Abstract
Purpose
To describe the histological development of the human central retina from fetal week (Fwk) 22 to 13 years.
Design
Retrospective observational case series
Methods
Retinal layers and neuronal substructures were delineated on foveal sections of fixed tissue stained in azure II-methylene blue and on frozen sections immunolabeled for cone, rod or glial proteins. Postmortem tissue was from 11 eyes at Fwk 20–27; 8 eyes at Fwk 28–37; 6 eyes at postnatal 1 day to 6 weeks; 3 eyes at 9–15 months; and 5 eyes at 28 months-13 years.
Results
At Fwk20–22 the fovea could be identified by the presence of a single layer of cones in the outer nuclear layer. Immunolabeling detected synaptic proteins, cone and rod opsins and Muller glial processes separating the photoreceptors. The foveal pit appeared at Fwk25, involving progressive peripheral displacement of ganglion cell, inner plexiform and inner nuclear layers. The pit became wider and shallower after birth, and appeared mature by 15months. Between Fwk25 and Fwk38, all photoreceptors developed more distinct inner and outer segments, but these were longer on peripheral than foveal cones. After birth the foveal outer nuclear layer became much thicker as cone packing occurred. Cone packing and neuronal migration during pit formation combined to form long central photoreceptor axons, which changed the outer plexiform layer from a thin sheet of synaptic pedicles into the thickest layer in the central retina by 15 months. Foveal inner and outer segment length matched peripheral cones by 15 months and was 4× longer by 13 years.
Conclusions
These data are necessary to understand the marked changes in human retina from late gestation to early adulthood. They provide qualitative and quantitative morphological information required to interpret the changes in hyper- and hypo-reflexive bands in pediatric spectral domain optical coherence tomography (SDOCT) images at the same ages.
INTRODUCTION
The fovea is the most critical portion of the primate retina for high visual acuity and color vision. Its early development begins at fetal week (Fwk) 12 with a complex expression sequence of cell-specific molecules1–7. After midgestation an outward displacement of inner retinal layers occurs to form a foveal pit, and after birth an inward displacement of the photoreceptors occurs to raise foveal cone density 10×8–17. Spectral domain optical coherence tomography (SDOCT) has become a valuable tool for clinical diagnosis of normal and diseased adult human retina18. Recently, Maldonado et al.19 used a portable research hand-held SDOCT unit to image human retinas from prematures through adults. They documented the immaturity of the neonatal human fovea and the marked difference in SDOCT layers from the standard adult SDOCT image. However, there is still little detailed description of histological development and almost no quantitative information about human central retina around birth. This paper provides the first detailed histological description of human central retinal development from midgestation to early adulthood. In particular, it shows the marked change in foveal photoreceptors from a single layer of short undeveloped cones up to birth to tightly-packed and highly elongated cones in the adult. Understanding these changes is essential to make the correct interpretation of neonatal SDOCT images.
METHODS
Tissue sections for this study were from eyes obtained under Human Subjects Approval #010447. Eyes younger than Fwk22 were from elective abortions; only normal-appearing fetuses were utilized. Older prenatal and neonatal eyes were from infants who survived days to weeks in the Neonatal ICU; babies with diagnosed conditions that might affect retinal development were not included in this report. Infant eyes were obtained from children who died of accidental causes, or from diseases that did not affect the retina.
Eyes were enucleated 2–8 hours after death, the cornea and lens removed and the globe immersed in a mixture of 4% paraformaldehyde and 0.5% glutaraldehyde in pH.7.4 phosphate buffer (PB) for 1day (d) to months. In one of the pair of eyes, the horizontal meridian was embedded in glycol methacrylate and cut serially at 2–3µm. This method gives excellent preservation and imaging of tissue. In a few eyes the entire globe was embedded in paraffin and sectioned serially at 12µm. This method was less time consuming, but the retina was usually detached from the pigment epithelium. In some eyes the horizontal meridian was frozen sectioned serially at 12µm, allowing immunolabeling at a known locus with a wide variety of antibodies. In each method, every 10th slide was stained with 1% azure II-methylene blue to locate the fovea.
Immunolabeling
Frozen sections were labeled as previously reported5, 7. The rod marker was mouse anti-rod opsin (4D2, 1/300; gift of R. Molday). Cone markers included rabbit anti-long-and-medium (L&M) wavelength-selective cone opsin (1/2000, gift of J. Saari), rabbit anti-short (S) wavelength-selective cone opsin (1/10,000, gift of J. Nathans), mouse anti-cone arrestin (7G6, 1/250–500; gift of P MacLeish); and mouse anti-cone alpha transducin (A1.1, 1/250–500; gift of J. Hurley). Mouse antisynaptophysin (1/1000; Sigma-Aldrich S5768) labels cone synaptic pedicles heavily and rod synaptic spherules lightly. Rabbit anti-cellular retinaldehyde-binding protein (CRALBP; 1/1000; gift of J. Saari) was used to label Müller glial cytoplasm.
Imaging
All photographs were from sections in which the foveal center could be reliably identified. Plastic or paraffin sections stained with azure II-methylene blue were imaged digitally using a Nikon E1000 wide-field digital microscope. Immunolabeled sections were imaged using a high sensitivity Hamatsu camera or in an Olympus Sluoview 1000 confocal microscope. All images were processed in Adobe Photoshop CS3 for size, color balance, sharpness and contrast.
Segmentation of light micrographs
A low power foveal montage was created at selected ages. One half was imaged in Photoshop and paths drawn along the inner surface of the retina and the borders between the ganglion cell (GCL) and inner plexiform layer (IPL); the IPL and inner nuclear layer (INL); the INL and outer plexiform layer (OPL); the OPL and outer nuclear layer (ONL); and the inner border of the retinal pigment epithelium (PE). Layers were differentially shaded (Figures 2–7). In young retinas, the OPL was thin and included in ONL, but in older retinas the OPL was distinguished as a separate layer. A dotted path was drawn along the external limiting membrane (ELM), separating the proximal ONL from the distal inner segment/outer segment (IS/OS) layer.
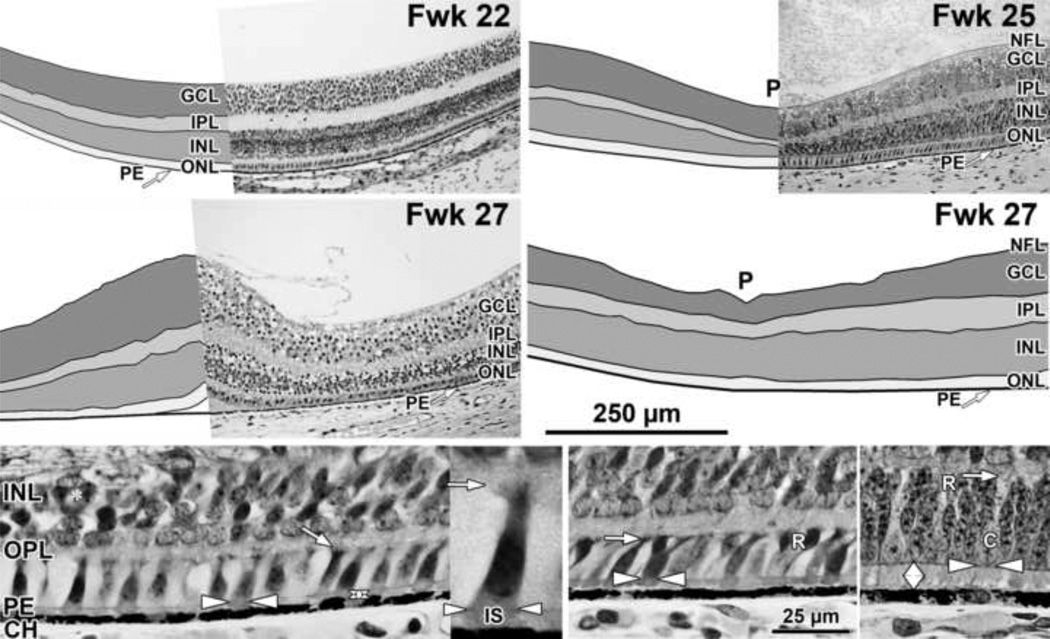
Figure 2. Layers and Histology of Mid-gestation Fetal Human Retina.
Development of the human fovea at (Top left) fetal week (Fwk) 22, (Top right) Fwk 25, and (middle left, middle right) Fwk 27. Retinal layers have been drawn from digital photographs using Adobe Photoshop (see Methods) and the layers shaded as indicated. Top left: At Fwk22 the foveal region is composed of five layers with a thick GCL and a thin ONL. Top right, middle right: After Fwk 25 the foveal pit (P) begins to invaginate the inner retinal layers. Bottom left: At Fwk25 there is only a very narrow space (vertical double arrow) between the ELM (horizontal white arrowheads) and the PE in central retina. The cone marked with arrowheads is shown at higher power in the inset to the right. A short thick IS extends distal to the ELM (white arrowheads) and a small synaptic pedicle (arrow) is present. Bottom middle: At 800µm from the fovea there is little difference from the fovea except two rods (R) are present. Bottom right: At 2mm from the fovea a slightly wider PE/ELM space is present (vertical double arrow) and is filled with cone and rod IS. OS are not obvious. Scale in middle right for Top and middle rows; in Bottom middle for Bottom row.
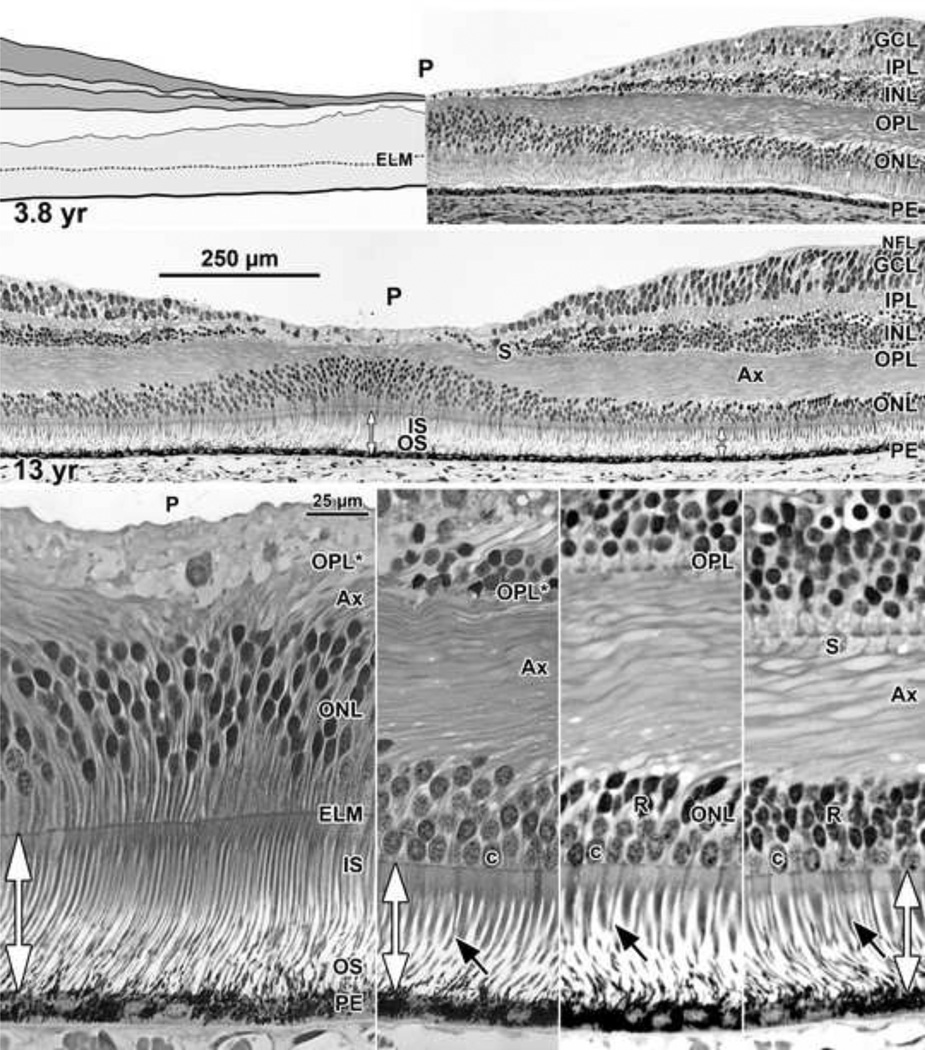
Figure 7. Layers and Histology of Human Retina during Childhood.
The final maturation stages of the human fovea are shown at (Top left) 3.8 years (yr) and (Top right) 13 years with higher magnification of the 13 years (middle) foveal center, (Bottom far left) first rods at 300µm, (Bottom middle left) 500µm, and (Bottom middle right) 2mm from the fovea. The foveal pit is wide and shallow. The foveal center is composed of long thin cone OS, IS, and cell bodies 8–12 deep. The central OPL contains a thick layer of Ax. The foveal OPL contains only Ax (OPL*) out to 500µm where synaptic pedicles (Bottom middle leftOPL; Bottom middle rights) are first encountered. Long thin rod OS (Bottom rowblack arrows) become more prominent with eccentricity. Note the marked increase in cone IS diameter from the foveal center to 300µm with a small further increase at 2mm. Scale in middle for Top and middle rows; scale in Bottom far left for Bottom row.
RESULTS
Normal Adult Retina
A section from adult human retina ~2mm nasal to the fovea is shown in Figure 1 for orientation to the layers and abbreviations used in the developing retina. A young adult fovea is shown in Figure 7 Bottom row.
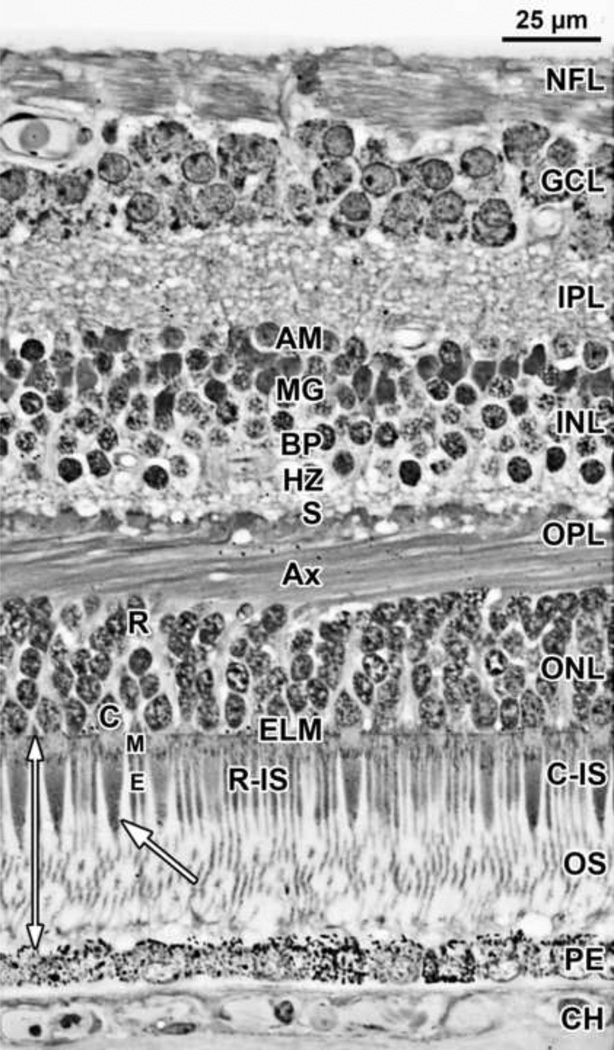
Figure 1. Layers and Histology of Normal Adult Human Retina.
Adult human retina 2mm nasal from the fovea center. The layers and their abbreviations used in this paper are choroid (CH); retinal pigment epithelium (PE); outer nuclear layer (ONL) which is subdivided into the photoreceptor outer segment (OS) and inner segment (IS) layers distal to the external limiting membrane (ELM), and the nuclear layer containing a single row of cone (C) cell bodies near the ELM and multiple rows of deeper rod (R) cell bodies. The thin rod IS are indicated. The rod OS are about half the thickness of the rod IS, are longer and reach the PE. The larger tapered cone IS have a shorter OS (arrow). The ELM marks the distal edge of the retina. The outer plexiform layer (OPL) contains a distal layer made up of the fibers of Henle or photoreceptor axons (Ax) and a proximal synaptic contact layer (S) containing cone pedicles and rod spherules. The inner nuclear layer (INL) contains the cell bodies of horizontal cells (HZ) lying most distal, bipolar cells (BP) in the middle, Müller glia (M), and amacrine cells (AM) lying most proximal. The inner plexiform (IPL), ganglion cell (GCL) and nerve fiber (NFL) layers complete the inner retina.
Development of the Human Fovea
Fetal Week 20–27 (11 retinas examined)
At midgestation, the future fovea had three distinctive differences from peripheral retina. First, the GCL is much thicker than in the periphery. Second, up to birth the ONL is formed by a single layer of cone photoreceptors (Figure 2 Bottom left, Figure 8 Top far left). This is in marked contrast to the thicker peripheral ONL containing both cones and rods (Figure 2 Bottom right; Figure 8 Top middle left and right). Third, the OPL is a narrow band of cone synaptic pedicles (Figure 2 Bottom left and insert, Bottom middle; Figure 8 Top far right Top and Bottom). These layers will undergo developmental changes that are critical to interpreting late prenatal and neonatal SDOCT images. In the following narrative, numbers like 800µm or 2mm indicate distance from the fovea center.
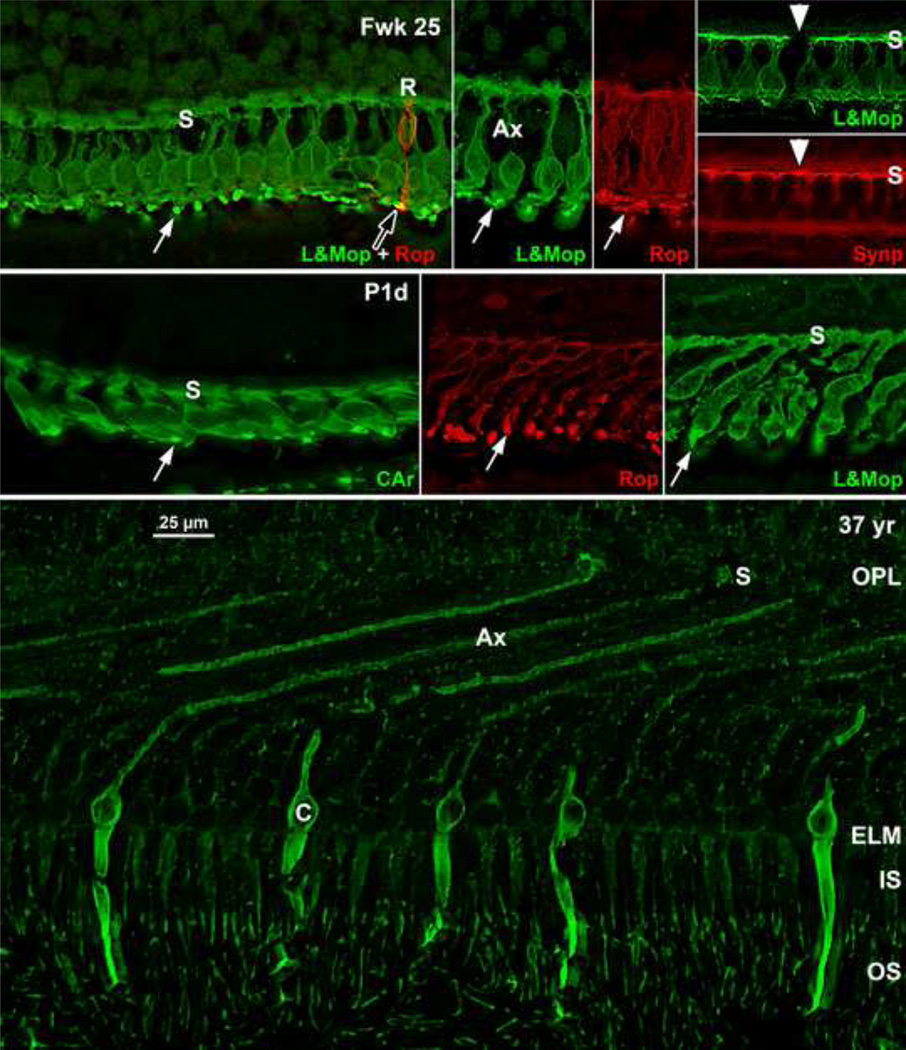
Figure 8. Immunocytochemistry of Photoreceptor Maturation.
Top row: Human Fwk24–25 retina immunocytochemically labeled for rod opsin (red) and cone L&M opsin (green). The entire cone and rod membrane are labeled at this age, outlining the cell shape. (Top far left) Each foveal cone has a short vertical axon ending in a row of synaptic pedicles (s) forming the OPL. A short cone OS is indicated by the white arrow. A single rod (R) with a tiny OS (arrowhead) is on the edge of the fovea. Top middle left and right: Photoreceptors at 2mm from the fovea. Note the longer OS (arrows). Top far right: Foveal cones stained for L&M opsin (green; Top) and synaptophysin (red; Bottom), a synaptic vesicle marker. The synaptic pedicle (s) is labeled in all L&M cones and in one short wavelength-selective cone (white arrowhead) unlabeled for L&M opsin. Middle row: P1d retina showing the change in photoreceptor morphology from the (middle left) foveal cones to (middle) rods and (middle right) cones 800µm from the fovea. The foveal cones are short and relatively flat while the peripheral photoreceptors have axons tilted away from the foveal center. Note the difference in length between foveal and peripheral OS (arrows). Bottom: A 37 years retina at 1mm labeled for short wavelength-selective cone opsin. The cone on the left can be traced from OS to synaptic pedicle (s). The striking change in central cone morphology from midgestation to adult can be appreciated by comparing Bottom to Top middle left. Scale in Bottom for all.
Rods are absent across the central 1500–1800µm forming a region called the rod-free zone underlying the thickened GCL5, 7. Central cones are 6–8µm wide with a large prominent nucleus (Figure 2 Bottom left insert, Figure 8 Top far left; Figure 9 Top right bottom). Each cone has a short axon (Ax) which ends below the cell body in a synaptic pedicle (Figure 2 Bottom left insert, arrow; Figure 8 Top middle left); these form the thin OPL (Figure 8 Top far left; Top row). Foveal cones are separated by Muller cell processes filling the space between cones. These end in small bulbs forming junctions with the cone cell bodies, creating the ELM (Figure 2 Bottom left white arrowhead; Figure 9 Top row).
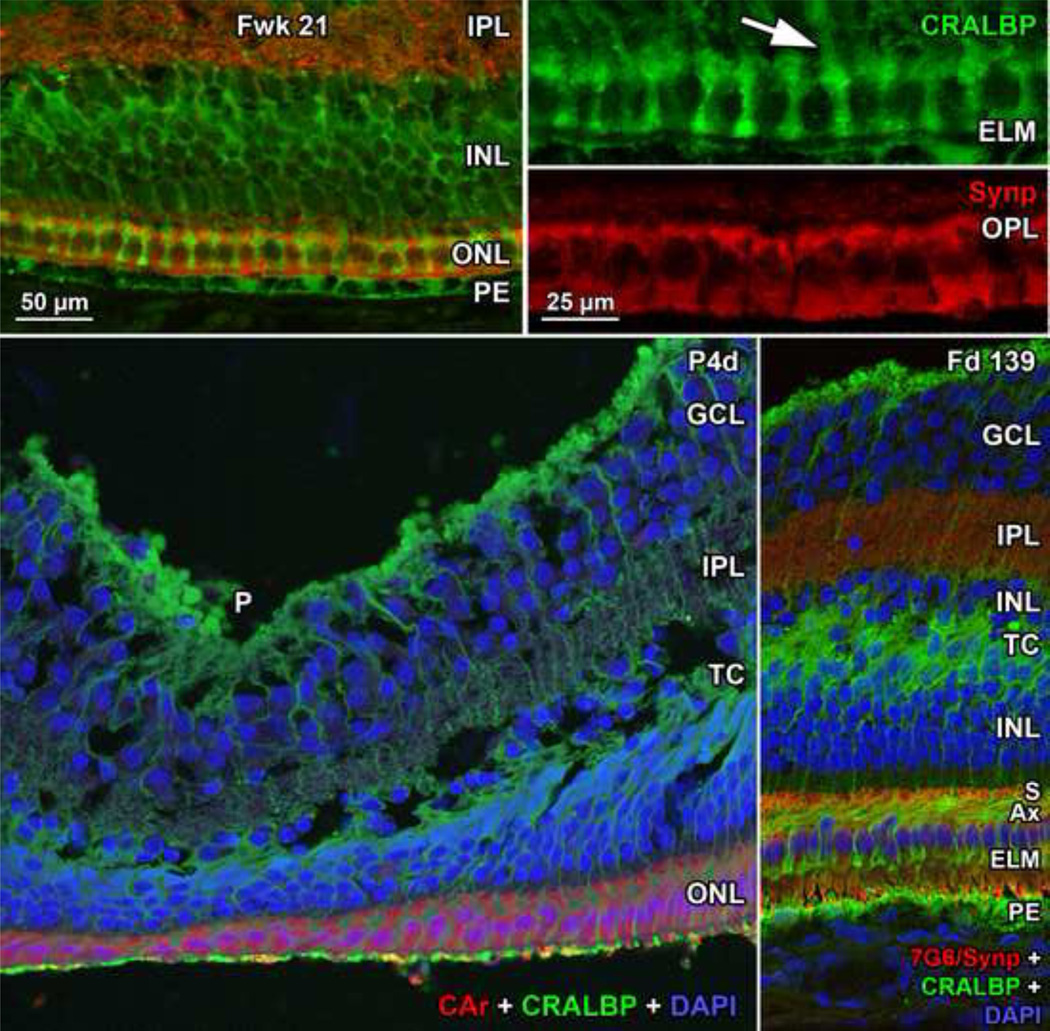
Figure 9. Immunocytochemistry of Müller Cell Morphology.
Top row: Fwk21 fovea immunocytochemically labeled for the Muller cell protein CRALBP (green) and synaptophysin (red). Müller cell processes extend from the cell body deep in the INL, run between each cone (Top right Toparrow) and form small clubs at the ELM. In the fovea Muller cytoplasm fills all space between foveal cones. Bottom left: P4d fovea triple labeled for cone arrestin (red), CRALBP (green) and nuclear label DAPI (blue). Cones over the pit (P) are in a thin single layer, but are longer on the slope. The TC is filled with Müller processes. Bottom right: The foveal slope from a late prenatal Macaca monkey fetus triple labeled for a mixture of cone transducin and synaptophysin (red), CRALBP (green) and nuclear label DAPI (blue). This retina more clearly shows the glial fiber makeup of the TC. Note how the Müller cell processes follow the angle of the cone axons (Ax) and then turn 90°to run between the cones to form the ELM. Scale in Top left for Top left and Bottom row; in Top right for Top right Top and Bottom.
A thick short extension of cone cytoplasm, the primitive IS, can be seen distal to the ELM (Figure 2 Bottom left insert), but OS are not obvious. However, immunolabeling for opsin reveals that all central cones and rods have short OS by Fwk25 (Figure 8 Top far left to Top middle right; also see5, 7). Immunolabeling for synaptic proteins shows that all Fwk20–25 cones have pedicles which contain many synaptic markers (Figure 8 Top far right Top and Bottom). These markers indicate that, despite their immature morphology, many of the essential molecular markers for photoreceptor function are expressed by Fwk25.
Outside the rod free zone, rods become much more numerous so that by 2mm (Figure 2 Bottom right; Figure 8 Top middle left and right) the ONL is formed by a single layer of large cones and 3–4 layers of rod cell bodies. The rod cell bodies are added between the cone nuclei and pedicles, causing cone axons to lengthen. Paradoxically both cone and rod IS are longer at 2mm than in the fovea (compare Figure 2 Bottom left and right).
In most Fwk25–27 eyes (Figure 2 Top and middle right) the inner layers are displaced peripherally to form a shallow foveal pit (P). The exception was a Fwk27 fovea (Figure 2 middle left) which had a prominent doming of all layers into the vitreous and a small detachment of the cones from the PE. No pit could be detected in multiple serial sections of this eye.
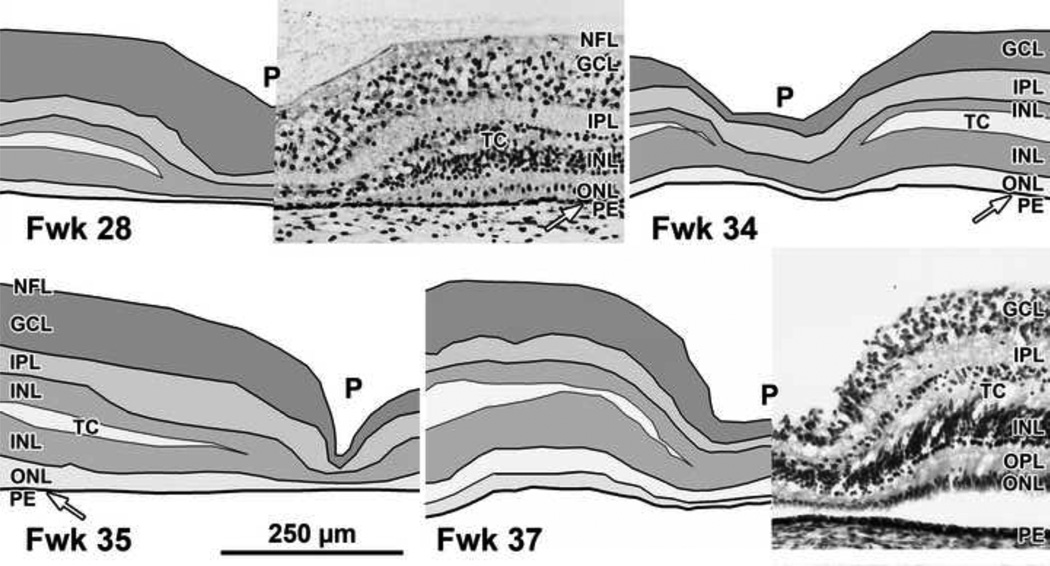
Fetal week 28–37 (8 eyes examined)
All retinas had a distinct foveal pit with an outward displacement of the GCL, IPL and INL, although the pit varied in width, depth and layers involved. On the pit edges (“slope”) the INL becomes divided by a layer of pale fibers and/or cytoplasm which separates the innermost cell bodies from the remainder of the INL. This layer is the transient layer of Chievitz (TC), a common feature in primate retinas around birth5, 9, 20. Immunocytochemical labeling indicates that the TC fibers are mainly Muller cell processes (Figure 9 Bottom row) which angle away from the foveal pit, reflecting the displacement of neurons and glia peripherally as the pit forms.
Up to birth the foveal cones still form a single layer under the developing pit (Figure 3). Cones are more tapered and the vertical axis of cones on the pit slope is tilted with the apical IS end pointing toward center and the synaptic axon and pedicle pointing away (Figure 4 Top row). This tilt and INL thinning indicates that the neurons postsynaptic to the foveal cones are being displaced peripherally by pit formation. The space between the ELM and RPE is still only ~5µm wide in the fovea (Figure 4 Top left, vertical double arrows). Foveal OS can be identified but are extremely short (Figure 4 Bottom left and middle, arrowhead). Peripheral to the foveal center (Figure 4 Top middle and right) the ONL now has 2–6 deeper layers of rod cell bodies. Axons form on the foveal cones and rods on the pit slope (Figure 4 Top middle) to maintain their synaptic contacts as pit formation forces inner neurons away from the fovea. This is reflected in the axon tilt away from the photoreceptor cell bodies (Figure 4 Top right, pit center to left).
Figure 3. Layers of Late-gestation Fetal Human Retina.
Development of the human fovea at (Top left) Fwk 28, (Top right) Fwk 34, (Bottom left) Fwk 35, and (Bottom right) Fwk 37. A transient layer of Chievitz (TC) is present as a gap in the INL of all eyes from this age group. The foveal pit (P) has thinned the GCL, IPL and INL compared to layers surrounding the pit (the foveal slope). Scale in Bottom left for all.
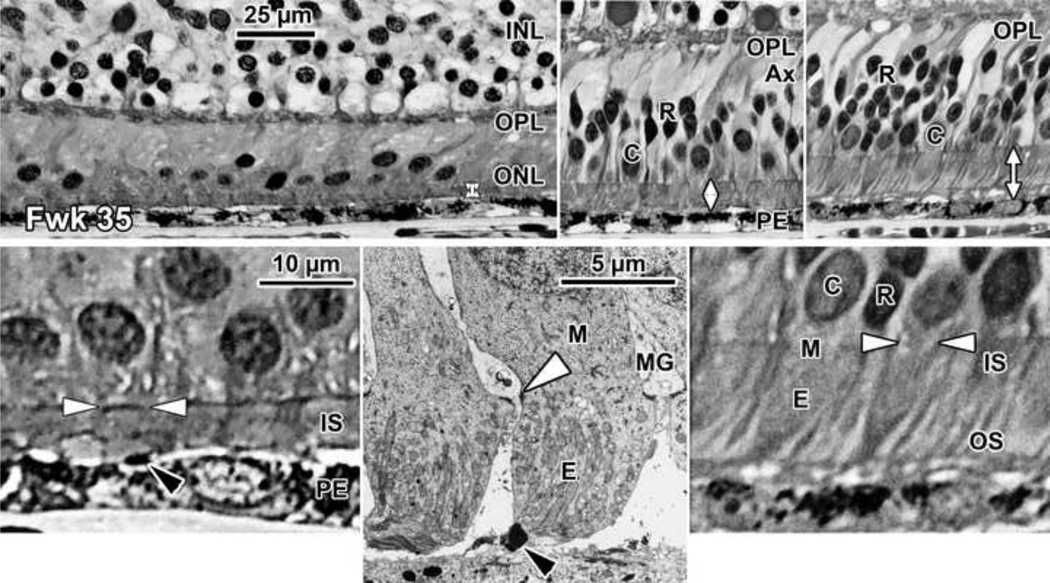
Figure 4. Histology of Late-gestation Fetal Human Retina.
A Fwk 35 retina is shown (Top left, Bottom left), at the fovea, (Top middle) at 800µm from the fovea where there are 2–3 layers of rods, and (Top right, Bottom right) at 2mm from the fovea. Longer axons (Ax) are appearing on cones and rods outside the fovea (Top middle, Top right) which tilt away from the pit center (fovea to left). Note that the space between the ELM and PE (vertical double arrows) is widest in the periphery. Short OS are present on both rods and cones in the periphery (Top right, Bottom right) but are difficult to recognize close to or in the fovea (Bottom left, Bottom rightblack arrowhead). Bottom middle: Two Fwk 30 foveal cones shown in a low magnification electron micrograph. The desmosomal junctions between Muller (M) glia and cones forming the ELM can be resolved (white arrowhead). Each cone is surrounded by pale M cytoplasm. Scale in Top left for Top row; scale in Bottom left for Bottom row.
The space between the ELM and PE is 12.5µm at 800µm (Figure 4 Top middle, white vertical arrow) and 20µm at 2mm (Figure 4 Top right, white vertical arrow). Peripheral rods and cones have tapered IS and short OS which are clearly more developed than on foveal cones (compare Figure 4 Bottom left and right).
The rod-free zone is narrower, with rods present ~600µm from the pit center. This suggests that cone packing has started11, 14, although cones remain in a single layer across central retina up to birth.
Postnatal 1day-6 weeks (6 retinas examined)
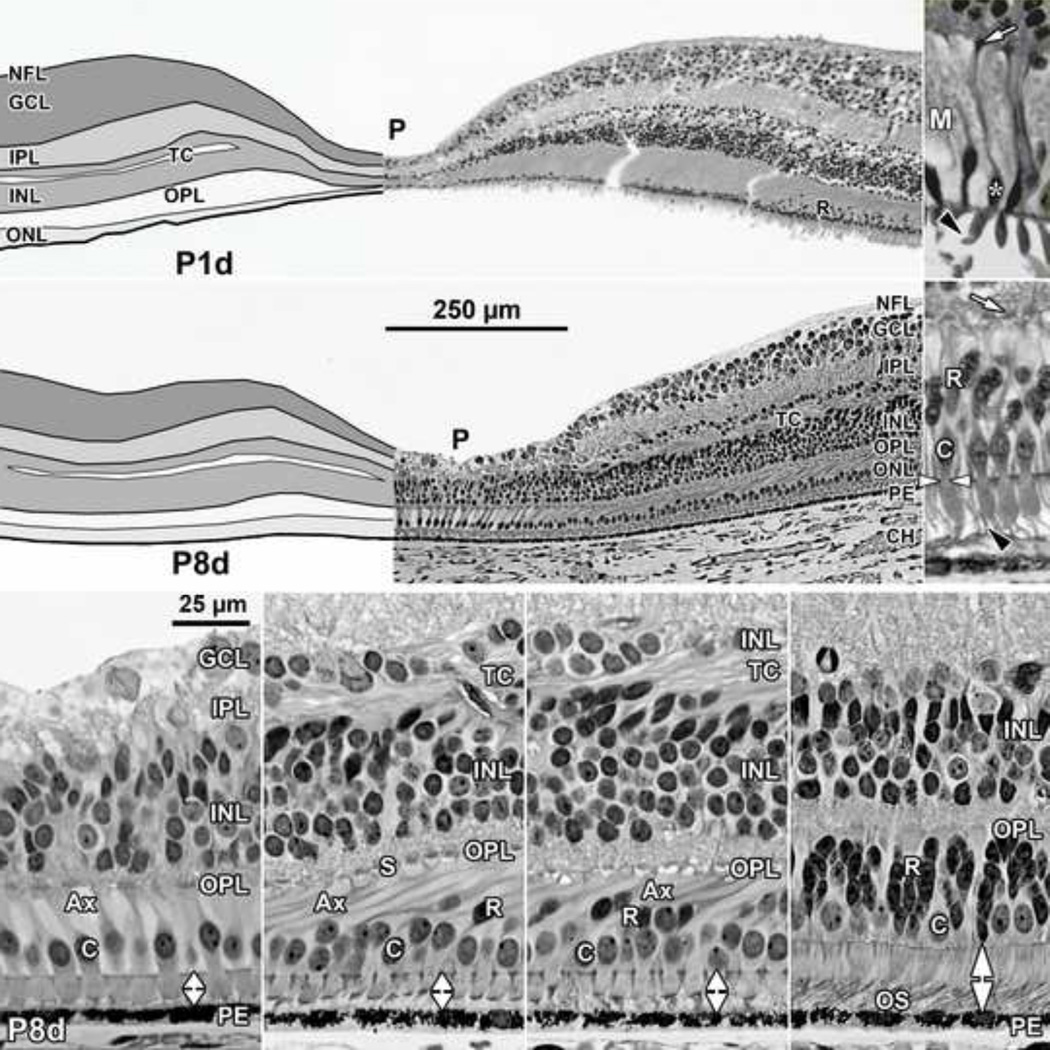
The pit becomes deeper and wider after birth with the INL, IPL and GCL only 1–2 cells deep (Figure 5 Top left). A TC is still present in the INL in some retinas, even those well fixed by histological criteria. Over the pit a single layer of short cones persists (Figure 5 Top right, middle left, Bottom far left; Figure 7 Bottom middle right; Figure 8 Top far right Top), but slope cones are thinner, elongated, and are packed into 2–3 layers (Figure 5 middle right). Changes in the OPL in and around the fovea are striking after birth. Long cone axons are prominent across central retina (Figure 5 Bottom far left to middle right; Figure 8 middle and middle right) making the OPL a much thicker layer. Most cones are angled with their cell bodies closer to the pit center than their synaptic axons and pedicles (Figure 5 Bottom middle left and right, pit center to left). Central cones (Figure 5 Bottom far left and middle left) have elongated tapered IS and short OS (Figure 5 Bottom far left, white vertical arrow), but peripheral IS and OS still are longer than foveal. The gap between ELM and PE is 14µm in the fovea, and 20µm at 1mm (Figure 5 Bottom middle right) and 30µm at 2mm (Figure 5 Bottom far right) from the fovea.
Figure 5. Layers and Histology of Post-natal Term Human Retina.
Development of the human fovea at (Top left) postnatal (P) 1 day (d), and (middle left) P8d. These retinas illustrate the range in development found around birth. In both retinas the pit is wider and more shallow than before birth and has displaced most of the inner layers and extends close to the cone synapses. A TC is present in the INL. Cones on the pit slope are 2–3 deep and have long Ax, but a single layer of cones still is present over the pit center. At P1d rods (R) are within 500µm of the foveal center. Top right: One of the cones (asterisk) on the slope from another P1d eye is shown at higher power. The IS is longer and narrower than before birth, and a short OS is present (arrowhead). The long axon ends in a synaptic spherule (arrow). Note the large amount of pale M cytoplasm surrounding each cone, also seen around foveal cones in (middle left). Middle right: A P8d retina at 1mm. Both cone (C) and rod (black arrowhead) OS are obvious. P8d photoreceptors (Bottom far left) at the foveal center, (Bottom middle left) on the foveal slope at the first rods, and (Bottom middle right) 800µm and (Bottom far right) 2mm from the fovea. Note that the distance between ELM and PE (vertical double arrows) has increased at the fovea, but is still narrower than in the periphery. OS are present at all locations but are much shorter in the fovea compared to the periphery. The fibrous acellular nature of the TC can be seen in Bottom middle left and right. Scale in middle left for Top and middle left; scale in Bottom far left for all others.
In this age group the two P1d retinas and one P8d were morphologically more mature than the other P8d retina (compare Figure 5 Top left and middle left). This may illustrate the expected variability both at birth and in postnatal development. The 5wk retina had increased growth in central IS and OS but in other respects resembled the P1d retina.
9–15 months (3 retinas examined)
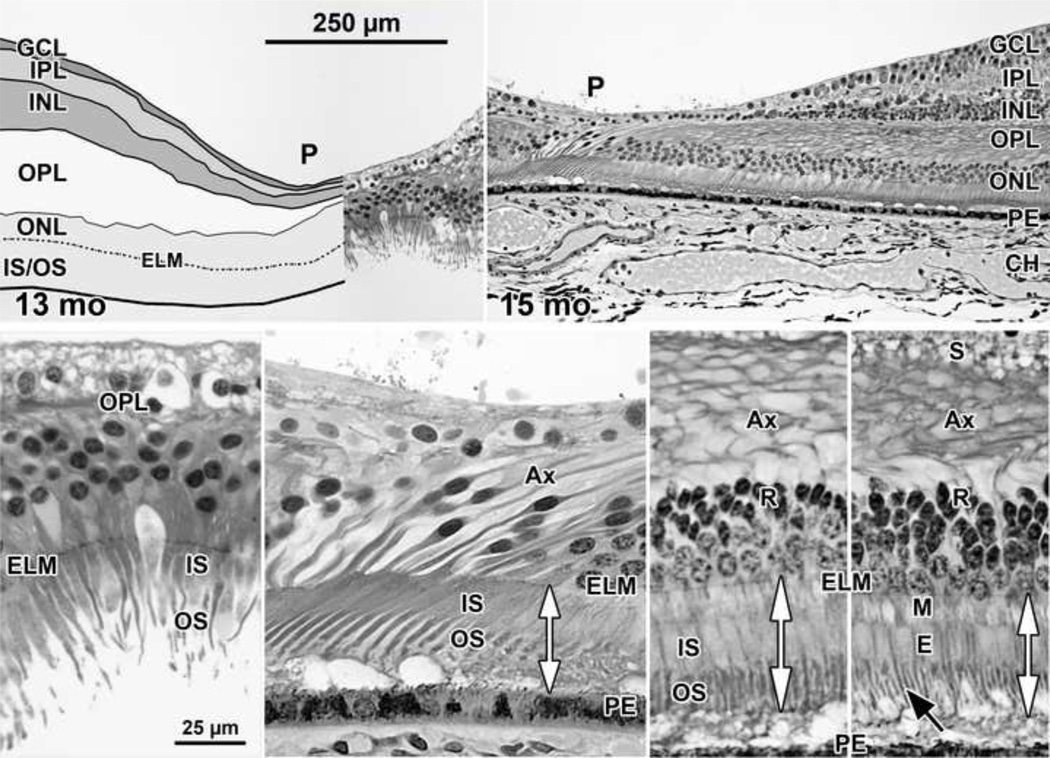
In 2 of the 3 retinas, in the pit center the GCL, IPL and INL have fused into a single thin layer (Figure 6 Top right). The pit is wider than at earlier ages. Foveal cones are packed 2–4 nuclei deep and have thin, long IS and OS (Figure 6 Bottom far left and middle left). The axons on foveal cones further elongate as cone packing into the fovea occurs after birth. Long axons are prominent across central retina (Figure 6 Top right, Bottom middle left and far right), This formation and elongation of photoreceptor axons around the fovea eventually makes the OPL a combination of a thick layer of axons and a single layer of cone synaptic pedicles. The OPL around the fovea is much thicker than in the periphery where it contains mainly synaptic contacts (Figure 1). In the first postnatal year, synaptic pedicles are disappearing over the pit (compare Figure 6 Bottom middle left to Bottom far right) as all INL neurons are displaced peripherally. Eventually these cellular migrations eliminate the OPL entirely from the foveal center (Figure 7 top and middle). For the first time, in this age group foveal and peripheral IS and OS are similar in length (compare double arrows in Figure 6 Bottom middle left to Bottom far right). In the P13 month specimen with a detached retina, foveal IS/OS are up to 46 µm long while slope cones are 36 µm long.
Figure 6. Layers and Histology of Human Retina during Infancy.
Postnatal maturation of the human fovea at (Top left) 13 months ; and (Top right) 15 months. Over the early postnatal months, the pit becomes wide and shallow with almost no neurons in the center except cone cell bodies. The drawing in Top left illustrates the large postnatal growth in thickness of the OPL, mainly due to the increased number and length of cone Ax. Bottom far left: Note the increase in ONL cone cell bodies over the 13 months pit center due to postnatal cone packing. These have long thin IS and OS. At 15 months, by comparing the double vertical arrows, it can be seen that (Bottom middle left) foveal, and IS/OS on photoreceptors at (Bottom middle right) 800µm and (Bottom far right) 2mm from the fovea now are similar in length. Long thin rod OS (Bottom far rightblack arrow) are prominent outside of the foveal center. Cone synapses (S) are absent from the foveal center (Bottom far and middle left) because they have been displaced onto the pit slope (Bottom middle and far rights). Scale in Top left for Top row; scale in Bottom far left for Bottom row.
These changes around 12 month indicate that pit formation is nearing its close, that cones are still packing into the foveal center, and foveal IS and OS are elongating compared to peripheral.
28 months-13 years (5 retinas examined)
The pit becomes wider and more shallow by 3.8 years (Figure 7 Top left) with virtually no neurons left in the pit center except cone cell bodies ~8 deep. Foveal IS are much thinner than slope IS and OS are longer. The 3.8 years fovea is morphologically similar to the 13 years but has not completed cone packing as indicated by its cone density of 108,400/mm2compared to 208,200/mm2 for a 37 years adult10.
At 13 years (Figure 7 middle) the fovea appears mature in all aspects. Cone cell bodies in the foveal ONL are up to 12 deep, and the floor of the pit contains Müller cytoplasm, cone axons and the occasional neuron. Foveal cone IS are very thin and so tightly packed that it is difficult to discern single IS. There is a marked IS width gradient from foveal center to IS at 2mm (compare Figure 7 Bottom row). The IS shape is also different, being almost straight in the fovea and tapered in the rest of the retina. Foveal cone IS are 168–189 µm long and ~3µm wide throughout their length while OS are 139–155 µm long. At the first rods (~300µm) cone IS are 92–105µm long and ~6µm wide with OS 126 µm long. At 2mm OS are ~40µm long, almost 4× shorter than foveal OS.
DISCUSSION
This study shows the pronounced change in human foveal morphology in both the inner and outer layers of the retina from midgestion to early adulthood. Because assignment of layers is critical to SDOCT interpretation18, 19, understanding these qualitiative and quantitative changes allows us to establish a standard reference for pediatric SDOCT. This correlation will be presented in the following paper. The second point that emerges is the marked similarity in human foveal development compared to Old World Macaca and New World marmoset monkeys (reviewed in12, 21–23). Human late fetal and infant necropsy retinas are scarce, especially with maximal preservation which allows detailed analysis of cellular development. There also is a marked similarity between adult monkey and human in SDOCT/histology24. This allows us to draw inferences about human development from well-preserved monkey fetal and infant retinas of known age. It also suggests that research into neonatal development versus SDOCT images would be feasible in monkeys.
This study documents the marked changes that occur within the central human retina after midgestation. Starting at Fwk25, outward displacements of the inner retinal layers begin to form the foveal pit. Molecular analysis of the macular region beginning at Fwk8 indicates the presence of axon guidance molecules such as pigment epithelium derived factor, natriuretic peptide precursor B, collagen type IV alpha 2 and ephrin A625, 26. These factors probably act first to repel axons and later blood vessels to form the foveal avascular zone20, 27–29. Pit formation begins shortly after Fwk24–25 when the foveal avascular zone is formed29, Modeling and quantitative morphology15, 16, 30 underscore the importance of the foveal avascular zone in pit development, with intraocular pressure acting on it to initiate invagination. By 13–15 months, pit formation appears complete with a single, broken layer of neurons in the pit center.
This paper documents the dramatic changes in the outer retina which mainly occur after birth. The foveal ONL contains a single layer of cones until after birth but then attains 10–12 deep thickness by 6–8 years9, 31, 32. Cone elongation and packing are mainly postnatal events, and it is just this period in which the pit shape changes from narrow and deep to wide and shallow, presumably due to retinal stretch during eye growth16. Cone density is 18,472 cones/mm2 at Fwk 22 before a pit is apparent, doubles to 36,294 at birth when the pit is narrow, rises to 52,787 at 15 months and at 108,439 cones/mm2 is within the Bottom end of the adult range at 3.8 years10. Thus pit remodeling may influence early phases of cone packing16, 33, but the cause for doubling of density between 15 months and 3.8 years is not obvious. A correlation of fibroblast growth factor expression and cone packing has been described34, but its exact role is unclear.
Of direct interest to SDOCT imaging is the observation that from Fwk22 to birth foveal cones have short thick IS and very short OS while cones at 1–2mm have longer IS and OS. This pattern was verified in vivo through 3D mapping of premature infant photoreceptor OS on SDOCT volumes19. This was first shown by Bach and Seefelder31 who provided the first description of human foveal development, and has been noted subsequently for monkeys8, 15, 20 and humans9, 32, 35. This pattern is unexpected in that all descriptions of expression of opsin, synaptic proteins and other photoreceptor molecules in humans2, 5–7, 36 find expression first in and around the fovea with a subsequent progression into the periphery. Thus photoreceptor differentiation begins very early in the fovea, but further maturation is delayed until well after birth.
At birth peripheral cone IS/OS are twice as long as foveal, by 15 months foveal and peripheral IS/OS are about the same length, and by 13 years foveal OS are 4× longer than at 2mm. IS and OS growth might be suppressed to facilitate cone displacement into the fovea. However, cone density rises 1.5× between birth and 15 months when elongation begins, and then doubles between 15 months and 13 years when it finishes10, demonstrating that very long IS and OS do not inhibit most cone packing.
An additional change within the outer retina is the postnatal elongation of cone and central rod axons. This changes the OPL from a thin layer of synaptic terminals to a layer as thick or thicker than the ONL. Photoreceptor axons are formed as a result of neuronal displacements in foveal development8, 13, 23. Foveal photoreceptors form synapses before midgestation36; (also see Figure 8 Top far right) with INL neurons. As these neurons are displaced peripherally by pit formation, photoreceptor axons elongate to keep contact. Axons must elongate further after birth when photoreceptors are displaced centrally to raise cone density. Thus photoreceptor axons form to retain synaptic integrity during these two opposing neuronal movements.
This paper expands the existing literature and shows that the human fovea develops over a very long period. Morphologically the incipient fovea can be identified at Fwk11–12 by its characteristic lamination. In the last half of gestation the pit forms by GCL, IPL and INL displacement which is finished by 1–2 years. Foveal cones change little in late gestation but in the first year after birth, they become elongated cells with long IS, OS and axons, and by 4–6 years develop the longest IS and OS in the retina. Concurrently, cones are packed into the fovea to raise cone density 10×. These processes are completed before 10 years when the fovea has its adult characteristics.
Now that technical advances in SDOCT imaging have made it possible to study pre-and neonatal human eyes, the data presented here will be critical in interpreting clinical images of retinal microstructures in young infants. These histological data provide a reference of normal development which will aid in the diagnosis of abnormal development detected in SDOCT imaging.
ACKNOWLEDGEMENTS/DISCLOSURE
Funding/Support:
Supported by the Heed Ophthalmic Foundation (LV). This research was made possible by the following grants: The Hartwell Foundation Biomedical Research Award (CAT); NIH Core Grants for Vision Research EY5722 and EY01730, Research to Prevent Blindness (RPB) Unrestricted Grant to Washington University, and RPB Physician Scientist Award (CAT).
Biographies

Cynthia A. Toth, M.D.
Professor of Ophthalmology and Biomedical Engineering Duke University
Cynthia A. Toth, Professor of Ophthalmology and Biomedical Engineering at Duke University, is a clinician-scientist, vitreoretinal surgeon. She directs the Duke Advanced Research in SDOCT Imaging Laboratory (DARSI Lab). Her translational research interests include ophthalmic diagnostics outside of conventional clinical settings, microsurgical instrumentation, and novel imaging biomarkers to improve the diagnosis, treatment, and outcomes for adults and children with vitreoretinal disease.

Anita Hendrickson, Ph.D.
University of Washington Department of Biology
Anita Hendrickson, Ph.D., is Professor Emerita of Ophthalmology and Biological Structure, University of Washington. She has published 175 peer-reviewed papers and reviews on primate brain and retinal development. Her work on primate foveal structure and development has been recognized by the Paul Kayser International Award of Merit in Retina Research (1998), Proctor Medal from Association for Research in Vision and Ophthalmology (2002), and University Distinguished Faculty Lecturer in Medicine (2004).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures:
Cynthia A. Toth receives royalties through her university from Alcon; obtained research support for other studies from Bioptigen, Genentech and Physical Sciences Inc. The other authors have no conflicts.
Contribution of Each Author:
Conception and design (AH, CAT)
Analysis and Interpretation of the data (AH, DP, LV, CAT)
Writing the article (AH, CAT)
Critical Revision of the article (AH, DP, LV, CAT)
Final approval of the article (AH, CAT)
Data Collection (AH, DP)
Provision of materials, patients, or resources (AH, CAT)
Statement about Conformity with Author Information: Postmortem specimens were obtained under University of Washington Human Subjects Approval #010447.
REFERENCES
- 1.Seiler MJ, Aramant RB. Photoreceptor and glial markers in human embryonic retina and in human embryonic retinal transplants to rat retina. Brain Res Dev Brain Res. 1994;80(1-2):81–95. doi: 10.1016/0165-3806(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 2.Nag TC, Wadhwa S. Expression of GABA in the fetal, postnatal, and adult human retinas: an immunohistochemical study. Vis Neurosci. 1997;14(3):425–432. doi: 10.1017/s0952523800012104. [DOI] [PubMed] [Google Scholar]
- 3.Nag TC, Wadhwa S. Developmental expression of calretinin immunoreactivity in the human retina and a comparison with two other EF-hand calcium binding proteins. Neuroscience. 1999;91(1):41–50. doi: 10.1016/s0306-4522(98)00654-x. [DOI] [PubMed] [Google Scholar]
- 4.Milam AH, Hendrickson AE, Xiao M, et al. Localization of tubby-like protein 1 in developing and adult human retinas. Invest Ophthalmol Vis Sci. 2000;41(8):2352–2356. [PubMed] [Google Scholar]
- 5.Xiao M, Hendrickson A. Spatial and temporal expression of short, long/medium, or both opsins in human fetal cones. J Comp Neurol. 2000;425(4):545–559. [PubMed] [Google Scholar]
- 6.O'Brien KM, Schulte D, Hendrickson AE. Expression of photoreceptor-associated molecules during human fetal eye development. Mol Vis. 2003;9:401–409. [PubMed] [Google Scholar]
- 7.Hendrickson A, Bumsted-O'Brien K, Natoli R, Ramamurthy V, Possin D, Provis J. Rod photoreceptor differentiation in fetal and infant human retina. Exp Eye Res. 2008;87(5):415–426. doi: 10.1016/j.exer.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendrickson A, Kupfer C. The histogenesis of the fovea in the macaque monkey. Invest Ophthalmol Vis Sci. 15(9):746–756. 976. [PubMed] [Google Scholar]
- 9.Hendrickson AE, Yuodelis C. The morphological development of the human fovea. Ophthalmology. 1984;91(6):603–612. doi: 10.1016/s0161-6420(84)34247-6. [DOI] [PubMed] [Google Scholar]
- 10.Yuodelis C, Hendrickson A. A qualitative and quantitative analysis of the human fovea during development. Vision Res. 1986;26(6):847–855. doi: 10.1016/0042-6989(86)90143-4. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Araya C, Provis JM. Evidence of photoreceptor migration during early foveal development: a quantitative analysis of human fetal retinae. Vis Neurosci. 1992;8(6):505–514. doi: 10.1017/s0952523800005605. [DOI] [PubMed] [Google Scholar]
- 12.Hendrickson A. A morphological comparison of foveal development in man and monkey. Eye (Lond) 1992;6(2):136–144. doi: 10.1038/eye.1992.29. [DOI] [PubMed] [Google Scholar]
- 13.Packer O, Hendrickson AE, Curcio CA. Development redistribution of photoreceptors across the Macaca nemestrina (pigtail macaque) retina. The J Comp Neurol. 1990;298(4):472–493. doi: 10.1002/cne.902980408. [DOI] [PubMed] [Google Scholar]
- 14.Robinson SR, Hendrickson A. Shifting relationships between photoreceptors and pigment epithelial cells in monkey retina: implications for the development of retinal topography. Vis Neurosci. 1995;12(4):767–778. doi: 10.1017/s0952523800009020. [DOI] [PubMed] [Google Scholar]
- 15.Springer AD, Hendrickson AE. Development of the primate area of high acuity. 2. Quantitative morphological changes associated with retinal and pars plana growth. Vis Neurosci. 2004;21(5):775–790. doi: 10.1017/S0952523804215115. [DOI] [PubMed] [Google Scholar]
- 16.Springer AD, Hendrickson AE. Development of the primate area of high acuity, 3: temporal relationships between pit formation, retinal elongation and cone packing. Vis Neurosci. 2005;22(2):171–185. doi: 10.1017/S095252380522206X. [DOI] [PubMed] [Google Scholar]
- 17.Springer AD, Troilo D, Possin D, Hendrickson AE. Foveal cone density shows a rapid postnatal maturation in the marmoset monkey. Vis Neurosci. 2011;28(6):473–484. doi: 10.1017/S0952523811000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011;31(8):1609–1619. doi: 10.1097/IAE.0b013e3182247535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldonado RS, O'Connell RV, Sarin N, et al. Dynamics of human foveal development after premature birth. Ophthalmology. 2011;118(12):2315–2325. doi: 10.1016/j.ophtha.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendrickson A, Troilo D, Possin D, Springer A. Development of the neural retina and its vasculature in the marmoset Callithrix jacchus. J Comp Neurol. 2006;497(2):270–286. doi: 10.1002/cne.20996. [DOI] [PubMed] [Google Scholar]
- 21.Curcio CA, Hendrickson A. Organization and Development of the Primate Photoreceptor Mosaic. In: Osborne N, Chader J, editors. Progress in retinal research. Oxford: Pergamon Press; 1991. pp. 89–120. [Google Scholar]
- 22.Provis JM, Diaz CM, Dreher B. Ontogeny of the primate fovea: a central issue in retinal development. Prog Neurobiol. 1998;54(5):549–580. doi: 10.1016/s0301-0082(97)00079-8. [DOI] [PubMed] [Google Scholar]
- 23.Hendrickson A, Provis J. Sernagor E. Retinal development. Cambridge: Cambridge University Press; 2006. Comparison of development of the primate fovea centralis with peripheral retina; pp. 126–149. [Google Scholar]
- 24.Anger EM, Unterhuber A, Hermann B, et al. Ultrahigh resolution optical coherence tomography of the monkey fovea. Identification of retinal sublayers by correlation with semithin histology sections. Exp Eye Res. 2004;78(6):1117–1125. doi: 10.1016/j.exer.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Kozulin P, Natoli R, O'Brien KM, Madigan MC, Provis JM. Differential expression of anti-angiogenic factors and guidance genes in the developing macula. Mol Vis. 2009;15:45–59. [PMC free article] [PubMed] [Google Scholar]
- 26.Kozulin P, Natoli R, Madigan MC, O'Brien KM, Provis JM. Gradients of Eph-A6 expression in primate retina suggest roles in both vascular and axon guidance. Mol Vis. 2009;15:2649–2662. [PMC free article] [PubMed] [Google Scholar]
- 27.Gariano RF, Iruela-Arispe ML, Hendrickson AE. Vascular development in primate retina: comparison of laminar plexus formation in monkey and human. Invest Ophthalmol Vis Sci. 1994;35(9):3442–3455. [PubMed] [Google Scholar]
- 28.Provis JM, Sandercoe T, Hendrickson AE. Astrocytes and blood vessels define the foveal rim during primate retinal development. Invest Ophthalmol Vis Sci. 2000;41(10):2827–2836. [PubMed] [Google Scholar]
- 29.Provis JM, Hendrickson AE. The foveal avascular region of developing human retina. Arch Ophthalmol. 2008;126(4):507–511. doi: 10.1001/archopht.126.4.507. [DOI] [PubMed] [Google Scholar]
- 30.Springer AD, Hendrickson AE. Development of the primate area of high acuity. 1. Use of finite element analysis models to identify mechanical variables affecting pit formation. Vis Neurosci. 2004;21(1):53–62. doi: 10.1017/s0952523804041057. [DOI] [PubMed] [Google Scholar]
- 31.Bach L, Seefelder R. Atlas zur entwicklungsgeschichte des menschlichen auges. Leipzig: W. Engelmann; 1914. pp. 1–148. [Google Scholar]
- 32.Abramov I, Gordon J, Hendrickson A, Hainline L, Dobson V, LaBossiere E. The retina of the newborn human infant. Science. 1982;217(4556):265–267. doi: 10.1126/science.6178160. [DOI] [PubMed] [Google Scholar]
- 33.Springer AD. New role for the primate fovea: a retinal excavation determines photoreceptor deployment and shape. Vis Neurosci. 1999;16(4):629–636. doi: 10.1017/s0952523899164034. [DOI] [PubMed] [Google Scholar]
- 34.Cornish EE, Natoli RC, Hendrickson A, Provis JM. Differential distribution of fibroblast growth factor receptors (FGFRs) on foveal cones: FGFR-4 is an early marker of cone photoreceptors. Mol Vis. 2004;10:1–14. [PubMed] [Google Scholar]
- 35.Hendrickson A, Drucker D. The development of parafoveal and mid-peripheral human retina. Behav Brain Res. 1992;49(1):21–31. doi: 10.1016/s0166-4328(05)80191-3. [DOI] [PubMed] [Google Scholar]
- 36.Nag TC, Wadhwa S. Differential expression of syntaxin-1 and synaptophysin in the developing and adult human retina. J Biosci. 2001;26(2):179–191. doi: 10.1007/BF02703642. [DOI] [PubMed] [Google Scholar]