Abstract
Optimal management of optic pit–related maculopathy remains to be determined. The fluid source for the maculopathy also remains controversial. In this article, we present a unique surgical technique for internal drainage of the intraretinal fluid and describe the intraoperative use of spectral-domain optical coherence tomography to assist in the surgical management of this condition. Pars plana vitrectomy was performed with elevation of the posterior hyaloid. Following an air-fluid exchange, aspiration over the optic nerve pit was performed. Following aspiration, intraoperative spectral-domain optical coherence tomography demonstrated collapse of the retinoschisis, strongly suggesting a connection between the vitreous cavity and the intraretinal fluid.
Optic nerve pit–associated maculopathy continues to pose a significant management challenge to clinicians. Optic pits are a congenitally anomalous disc abnormality secondary to colobomatous malformation of the optic nerve head.1 They often pose no significant clinical manifestation, but in approximately 40% to 50% of patients they may result in a maculopathy (eg, serous retinal detachment, retinoschisis).1 The pathogenesis of the macular abnormalities as well as the source of the fluid continue to be controversial, with various theories proposed for both the source of the abnormal fluid as well as the conduit for the fluid.2,3 Advances in optical coherence tomography (OCT) are helping to advance our understanding of the ultrastructural characteristics of optic pits and associated maculopathy as well as assisting clinicians in evaluating clinical outcome following treatment.4-6 Additionally, intraoperative spectral-domain OCT (SD-OCT) has advanced our understanding of various vitreoretinal diseases and the effects of surgical interventions on the anatomical configuration of those conditions.7,8
In this study, we used a handheld intraoperative SD-OCT device (Bioptigen, Research Triangle Park, North Carolina) to analyze the effects of surgical maneuvers during pars plana vitrectomy (PPV) for macular retinoschisis associated with an optic pit. High-resolution images of the optic nerve head and the macula were obtained with serial volumetric analysis. Intraoperative imaging established a likely connection between the vitreous cavity and the macular retinoschisis. We were unable to identify any other published cases demonstrating the probable in vivo connection between the vitreous cavity and the intraretinal fluid as well as the use of intraoperative SD-OCT in the analysis of PPV for this condition.
METHODS
REPORT OF A CASE
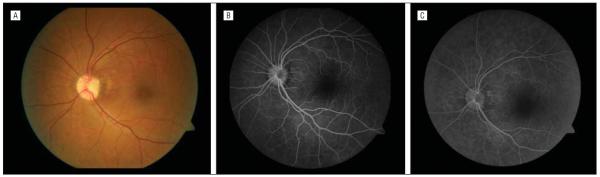
A 50-year-old man was referred for chronic macular edema in his left eye. Results from examination of the right eye were normal. Visual acuity was 20/50 OS. An anomalous optic disc with an optic pit, focal peripapillary pigmentary changes, and diffuse macular swelling consistent with edema or retinoschisis were present (Figure 1A). Fluorescein angiography findings were within normal limits in both eyes except for a mild window defect in the area of the pigmentary changes in the left eye (Figure 1B and C). Imaging with SD-OCT showed diffuse macular retinoschisis with increased prominence in the peripapillary region in the left eye (Figure 2A-C). The patient consented to intraoperative imaging under an institutional review board-approved protocol.
Figure 1.
Fundus photograph and fluorescein angiograms. A, Color fundus photograph of the left eye showing the optic nerve pit and peripapillary pigmentary changes with blunting of the foveal light reflex. B, Midphase fluorescein angiogram showing a mild window defect in the area of the pigmentary changes. C, Late-phase fluorescein angiogram without evidence of leakage.
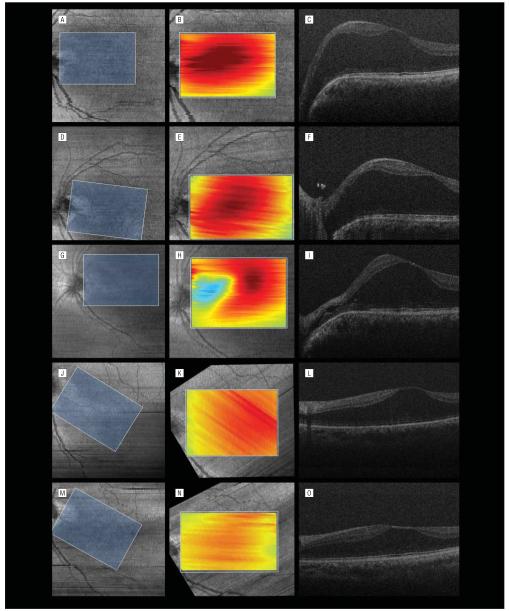
Figure 2.
Serial volumetric analysis of overlapping macular areas with spectral-domain optical coherence tomography (SD-OCT). A, D, G, J, and M, The first column shows summed voxel projections with corresponding overlapping areas (blue rectangles). The summed voxel projections are rotated because of varied orientations of the handheld SD-OCT probe. B, E, H, K, and N, In the second column, each summed voxel projection is rotated to align with the overlapped area. Included in the overlapped area is the overlay of a tomographic map of the volumetric analysis. Red corresponds to the highest retinal thickness; blue, the lowest retinal thickness. C, F, I, L, and O, The third column shows corresponding B-scans taken at the various times. A-C, Preoperative SD-OCT imaging with significant diffuse macular thickening, more prominent on the nasal side of the fovea. D-F, Intraoperative SD-OCT imaging following elevation of the posterior hyaloid. Prominent nasal elevation and diffuse retinoschisis are present with residual hyaloidal elements present at the optic nerve. E, The thickness map is similar to the preoperative scan. G-I, Intraoperative SD-OCT imaging following air-fluid exchange with aspiration over the optic pit. H and I, Imaging is performed after refilling the eye with fluid. Collapse of the schisis in the nasal aspect of the macula is noted following aspiration, verifying aspiration of intraretinal fluid. H, The thickness map demonstrates decreased retinal thickening in the peripapillary region. J-L, Postoperative SD-OCT imaging at 1 month revealing decreased foveal height and macular retinoschisis with some nasal redistribution of the fluid from the temporal macula. M-O, Postoperative SD-OCT imaging at 9 months revealing continued resolution of the intraretinal fluid.
SURGICAL TECHNIQUE
During surgery but prior to PPV, Doppler SD-OCT imaging was performed to look for a lumen connecting the optic pit to the retinoschisis; however, none were found. All hyporeflective tubular areas in the region of interest were verified to be vascular structures on Doppler SD-OCT imaging. Small-gauge (25-gauge) PPV was performed with elevation of the posterior hyaloid, which was firmly attached at the optic nerve and throughout the posteriorpole. Imaging with SD-OCT showed no resolution of schisis fluid after this maneuver (Figure 2D-F). Fluid-air exchange was performed with aspiration of fluid via a soft-tipped cannula at the optic disc immediately over the site of the optic pit. The vitreous cavity was then refilled with fluid, and SD-OCT imaging documented the collapsed configuration of the schisis (Figure 2G-I). A partial fluid-air exchange (<50% air)was performed for 25-gauge sclerotomy closure. This did not tamponade the macula or disc and there was no face-down positioning. At 1 month, visual acuity was 20/40 with improvement in the preoperative architecture and redistribution of the temporal intraretinal fluid to include the peripapillary retina compared with the final intraoperative imaging findings (Figure 2J-L). At 9 months, visual acuity remained 20/40 with further reduction in retinoschisis (Figure 2M-O).
Serial volumetric analysis was performed using MATLAB (MathWorks, Natick, Massachusetts) and PowerPoint (Microsoft Corp, Redmond, Washington) software. Summed voxel projection images were stacked in PowerPoint to overlap identical areas on each of the scans using landmarks such as retinal vessels, optic nerve, and fovea. Because of small rotation of the handheld probe between imaging sessions, alignment of the rectangles required rotating and laterally shifting each rectangle. A 4.5×6.6-mm rectangular area was common to all scans and was traced over the stack in PowerPoint. For each scan, the boundaries of the rectangle were determined on the summed voxel projections, and the B-scans in the common area for each session were entered into MATLAB to determine the macular volume of each scan through summation of the corresponding segmented areas of retinal thickness. Macular volume was 8.49 mm3 immediately prior to surgery and 7.86 mm3 after posterior hyaloid elevation. Following air-fluid exchange with optic pit aspiration, the volume decreased to 7.25 mm3. At 1 and 9 months postoperatively, the macular volume was 7.00 and 6.15 mm3, respectively.
COMMENT
This article describes a unique technique for internal drainage of intraretinal fluid secondary to an optic pit. Additionally, we used real-time anatomical evaluation of optic nerve pit surgery by performing intraoperative SD-OCT for examination and measurements prior to and after surgical manipulations (ie, elevating the posterior hyaloid, fluid-air exchange with optic pit aspiration).
The observations in this case support a functional connection between the vitreous cavity and the intraretinal schisis, even though no lumen was visible on high-resolution SD-OCT. Traction on the inner retina during posterior hyaloid separation appeared to increase the nasal height of the schisis, while aspiration at the optic pit clearly decreased the macular volume and caused a localized collapse of the schisis at the nasal aspect of the macula, proximal to the optic nerve pit. The macular volume decreased both following posterior hyaloid elevation and following aspiration at the optic pit. High aspiration during engagement of the hyaloid may have resulted in fluid aspiration through the pit. Once the tractional force of the hyaloid was removed, direct aspiration appeared to result in the collapse of a portion of the macular schisis. This along with the absence of any visible connecting channel on SD-OCT suggest a more sievelike connection between the vitreous cavity and the intraretinal space at the pit. As previously reported, the ability to internally drain fluid also supports a rhegmatogenous component to the fluid accumulation.1
Although a connection between the vitreous cavity and the intraretinal space appears most likely given our findings, additional mechanisms for the observations should be considered. Rather than fluid moving to the vitreous, the fluid could shift away from the peripapillary region either anteriorly into the more peripheral retina or posteriorly into the retrolaminar subarachnoid space of the optic nerve. The former is less likely because of the supine position of the patient and the specific gravity of fluid vs air. A decrease in the macular volume would require the anterior shift to be significant enough to shift the fluid outside the analyzed area. We cannot rule out the possibility that there may have been a posterior shift in fluid to the subarachnoid space of the optic nerve rather than a drainage from the vitreous cavity. Our measurements indicate that the macular volume decreased, suggesting that fluid was likely removed; also, we found no changes in the images of the nerve, although these do not extend deep beyond the globe. Note that the intraoperative OCT scan was completed with a fluid-filled eye following a fluid-air/air-fluid exchange.
Optimal treatment of the maculopathy is unclear. Multiple approaches have been suggested, including laser photocoagulation and PPV with various adjuncts (eg, laser, gas exchange, internal limiting membrane peeling, inner retinal fenestration).1,9-13 However, we were unable to identify other reported cases that documented intraoperative removal of intraretinal fluid through the region of the pit. Our patient demonstrated a progressive decrease in schisis volume with stability of visual acuity following vitrectomy with separation of the posterior hyaloid and fluid-air exchange with localized aspiration at the optic pit. As seen in this case, intraoperative imaging and SD-OCT can play an important role in our understanding of vitreoretinal diseases and the early effect of our surgical interventions.
Acknowledgments
Funding/Support: This work was supported by grant R21-EY-019411 from the National Eye Institute.
Footnotes
Financial Disclosure: Dr Toth has received research support and royalties from Alcon Laboratories and Bioptigen. Duke University has an equity interest in Bioptigen. Joseph Izatt, PhD, principal investigator on grant R21-EY-019411, also has equity interest in Bioptigen.
Contributor Information
Dr Justis P. Ehlers, Cole Eye Institute, Cleveland, Ohio; Duke Eye Center, Durham, North Carolina.
Ms Kendal Kernstine, Duke Eye Center, Durham, North Carolina.
Sina Farsiu, Duke Eye Center, Durham, North Carolina.
Neeru Sarin, Duke Eye Center, Durham, North Carolina.
Ramiro Maldonado, Duke Eye Center, Durham, North Carolina.
Cynthia A. Toth, Duke Eye Center, Durham, North Carolina.
REFERENCES
- 1.Postel EA, Pulido JS, McNamara JA, Johnson MW. The etiology and treatment of macular detachment associated with optic nerve pits and related anomalies. Trans Am Ophthalmol Soc. 1998;96:73–93. [PMC free article] [PubMed] [Google Scholar]
- 2.Brown GC, Shields JA, Patty BE, Goldberg RE. Congenital pits of the optic nerve head, I: experimental studies in collie dogs. Arch Ophthalmol. 1979;97(7):1341–1344. doi: 10.1001/archopht.1979.01020020083020. [DOI] [PubMed] [Google Scholar]
- 3.Gass JD. Serous detachment of the macula: secondary to congenital pit of the optic nervehead. Am J Ophthalmol. 1969;67(6):821–841. doi: 10.1016/0002-9394(69)90075-0. [DOI] [PubMed] [Google Scholar]
- 4.Konno S, Akiba J, Sato E, Kuriyama S, Yoshida A. OCT in successful surgery of retinal detachment associated with optic nerve head pit. Ophthalmic Surg Lasers. 2000;31(3):236–239. [PubMed] [Google Scholar]
- 5.Rutledge BK, Puliafito CA, Duker JS, Hee MR, Cox MS. Optical coherence tomography of macular lesions associated with optic nerve head pits. Ophthalmology. 1996;103(7):1047–1053. doi: 10.1016/s0161-6420(96)30568-x. [DOI] [PubMed] [Google Scholar]
- 6.Theodossiadis PG, Grigoropoulos VG, Emfietzoglou J, Theodossiadis GP. Vitreous findings in optic disc pit maculopathy based on optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2007;245(9):1311–1318. doi: 10.1007/s00417-007-0534-4. [DOI] [PubMed] [Google Scholar]
- 7.Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116(12):2448–2456. doi: 10.1016/j.ophtha.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dayani PN, Maldonado R, Farsiu S, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009;29(10):1457–1468. doi: 10.1097/IAE.0b013e3181b266bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annesley W, Brown G, Bolling J, Goldberg R, Fischer D. Treatment of retinal detachment with congenital optic pit by krypton laser photocoagulation. Graefes Arch Clin Exp Ophthalmol. 1987;225(5):311–314. doi: 10.1007/BF02153395. [DOI] [PubMed] [Google Scholar]
- 10.Poulson AV, Snead DR, Jacobs PM, Ahmad N, Snead MP. Intraocular surgery for optic nerve disorders. Eye (Lond) 2004;18(11):1056–1065. doi: 10.1038/sj.eye.6701572. [DOI] [PubMed] [Google Scholar]
- 11.Schaal KB, Wrede J, Dithmar S. Internal drainage in optic pit maculopathy. Br J Ophthalmol. 2007;91(8):1093. doi: 10.1136/bjo.2006.110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaide RF, Fisher Y, Ober M, Stoller G. Surgical hypothesis: inner retinal fenestration as a treatment for optic disc pit maculopathy. Retina. 2006;26(1):89–91. doi: 10.1097/00006982-200601000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Ziahosseini K, Sanghvi C, Muzaffar W, Stanga PE. Successful surgical treatment of optic disc pit maculopathy. Eye (Lond) 2009;23(6):1477–1479. doi: 10.1038/eye.2008.211. [DOI] [PubMed] [Google Scholar]