Abstract
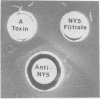
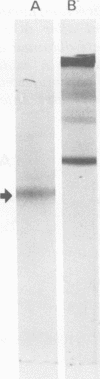
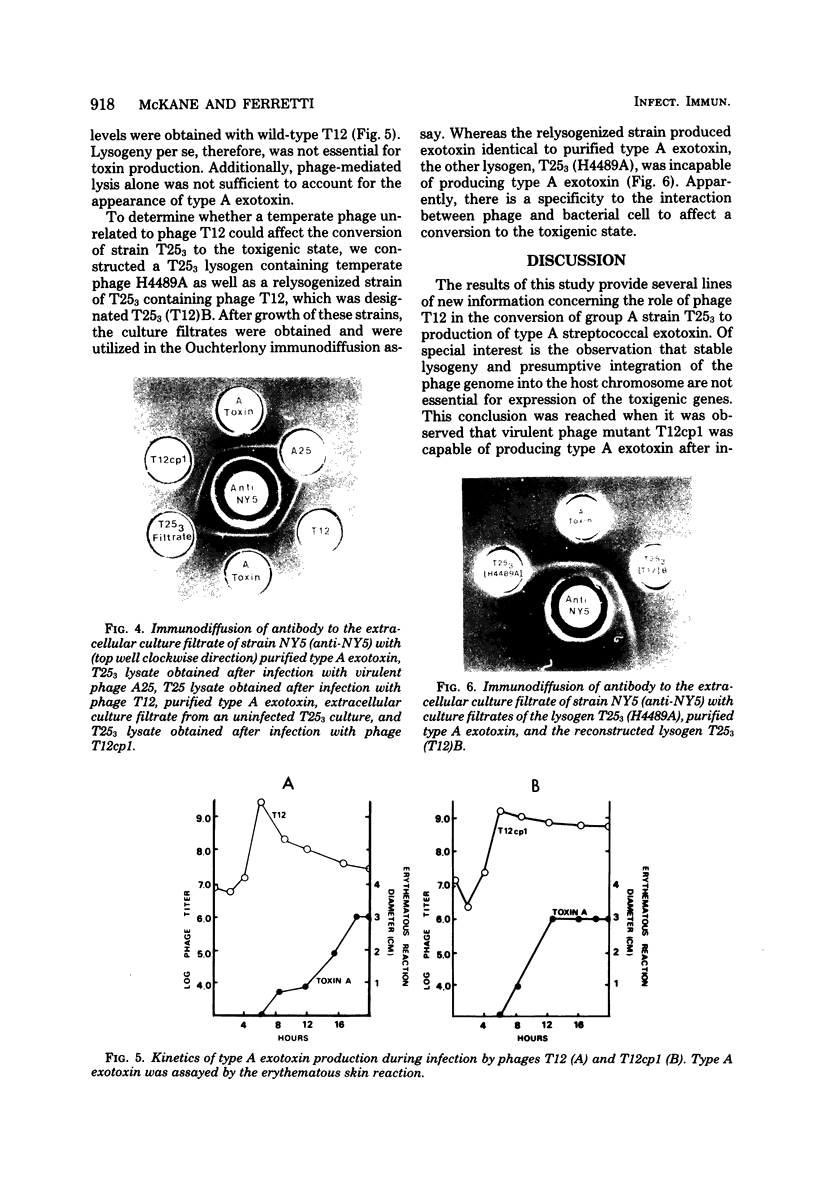
The infection of Streptococcus pyogenes nontoxigenic strain T 253 with bacteriophage T12 to form lysogen T 253 (T12) resulted in the production of type A streptococcal exotoxin (erythrogenic toxin or streptococcal pyrogenic exotoxin). Two lines of evidence indicated that lysogeny per se was not sufficient to promote toxigenic conversion of strain T 253. First, a virulent mutant of phage T12, unable to form stable lysogens, was able to affect type A exotoxin production by strain T 253. An unrelated virulent phage A25 did not affect type A exotoxin production after infection of strain T 253. Second, the temperate phage H4489A, which established stable lysogens with strain T 253 did not promote type A exotoxin production. These results suggest that there is a strain specificity to the phage-host interaction which affects type A exotoxin synthesis. Additional evidence is presented which indicates that type A streptococcal exotoxin was not a structural component of phage T12.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behnke D., Ferretti J. J. Molecular cloning of an erythromycin resistance determinant in streptococci. J Bacteriol. 1980 Nov;144(2):806–813. doi: 10.1128/jb.144.2.806-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke D., Ferretti J. J. Physical mapping of plasmid pDB101: a potential vector plasmid for molecular cloning in streptococci. Plasmid. 1980 Sep;4(2):130–138. doi: 10.1016/0147-619x(80)90002-5. [DOI] [PubMed] [Google Scholar]
- Cunningham C. M., Barsumian E. L., Watson D. W. Further purification of group A streptococcal pyrogenic exotoxin and characterization of the purified toxin. Infect Immun. 1976 Sep;14(3):767–775. doi: 10.1128/iai.14.3.767-775.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Diezel W., Kopperschläger G., Hofmann E. An improved procedure for protein staining in polyacrylamide gels with a new type of Coomassie Brilliant Blue. Anal Biochem. 1972 Aug;48(2):617–620. doi: 10.1016/0003-2697(72)90117-0. [DOI] [PubMed] [Google Scholar]
- Houston C. W., Ferretti J. J. Enzyme-linked immunosorbent assay for detection of type A streptococcal exotoxin: kinetics and regulation during growth of Streptococcus pyogenes. Infect Immun. 1981 Sep;33(3):862–869. doi: 10.1128/iai.33.3.862-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. P., Schlievert P. M., Watson D. W. Transfer of group A streptococcal pyrogenic exotoxin production to nontoxigenic strains of lysogenic conversion. Infect Immun. 1980 Apr;28(1):254–257. doi: 10.1128/iai.28.1.254-257.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. B., Watson D. W. A purified group A streptococcal pyrogenic exotoxin. Physiochemical and biological properties including the enhancement of susceptibility to endotoxin lethal shock. J Exp Med. 1970 Mar 1;131(3):611–622. doi: 10.1084/jem.131.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macrina F. L., Jones K. R., Wood P. H. Chimeric streptococcal plasmids and their use as molecular cloning vehicles in Streptococcus sanguis (Challis). J Bacteriol. 1980 Sep;143(3):1425–1435. doi: 10.1128/jb.143.3.1425-1435.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauciel C., Blass J., Mangalo R., Raynaud M. Evidence for two molecular forms of streptococcal erythrogenic toxin. Conversion to a single form by 2-mercaptoethanol. Eur J Biochem. 1969 Nov;11(1):160–164. doi: 10.1111/j.1432-1033.1969.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Nauciel C., Raynaud M., Bizzini B. Purification et propriétés de la toxine érythrogèbe du streptocoque. Ann Inst Pasteur (Paris) 1968 Jun;114(6):796–811. [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Wannamaker L. W., Almquist S., Skjold S. Intergroup phage reactions and transduction between group C and group A streptococci. J Exp Med. 1973 Jun 1;137(6):1338–1353. doi: 10.1084/jem.137.6.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannamaker L. W., Skjold S., Maxted W. R. Characterization of bacteriophages from nephritogenic group A streptococci. J Infect Dis. 1970 Apr;121(4):407–418. doi: 10.1093/infdis/121.4.407. [DOI] [PubMed] [Google Scholar]
- ZABRISKIE J. B. THE ROLE OF TEMPERATE BACTERIOPHAGE IN THE PRODUCTION OF ERYTHROGENIC TOXIN BY GROUP A STREPTOCOCCI. J Exp Med. 1964 May 1;119:761–780. doi: 10.1084/jem.119.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]