 262, 17703–17711. [PubMed] [Google Scholar]
- Clark E. A., Trikha M., Markland F. S., Brugge J. S. (1994). Structurally distinct disintegrins contortrostatin and multisquamatin differentially regulate platelet tyrosine phosphorylation. J. Biol. Chem. 269, 21940–21943. [PubMed] [Google Scholar]
- Cox D., Brennan M., Moran N. (2010). Integrins as therapeutic targets: lessons and opportunities. Nat. Rev. Drug Discov. 9, 804–820 10.1038/nrd3266 [DOI] [PubMed] [Google Scholar]
- Dechantsreiter M., Planker E., Mathä B., Lohof E., Hölzemann G., Jonczyk A., Goodman S. L., Kessler H. (1999). N-methylated cyclic RGD peptides as highly active and selective αvβ3 integrin antagonists. J. Med. Chem. 42, 3033–3040 10.1021/jm970832g [DOI] [PubMed] [Google Scholar]
- Deryugina E. I., Bourdon M. A., Jungwirth K., Smith J. W., Strongin A. Y. (2000). Functional activation of integrin alpha V beta 3 in tumor cells expressing membrane-type 1 matrix metalloproteinase. Int. J. Cancer 86, 15–23 [DOI] [PubMed] [Google Scholar]
- Desgrosellier J. S., Cheresh D. A. (2010). Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 10, 9–22 10.1038/nrc2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du X., Gu M., Weisel J. W., Nagaswami C., Bennett J. S., Bowditch R., Ginsberg M. H. (1993). Long range propagation of conformational changes in integrin alpha IIb beta 3. J. Biol. Chem. 268, 23087–23092. [PubMed] [Google Scholar]
- Epitomics. (2010). Epitomics Rabbit Monoclonal Antibodies Burlingam, CA, USA: Epitomics. [Google Scholar]
- Falcioni R., Cimino L., Gentileschi M. P., D'Agnano I., Zupi G., Kennel S. J., Sacchi A. (1994). Expression of beta 1, beta 3, beta 4, and beta 5 integrins by human lung carcinoma cells of different histotypes. Exp. Cell Res. 210, 113–122 10.1006/excr.1994.1017 [DOI] [PubMed] [Google Scholar]
- Fang L., Deng Z., Shatseva T., Yang J., Peng C., Du W. W., Yee A. J., Ang L. C., He C., Shan S. W. et al. (2011). MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-[beta]8. Oncogene 30, 806–821 10.1038/onc.2010.465 [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B., Mueller B. M., Romerdahl C. A., Cheresh D. A. (1992). Involvement of integrin alpha V gene expression in human melanoma tumorigenicity. J. Clin. Invest. 89, 2018–2022 10.1172/JCI115811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornaro M., Languino L. R. (1997). Alternatively spliced variants: A new view of the integrin cytoplasmic domain. Matrix Biol. 16, 185–193 10.1016/S0945-053X(97)90007-X [DOI] [PubMed] [Google Scholar]
- Frelinger A. L., III Cohen I., Plow E. F., Smith M. A., Roberts J., Lam S. C. T., Ginsberg M. H. (1990). Selective Inhibition of Integrin function by antibodies for ligand-occupied receptor conformers. J. Biol. Chem. 265, 6346–6352. [PubMed] [Google Scholar]
- Frelinger A. L., Du X. P., Plow E. F., Ginsberg M. H. (1991). Monoclonal antibodies to ligand-occupied conformers of integrin alpha IIb beta 3 (glycoprotein IIb-IIIa) alter receptor affinity, specificity, and function. J. Biol. Chem. 266, 17106–17111. [PubMed] [Google Scholar]
- Godefroy E., Moreau-Aubry A., Diez E., Dreno B., Jotereau F., Guilloux Y. (2005). αvβ3-dependent cross-presentation of matrix metalloproteinaseΓÇô2 by melanoma cells gives rise to a new tumor antigen. J. Exp. Med. 202, 61–72 10.1084/jem.20042138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. L., Holzemann G., Sulyok G. A., Kessler H. (2002). Nanomolar small molecule inhibitors for alphav(beta)6, alphav(beta)5, and alphav(beta)3 integrins. J. Med. Chem. 45, 1045–1051 10.1021/jm0102598 [DOI] [PubMed] [Google Scholar]
- Gu J., Isaji T., Sato Y., Kariya Y., Fukuda T. (2009). Importance of N-glycosylation on alpha5beta1 integrin for its biological functions. Biol. Pharm. Bull. 32, 780–785 10.1248/bpb.32.780 [DOI] [PubMed] [Google Scholar]
- Hart I. R., Birch M., Marshall J. F. (1991). Cell adhesion receptor expression during melanoma progression and metastasis. Cancer Metastasis Rev. 10, 115–128 10.1007/BF00049409 [DOI] [PubMed] [Google Scholar]
- Haywood-Reid P. L., Zipf D. R., Springer W. R. (1997). Quantification of integrin subunits on human prostatic cell lines-comparison of nontumorigenic and tumorigenic lines. Prostate. 31, 1–8 [DOI] [PubMed] [Google Scholar]
- Honda S., Tomiyama Y., Pelletier A. J., Annis D., Honda Y., Orchekowski R., Ruggeri Z., Kunicki T. J. (1995). Topography of ligand-induced binding sites, including a novel cation-sensitive epitope (AP5) at the amino terminus, of the human integrin beta 3 subunit. J. Biol. Chem. 270, 11,947–11,954. [DOI] [PubMed] [Google Scholar]
- Horan G. S., Wood S., Ona V., Li D. J., Lukashev M. E., Weinreb P. H., Simon K. J., Hahm K., Allaire N. E., Rinaldi N. J. et al. (2008). Partial Inhibition of Integrin {alpha}v{beta}6 Prevents Pulmonary Fibrosis Without Exacerbating Inflammation. Am. J. Respir. Crit. Care Med. 177, 56–65 10.1164/rccm.200706-805OC [DOI] [PubMed] [Google Scholar]
- Hynes R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- Janes S. M., Watt F. M. (2004). Switch from alphavbeta5 to alphavbeta6 integrin expression protects squamous cell carcinomas from anoikis. J. Cell Biol. 166, 419–431 10.1083/jcb.200312074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemperman H., Wijnands Y. M., Roos E. (1997). alphaV Integrins on HT-29 colon carcinoma cells: adhesion to fibronectin is mediated solely by small amounts of alphaVbeta6, and alphaVbeta5 is codistributed with actin fibers. Exp. Cell Res. 234, 156–164 10.1006/excr.1997.3599 [DOI] [PubMed] [Google Scholar]
- Kieffer N., Fitzgerald L. A., Wolf D., Cheresh D. A., Phillips D. R. (1991). Adhesive properties of the beta 3 integrins: comparison of GP IIb-IIIa and the vitronectin receptor individually expressed in human melanoma cells. J. Cell Biol. 113, 451–461 10.1083/jcb.113.2.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretz K., Bruderlein S., Henne C., Fietz T., Laque M., Moller P. (1994). Comparative evaluation of integrin alpha- and beta-chain expression in colorectal carcinoma cell lines and in their tumours of origin. Virchows Arch. 425, 229–236 10.1007/BF00196144 [DOI] [PubMed] [Google Scholar]
- Kraft S., Diefenbach B., Mehta R., Jonczyk A., Luckenbach G. A., Goodman S. L. (1999). Definition of an unexpected ligand recognition motif for alphav beta6 integrin. J. Biol. Chem. 274, 1979–1985 10.1074/jbc.274.4.1979 [DOI] [PubMed] [Google Scholar]
- Lacy-Hulbert A., Smith A. M., Tissire H., Barry M., Crowley D., Bronson R. T., Roes J. T., Savill J. S., Hynes R. O. (2007). Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc. Natl. Acad. Sci USA 104, 15,823–15,828 10.1073/pnas.0707421104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landen C. N., Kim T. J., Lin Y. G., Merritt W. M., Kamat A. A., Han L. Y., Spannuth W. A., Nick A. M., Jennnings N. B., Kinch M. S. et al. (2008). Tumor-selective response to antibody-mediated targeting of alphavbeta3 integrin in ovarian cancer. Neoplasia 10, 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. (1989). An Integrin Receptor on Normal and Thrombasthenic Platelets That Binds Thrombospondin. Blood 74, 2022–2027. [PubMed] [Google Scholar]
- Lin E. C., Carron C. P., Meyer D. M., Smith J. W. (1998). A series of function blocking antibodies against the alpha v beta 3 integrin bind allosteric to the ligand binding site and induce ligand dissociation. Cell Adhes. Commun. 6, 451–464 10.3109/15419069809010794 [DOI] [PubMed] [Google Scholar]
- Liu J., Wolf B. (1998). Co-existence of somatic hypermutation and gene conversion in hypervariable regions of single Igkappa clones. Immunology 95, 291–301 10.1046/j.1365-2567.1998.00590.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo B. H., Carman C. V., Takagi J., Springer T. A. (2005). Disrupting integrin transmembrane domain heterodimerization increases ligand binding affinity, not valency or clustering. Proc. Natl. Acad. Sci. USA 102, 3679–3684 10.1073/pnas.0409440102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C., Sonnenberg A. (2010). Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 11, 97–105 10.1038/embor.2009.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maubant S., Cruet-Hennequart S., Poulain L., Carreiras F., Sichel F., Luis J., Staedel C., Gauduchon P. (2002). Altered adhesion properties and alphav integrin expression in a cisplatin-resistant human ovarian carcinoma cell line. Int. J. Cance. 97, 186–194 10.1002/ijc.1600 [DOI] [PubMed] [Google Scholar]
- Mehta R. J., Diefenbach B., Brown A., Cullen E., Jonczyk A., Gussow D., Luckenbach G. A., Goodman S. L. (1998). Transmembrane-truncated alphavbeta3 integrin retains high affinity for ligand binding: evidence for an ‘inside-out’ suppressor? Biochem. J. 330, 861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Marshall J. F., Hart I. R. (1998). Expression of alphav integrins and vitronectin receptor identity in breast cancer cells. Br. J. Cancer 77, 530–536 10.1038/bjc.1998.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen T., Brodie C., Finniss S., Berens M. E., Rennert J. L., Nelson K., Lemke N., Goodman Brown S. L., Hahn D., Neuteboom B. et al. (2008). Radiation sensitization of glioblastoma by cilengitide has unanticipated schedule-dependency. Int. J. Cancer 124, 2719–2727 10.1002/ijc.24240 [DOI] [PubMed] [Google Scholar]
- Milner R., Huang X., Wu J., Nishimura S., Pytela R., Sheppard D., ffrench-Constant C. (1999). Distinct roles for astrocyte alphavbeta5 and alphavbeta8 integrins in adhesion and migration. J. Cell Sci. 112, 4271–4279. [DOI] [PubMed] [Google Scholar]
- Mitjans F., Sander D., Adan J., Sutter A., Martinez J. M., Jaggle C. S., Moyano J. M., Kreysch H. G., Piulats J., Goodman S. L. (1995). An anti-alpha v-integrin antibody that blocks integrin function inhibits the development of a human melanoma in nude mice. J. Cell Sci. 108, 2825–2838. [DOI] [PubMed] [Google Scholar]
- Mould A. P., Garratt A. N., Askari J. A., Akiyama S. K., Humphries M. J. (1995). Identification of a novel anti-integrin monoclonal antibody that recognises a ligand-induced binding site epitope on the beta 1 subunit. FEBS Lett. 363, 118–122 10.1016/0014-5793(95)00301-O [DOI] [PubMed] [Google Scholar]
- Nagel H., Maag S., Tassis A., Nestle F. O., Greber U. F., Hemmi S. (2003). The [alpha]v[beta]5 integrin of hematopoietic and nonhematopoietic cells is a transduction receptor of RGD-4C fiber-modified adenoviruses. Gene Ther. 10, 1643–1653 10.1038/sj.gt.3302058 [DOI] [PubMed] [Google Scholar]
- Nishimura S. L., Sheppard D., Pytela R. (1994). Integrin alpha v beta 8. Interaction with vitronectin and functional divergence of the beta 8 cytoplasmic domain. J. Biol. Chem. 269, 28,708–28,715. [PubMed] [Google Scholar]
- Nishimura S. L., Boylen K. P., Einheber S., Milner T. A., Ramos D. M., Pytela R. (1998). Synaptic and glial localization of the integrin alphavbeta8 in mouse and rat brain. Brain Res. 791, 271–282 10.1016/S0006-8993(98)00118-8 [DOI] [PubMed] [Google Scholar]
- Pampori N., Hato T., Stupack D. G., Aidoudi S., Cheresh D. A., Nemerow G. R., Shattil S. J. (1999). Mechanisms and consequences of affinity modulation of integrin alpha(V)beta(3) detected with a novel patch-engineered monovalent ligand. J. Biol. Chem. 274, 21,609–21,616 10.1074/jbc.274.31.21609 [DOI] [PubMed] [Google Scholar]
- Pasqualini R., Bodorova J., Ye S., Hemler M. E. (1993). A study of the structure, function and distribution of beta 5 integrins using novel anti-beta 5 monoclonal antibodies. J. Cell Sci. 105, 101–111. [DOI] [PubMed] [Google Scholar]
- Pidgeon G. P., Tang K., Cai Y. L., Piasentin E., Honn K. V. (2003). Overexpression of Platelet-type 12-Lipoxygenase Promotes Tumor Cell Survival by enhancing alpha(v)beta(3) and alpha(v)beta(5) integrin expression. Cancer Res. 63, 4258–4267. [PubMed] [Google Scholar]
- Putnam A. J., Schulz V. V., Freiter E. M., Bill H. M., Miranti C. K. (2009). Src, PKCalpha, and PKCdelta are required for alphavbeta3 integrin-mediated metastatic melanoma invasion. Cell Commun. Signal. 7, 10 10.1186/1478-811X-7-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Zhu W., Ke Y., Qian Q., Au H. C. (2008). Fusion partner for production of rabbit monoclonal antibodies, US patent 7, 429,487. [Google Scholar]
- Schwartz M. A., Ginsberg M. H. (2002). Networks and crosstalk: integrin signalling spreads. Nat. Cell Biol. 4, E65–E68 10.1038/ncb0402-e65 [DOI] [PubMed] [Google Scholar]
- Sheppard D. (1996). Epithelial integrins. BioEssays 18, 655–660 10.1002/bies.950180809 [DOI] [PubMed] [Google Scholar]
- Sheppard D. (2005). Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev. 24, 395–402 10.1007/s10555-005-5131-6 [DOI] [PubMed] [Google Scholar]
- Sipos B., Hahn D., Carceller A., Piulats J., Hedderich J., Kalthoff H., Goodman S. L., Kosmahl M., Kloppel G. (2004). Immunohistochemical screening for beta6-integrin subunit expression in adenocarcinomas using a novel monoclonal antibody reveals strong up-regulation in pancreatic ductal adenocarcinomas in vivo and in vitro. Histopathology 45, 226–236 10.1111/j.1365-2559.2004.01919.x [DOI] [PubMed] [Google Scholar]
- Spieker-Polet H., Sethupathi P., Yam P. C., Knight K. L. (1995). Rabbit monoclonal antibodies: generating a fusion partner to produce rabbit-rabbit hybridomas. Proc. Natl. Acad. Sci. USA 92, 9348–9352 10.1073/pnas.92.20.9348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga T., Suzuki A., Gonzalez-Gomez I., Gilles F. H., Stins M., Shimada H., Barsky L., Weinberg K. I., Laug W. E. (2002). alpha v-Integrin antagonist EMD 121974 induces apoptosis in brain tumor cells growing on vitronectin and tenascin. Int. J. Cancer 98, 690–697 10.1002/ijc.10265 [DOI] [PubMed] [Google Scholar]
- Tchaicha J. H., Reyes S. B., Shin J., Hossain M. G., Lang F. F., McCarty J. H. (2011). Glioblastoma angiogenesis and tumor cell invasiveness are differentially regulated by β8 integrin. Cancer Res. 71, 6371–6381 10.1158/0008-5472.CAN-11-0991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. J., Nystrom M. L., Marshall J. F. (2006). Alphavbeta6 integrin in wound healing and cancer of the oral cavity. J. Oral Pathol. Med. 35, 1–10 10.1111/j.1600-0714.2005.00374.x [DOI] [PubMed] [Google Scholar]
- Tomiya N., Narang S., Lee Y. C., Betenbaugh M. J. (2004). Comparing N-glycan processing in mammalian cell lines to native and engineered lepidopteran insect cell lines. Glycoconj. J. 21, 343–360 10.1023/B:GLYC.0000046275.28315.87 [DOI] [PubMed] [Google Scholar]
- Trikha M., De Clerck Y. A., Markland F. S. (1994). Contortrostatin, a snake venom disintegrin, inhibits beta 1 integrin-mediated human metastatic melanoma cell adhesion and blocks experimental metastasis. Cancer Res. 54, 4993–4998. [PubMed] [Google Scholar]
- Ulmer T. S. (2010). Structural basis of transmembrane domain interactions in integrin signaling. Cell Adh. Migr. 4, 243–248 10.4161/cam.4.2.10592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Orlando R. A., Cheresh D. A. (1991). Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J. Cell Biol. 113, 919–929 10.1083/jcb.113.4.919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinacker A., Chen A., Agrez M., Cone R. I., Nishimura S., Wayner E., Pytela R., Sheppard D. (1994). Role of the Integrin alpha v beta 6 in Cell Attachment to Fibronectin - Heterologous Expression of Intact and Secreted Forms of the Receptor. J. Biol. Chem. 269, 6940–6948. [PubMed] [Google Scholar]
- Wilson I. B., Harthill J. E., Mullin N. P., Ashford D. A., Altmann F. (1998). Core alpha1,3-fucose is a key part of the epitope recognized by antibodies reacting against plant N-linked oligosaccharides and is present in a wide variety of plant extracts. Glycobiology 8, 651–661 10.1093/glycob/8.7.651 [DOI] [PubMed] [Google Scholar]
- Witkowski C. M., Rabinovitz I., Nagle R. B., Affinito K. S., Cress A. E. (1993). Characterization of integrin subunits, cellular adhesion and tumorgenicity of four human prostate cell lines. J. Cancer Res. Clin. Oncol. 119, 637–644 10.1007/BF01215981 [DOI] [PubMed] [Google Scholar]
- Xiong J. P., Stehle T., Zhang R., Joachimiak A., Frech M., Goodman S. L., Arnaout M. A. (2002). Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science 296, 151–155 10.1126/science.1069040 [DOI] [PubMed] [Google Scholar]
- Xiong J. P., Goodman S. L., Arnaout M. A. (2007). Purification, analysis, and crystal structure of integrins. Methods Enzymol. 426, 307–336 10.1016/S0076-6879(07)26014-8 [DOI] [PubMed] [Google Scholar]
- Xue H., Atakilit A., Zhu W., Li X., Ramos D. M., Pytela R. (2001). Role of the alpha(v)beta6 integrin in human oral squamous cell carcinoma growth in vivo and in vitro. Biochem. Biophys. Res. Commun. 288, 610–618 10.1006/bbrc.2001.5813 [DOI] [PubMed] [Google Scholar]
- Zheng D. Q., Woodard A. S., Tallini G., Languino L. R. (2000). Substrate specificity of alpha(v)beta(3) integrin-mediated cell migration and phosphatidylinositol 3-kinase/AKT pathway activation. J. Biol. Chem. 275, 24565–24574 10.1074/jbc.M002646200 [DOI] [PubMed] [Google Scholar]