Abstract
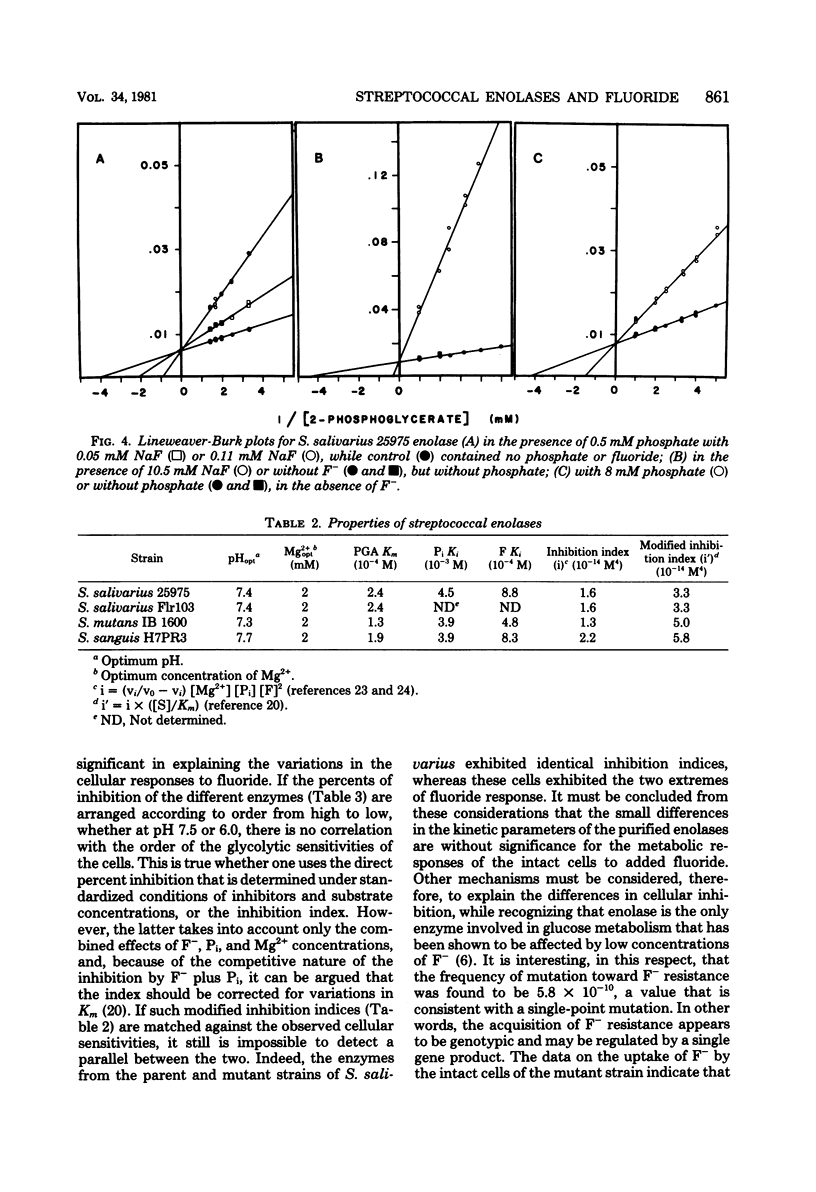
The enolase from a highly fluoride-sensitive strain of Streptococcus salivarius and its fluoride-resistant mutant, as well as those from strains of Streptococcus sanguis and Streptococcus mutans with intermediate and low sensitivities to fluoride have been shown to be inhibited by fluoride. Comparisons of the purified, strain-specific enzymes showed a high degree of similarity for all preparations. The Michaelis constants for the substrate 2-phosphoglycerate were 1.3 x 10(-4) to 2.4 x 10(-4) M, pH optima were 7.3 to 7.7, and Mg2+ optima were 2 mEq/liter for all. Inhibition by fluoride required the presence of inorganic phosphate and was competitive in nature, and the calculated modified inhibition indices were found to be in the range from 3.3 x 10(-14) to 5.8 x 10(-14) M4. Percent inhibitions were determined under standardized conditions (0.16 mM NaF, 2 mM MgSO4, 0.5 mM Pi, and 0.5 mM 2-phosphoglycerate) and were found to range from 53.3 to 65.9% for all of the purified enzymes. The differences do not appear to be meaningful metabolically. Inhibition was reduced to about 14% at pH 6.0. From the similarities in the behavior of the strain-specific enzymes it is concluded that the differences in the glycolytic sensitivities of the different strains of streptococci to fluoride are not the consequence of any kinetic differences between the respective enolases.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brudevold F., Moreno E., Bakhos Y. Fluoride complexes in drinking water. Arch Oral Biol. 1972 Aug;17(8):1155–1163. doi: 10.1016/0003-9969(72)90086-6. [DOI] [PubMed] [Google Scholar]
- Cimasoni G. The inhibition of enolase by fluoride in vitro. Caries Res. 1972;6(2):93–102. doi: 10.1159/000259782. [DOI] [PubMed] [Google Scholar]
- Cory R. P., Wold F. Isolation and characterization of enolase from rainbow trout (Salmo gairdnerii gairdnerii). Biochemistry. 1966 Oct;5(10):3131–3137. doi: 10.1021/bi00874a008. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R. Effects of fluoride on enzymatic regulation of bacterial carbohydrate metabolism. Caries Res. 1977;11 (Suppl 1):262–291. doi: 10.1159/000260304. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Spitz E. Accumulation of arsenate, phosphate, and aspartate by Sreptococcus faecalis. J Bacteriol. 1975 Apr;122(1):266–277. doi: 10.1128/jb.122.1.266-277.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- JENKINS G. N. The effect of pH on the fluoride inhibition of salivary acid production. Arch Oral Biol. 1959 Aug;1:33–41. doi: 10.1016/0003-9969(59)90019-6. [DOI] [PubMed] [Google Scholar]
- Jenkins G. N., Edgar W. M. The distribution and metabolic effects of human plaque fluorine. Arch Oral Biol. 1969 Jan;14(1):105–119. doi: 10.1016/0003-9969(69)90025-9. [DOI] [PubMed] [Google Scholar]
- Jenkins G. N. The mechanism of action of fluoride in reducing caries incidence. Int Dent J. 1967 Sep;17(3):552–563. [PubMed] [Google Scholar]
- Kashket S., Bunick F. J. Binding of fluoride in oral streptococci. Arch Oral Biol. 1978;23(11):993–996. doi: 10.1016/0003-9969(78)90255-8. [DOI] [PubMed] [Google Scholar]
- Kashket S., Rodriguez V. M., Bunick F. J. Inhibition of glucose utilization in oral streptococci by low concentrations of fluoride. Caries Res. 1977;11(6):301–307. doi: 10.1159/000260283. [DOI] [PubMed] [Google Scholar]
- Kashket S., Rodriguez V. M. Fluoride accumulation by a strain of human oral Streptococcus sanguis. Arch Oral Biol. 1976;21(8):459–464. doi: 10.1016/0003-9969(76)90103-5. [DOI] [PubMed] [Google Scholar]
- Larsen M. J. Dissolution of enamel. Scand J Dent Res. 1973;81(7):518–522. doi: 10.1111/j.1600-0722.1973.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Rider C. C., Taylor C. B. Enolase isoenzymes in rat tissues. Electrophoretic, chromatographic, immunological and kinetic properties. Biochim Biophys Acta. 1974 Sep 13;365(1):285–300. doi: 10.1016/0005-2795(74)90273-6. [DOI] [PubMed] [Google Scholar]
- Ruth R. C., Soja D. M., Wold F. Purification and characterization of enolases from coho (Oncorhynchus kisutch) and chum (Oncorhynchus keta) salmon. Arch Biochem Biophys. 1970 Sep;140(1):1–10. doi: 10.1016/0003-9861(70)90002-0. [DOI] [PubMed] [Google Scholar]
- Sandham H. J., Kleinberg I. Effect of fluoride on carbon dioxide and acid formation in salivary sediment mixtures incubated with glucose. Arch Oral Biol. 1973 Feb;18(2):211–225. doi: 10.1016/0003-9969(73)90141-6. [DOI] [PubMed] [Google Scholar]
- Singh R. P., Setlow P. Enolase from spores and cells of Bacillus megaterium: two-step purification of the enzyme and some of its properties. J Bacteriol. 1978 Apr;134(1):353–355. doi: 10.1128/jb.134.1.353-355.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring T. G., Wold F. The purification and characterization of Escherichia coli enolase. J Biol Chem. 1971 Nov 25;246(22):6797–6802. [PubMed] [Google Scholar]
- Wang T., Himoe A. Kinetics of the rabbit muscle enolase-catalyzed dehydration of 2-phosphoglycerate. Fluoride and phosphate inhibition. J Biol Chem. 1974 Jun 25;249(12):3895–3902. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


