Abstract
γ-secretase is a protease complex with at least four components: presenilin, nicastrin (NCT), anterior pharynx-defective 1 (Aph-1), and presenilin enhancer 2 (Pen-2). In this study, using knockout cell lines and small interfering RNA technology, our data demonstrated that the disappeared presenilin 1 C-terminal fragment (PS1C) caused by knockdown of pen-2 or knockout of NCT or Aph-1 was recovered by the addition of proteasome inhibitors, indicating that Pen-2, as well as NCT and Aph-1α , is dispensable for presenilin endoproteolysis. Our data also demonstrate that the formation of the nicastrin-Aph-1 subcomplex plays not only an important role in γ-secretase complex assembly but also in recruiting substrate C-terminal fragment of amyloid precursor protein generated by β-cleavage (CTFβ). Ablating any one component resulted in the instability of other components of the γ-secretase complex, and the presence of all three of the other components is required for full maturation of NCT.
Keywords: Alzheimer’s disease, gamma-secretase, presenilin, Pen-2, APP
β-amyloid peptide (Aβ) is produced from a large amyloid precursor protein (APP) by β-secretase and γ-secretase; the latter cleaves APP within its transmembrane domain at multiple sites in a sequential manner: first at ε-cleavage at Aβ49, rapidly followed by ζ-cleavage at Aβ46 and γ-cleavage at Aβ40/42 (Xu, 2009).. According to the “amyloid cascade hypothesis,” the ratio of long Aβ vs short Aβ, Aβ42/Aβ40, is a key factor in the development and pathogenesis of AD (Zhang and Xu, 2007). Mutations in the presenilin (PS) protein, which functions as the catalytic core of the γ-secretase complex, have been found either to increase the total Aβ load or increase the Aβ42/Aβ40 ratio (Borchelt et al., 1996; De Strooper and Annaert, 2000). Therefore, understanding the molecular nature of the γ-secretase complex and its biological function with respect to processing of APP and Aβ formation is critical for understanding AD pathology. A functional γ-secretase complex consists of presenilin (PS1 or PS2) and three other transmembrane proteins: nicastrin (NCT), anterior pharynx defective 1 (Aph-1), and PS enhancer 2 (PEN-2) (Dries and Yu, 2008). Presenilins are believed to be nine-pass transmembrane proteins that undergo endoproteolytic processing between the 6th and 7th transmembrane domains resulting in a 28 kDa N-terminal fragment (PSN) and a 17 kDa C-terminal fragment (PSC) (Thinakaran et al., 1996). The discoveries that knockout of both PS1 and PS2 results in the abolishment of γ-secretase activity (De Strooper et al., 1998; Herreman et al., 2000; Zhang et al., 2000) and that two conserved aspartate residues in the 6th and 7th transmembrane domains of PS have been identified as essential for γ-secretase activity (Kimberly et al., 2000; Wolfe et al., 1999) suggest that PS bear the γ-secretase active site. NCT has been suggested to function as the substrate receptor (Shah et al., 2005). Using siRNA technology, studies suggested that Aph-1 is required for stabilization of the PS1 endoproteolysis products PS1N and PS1C (Francis et al., 2002; Lee et al., 2002; Steiner et al., 2002) and that Pen-2 is required for endoproteolysis of PS1 (Luo et al., 2003; Takasugi et al., 2003). However, data from our current study, using knockout cell lines and siRNA technology, indicate Pen-2 is dispensable for the endoproteolysis of PS1. Our study also revealed several other interesting findings that contribute to a better understanding of the role of each γ-secretase component in the assembly and functional activity of the γ-secretase complex.
Methods
Cell culture
Mouse embryonic fibroblast (MEF) cells obtained from PS1/PS2-KO (Herreman et al., 2000), NCT-KO (Li et al., 2003), APH-KO (Ma et al., 2005), and wild type MEFs were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum.
Immunoprecipitation and Western blotting
Immunoprecipitation and Western blotting were carried out as described previously (Zhao et al., 2004).
siRNA treatment
Both siRNAs and delivery reagent were purchased from Qiagen (Valencia, CA, USA), and treatment of cells with siRNAs was carried out according to the manufacturer’s instruction.
Materials
Proteasome inhibitor MG132 was purchased from Peptides International (Louisville, KY, USA). γ-secretase inhibitors compound E and L685, 458 were from EMD Chemicals (Gibbstown, NJ, USA). Polyclonal antibodies against components of γ-secretase were raised or purchased as follows: Anti-PS1N and anti-PS1C were raised N-terminal (residues 27-50) and C-terminal (residues 307-321) peptides of PS1 as described previously (Xu et al., 2002; Zhao et al., 2004); Ab14, a PS1N-specific antibody used in a previous study (Luo et al., 2003) was received as a gift from Dr. Huaxi Xu (Sanford-Burnham Medical Research Institute, San Diego, CA, USA); Commercial anti-PS1N (N-19) and anti-PS1C (C-20) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA); Anti-NCT from Sigma-Aldrich (St. Louis, MO, USA); Polyclonal antibodies anti-APH1aL and anti-PEN-2N and the monoclonal antibody 6E10 against the first 17 amino acids of Aβ were from Covance (Emeryville, CA, USA). The other anti-PEN-2 antibody used, anti-PEN2C, was raised against a peptide with corresponding PEN-2 residues from 86 to 101; A commercial anti-PEN-2 raised against the full length of PEN-2 was from Santa Cruz.
Results
Pen-2, NCT, and Aph-1 are not required for PS endoproteolytic processing, but are required for stabilizing the endoproteolytic products of PS
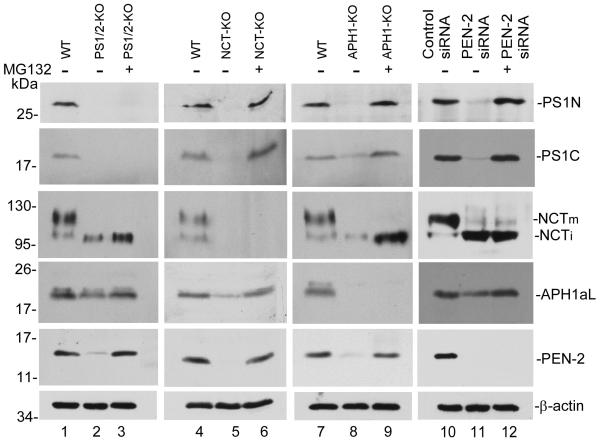
To achieve knockdown of Pen-2, the wild type MEFs stably expressing human Swedish mutant APP were treated with Pen-2 siRNA. As shown in Fig. 1A, after 6 days’ treatment (transfected every 2 days), Pen-2 protein decreased to a level almost undetectable (lanes 11 and 12). After confirming complete Pen-2 knockdown, the Pen-2 knockdown (Pen-2-KD) cells, along with other knockout cells (i.e., PS1 and PS2 double knockout [PS1/2-KO], NCT knockout [NCT-KO], and Aph-1 knockout [Aph-1-KO] cells), were incubated in the presence or absence of proteasome inhibitor MG132 for an additional 10 h. As shown in Fig. 1A, ablation of one of the four components, either by genetic knockout or by siRNA knockdown, resulted in instability of the other components. Ablation of any other component resulted in a striking reduction in the levels of PS1 N-terminal fragment (PS1N) and PS1 C-terminal fragment (PS1C) (lanes 5, 8, and 11, top and second rows) and Pen-2 protein (lanes 2, 5, and 8, fifth row); a strong inhibition of NCT maturation and a reduction in the level of NCT (lanes 2, 8, and 11, third row); and a significant reduction in the level of Aph-1 protein (lanes 2, 5, and 11, fourth row). Interestingly, when the proteasome inhibitor MG132 (or lactacystin, data not shown) was added, the decrease of these proteins caused by ablation of other components of γ-secretase was strongly inhibited (third lane of each column). This result indicates that when one component is ablated, the other three components undergo proteasomal degradation. It is particularly noteworthy that PS1N and PS1C, which disappeared in Pen-2-knockdown cells, were recovered by addition of proteasome inhibitors. This finding strongly indicates that PS1 was endoproteolytically processed in the absence of Pen-2 just as in the case of knockout of NCT and Aph-1, i.e. Pen-2 is dispensable for PS endoproteolysis. A similar result was also observed when human HEK293 was used (data not shown).
Fig. 1.
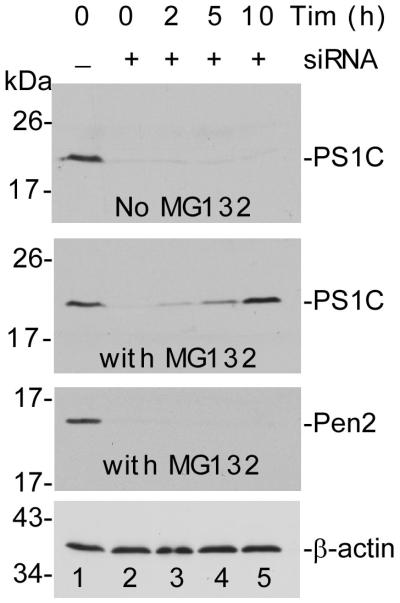
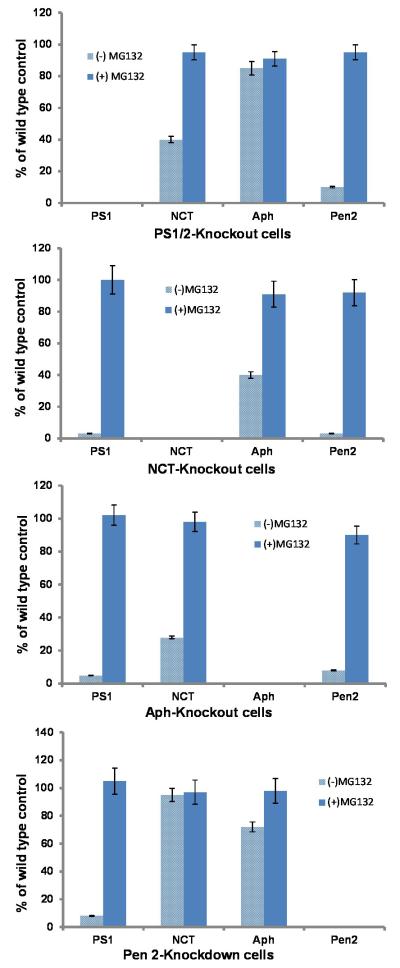
PS1C is generated in Pen-2 knockdown cells. (a) Wild type MEF cells (WT), nicastrin knockout cells (NCT-KO), Aph-1 knockout cells (Aph-KO), Pen-2 siRNA-treated (Pen-2 siRNA), and non-silenced siRNA-treated wild type MEF cells were cultured in the presence (+) or absence (−) of proteasome inhibitor MG132 for 10 h. Cell lysates were analyzed by SDS-PAGE followed by Western blotting using antibodies specific to each γ-secretase component. NCTm indicates the mature and NCTi indicates the immature form of NCT. The bottom row is the reprobe of the first row for β-actin to confirm equal loading of samples. (b) Time course of PS1C generation after knockdown of Pen-2. The bottom panel is the reprobe of the second panel for β-actin to confirm equal loading of samples. (c) Graphs represent the levels of each γ-secretase component under different conditions expressed as percentage of that detected in the wild type cells in the same blot based on the densitometric analysis of three repeated Western blots shown in (a) using Gel Digitizing Software UN-SCAN-IT (Silk Scientific, Orem, UT, USA). The density of each band was measured and normalized against actin. Each bar is a mean ± SD (n = 30). Since the full-length APP is undetectable under our experimental conditions, the PS1 level was estimated as the sum of PS1N and PS1C.
To rule out the possibility that the PS1N and PS1C detected in the presence of MG132 are produced before knockdown of Pen-2, we performed a time course experiment. As shown in Fig. 1B, at the time at which knockdown of Pen-2 was confirmed after 6 days of treatment with Pen-2 siRNA (lane 2, third panel), upon addition of MG132, the PS1C that had disappeared was recovered in a time-dependent manner, and fully recovery at 10 h (compare lanes 5 and 1, second panel). However, in the absence of MG132, PS1C was almost undetectable through the time course (lanes 2 to 5, top panel). This result clearly indicates that the accumulated PS1C in the presence of MG132 was newly generated after Pen-2 knockdown. A similar result was also observed for PS1N (data not shown). Fig. 1C summarizes the quantitative analysis of the levels of each γ-secretase component under different conditions. It is clear that APH-1α is the most stable one in comparison with others. Previous studies showed that NCT and Aph-1α can form a subcomplex (Fraering et al., 2004; LaVoie et al., 2003) and this high affinity between these two molecules may explain why Aph-1α degradation is more severe in NCT-knockout cells and vice versa because of the loss of protection from each other (compare lane 5 with lanes 2 and 11). In the presence of both Aph and PS, the level of NCT only slightly decreased (fourth panel). Similarly, in the presence of both NCT and Pen-2, Aph only slightly decreased (top panel), suggesting PS1 and Pen-2 provided additional protection to NCT and Aph-1α respectively.
Aph-1 interacts with NCT without PS, and PS interacts with NCT without Aph-1 and vice versa
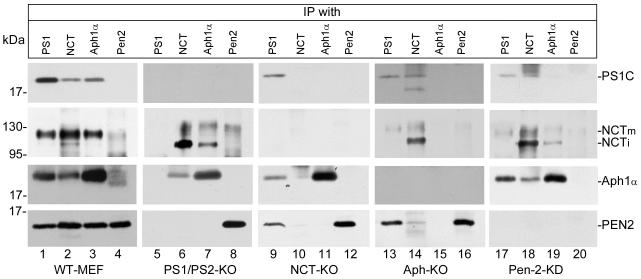
Next, we examined the effect of ablation of one of the components on the interaction of other components of the γ-secretase complex. After 12 h culture in the presence of MG132, co-immunoprecipitation was performed using antibodies against each of the γ-secretase components followed by Western blot analysis. As shown in the lane 1 of Fig. 2, PS1C, NCT, Aph-1α, and Pen-2 were co-immunoprecipitated from wild type cells by PS1-N-terminal-specific antibody, which was raised against residues 27-50 (Zhao et al., 2004). As shown in lane 2, the NCT-specific antibody immunoprecipitated both mature and immature forms of NCT (row 2) and co-immunoprecipitated PS1C, Aph-1α, and Pen-2. As shown in lane 3, Aph-1α-specific antibody immunoprecipitated Aph-1α and co-immunoprecipitated PS1C, the mature form of NCT, and Pen-2. Anti-Pen-2-specific antibody, which was raised against residues 86-101, immunoprecipitated Pen-2 and a trace amount of NCT but not Aph-1α and PS1C (lane 4). We have also used anti-Pen-2 N-terminal antibody from Covance and an antibody from Santa Cruz, which was raised using full length Pen-2, but these two antibodies also failed to co-immunoprecipitate other components. Pen-2 is a small protein (101 amino acids) with two transmembrane domains. The short soluble N-terminal and C-terminal domains of Pen-2 may play a role in interacting with other proteins and may also function as an epitope in raising antibodies. Thus, the inability of Pen-2 to co-immunoprecipitate other components is possibly because the anti-Pen-2 antibodies used in this study, upon binding to an epitope, result in disruption of the γ-secretase complex under our experimental conditions.
Fig. 2.
NCT and Aph-1 form a complex in the absence of PS, and this NCT-Aph-1 subcomplex is not required for the interaction of NCT and Aph-1 with PS1, but may be required for their interaction with Pen-2 mediated by PS1. Cell lysates of knockout and knockdown cells (indicated on the bottom of each column) were immunoprecipitated with antibodies specific to each γ-secretase component, as indicated on top of each column, and probed with antibody against each component, as indicated at right of each row. NCTm indicates the mature and NCTi indicates the immature form of NCT. Note that the spot in lane 4 of row 3 is not a specific band, but more likely a non-specific stain.
As shown in the second column of Fig. 2, in the PS1/PS2 double knockout cells, anti-PS1N antibody did not immunoprecipitate anything (lane 5). Anti-NCT antibody immunoprecipitated NCT (lane 6, second row) and a significant amount of Aph-1α (third row), but not Pen-2. When the anti-Aph-1α was used, in addition to Aph-1α itself, a significant amount of NCT, but not Pen-2, was co-immunoprecipitated (lane 7). Pen-2-specific antibody immunoprecipitated Pen-2 itself, but not any others. In the NCT knockout cells (third column), anti-PS1N antibody immunoprecipitated PS1C and co-immunoprecipitated low amounts of Aph-1α and Pen-2 (lane 9). This result indicates that interaction of PS1 with Aph-1α and Pen-2 does not require the presence of nicastrin. As expected, anti-NCT antibody did not bring down anything (lane 10). Anti-Aph-1α antibody immunoprecipitated Aph-1α itself but no other components of the γ-secretase complex (lane 11). Similarly, anti-Pen-2 antibody immunoprecipitated only Pen-2 (lane 12). In the Aph-KO cells, as shown in the fourth column, as expected, anti-Aph antibody did not bring down anything (lane 15). As shown in lane 13, anti-PS1N antibody immunoprecipitated PS1C and co-immunoprecipitated a small amount of the mature form of NCT and a significant amount of Pen-2. This result indicates that interaction of PS1 with NCT and Pen-2 does not require the presence of Aph. It is notable that, even though the level of the mature form of NCT was very low, the NCT co-immunoprecipitated with PS1 was only the mature form and not the immature form of NCT, indicating that PS1 has high affinity to mature NCT over immature NCT. Anti-NCT antibody immunoprecipitated both the mature and immature form of NCT and also co-immunoprecipitated a low amount of PS1C and a very low amount of Pen-2 (lane 14). Anti-pen-2 antibody immunoprecipitated itself, but no other components (lane 16). As shown in the fifth column, in the Pen-2 knockdown cells, anti-PS1N antibody immunoprecipitated PS1C and co-immunoprecipitated a very low amount of the mature form of NCT and a significantly high amount of Aph-1α (lane 17). Anti-NCT antibody immunoprecipitated NCT and co-immunoprecipitated a low amount of Aph-1α and a very low amount of PS1C (lane 18). Anti-Aph-1α antibody immunoprecipitated Aph-1α and co-immunoprecipitated a low amount of NCT (lane 19). These data indicate that Pen-2 is not required for PS interaction with NCT and Aph-1 and that NCT interacts with Aph-1. As expected, anti-Pen-2 antibody did not bring down anything (lane 20). In NCT-KO and Pen-2-KD cells, anti-PS1 antibody co-immunoprecipitated Aph-1, but anti-Aph-1 antibody did not co-immunoprecipitate PS1C. One possible reason for this result is that, in the absence of other components, the Aph-1 protein adapted a conformation that blocked the epitope of anti-Aph-1 antibody.
Both NCT Aph-1 are required for the interaction of PS1 with CTFβ
Next, we determined the effect of elimination of one of the components of the γ-secretase complex on the interaction of the complex with substrate CTFβ. To do so, PS1/2-KO, NCT-KO, Aph-KO, Pen-2-KD, and wild type cells were cultured in the presence of both the proteasome inhibitor MG132 and γ-secretase inhibitor compound E, which causes accumulation of both CTFβ and Aβ46 (Zhao et al., 2004). As shown in the first column of Fig. 3, in wild type cells, antibodies against PS1N, NCT, and Aph-1α co-immunoprecipitated both CTFβ and Aβ46, confirming our previous observation that the intermediate product Aβ46 is tightly associated with the γ-secretase complex (Zhao et al., 2005). However, antibody against Pen-2 co-immunoprecipitated a very small amount of CFTβ (lane 4) only. Interestingly, as shown in the second column, in the PS1/PS2-double knockout cells, anti-NCT antibody co-immunoprecipitated a significant amount of CTFβ (lane 8), anti-Aph-1α antibody co-immunoprecipitated a trace amount of CTFβ (lane 9), and nothing was co-immunoprecipitated by anti-Pen-2 antibody (lane 10). These results indicate that NCT and Aph-1α interact with CTFβ and do not require PS1. These results also indicate that NCT has stronger affinity to CTFβ than does Aph-1α. As shown in the third column of Fig. 3, in Pen-2-KD cells, a small amount of CTFβ was co-immunoprecipitated by anti-PS1N (lane 13), anti-NCT (lane 14), and anti-Aph-1α (lane 15). However, in the NCT-KO cells (fourth column) and Aph-1α-KO cells (fifth column), no CTFβ was co-immunoprecipitated by any of the antibodies against the γ-secretase complex components including PS1. These data suggest that the NCT-Aph-1 subcomplex is required for γ-secretase to interact with CTFβ.
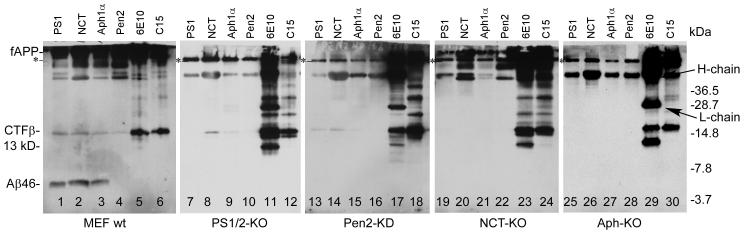
Fig. 3.
Interaction of PS1 with CTFβ requires the presence of both NCT and Aph-1. PS1/2-KO, NCT-KO, Ahp-1-KO, and Pen-2-KD cells were cultured for 12 h in the presence of both γ-secretase inhibitor compound E and proteasome inhibitor MG132. Cell lysates were subjected to co-immunoprecipitation using antibodies against each γ-secretase component, and the blots were probed with 6E10. As controls, 6E10 and C15 were employed. Since 6E10 was used for both immunoprecipitation and probing, both heavy chain and light chain IgG was detected in 6E10 immunoprecipitate, and the heavy chains of other polyclonal antibodies used were also detected throughout the immunoprecipitates. The band under the putative fAPP band detected in wild type cells and indicated with * is apparently nonspecific because it appears in all immunoprecipitates, including those from knockout cells.
The above cell lysates were also subjected to immunoprecipitation using 6E10, which is specific to the Aβ sequence, and C15, which is specific to the C-terminal of APP (Zhao et al., 2005). In wild type cells, both 6E10 and C15 immunoprecipitated CTFβ, but not Aβ46 (lanes 5 and 6), suggesting that, in the presence of compound E, the γ-secretase complex might have blocked the interaction of 6E10 with Aβ46, which is tightly associated with the γ-secretase complex (Zhao et al., 2005). In the knockout and knockdown cells, in addition to CTFβ, several bands with a migration rate either slower or faster than that of the CTFβ band were detected (lanes 11, 12, 17, 18, 23, 24 29, and 30). It is known that APP can be randomly degraded or processed in a secretase-independent manner (Haass et al., 2012). It should also be noted that γ-secretase may also be involved in APP trafficking (Vetrivel et al., 2006). Thus, these bands are likely the products of partial processing or random degradation of APP possibly because of disturbed intracellular trafficking of APP due to knockout or knockdown of the γ-secretase components in these cells.
Discussion
In the present study, using genetic knockout and siRNA-mediated knockdown cells, we systematically investigated the role of each component of the γ-secretase complex during assembly, maturation, and APP processing activity. Our data revealed several new findings. First, Pen-2 is dispensable for PS endoproteolysis. The data presented here clearly demonstrate that in Pen-2-KD cells, similar to what is observed in NCT-KO and Aph-KO cells, the disappearance of PS1C was prevented by the addition of the proteasome inhibitor MG132 or lactacystin. In particular, our results from the time course experiments undoubtedly show that the accumulated PS1C in Pen-2-KD cells, in the presence of proteasome inhibitors, was newly generated after knockdown of Pen-2. These findings indicate that endoproteolysis of PS occurred in the absence of Pen-2; however, in the absence of MG132, the resulting PS1C was degraded by proteasome. It has been reported that PS1 undergoes endoproteolysis between Thr291 and Ala299 (Podlisny et al., 1997). The PS1C fragment observed in this study was detected by two specific antibodies: one was raised against a peptide corresponding to residues 307-321 of PS1 (Xu et al., 2002) as shown in Figs. 1 and 2; the other is specific to the last 20 amino acids of PS1 (from Santa Cruz, data not shown). This result indicates both the N-terminus and the C-terminus of the PS1C fragment observed here remain intact. In addition, the PS1N fragment observed in this study was detected by three PS1N-specific antibodies: anti-PS1N (against residues 27-50 (Zhao et al., 2004)], Figs. 1 and 2), N-19 (Santa Cruz, data not shown), and Ab14 ((Luo et al., 2003)],, data not shown). These data strongly indicate that the PS1N and PS1C observed in knockout cells are the same as those detected in wild type cells, i.e., knockout of other components has no effect on the cleavage site of PS1 endoproteolytic processing. Why, in some cases, could full-length PS1 be detected in the Pen-2 knockout cells? In this regard, it is notable that APH-1 and NCT have been shown to form a stable subcomplex that binds to and stabilizes the PS1 holoprotein (LaVoie et al., 2003; Takasugi et al., 2003). Therefore, it is very possible that, in the Pen-2 knockdown cells, the residual unprocessed full-length PS1 resulting from inefficient processing was protected from degradation by the NCT-Aph-1 subcomplex and can be detected under certain experimental conditions. However, in the NCT-KO or Aph-1-KO cells, the knockout of NCT or Aph-1 abolishes the formation of the NCT-Aph-1 subcomplex, resulting in the degradation of the unprotected full-length PS1. Thus, in conclusion, though it cannot be ruled out that the presence of all of other components may accelerate the endoproteolytic processing of PS, the observations that depletion of other components of γ-secretase did not abolish the production of PS1N and PS1C strongly indicate that none of these γ-secretase components is absolutely required for endoproteolysis of PS, but they all are required to stabilize the processing product.
Second, formation of the NCT-Aph-1 subcomplex is not required for NCT and Aph-1 to interact with PS but, it is required for them to interact with Pen-2. Using two-dimensional PAGE and differential detergent dissociation approaches, it was revealed that NCT-Aph-1 forms an intermediate subcomplex (Fraering et al., 2004; LaVoie et al., 2003). However, the role of this subcomplex in γ-secretase assembly remains elusive. In the current study, using knockout cells and siRNA approaches, our data confirmed that NCT and Aph-1 can form a complex in the absence of both PS1 and PS2. In investigating the role of this subcomplex in γ-secretase assembly, our co-immunoprecipitation data demonstrated that PS1 can be co-immunoprecipitated with Aph-1 in the absence of NCT and vice versa. Our data also demonstrated that Pen-2 can be co-immunoprecipitated with PS1 in the absence of NCT or Aph-1. These data indicate that the NCT-Aph-1 subcomplex is not required for PS to interact with NCT, Aph-1, or Pen-2. Our data further demonstrate that in wild type cells both NCT and Aph-1 can be co-immunoprecipitated with Pen-2, but neither NCT nor Aph-1 can be co-immunoprecipitated with Pen-2 in the absence of PS protein, indicating this subcomplex is not sufficient to interact with Pen-2, and its interaction with Pen-2 may be mediated by PS protein. However, our data demonstrated that knockout of NCT abolished the interaction of Aph-1 with Pen-2, and only a trace amount of Pen-2 was co-immunoprecipitated with NCT in the absence of Aph-1, indicating that formation of the NCT-Aph-1 subcomplex is required for NCT and Aph-1 to efficiently interact with Pen-2 in the presence of PS1.
Third, our data suggest that formation of the NCT-Aph-1 subcomplex plays not only an important role in γ-secretase complex assembly but also in recruiting substrate CTFβ to the complex. It is interesting to note that our data clearly demonstrated that both NCT and Aph-1 can be co-immunoprecipitated with CTFβ in the PS1 and PS2 double knockout cells and also in Pen-2-KD cells, indicating that formation of the NCT-Aph-1 subcomplex is sufficient for interaction with CTFβ, the initial substrate of γ-secretase. Moreover, no CTFβ can be co-immunoprecipitated with PS1 in the NCT-KO or Aph-KO cells, indicating that the interaction between PS and CTFβ is mediated by the formation of the NCT-Aph subcomplex, i.e., NCT-Aph subcomplex formation is necessary for γ-secretase to interact with its initial substrate CTFβ. It was also notable that more CTFβ was co-immunoprecipitated with NCT than with other γ-secretase components, suggesting that NCT has the highest affinity for CTFβ and plays an important role in recruiting substrates to γ-secretase. This finding is consistent with a previous observation that suggests NCT may function as a substrate receptor (Shah et al., 2005). On the other hand, Aph-1α may contribute to recruiting CTFβ by interacting with CTFβ through the GxxxG motif (Mao et al., 2009).
Acknowledgments
We thank Dr. Bart De Strooper (Center for Human Genetics, K.U. Leuven, Herestraat 49, B-3000 Leuven, Belgium) for providing presenilin-knockout cell lines. We thank Dr. Huaxi Xu (Sanford-Burnham Medical Research Institute, San Diego, CA, USA) for the gift of an anti-PS1N antibody. We also thank Ms. Misty R. Bailey for her critical reading of the manuscript. This work was supported by National Institutes of Health (NIH) grants R181741110 and R21AG039596, an Alzheimer’s Association grant, and a grant from the American Health Assistance Foundation (to XX) and by NIH grant 0355339B (to M-Z C). The authors declare no conflicts of interest.
Abbreviations used
- Aβ
β-amyloid peptide
- AD
Alzheimer’s disease
- Aph1
anterior pharynx-defective 1
- Aph-KO
Aph-1a, b, and c triple knockout cell
- APP
amyloid precursor protein
- CTFβ
C-terminal fragment of APP generated by β-cleavage
- DMEM
Dulbecco’s modified Eagle’s medium
- MEF
mouse embryonic fibroblast
- NCT
nicastrin
- NCT-KO
Nicastrin knockout cell
- Pen-2
presenilin enhancer 2
- Pen-2-KD
Pen-2 knockdown cell
- PS
presenilin
- PS1C
C-terminal fragment of PS1
- PS1N
N-terminal fragment of PS1
- PS1/2-KO
PS1 and PS2 double knockout cells
- siRNA
small interfering RNA.
References
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/s0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W. Proteolytic processing and cell biological functions of the amyloid precursor protein. J. Cell Sci. 2000;113(Pt 11):1857–1870. doi: 10.1242/jcs.113.11.1857. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- Dries DR, Yu G. Assembly, maturation, and trafficking of the gamma-secretase complex in Alzheimer’s disease. Current Alzheimer research. 2008;5:132–146. doi: 10.2174/156720508783954695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraering PC, LaVoie MJ, Ye W, Ostaszewski BL, Kimberly WT, Selkoe DJ, Wolfe MS. Detergent-dependent dissociation of active gamma-secretase reveals an interaction between Pen-2 and PS1-NTF and offers a model for subunit organization within the complex. Biochemistry (Mosc) 2004;43:323–333. doi: 10.1021/bi035748j. [DOI] [PubMed] [Google Scholar]
- Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, Parks AL, Xu W, Li J, Gurney M, Myers RL, Himes CS, Hiebsch R, Ruble C, Nye JS, Curtis D. aph-1 and pen-2 are required for Notch pathway signaling, gamma-secretase cleavage of betaAPP, and presenilin protein accumulation. Dev Cell. 2002;3:85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- Haass C, Kaether C, Thinakaran G, Sisodia S. Trafficking and Proteolytic Processing of APP. Cold Spring Harbor perspectives in medicine. 2012;2:a006270. doi: 10.1101/cshperspect.a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreman A, Serneels L, Annaert W, Collen D, Schoonjans L, De Strooper B. Total inactivation of gamma-secretase activity in presenilin-deficient embryonic stem cells. Nat Cell Biol. 2000;2:461–462. doi: 10.1038/35017105. [DOI] [PubMed] [Google Scholar]
- Kimberly WT, Xia W, Rahmati T, Wolfe MS, Selkoe DJ. The transmembrane aspartates in presenilin 1 and 2 are obligatory for gamma-secretase activity and amyloid beta-protein generation. J. Biol. Chem. 2000;275:3173–3178. doi: 10.1074/jbc.275.5.3173. [DOI] [PubMed] [Google Scholar]
- LaVoie MJ, Fraering PC, Ostaszewski BL, Ye W, Kimberly WT, Wolfe MS, Selkoe DJ. Assembly of the gamma-secretase complex involves early formation of an intermediate subcomplex of Aph-1 and nicastrin. J. Biol. Chem. 2003;278:37213–37222. doi: 10.1074/jbc.M303941200. [DOI] [PubMed] [Google Scholar]
- Lee SF, Shah S, Li H, Yu C, Han W, Yu G. Mammalian APH-1 interacts with presenilin and nicastrin and is required for intramembrane proteolysis of amyloid-beta precursor protein and Notch. J. Biol. Chem. 2002;277:45013–45019. doi: 10.1074/jbc.M208164200. [DOI] [PubMed] [Google Scholar]
- Li T, Ma G, Cai H, Price DL, Wong PC. Nicastrin is required for assembly of presenilin/gamma-secretase complexes to mediate Notch signaling and for processing and trafficking of beta-amyloid precursor protein in mammals. J. Neurosci. 2003;23:3272–3277. doi: 10.1523/JNEUROSCI.23-08-03272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo WJ, Wang H, Li H, Kim BS, Shah S, Lee HJ, Thinakaran G, Kim TW, Yu G, Xu H. PEN-2 and APH-1 coordinately regulate proteolytic processing of presenilin 1. J. Biol. Chem. 2003;278:7850–7854. doi: 10.1074/jbc.C200648200. [DOI] [PubMed] [Google Scholar]
- Ma G, Li T, Price DL, Wong PC. APH-1a is the principal mammalian APH-1 isoform present in gamma-secretase complexes during embryonic development. J. Neurosci. 2005;25:192–198. doi: 10.1523/JNEUROSCI.3814-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G, Tan J, Cui MZ, Chui D, Xu X. The GxxxG motif in the transmembrane domain of AbetaPP plays an essential role in the interaction of CTFbeta with the gamma-secretase complex and the formation of amyloid-beta. J Alzheimers Dis. 2009;18:167–176. doi: 10.3233/JAD-2009-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podlisny MB, Citron M, Amarante P, Sherrington R, Xia W, Zhang J, Diehl T, Levesque G, Fraser P, Haass C, Koo EH, Seubert P, St George-Hyslop P, Teplow DB, Selkoe DJ. Presenilin proteins undergo heterogeneous endoproteolysis between Thr291 and Ala299 and occur as stable N- and C-terminal fragments in normal and Alzheimer brain tissue. Neurobiol. Dis. 1997;3:325–337. doi: 10.1006/nbdi.1997.0129. [DOI] [PubMed] [Google Scholar]
- Shah S, Lee SF, Tabuchi K, Hao YH, Yu C, LaPlant Q, Ball H, Dann CE, 3rd, Sudhof T, Yu G. Nicastrin functions as a gamma-secretase-substrate receptor. Cell. 2005;122:435–447. doi: 10.1016/j.cell.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Steiner H, Winkler E, Edbauer D, Prokop S, Basset G, Yamasaki A, Kostka M, Haass C. PEN-2 is an integral component of the gamma-secretase complex required for coordinated expression of presenilin and nicastrin. J. Biol. Chem. 2002;277:39062–39065. doi: 10.1074/jbc.C200469200. [DOI] [PubMed] [Google Scholar]
- Takasugi N, Tomita T, Hayashi I, Tsuruoka M, Niimura M, Takahashi Y, Thinakaran G, Iwatsubo T. The role of presenilin cofactors in the gamma-secretase complex. Nature. 2003;422:438–441. doi: 10.1038/nature01506. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, Hardy J, Levey AI, Gandy SE, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- Vetrivel K, Zhang Y.-w., Xu H, Thinakaran G. Pathological and physiological functions of presenilins. Molecular Neurodegeneration. 2006;1:4. doi: 10.1186/1750-1326-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Xu X. γ-Secretase Catalyzes Sequential Cleavages of the AβPP Transmembrane Domain. Journal of Alzheimer’s Disease. 2009;16:211–224. doi: 10.3233/JAD-2009-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Shi YC, Gao W, Mao G, Zhao G, Agrawal S, Chisolm GM, Sui D, Cui MZ. The novel presenilin-1-associated protein is a proapoptotic mitochondrial protein. J. Biol. Chem. 2002;277:48913–48922. doi: 10.1074/jbc.M209613200. [DOI] [PubMed] [Google Scholar]
- Zhang YW, Xu H. Molecular and cellular mechanisms for Alzheimer’s disease: understanding APP metabolism. Current molecular medicine. 2007;7:687–696. doi: 10.2174/156652407782564462. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Nadeau P, Song W, Donoviel D, Yuan M, Bernstein A, Yankner BA. Presenilins are required for gamma-secretase cleavage of beta-APP and transmembrane cleavage of Notch-1. Nat Cell Biol. 2000;2:463–465. doi: 10.1038/35017108. [DOI] [PubMed] [Google Scholar]
- Zhao G, Cui MZ, Mao G, Dong Y, Tan J, Sun L, Xu X. g-Cleavage is dependent on z-cleavage during the proteolytic processing of amyloid precursor protein within its transmembrane domain. J. Biol. Chem. 2005;280:37689–37697. doi: 10.1074/jbc.M507993200. [DOI] [PubMed] [Google Scholar]
- Zhao G, Mao G, Tan J, Dong Y, Cui M-Z, Kim S-H, Xu X. Identification of a New Presenilin-dependent z-Cleavage Site within the Transmembrane Domain of Amyloid Precursor Protein. J. Biol. Chem. 2004;279:50647–50650. doi: 10.1074/jbc.C400473200. [DOI] [PubMed] [Google Scholar]