Abstract
The effect of foliar application of salicylic acid (SA) at different concentrations (10−3 M and 10−5 M) was investigated on the production of secondary metabolites (flavonoids), chalcone synthase (CHS) activity, antioxidant activity and anticancer activity (against breast cancer cell lines MCF-7 and MDA-MB-231) in two varieties of Malaysian ginger, namely Halia Bentong and Halia Bara. The results of high performance liquid chromatography (HPLC) analysis showed that application of SA induced the synthesis of anthocyanin and fisetin in both varieties. Anthocyanin and fisetin were not detected in the control plants. Accordingly, the concentrations of some flavonoids (rutin and apigenin) decreased significantly in plants treated with different concentrations of SA. The present study showed that SA enhanced the chalcone synthase (CHS) enzyme activity (involving flavonoid synthesis) and recorded the highest activity value of 5.77 nkat /mg protein in Halia Bara with the 10−5 M SA treatment. As the SA concentration was decreased from 10−3 M to 10−5 M, the free radical scavenging power (FRAP) increased about 23% in Halia Bentong and 10.6% in Halia Bara. At a concentration of 350 μg mL−1, the DPPH antioxidant activity recorded the highest value of 58.30%–72.90% with the 10−5 M SA treatment followed by the 10−3 M SA (52.14%–63.66%) treatment. The lowest value was recorded in the untreated control plants (42.5%–46.7%). These results indicate that SA can act not only as an inducer but also as an inhibitor of secondary metabolites. Meanwhile, the highest anticancer activity against MCF-7 and MDA-MB-231 cell lines was observed for H. Bara extracts treated with 10−5 M SA with values of 61.53 and 59.88%, respectively. The results suggest that the high anticancer activity in these varieties may be related to the high concentration of potent anticancer components including fisetin and anthocyanin. The results thus indicate that the synthesis of flavonoids in ginger can be increased by foliar application of SA in a controlled environment and that the anticancer activity in young ginger extracts could be improved.
Keywords: flavonoids, salicylic acid, chalcone synthase, HPLC, FRAP, DPPH, anticancer, Halia Bara
1. Introduction
Salicylic acid (SA) is a phenolic compound capable of enhancing plant growth and yield in some plants. SA acts as a potential non-enzymatic antioxidant, as well as a plant growth regulator, and plays an important role in regulating a number of plant physiological processes and bioactive compounds production [1]. Plants are potential sources of natural bioactive compounds which occur as secondary metabolites. They absorb sun light and produce high levels of oxygen and secondary metabolites during photosynthesis. Flavonoids are an important group of secondary metabolites and are a source of bioactive compounds in plants [2]. They are also a kind of natural product with antioxidant properties capable of scavenging free superoxide radicals, having anti-aging properties as well as reducing the risk of cancer. Sung-jin et al.[3] showed that some flavonoid components in green tea are effective in inhibiting cancer or induce mechanisms that may kill cancer cells and inhibit tumor invasion. It was found that flavonoids reduced blood-lipids and glucose, and enhanced human immunity [4]. Currently, flavonoids are extracted among other sources, from ginkgo leaves [5], kudzu roots [6], lotus leaves [7] and ginger rhizomes and leaves [8]. Chalcone synthase enzyme (CHS) is a key entry enzyme committed to the production of flavonoids in plants. Naringenin, the product of CHS activity is the initiator of a large variety of secondary metabolites including flavonoids, isoflavonoids, anthocyanins and phloroglucinols [9]. CHS condenses three malonyl-CoA molecules with cinnamoyl-CoA to produce chalcone. This condensation is the main pathway for the production of flavonoids (Figure 1), [10]. Ginger is one of the traditional folk medicines and it is widely used in cooking in Malaysia. It is extensively used overall especially in Asia and contains several interesting bioactive constituents which possess health promoting properties. Ginger is regarded as an important resource with a wide range of high flavonoid components, is low priced [11,12], and therefore serves as a cheap and important food material. Food composition and food additives play a major role in providing the required antioxidants for the body, although traditionally, spices such as ginger are commonly used in food preparations to improve flavor and taste. Previous studies by the current authors demonstrated potent antioxidant and anticancer activities of Malaysian ginger (Halia Bara and Halia Bentong) [13–15]. However, the impact of foliar application of SA on the production of biopharmaceuticals in Malaysian herbs has not been widely investigated. This needs to be understood, especially when the objective is production optimization with regard to herbal chemistry. Furthermore, information on SA effects on the chemistry of Malaysian ginger varieties is not available. Such data would be useful to provide information on the availability of high levels of beneficial components.
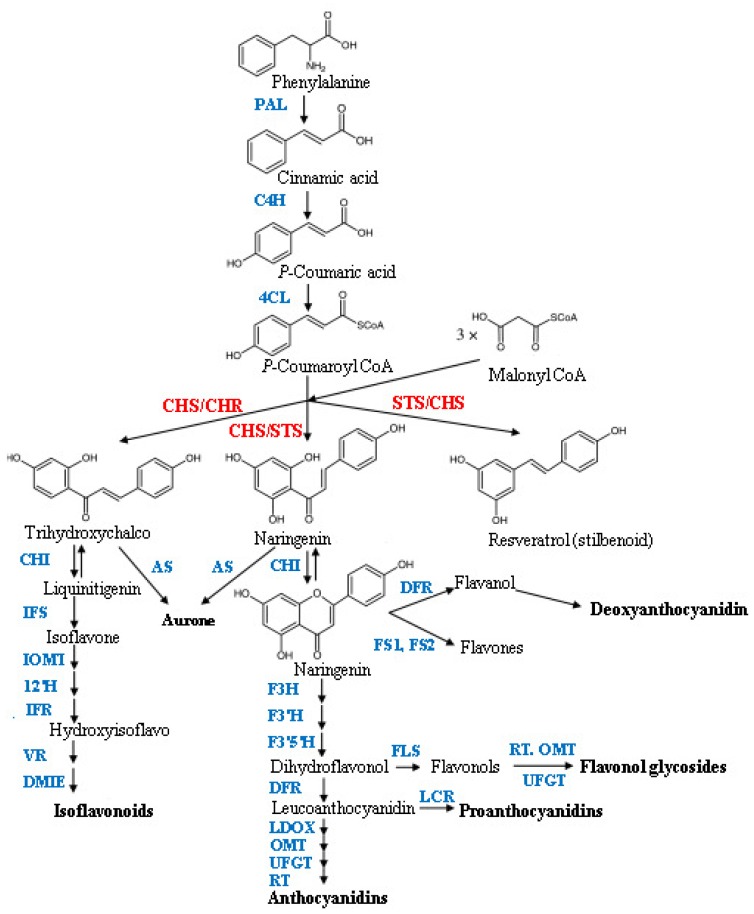
Figure 1.
Flavonoid biosynthetic pathway. ANS, anthocyanidin synthase; AS, aureusidin synthase; C4H, cinnamate-4-hydroxylase; CHR, chalcone reductase; DFR, dihydroflavonol 4-reductase; DMID, 7,2′-dihydroxy,4′-methoxyisoflavanol dehydratase; F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′ hydroxylase; F3′5′H, flavonoid 3′5′ hydroxylase; FS1/FS2, flavone synthase; I2′H, isoflavone 2′-hydroxylase; IFR, isoflavone reductase; IFS, isoflavone synthase; IOMT, isoflavone O-methyltransferase; LCR, leucoanthocyanidin reductase; LDOX, leucoanthocyanidin dioxygenase OMTO-methyltransferase; PAL, phenylalanine ammonia-lyase; RT, rhamnosyl transferase; UFGT, UDP flavonoid glucosyl transferase; VR, vestitone reductase; STS, stilbene synthase; FLS, flavanol synthase [16].
This study was therefore designed to examine the effects of foliar application of SA on the production of secondary metabolites in two varieties of Malaysian ginger (Zingiber officinale), namely H. Bentong and H. Bara and to evaluate CHS enzyme activity. In addition in vitro antioxidant and anticancer properties of the extracts against breast cancer cell lines were also investigated.
2. Results and Discussion
2.1. HPLC Analysis of Flavonoid Compounds
Results of high performance liquid chromatography (HPLC) analysis of flavonoids and phenolic acids are present in Table 1. Leaf extracts of Malaysian ginger, especially the variety H. Bara contained considerably (p ≤ 0.05) high amounts of rutin (0.893 mg g−1 DW) and apigenin (0.384 mg g−1 DW). SA application reduced rutin production in H. Bara (6.8%) and H. Bentong (21.8%). According to the data obtained, the concentration of some flavonoids (e.g., rutin, apigenin) decreased significantly in plants treated with different concentrations of SA (Table 1). High concentrations of these flavonoids were found in the control plants. Conversely, concentration of naringenin, fisetin and morin increased significantly in both varieties when treated with different concentrations of SA. The interesting finding was that application of SA in both varieties induced synthesis of fisetin and anthocyanin which were not detected in the control plants. Highest levels of anthocyanin (0.442 mg g−1 DW) and fisetin (0.359 mg g−1 DW) were observed in leaf extracts of H. Bara treated with 10−5 M of SA. Obinat et al. [17] also reported that production of anthocyanin was induced in cultured grape cells following foliar application of SA. Myricetin has antioxidant properties and in vitro research suggests that various concentrations of myricetin can modify LDL cholesterol and enable increased uptake by white blood cells [18]. In the present study production of myricetin was enhanced in ginger varieties treated with SA compared to control plants. High levels of this potent antioxidant compound were observed in H. Bara (0.112 mg g−1 DW) treated with 10−5 M SA. Morien is a rare yet well known flavonoid component of plants and acts as a chemo-preventive agent in vitro and in vivo against oral carcinogenesis [19,20]. The importance of morin and related compounds as anti-tumour drugs has also been widely recognized [21]. A high content of morin (0.193 mg g−1 DW) was obtained in extracts of H. Bara treated with 10−5 M SA. According to HPLC analysis, it could be concluded that the application of SA induced synthesis of some flavonoids, while conversely inhibiting production of some other flavonoids in ginger. Our results suggest the ability of SA application to modify or alter both the profile and the concentration of flavonoids in ginger.
Table 1.
High performance liquid chromatography analysis of ginger (Zingiber officinale) varieties treated with salicylic acid (SA).
| Compounds | Halia Bentong | Halia Bara | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Control | SA 10−5 | SA 10−3 | Control | SA 10−5 | SA 10−3 | |
| Rutin | 0.893 ± 0.03 b,c | 0.736 ± 0.09 c | 0.79 ± 0.06 c | 1.13 ± 0.12 a | 0.883 ± 0.07 b,c | 0.993 ± 0.07 a,b |
| Apigenin | 0.384 ± 0.049 c | 0.276 ± 0.08 d | 0.305 ± 0.09 d | 0.553 ± 0.06 a | 0.45 ± 0.06 b | 0.55 ± 0.04 a |
| Myricetin | 0.04 ± 0.009 b | 0.088 ± 0.009 a,b | 0.059 ± 0.018 b | 0.06 ± 0.001 b | 0.112 ± 0.004 a | 0.074 ± 0.006 a,b |
| Naringenin | 0.227 ± 0.049 a | 0.29 ± 0.1 a | 0.259 ± 0.045 a | 0.259 ± 0.033 a | 0.303 ± 0.097 a | 0.304 ± 0.02 a |
| Fisetin | ND | 0.237 ± 0.017 c | 0.228 ± 0.03 c | ND | 0.359 ± 0.046 a | 0.304 ± 0.01 b |
| Morien | 0.117 ± 0.02 a,b | 0.173 ± 0.055 a,b | 0.158 ± 0.042 a,b | 0.102 ± 0.042 b | 0.193 ± 0.03 a | 0.182 ± 0.017 a |
| Anthocyanin | ND | 0.381 ± 0.05 b | 0.369 ± 0.053 b | ND | 0.442 ± 0.041 a | 0.426 ± 0.122 a |
All analyses are the mean of triplicate measurements ±standard deviation; Results expressed in mg g−1 DW; Means not sharing a common single letter were significantly different at p ≤ 0.05.; ND: not detected.
2.2. Chalcone Synthase Enzyme (CHS) Activity
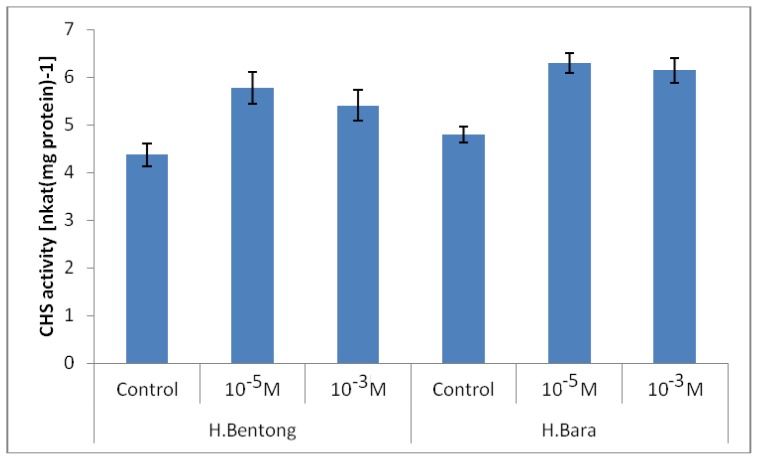
According to the results CHS activity was influenced by SA concentration (p ≤ 0.01; Figure 2). In both varieties treated with SA, CHS activity was found to be consistently higher in ginger treated with 10−5 M SA with values ranging between 5.77 and 6.30 nkat mg protein−1 than in gingers treated with 10−3 M SA, which recorded CHS activity of 5.40 to 6.14 nkat mg protein−1. Plants not treated with SA showed the lowest values for CHS activity which were registered between 4.37 and 4.80 nkat mg protein−1. The present study showed that with SA application the activity of CHS was enhanced. This is basically due to the fact that CHS is a precursor to flavonoids biosynthesis [22,23]. The increase in CHS activity is usually followed by an increase in C/N ratio due to the enhanced growth rate using SA. Results of recent studies suggest that increases in the C/N ratio in plants are an indication of increases in the synthesis of secondary plant metabolites, especially flavonoids [24,25].
Figure 2.
Chalcone synthase enzyme (CHS) activity in two ginger varieties treated with different concentration of salicylic acid (SA). Error bar represents standard error of means.
It is hypothesized that the increase in production of anthocyanin and flavonoids in the present work could be attributed to an increase in CHS activity in SA treated plants. Our results are consistent with Compos et al. [10] who reported SA induced CHS activity in beans. Ozeki et al.[26] pointed out that change in CHS activity rather than PAL activity (also involving enzymes for flavonoid synthesis) was correlated with changes in anthocyanin accumulation under various culture conditions. CHS is the first enzyme to switch from phenylpropanoid metabolism to flavonoid metabolism and is believed to be a key enzyme in this system [27]. These findings together with evidence for channeling between SA concentration and CHS activity in the general phenylpropanoid pathway [16], indicate that the organization of these systems are important in the understanding of how plant metabolism is regulated.
2.3. Antioxidant Activity
2.3.1. Ferric Reducing Antioxidant Potential (FRAP) Assay
Several methods have been used to measure the total antioxidant capacity of herbs, including FRAP assay, which has been adopted in this study. The FRAP assay depends on the reduction of ferric tripyridyltriazine (Fe (III)-TPTZ) complex to the ferrous tripyridyltriazine (Fe (II)-TPTZ) by a reductant at low pH. The reducing power of the leaf extract of young ginger was in the range of 476.22–793.26 μm of Fe (II)/g dry weight (Table 2). Increasing SA concentration had a significant effect on FRAP activity of young ginger. The FRAP values for the leaf extracts of both varieties treated with SA were significantly lower than α-tocopherol (953 μmol Fe (II)/g), but higher than that of BHT (611.82 μmol Fe (II)/g). It was reported that the effect of antioxidant scavenging is due to hydrogen donating ability [28,29]. The FRAP assay has been used widely to estimate the antioxidant component/power of dietary polyphenols [30]. It is evident that foliar application of SA significantly enhanced the content of some flavonoids in both ginger varieties, and the high flavonoid content was associated with high antioxidant activity. In a previous study, a strong positive relationship between total flavonoid content and antioxidant activity was reported, which appears to be the trend in many plant species [31].
Table 2.
Antioxidant activity (FRAP) in two varieties of Zingiber officinale treated with different concentration of salicylic acid (SA).
| Variety | SA (M) | FRAP (μmol Fe (II)/g) |
|---|---|---|
| H. Bentong | 0 | 476.22 + 10.19 e |
| 10−5 | 644.7 + 32.7 c | |
| 10−3 | 521.3 + 27.1 d | |
| 0 | 509.4 + 14.26 d | |
| H. Bara | 10−5 | 793.26 + 33.2 a |
| 10−3 | 716.62 + 32.5 b | |
| Positive controls | BHT | 611.82 + 15.2 |
| α-tocopherol | 887.34 + 29.5 |
All analyses are the mean of triplicate measurements ± standard deviation. Means not sharing a common letter were significantly different at p ≤ 0.05.
This study has shown that ginger has good free radical scavenging ability and therefore can be used as a radical inhibitor or scavenger, possibly acting as a primary antioxidant. Additionally, foliar application of SA can enhance the antioxidant activity of the ginger leaf extracts.
2.3.2. DPPH Antioxidant Activity
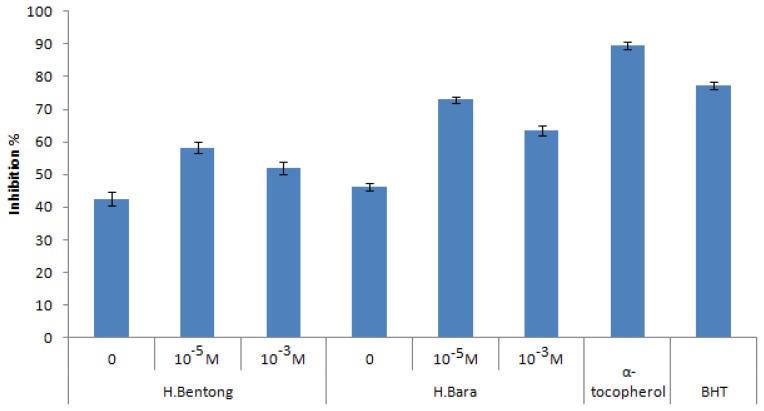
DPPH antioxidant activity was highest in the leaves of both varieties treated with SA compared to non treated plants (Figure 3). The effects on DPPH were contributed by SA treatments (p ≤ 0.05). At a concentration of 350 μg mL−1, the DPPH antioxidant activity recorded the highest value of 58.30%–72.90% with the 10−5 M SA treatment, followed by the 10−3 M SA (52.14%–63.66%) treatment, while the lowest value was obtained in the nontreated plants (42.50%–46.73%). However, DPPH radical scavenging ability of the plant extracts was lower than those of butylated hydroxyl toluene (BHT; 77.17%) and α-tocopherol (89.6%) registered at 350 μg mL−1. The highest value of DPPH activity was observed in H. Bara extracts (72.90%) when plants were treated with 10−5 M SA. This study showed that H. Bara extract had a good free radical scavenging activity, and hence can be used as a radical scavenger, acting possibly as a primary antioxidant. These results also imply that the 10−5 M SA treatment could significantly reduce the DPPH radical scavenging activity of the Malaysian ginger varieties. The principle involved is that in the presence of a molecule consisting of a stable free radical (DPPH), an antioxidant with the ability to donate a hydrogen atom will quench the stable free radical, a process which is associated with a change in absorption that can be translated spectrophotometrically.
Figure 3.
1,1-Diphenyl-2-picrylhydrazyl (DPPH) scavenging activities of two varieties of ginger treated with different concentration of SA compared with positive controls (α-tocopherol and BHT). Error bar represents standard error of means.
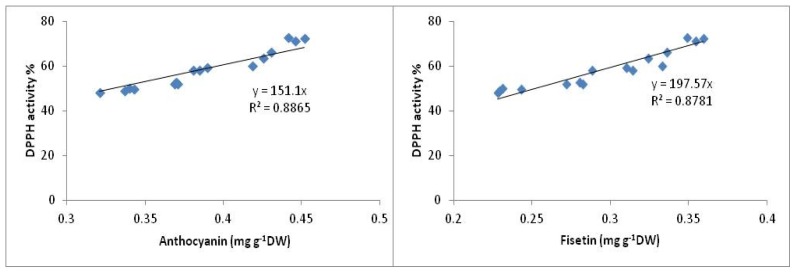
To date, more than 8000 phenolic compounds are known in plants, of which almost two-thirds belong to the predominantly water soluble flavonoid antioxidant family. Fisetin and anthocyanin were reported as potent antioxidant compounds with high antioxidant activity (DPPH activity) [32–34], and in the current study high concentrations of fisetin and anthocyanin were observed in ginger treated with 10−5 M SA. The high antioxidant activity in ginger varieties observed with the 10−5 M SA treatment is attributed to the high concentrations of anthocyanin and fisetin in these varieties. In this study, positive and significant (p < 0.05) correlations have been observed between anthocyanin (R2 = 0.88) and fisetin (R2 = 0.87) content with DPPH activity (Figure 4). The present findings are consistent with other research reports which found positive and significant correlations between anthocyanin and fisetin with antioxidant activity in medicinal plants [35,36]. This effect maybe due to the electron-donating ability of these compounds.
Figure 4.
Relationship between anthocyanin and fisetin with DPPH activity in ginger varieties.
2.4. Anticancer Activity
The two ginger varieties were found to express MCF-7 and MDA-MB-231 cancer inhibitory activity when tested at concentrations of 4.6875–300 μg mL−1 (Table 3). Significant tumor inhibition was observed at 37.5 μg mL−1. At a concentration of 37.5 μg mL−1 most of the extracts exhibited strong anticancer activity towards MCF-7 and MDA-MB-231 cells. Maximum MCF-7 and MDA-MB-231 cell line inhibition was observed with H. Bara with values of 61.53% and 59.88%, respectively when treated with 10−5 M SA, while minimum MDA-MB-231 cell line inhibition was observed with the untreated H. Bentong (with values of 45.30% and 40.64%, respectively). MCF-7 and MDA-MB-231 cell lines treated with tamoxifen (positive control) showed 77.44% and 73.82% inhibition at the same concentration. Based on several in vivo and in vitro studies, many mechanisms of anticancer action may be involved. These include cell cycle arrest, carcinogen inactivation, antiproliferation, inhibition of angiogenesis, induction of apoptosis and differentiation, antioxidation and reversal of multi-drug resistance or a combination of these mechanisms [37–39]. Flavonoids are among the best candidates for mediating the protective effect of diets rich in fruits and vegetables with respect to colorectal cancer. Additional information on their effects on cancer cells and their mechanisms of action were obtained by adding, a series of related flavonoids to cultures of cancer cells; all flavonoid compounds were found to increase growth inhibition and cell loss at concentrations of 1 to 100 mM, with relative effectiveness being fisetin > kaempferol [40]. Hence, flavonoid compounds could probably be responsible for the anticancer activity of Z. officinale. According to the American National Cancer Institute, the criterion of normal cell viability for crude extracts of herbs is 76% [41]. This implies that extracts of herbs that show cell viability lower than this range are suitable for human consumption and are not harmful. Anthocyanin is a potent antioxidant [42–45]. Anthocyanin fractions extracted from different sources, including grape rinds and red rice [46], flower petals [47], Vaccinium species [48], red soyabeans and red beans [49], different cherry and berry extracts [50–52], and purple corn [53], have demonstrated anticancer activity. Katsube et al.[51] reported HCT-116 colon cancer cells were inhibited by anthocyanin-containing berry extracts including cowberry, blueberry, strawberry and bilberry extracts [51]. Similarly, tart cherry anthocyanins were shown to inhibit the growth of human colon cancer cell lines HCT116 and HT-29 [52]. Fisetin is found in several plants, fruits, vegetables, nuts and wine [54] and displays a variety of biological effects including anti-inflammatory, antioxidant [55,56], anti-carcinogenic and in vitro anti-angiogenesis [57]. In vivo, fisetin has recently been shown to possess interesting anticancer activity in mice bearing prostate tumours [58], lung carcinoma [59] and human embryonal carcinoma [60]. The in vivo mechanism of action appears rather complex and may include anti-angiogenic, anti-metastatic and anti-androgenic activities [61].
Table 3.
Anticancer activities (cell viability) and percent of inhibition of ginger extracts towards MCF-7 and MDA-MB-231 cell lines as determined by the MTT assay (at concentration 37.5 μg mL−1).
| Variety | SA (M) | MCF-7 | MDA-MB-231 | Inhibition % (MCF-7) | Inhibition % (MDA-MB-231) |
|---|---|---|---|---|---|
| H. Bentong | 0 | 54.7 ± 2.17 a | 59.36 ± 2.2 a | 45.3 | 40.64 |
| 10−5 | 41.08 ± 1.56 b | 45.3 ± 1.63 b | 58.92 | 54.7 | |
| 10−3 | 47.44 ± 1.64 b | 48.69 ± 1.77 b | 52.56 | 51.31 | |
| H. Bara | 0 | 51.63 ± 1.92 a | 56.2 ± 2.11 a | 48.37 | 43.8 |
| 10−5 | 38.47 ± 1.15 c | 40.12 ± 1.4 c | 61.53 | 59.88 | |
| 10−3 | 42.28 ± 1.68 b | 47.18 ± 1.58 c | 58.72 | 52.82 | |
|
| |||||
| Positive control | Tamoxifen | 22.56 ± 1.07 | 26.18 ± 1.27 | 77.44 | 73.82 |
All analyses are the mean of triplicate measurements ± standard deviation. Results expressed in percent of cell viability. Means not sharing a common letter were significantly different at p ≤ 0.05.
In the current study the highest values of both fisetin and athocyanin were detected in ginger varieties treated with 10−5 M SA. Meanwhile, the highest anticancer activity against MCF-7 and MDA-MB-231 cell lines has been observed with extracts of H. Bara treated with 10−5 M SA. This suggests that high anticancer activity in these varieties may be attributed to the high concentrations of potent anticancer components such as fisetin and anthocyanin. However, more research needs to be undertaken before the association between these flavonoids and anticancer activity in ginger varieties is more clearly understood. In varieties treated with SA the value of IC50 decreased significantly compared to non treated plants. The IC50 values of Halia Bara extract (not treated with SA) against MCF-7 and MDA-MB-231 cells were 39.1 and 42.1 μg mL−1, respectively (Table 4). In H. Bara treated with 10−5 M SA, the IC50 value decreased to 31.5 and 40.6 μg mL−1 for MCF-7 and MDA-MB-231 respectively; while the IC50 values of tamoxifen as a positive control for MCF-7 and MDA-MB-231 cells were 17.4 and 19.5 μg mL−1, respectively.
Table 4.
IC50 values of ginger extracts towards MCF-7 and MDA-MB-231 cancer cell lines as determined by the MTT assay.
| Variety | SA (M) | MCF-7 | MDA-MB-231 |
|---|---|---|---|
| H. Bentong | 0 | 50.6 ± 1.45 a | 54.7 ± 1.74 a |
| 10−5 | 42.5 ± 1.33 b | 48.8 ± 1.3b c | |
| 10−3 | 44.4 ± 1.72 b | 50.6 ± 1.28 b | |
| H. Bara | 0 | 39.1 ± 1.18 c | 45.2 ± 1.14 d |
| 10−5 | 31.5 ± 1.66 d | 40.6 ± 1.06 f | |
| 10−3 | 33.6 ± 1.2 d | 44.5 ± 1.66 e | |
|
| |||
| Tamoxifen | 17.4 ± 2.16 | 19.5 ± 1.88 | |
All analyses are the mean of triplicate measurements ± standard deviation. Results expressed in percent of cell viability. Means not sharing a common letter were significantly different at p ≤ 0.05.
3. Experimental Section
3.1. Plant Material and Maintenance
The rhizomes of ginger varieties Halia Bentong and Halia Bara (Zingiber officinale) were collected from the ginger planting area in Bentong Village, Malaysia. Voucher specimens were identified by the herbarium of the University Putra Malaysia, Selangor, Malaysia (H. Bentong Stone 6030 (KLU) and H. Bara Stone 7233 (KLU)). Rhizomes were sprouted for two weeks in 10 cm diameter pots filled with peat. They were then transferred to polyethylene bags filled with a soilless mixture consisting of burnt rice husk and coco peat (1:1). The plants were grown in a glasshouse at the Universiti Putra Malaysia (UPM) glasshouse complex. The seedlings were raised in specially constructed growth chambers receiving 12-h photoperiod and average photosynthetic photon flux density of 310 μmol m−2 s−1. Day and night temperatures were maintained at 30 ± 1.0 °C and 20 ± 1.5 °C, respectively, and relative humidity of between 70% and 80%. At the second leaf stage, the ginger seedlings were sprayed with two concentrations (10−3 or 10−5 M) of salicylic acid solution (SA; 2-hydroxybenzoic acid +100 μL dimethyl sulfoxide +0.02% polyoxyethylenesorbitan monolaurate, Tween 20, Sigma Chemicals; pH 6.5). Control plants were sprayed with the same solution but without SA. Plants were sprayed early in the morning with 10 mL of the respective solutions once a week for four weeks.
3.2. Extract Preparation
Leaf samples (0.25 g) were extracted with 20 mL of methanol, and 5 mL of 6 M HCl was added to each extract to give a total volume of 25 mL. The extracts were then refluxed at 90 °C for 2 h. Aliquots of 500 μL were taken before and after hydrolysis, filtered through a 0.45 μm filter, and analyzed for flavonoids using High Performance Liquid Chromatography (HPLC) [62].
3.3. High Performance Liquid Chromatography (HPLC) Analysis
3.3.1. Standard Curve Preparation
The standard stock solutions were prepared by dissolving standards in methanol to 100 μg mL−1. For the calibration curves, four additional concentrations (20, 40, 60 and 80 μg mL−1) were prepared by the dilution of the stock solutions with methanol.
3.3.2. Chromatography Conditions
Reversed-phase HPLC was used to assay flavonoid composition. The Agilent HPLC system used consisted of a Model 1100 pump equipped with a multi-solvent delivery system, an L-7400 ultraviolet (UV) detector, and fitted with an Agilent C18 (5 μm, 4.6 mm internal diameter 250 mm) column. The mobile phase consisted of: (A) 2% acetic acid (CH3COOH) and (B) 0.5% acetic acid-acetonitrile (CH3CN), (50:50 v/v). The mobile phase was filtered under vacuum through a 0.45 um membrane filter before use. Gradient elution was performed as follows: 0 min, 95:5; 10 min, 90:10; 40 min, 60:40, 55 min, 45:55; 60 min, 20:80; and 65 min, 0:100. The flow rate was maintained at 1 mL min−1 and UV absorbance was measured at 280–365 nm. The operating temperature was maintained at room temperature [63]. Identification of the flavonoids was achieved by comparison of retention times with standards, UV spectra and UV absorbance ratios after co-injection of samples and standards. The standards were purchased from Sigma–Aldrich (St. Louis, MO, USA).
3.4. Chalcone Synthase (CHS) Assay
CHS activity was assayed spectrophotometrically as described in Obinata et al.[17]. Enzymes were extracted at 4 °C by homogenizing the frozen harvested cells (0.4 g) in 1 mL of 0.1 M borate buffer (pH 8.8) containing 1 mM 2-mercaptoethanol with a homogenizer (Polytron). The homogenates were treated with 0.1 g of Dowex l × 4 for 10 min and the cell debris and resin were removed by centrifugation at 15,000 rpm for 10 min. A 0.2 g sample of Dowex l × 4 resin was added to the supernatant and treated for another 20 min. The resin was then removed by centrifugation at 15,000 rpm for 15 min. The resultant supernatant was used in the CHS assay. The CHS assay was performed with 100 μL of enzyme extract mixed with 1.89 mL of 50 mM Tris-HCI buffer, pH 7.6, containing 10 mM KCN. The enzyme reaction was allowed to proceed for 1 min at 30 °C after adding 10 mg chalcone to 10 μL ethylene glycol monomethylether. The activity was determined by measuring the absorbance at 370 nm.
3.5. Determination of Antioxidant Activities
3.5.1. Ferric Reducing Antioxidant Potential (FRAP) Assay
The stock solutions consisted of 300 mM acetate buffer, 10 mM TPTZ (2,4,6-tripyridyl-S-triazine) solution in 40 mM HCl, and 20 mM FeCl3 solution. Acetate buffer (25 mL) and TPTZ (2.5 mL) were mixed, and 2.5 mL FeCl3 added. Leaf extract (150 μL) was added to 2850 μL of the FRAP solution and kept for 30 min in the dark place. The absorbance of solution was measured at 593 nm using a spectrophotometer (U-2001, Hitachi Instruments Inc., Tokyo, Japan) [64].
3.5.2. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
1,1-Diphenyl-2-picrylhydrazyl (DPPH) was purchased from Sigma–Aldrich. Butylated hydroxytoluene (BHT) and α-tocopherol were purchased from Merck (India). The radical scavenging ability was determined using the method described in Mensor et al.[65]. Briefly, an alcohol solution of DPPH (1 mL, 3 mg mL−1) was added to 2.5 mL samples containing different concentrations of extracts originating from different parts of the ginger varieties. The samples were first kept in the dark at room temperature and their absorbance was read at 518 nm after 30 min. The antiradical activity was determined using the following formula:
| (1) |
Where A0 is the absorbance value of the blank sample or control reaction, and A1 is the absorbance value of the test sample. The optic density of the samples and controls were measured in comparison to ethanol. BHT (butylhydroxytoluene) and α-tocopherol, were used as positive controls.
3.6. Determination of Anticancer Activity
3.6.1. Cell Culture and Treatment
Human breast carcinoma cell lines (MCF-7 and MDA-MB-231) were cultured in 100 μL of RPMI 1640 media (Roswell Park Memorial Institute) containing 10% fetal bovine serum (FBS). MCF-7 and MDA-MB-231 cells were incubated overnight at 37 °C in 5% CO2 for cell attachment.
3.6.2. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) Assay
The assay was conducted as follows: Cancer cells were seeded in 96-well plates at a density of 1 × 104 cells/well in 100 μL RPMI. At 24 h after seeding, the medium was removed and the cells were incubated for 3 days with RPMI in the absence or presence of various concentrations of ginger extracts. Ginger extract concentrations used ranged from 4.6875, 9.375, 18.75, 37.5, 75, 150 to 300 μg mL−1. After incubation, 20 μL of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] reagent was added into each well. The plate was incubated again for 4 h in a CO2 incubator at 37 °C. The resulting MTT–products were determined by measuring the absorbance at 570 nm using ELISA reader [66]. Each point represents the mean of triplicate experiments. The cell viability was determined using the formula:
| (2) |
3.7. Statistical Analysis
Data were analyzed using the Statistical Analysis System (SAS, system 9.0; SAS Institute, Inc.: Cary, NC, USA, 2002). Means separation was performed using the Duncan multiple range test. The experimental results were expressed as mean ± standard deviation of three replicates. Mean differences at p ≤ 0.05 were regarded as significant.
4. Conclusions
This study demonstrated that foliar application of SA could be promoting the production of secondary metabolites and as a consequence, the antioxidant and anticancer properties of ginger varieties have been improved. SA is one of the special elicitors in the study of elicitation of secondary compounds through physiological pathways. Treatment of H. Bentong and H. Bara with SA improved production of fisetin and anthocyanin who have potent antioxidant activity and which were not detected in control plants. SA can act not only as an inducer but also as an inhibitor of secondary compound production [67] and in this study reduction of rutin and apigenin in both varieties was observed. In agreement with Nicholson and Hammerschmidt [68], an increase in the activity of CHS can be considered as a biochemical marker for resistance, given that this enzyme is the key for the necessary synthesis of flavonoid compounds. It would appear that foliar application of SA not only significantly enhances biomass production in ginger varieties, but that it also slightly increases the concentrations of several therapeutic compounds. Halia Bentong and Halia Bara exhibited promising anticancer activity on human breast cancer cell lines (MCF-7 and MDA-MB-231). The leaf extracts of these varieties contained appreciable amounts of effective flavonoid compounds like fisetin, morien, myreciten and anthocyanin, which is a potent agent for breast cancer growth inhibition. Subsequently, our MTT assay indicated that enriched Halia Bara leaf with 10−5 M of SA is a potential source of anticancer therapeutic compounds. It seems that SA could be used to enhance phyrochemical production and the pharmaceutical quality of ginger.
Acknowledgments
The authors are grateful to the Research Management Centre of University Putra Malaysia for financing and supporting this work.
References
- 1.Amanullah M.M., Sekar S., Vincent S. Plant growth substances in crop production. Asian J. Plant Sci. 2010;9:215–222. [Google Scholar]
- 2.Karimi E., Jaafar H.Z., Ahmad S. Phytochemical analysis and antimicrobial activities of methanolic extracts of leaf, stem and root from different varieties of Labisa pumila Benth. Molecules. 2011;16:4438–4450. doi: 10.3390/molecules16064438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park S.J., Myoung H., Kim Y.Y., Paeng J.Y., Park J.W., Kim M.J., Hong S.M. Anticancer effects of genistein, green tea catechins, and cordycepin on oral squamous cell carcinoma. J. Korean Oral Maxillofac. Surg. 2008;34:1–10. [Google Scholar]
- 4.Chan E.W.C., Lim Y.Y., Wong L.F., Lianto F.S., Wong S.K. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008;109:477–483. [Google Scholar]
- 5.Xie J., Zhu L., Luo H., Zhou L., Li C., Xu X. Direct extraction of specific pharmacophoric flavonoids from gingko leaves using a molecularly imprinted polymer for quercetin. J. Chromatogr. A. 2001;934:1–11. doi: 10.1016/s0021-9673(01)01294-8. [DOI] [PubMed] [Google Scholar]
- 6.Liao H.B., He Z.E. Research and prospect of kudzu root. Natl. Food Assoc. 2003;24:81–83. [Google Scholar]
- 7.Chen H.G., Yu Y.G., Zeng O.X. Study on extraction of flavonoids and alkaloids from lotus leaf. Food Sci. 2002;23:69–71. [Google Scholar]
- 8.Ghasemzadeh A., Jaafar H.Z.E., Rahmat A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe) Molecules. 2010;15:4324–4333. doi: 10.3390/molecules15064324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dao T.T., Linthorst H.J., Verpoorte R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011;10:397–412. doi: 10.1007/s11101-011-9211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campos A.D., Ferreira A.G., Hampe M.M.V., Antunes I.F., Brancão N., Silveira E.P., da Silva J.B., Osório V.A. Induction of chalcone synthase and phenylalanine ammonia-lyase by salicylic acid and Colletotrichum lindemuthianum in common bean Determination of Antioxidant Activities. Braz. J. Plant Physiol. 2003;15:129–134. [Google Scholar]
- 11.Ghasemzadeh A., Jaafar H.Z., Rahmat A. Elevated Carbon Dioxide Increases Contents of Flavonoids and Phenolic Compounds, and Antioxidant Activities in Malaysian Young Ginger (Zingiber officinale Roscoe) Varieties. Molecules. 2010;15:7907–7922. doi: 10.3390/molecules15117907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang Y., Zhao A. Study on the flavone content of buckwheat. J. Sichuan Agric. Univ. 2001;19:352–354. [Google Scholar]
- 13.Ghasemzadeh A., Jaafar H.Z.E. Anticancer and antioxidant activities of Malaysian young ginger (Zingiber officinale Roscoe) varieties grown under different CO2 concentration. J. Med. Plant Res. 2011;5:3247–3255. [Google Scholar]
- 14.Ghasemzadeh A., Jaafar H.Z.E. Effect of CO2 Enrichment on Some Primary and Secondary Metabolites synthesis in Ginger (Zingiber officinale Roscoe) Int. J. Mol. Sci. 2011;12:1101–1114. doi: 10.3390/ijms12021101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghasemzadeh A., Jaafar H.Z.E., Rahmat A., Megat Wahab P.E., Halim M.R. Effect of Different Light Intensities on Total Phenolics and Flavonoids Synthesis and Anti-oxidant Activities in Young Ginger Varieties (Zingiber officinale Roscoe) Int. J. Mol. Sci. 2010;11:3885–3897. doi: 10.3390/ijms11103885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winkel-Shirley B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001;126:485–493. doi: 10.1104/pp.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obinata N., Yamakawat T., Takamiya M., Tanaka N., Ishimaru K., Kodama T. Effects of Salicylic Acid on the Production of Procyanidin and Anthocyanin in Cultured Grape Cell. Plant Biotechnol. 2003;20:105–111. [Google Scholar]
- 18.Hayder N., Bouhlel I., Skandrani I., Kadri M., Steiman R., Guiraud P., Mariotte A.M., Ghedira K., Dijoux-Franca M.G., Chekir-Ghedira L. In vitro antioxidant and antigenotoxic potentials of myricetin-3-O-galactoside and myricetin-3-O-rhamnoside from Myrtus communis: Modulation of expression of genes involved in cell defence system using cDNA microarray. Toxicol. In Vitro. 2008;22:567–581. doi: 10.1016/j.tiv.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Brown J., Prey J., Harrison P.R. Enhanced sensitivity of human oral tumours to the flavonol, morin, during cancer progression: Involvement of the Akt and stress kinase pathways. Carcinogenesis. 2003;24:171–177. doi: 10.1093/carcin/24.2.171. [DOI] [PubMed] [Google Scholar]
- 20.Kawabata K., Tanaka T., Honjo S., Kakumoto M., Hara A., Makita H., Tatematsu N., Ushida J., Tsuda H., Mori H. Chemopreventive effect of dietary flavonoid morin on chemically induced rat tongue carcinogenesis. Int. J. Cancer. 1999;83:381–386. doi: 10.1002/(sici)1097-0215(19991029)83:3<381::aid-ijc14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 21.Song Y.M., Kang J.W., Zhou J., Wang Z.H., Lua X.Q., Wang L.F., Gao J.Z. Study on the fluorescence spectra and electrochemical behavior of ZnL2 and morin with DNA. Spectrochim. Acta A. 2000;56:2491–2497. doi: 10.1016/s1386-1425(00)00340-1. [DOI] [PubMed] [Google Scholar]
- 22.Muzika R.M. Terpenes and phenolics in response to nitrogen fertilization: A test of the carbon/nutrient balance hypothesis. Chemoecology. 1993;4:3–7. [Google Scholar]
- 23.Fajer E.D., Bowers M.D., Bazaaz F.A. The effects of nutrients and enriched CO2 environment on the production of carbon based allelochemicals in Plantago: A test of the carbon/nutrient balance hypothesis. Am. Nat. 1992;140:707–723. doi: 10.1086/285436. [DOI] [PubMed] [Google Scholar]
- 24.Winger A., Purdy S., Maclean A., Pourtau N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. New Phytol. 2006;161:781–789. doi: 10.1093/jxb/eri279. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim M.H., Jaafar H.Z.E. Impact of Elevated Carbon Dioxide on Primary, Secondary Metabolites and Antioxidant Responses of Eleais guineensis Jacq. (Oil Palm) Seedlings. Molecules. 2012;17:5195–5211. doi: 10.3390/molecules17055195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozeki Y., Komamine A., Noguchi H., Sankawa U. Changes in activities of enzymes involved in flavonoid metabolism during the initiation and suppression of anthocyanin synthesis in carrot suspension cultures regulated by 2,4-dichlorophenoxyacetic acid. Physiol. Plant. 2006;69:123–128. [Google Scholar]
- 27.Takeda J. Ligh induced synthesis of anthocyanin in carrot cells in suspension. J. Exp. Bot. 1990;41:749–755. [Google Scholar]
- 28.Karimi E., Oskoueian E., Hendra R., Jaafar H.Z. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules. 2010;15:6244–6256. doi: 10.3390/molecules15096244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luximon-Ramma A., Bahorun T., Soobrattee A.M., Aruoma O.I. Antioxidant activities of phenolic, proanthocyanidin and flavonoid components in extracts of Acacia fistula. J. Agric. Food Chem. 2005;50:5042–5047. doi: 10.1021/jf0201172. [DOI] [PubMed] [Google Scholar]
- 30.Wojdy A., Oszmiański J., Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2006;105:940–947. [Google Scholar]
- 31.Karimi E., Jaafar H.Z. HPLC and GC-MS determination of bioactive compounds in microwave obtained extracts of three varieties of Labisia pumila Benth. Molecules. 2011;16:6791–6805. doi: 10.3390/molecules16086791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasko P., Barton H., Zagrodzki P., Gorinstein S., Fołta M., Zachwieja Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 2009;115:994–998. [Google Scholar]
- 33.Astadi I.R., Astuti M., Santoso U., Nugraheni P.S. In vitro antioxidant activity of anthocyanins of black soybean seed coat in human low density lipoprotein (LDL) Food Chem. 2009;112:652–663. [Google Scholar]
- 34.Khanduja K.L., Bhardwaj A. Stable free radical scavenging and antiperoxidative properties of resveratrol compared in vitro with some other bioflavonoids. Indian J. Biochem. Biophys. 2003;40:416–422. [PubMed] [Google Scholar]
- 35.Shih P.H., Chan Y.C., Liao J.W., Wang M.F., Yen G.C. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer's disease. J. Nutr. Biochem. 2010;21:598–605. doi: 10.1016/j.jnutbio.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Sengupta B., Banerjee A., Sengupta P.K. Investigations on the binding and antioxidant properties of the plant flavonoid fisetin in model biomembranes. FEBS Lett. 2004;570:77–81. doi: 10.1016/j.febslet.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 37.Cragg G.B., Newman D.G. Plants as a source of anti-cancer and anti-HIV agents. Ann. Appl. Biol. 2003;143:127–133. [Google Scholar]
- 38.Davis W., Lamson M.S., Matthew S., Brignall N.D. Antioxidants and Cancer III: Quercetin. Alter. Med. Rev. 2000;5:196–204. [PubMed] [Google Scholar]
- 39.Gibellini L., Pinti M., Nasi M., De B.S., Roat E., Bertoncelli L., Cossarizza A. Interfering with ROS Metabolism in Cancer Cells: The Potential Role of Quercetin. Cancers. 2010;2:1288–1311. doi: 10.3390/cancers2021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verschoyle R.D., Steward W.P., Gescher A.J. Putative cancer chemopreventive agents of dietary origin-how safe are they? Nutr. Cancer. 2007;59:152–162. doi: 10.1080/01635580701458186. [DOI] [PubMed] [Google Scholar]
- 41.Itharat A., Houghton P.J., Eno A.E., Burke P.J., Sampson J.H., Raman A. In vitro cytotoxic activity of Thai medicinal plants used traditionally to treat cancer. J. Ethnopharm. 2004;90:33–38. doi: 10.1016/j.jep.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Wang H., Nair M.G., Strasburg G.M., Chang Y., Booren A.M., Gray J.I., DeWitt D.L. Antioxidant and anti-inflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999;62:294–296. doi: 10.1021/np980501m. [DOI] [PubMed] [Google Scholar]
- 43.Pool-Zobel B.L., Bub A., Schroder N., Rechkemmer G. Anthocyanins are potent antioxidants in model systems but do not reduce endogenous oxidative DNA damage in human colon cells. Eur. J. Nutr. 1999;38:227–234. doi: 10.1007/s003940050065. [DOI] [PubMed] [Google Scholar]
- 44.Seeram N.P., Momin R.A., Nair M.G., Bourquin L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine. 2001;8:362–369. doi: 10.1078/0944-7113-00053. [DOI] [PubMed] [Google Scholar]
- 45.Mazza G., Kay C.D., Cottrell T., Holub B.J. Absorption of anthocyanins from blueberries and serum antioxidant status in human subjects. J. Agric. Food Chem. 2002;50:7731–7737. doi: 10.1021/jf020690l. [DOI] [PubMed] [Google Scholar]
- 46.Koide T., Kamei H., Hashimoto Y., Kojima T., Hasegawa M. Antitumor effect of hydrolyzed anthocyanin from grape rinds and red rice. Cancer Biother. Radiopharm. 1996;11:273–277. doi: 10.1089/cbr.1996.11.273. [DOI] [PubMed] [Google Scholar]
- 47.Kamei H., Kojima T., Hasegawa M., Koide T., Umeda T., Yukawa T., Terabe K. Suppression of tumor cell growth by anthocyanins in vitro. Cancer Invest. 1995;13:590–594. doi: 10.3109/07357909509024927. [DOI] [PubMed] [Google Scholar]
- 48.Bomser J., Madhavi D.L., Singletary K., Smith M.A. In vitro anticancer activity of fruit extracts from Vaccinium species. Planta Med. 1996;62:212–216. doi: 10.1055/s-2006-957862. [DOI] [PubMed] [Google Scholar]
- 49.Koide T., Hashimoto Y., Kamei H., Kojima T., Hasegawa M., Terabe K. Antitumor effect of anthocyanin fractions extracted from red soybeans and red beans in vitro and in vivo. Cancer Biother. Radiopharm. 1997;12:277–280. doi: 10.1089/cbr.1997.12.277. [DOI] [PubMed] [Google Scholar]
- 50.Harris G.K., Gupta A., Nines R.G., Kresty L.A., Habib S.G., Frankel W.L., LaPerle K., Gallaher D.D., Schwartz S.J., Stoner G.D. Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2′-deoxyguanosine levels in the Fischer 344 rat. Nutr. Cancer. 2001;40:125–133. doi: 10.1207/S15327914NC402_8. [DOI] [PubMed] [Google Scholar]
- 51.Katsube N., Iwashita K., Tsushida T., Yamaki K., Kobori M. Induction of apoptosis in cancer cells by bilberry (Vaccinium myrtillus) and the anthocyanins. J. Agric. Food Chem. 2003;51:68–75. doi: 10.1021/jf025781x. [DOI] [PubMed] [Google Scholar]
- 52.Kang S.Y., Seeram N.P., Nair M.G., Bourquin L.D. Tart cherry anthocyanins inhibit tumor development in Apc(Min) mice and reduce proliferation of human colon cancer cells. Cancer Lett. 2003;194:13–19. [PubMed] [Google Scholar]
- 53.Hagiwara A., Miyashita K., Nakanishi T., Sano M., Tamano S., Kadota T., Koda T., Nakamura M., Imaida K., Ito N. Anthocyanin-Rich Extracts and Colonic Cell Growth. J. Agric. Food Chem. 2004;52:6120–6127. [Google Scholar]
- 54.Arai Y., Watanabe S., Kimira M., Shimoi K., Mochizuki R., Kinae N. Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000;130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 55.Park H.H., Lee S., Oh J.M., Lee M.S., Yoon K.H., Park B.H., Kim J.W., Song H., Kim S.H. Anti-inflammatory activity of fisetin in human mast cells (HMC-1) Pharmacol. Res. 2007;55:31–37. doi: 10.1016/j.phrs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 56.Woodman O.L., Chan E.C. Vascular and anti-oxidant actions of flavonols and flavones. Clin. Exp. Pharmacol. Physiol. 2004;31:786–790. doi: 10.1111/j.1440-1681.2004.04072.x. [DOI] [PubMed] [Google Scholar]
- 57.Fotsis T., Pepper M.S., Aktas E., Breit S., Rasku S., Adlercreutz H., Wahala K., Montesano R., Schweigerer L. Flavonoids, dietary-derived inhibitors of cell proliferation and in vitro angiogenesis. Cancer Res. 1997;57:2916–2921. [PubMed] [Google Scholar]
- 58.Touil Y.S., Fellous A., Scherman D., Chabot G.G. Flavonoid-induced morphological modifications of endothelial cells through microtubule stabilization. Nutr. Cancer. 2009;6:310–321. doi: 10.1080/01635580802521346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tripathi R., Samadder T., Gupta S., Surolia A., Shaha C. Anticancer activity of a combination of cisplatin and fisetin in embryonal carcinoma cells and xenograft tumors. Mol. Cancer Ther. 2011;10:255–268. doi: 10.1158/1535-7163.MCT-10-0606. [DOI] [PubMed] [Google Scholar]
- 60.Khan N., Asim M., Afaq F., Abu Z.M., Mukhtar H. A novel dietary flavonoid fisetin inhibits androgen receptor signaling and tumor growth in athymic nude mice. Cancer Res. 2008;68:8555–8563. doi: 10.1158/0008-5472.CAN-08-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chien C.S., Shen K.H., Huang J.S., Ko S.C., Shih Y.W. Antimetastatic potential of fisetin involves inactivation of the PI3K/Akt and JNK signaling pathways with downregulation of MMP-2/9 expressions in prostate cancer PC-3 cells. Mol. Cell Biochem. 2010;333:169–180. doi: 10.1007/s11010-009-0217-z. [DOI] [PubMed] [Google Scholar]
- 62.Crozier A., Jensen E., Lean M.E.J., Mc Donald M.S. Quantitative analysis of flavonoids by reversed-phase high performance liquid chromatography. J. Chromatogr. 1997;761:315–321. [Google Scholar]
- 63.Wang T.C., Chuang Y.C., Ku Y.H. Quantification of bioactive compounds in citrus fruits cultivated in Taiwan. Food Chem. 2007;102:1163–1171. [Google Scholar]
- 64.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 65.Mensor L.L., Menezes F.S., Leitao G.G., Reis A.S., Santos T.S., Coube C.S. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 66.Lau C.S., Ho C.Y., Kim C.F., Leung K.N., Fung K.P., Tse T.F., Chan H.L., Chow M.S. Cytotoxic activities of Coriolus versicolor (Yunzhi) extract on human leukemia and lymphoma cells by induction of apoptosis. Life Sci. 2004;75:797–808. doi: 10.1016/j.lfs.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 67.Doares S.H., Narvaez-Vasquez J., Conconi A., Ryan C.A. Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol. 1995;108:1741–1746. doi: 10.1104/pp.108.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Niccholson R.L., Hammerschmidt R. Phenolic compounds and their role disease resistance. Ann. Rev. Phytopathol. 1992;30:369–389. [Google Scholar]