Abstract
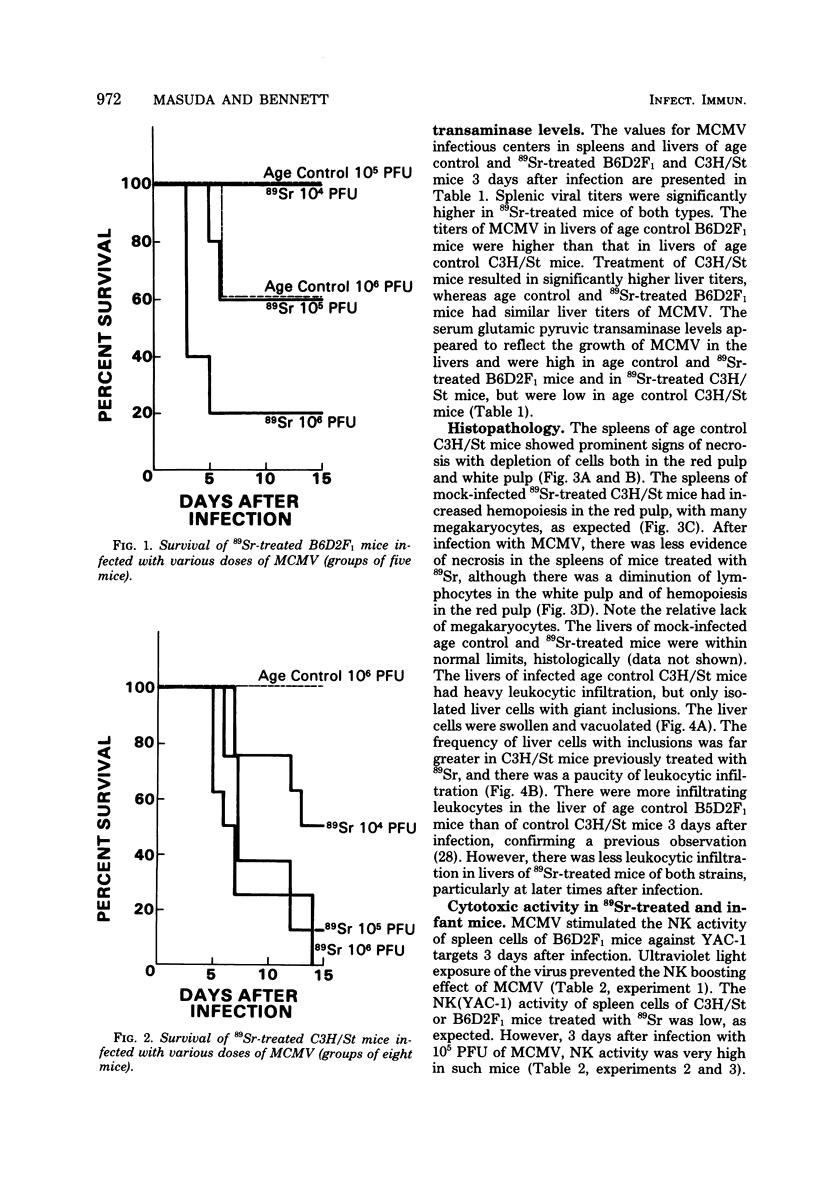
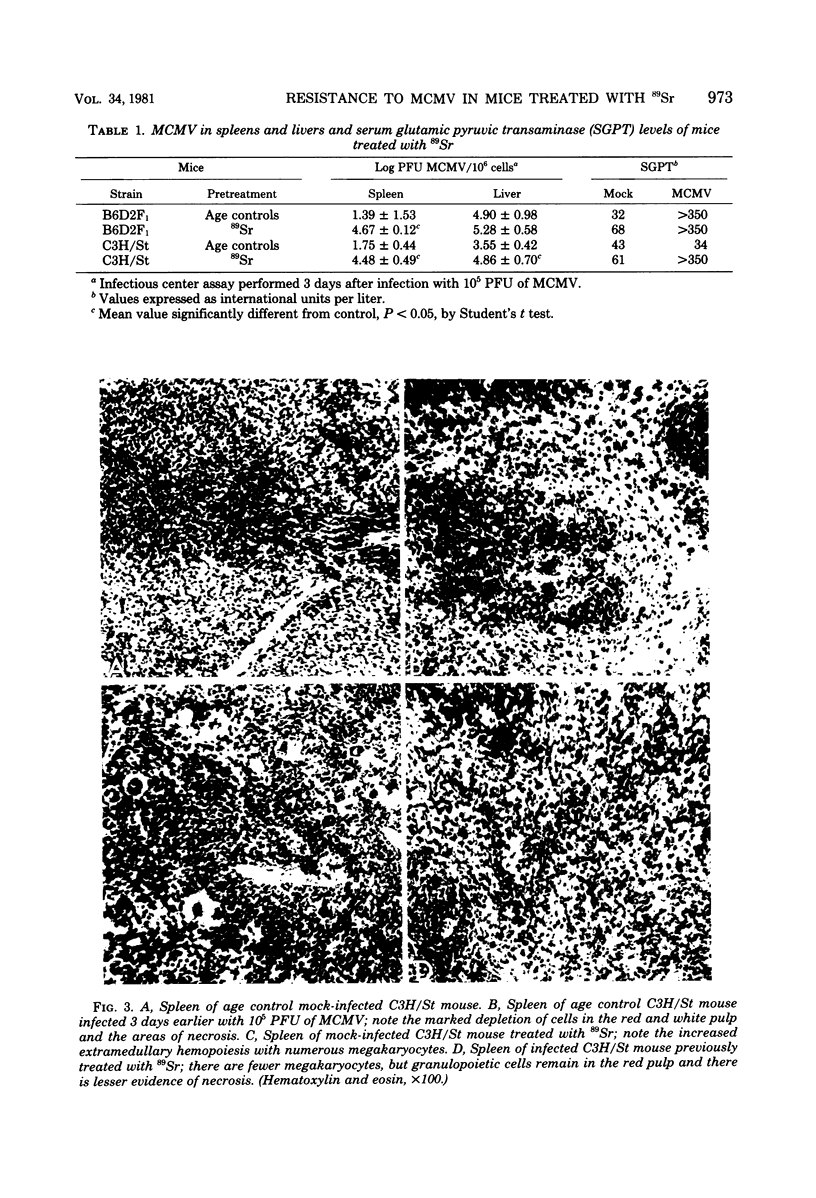
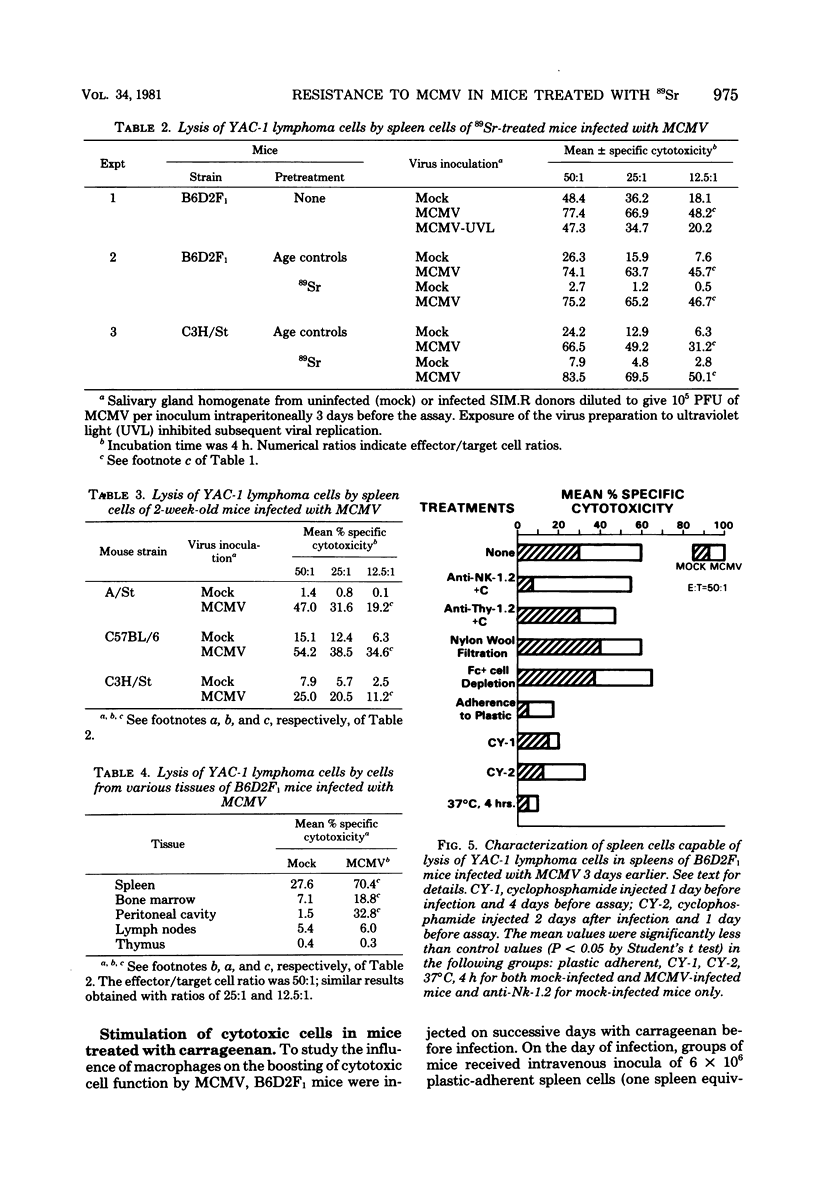
Treatment of C3H/St mice with 100 microCi of 89Sr weakened their genetic resistance to murine cytomegalovirus (MCMV) infection. The criteria utilized to detect increased susceptibility were: (i) survival of mice; (ii) numbers of MCMV-infected cells in the spleens and liver; and (iii) serum glutamic pyruvic transaminase levels. The natural killer (NK) cell activity of spleen cells from mice treated with 89Sr is very low. However, the NK activities of spleen cells of both normal and 89Sr-treated mice were greatly augmented 3 days after infection with MCMV. These NK cells lysed a variety of tumor cells and shared several features with conventional NK cells, but were not lysed by anti-Nk-1.2 serum (specific for NK cells) plus complement. Splenic adherent cells did not lyse tumor cells themselves but were necessary for the stimulation of NK cells by MCMV. The paradox of high NK cell function and poor survival in 89Sr-treated mice infected with MCMV was a surprise. We conclude that these augmented NK cells, of themselves, cannot account for the genetic resistance of C3H/St mice to infection with MCMV.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Shellam G. R., Chalmer J. E. Genetic influences on the augmentation of natural killer (NK) cells during murine cytomegalovirus infection: correlation with patterns of resistance. J Immunol. 1981 Mar;126(3):988–994. [PubMed] [Google Scholar]
- Bennett M., Baker E. E., Eastcott J. W., Kumar V., Yonkosky D. Selective elimination of marrow precursors with the bone-seeking isotope 89Sr: implications for hemopoiesis, lymphopoiesis, viral leukemogenesis and infection. J Reticuloendothel Soc. 1976 Jul;20(1):71–87. [PubMed] [Google Scholar]
- Bennett M., Baker E. E. Marrow-dependent cell function in early stages of infection with Listeria monocytogenes. Cell Immunol. 1977 Sep;33(1):203–210. doi: 10.1016/0008-8749(77)90147-2. [DOI] [PubMed] [Google Scholar]
- Bennett M. Prevention of marrow allograft rejection with radioactive strontium: evidence for marrow-dependent effector cells. J Immunol. 1973 Feb;110(2):510–516. [PubMed] [Google Scholar]
- Burton R. C., Winn H. J. Studies on natural killer (NK) cells. I. NK cell specific antibodies in CE anti-CBA serum. J Immunol. 1981 May;126(5):1985–1989. [PubMed] [Google Scholar]
- Fiala M., Payne J. E., Berne T. V., Moore T. C., Henle W., Montgomerie J. Z., Chatterjee S. N., Guze L. B. Epidemiology of cytomegalovirus infection after transplantation and immunosuppression. J Infect Dis. 1975 Oct;132(4):421–433. doi: 10.1093/infdis/132.4.421. [DOI] [PubMed] [Google Scholar]
- Gruber D. F., Zucali J. R., Wleklinski J., LaRussa V., Mirand E. A. Temporal transition in the site of rat erythropoietin production. Exp Hematol. 1977 Sep;5(5):399–407. [PubMed] [Google Scholar]
- Ho M. Role of specific cytotoxic lymphocytes in cellular immunity against murine cytomegalovirus. Infect Immun. 1980 Mar;27(3):767–776. doi: 10.1128/iai.27.3.767-776.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Najarian J. S. Cytomegalovirus-induced immune suppression. I. Humoral immunity. Clin Exp Immunol. 1974 Sep;18(1):109–118. [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Ben-Ezra J., Bennett M., Sonnenfeld G. Natural killer cells in mice treated with 89strontium: normal target-binding cell numbers but inability to kill even after interferon administration. J Immunol. 1979 Oct;123(4):1832–1838. [PubMed] [Google Scholar]
- Kumar V., Bennett M., Eckner R. J. Mechanisms of genetic resistance to friend virus leukemia in mice. J Exp Med. 1974 May 1;139(5):1093–1109. doi: 10.1084/jem.139.5.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Bennett M. Mechanisms of genetic resistance to Friend virus leukemia in mice. II. Resistance of mitogen-responsive lymphocytes mediated by marrow-dependent cells. J Exp Med. 1976 Apr 1;143(4):713–727. doi: 10.1084/jem.143.4.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Caruso T., Bennett M. Mechanisms of genetic resistance to Friend virus leukemia. III. Susceptibility of mitogen-responsive lymphocytes mediated by T cells. J Exp Med. 1976 Apr 1;143(4):728–740. doi: 10.1084/jem.143.4.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Luevano E., Bennett M. Hybrid resistance to EL-4 lymphoma cells. I. Characterization of natural killer cells that lyse EL-4 cells and their distinction from marrow-dependent natural killer cells. J Exp Med. 1979 Sep 19;150(3):531–547. doi: 10.1084/jem.150.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuvano E., Kumar V., Bennett M. Hybrid resistance to EL-4 lymphoma cells. II. Association between loss of hybrid resistance and detection of suppressor cells after treatment of mice with 89Sr. Scand J Immunol. 1981;13(6):563–571. doi: 10.1111/j.1365-3083.1981.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Loh L., Hudson J. B. Immunosuppressive effect of murine cytomegalovirus. Infect Immun. 1980 Jan;27(1):54–60. doi: 10.1128/iai.27.1.54-60.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann-Matthes M. L., Domzig W., Roder J. Promonocytes have the functional characteristics of natural killer cells. J Immunol. 1979 Oct;123(4):1883–1886. [PubMed] [Google Scholar]
- Lopez C., Ryshke R., Bennett M. Marrow-dependent cells depleted by 89Sr mediate genetic resistance to herpes simplex virus type 1 infection in mice. Infect Immun. 1980 Jun;28(3):1028–1032. doi: 10.1128/iai.28.3.1028-1032.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lust J. A., Kumar V., Burton R. C., Bartlett S. P., Bennett M. Heterogeneity of natural killer cells in the mouse. J Exp Med. 1981 Aug 1;154(2):306–317. doi: 10.1084/jem.154.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo D. R., Armstrong J. A., Ho M. Reactivation of murine cytomegalovirus by cyclophosphamide. Nature. 1977 Jun 23;267(5613):721–723. doi: 10.1038/267721a0. [DOI] [PubMed] [Google Scholar]
- Peschle C., Marone G., Genovese A., Rappaport I. A., Condorelli M. Increased erythropoietin production in anephric rats with hyperplasia of the reticuloendothelial system induced by colloidal carbon or zymosan. Blood. 1976 Feb;47(2):325–337. [PubMed] [Google Scholar]
- Quinnan G. V., Manischewitz J. E. The role of natural killer cells and antibody-dependent cell-mediated cytotoxicity during murine cytomegalovirus infection. J Exp Med. 1979 Dec 1;150(6):1549–1554. doi: 10.1084/jem.150.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand K. H., Pollard R. B., Merigan T. C. Increased pulmonary superinfections in cardiac-transplant patients undergoing primary cytomegalovirus infection. N Engl J Med. 1978 Apr 27;298(17):951–953. doi: 10.1056/NEJM197804272981705. [DOI] [PubMed] [Google Scholar]
- Roder J. C., Lohmann-Matthes M. L., Domzig W., Kiessling R., Haller O. A functional comparison of tumor cell killing by activated macrophages and natural killer cells. Eur J Immunol. 1979 Apr;9(4):283–288. doi: 10.1002/eji.1830090407. [DOI] [PubMed] [Google Scholar]
- Seaman W. E., Gindhart T. D., Greenspan J. S., Blackman M. A., Talal N. Natural killer cells, bone, and the bone marrow: studies in estrogen-treated mice and in congenitally osteopetrotic (mi/mi) mice. J Immunol. 1979 Jun;122(6):2541–2547. [PubMed] [Google Scholar]
- Seaman W. E., Merigan T. C., Talal N. Natural killing in estrogen-treated mice responds poorly to poly I.C despite normal stimulation of circulating interferon. J Immunol. 1979 Dec;123(6):2903–2905. [PubMed] [Google Scholar]
- Selgrade M. K., Osborn J. E. Role of macrophages in resistance to murine cytomegalovirus. Infect Immun. 1974 Dec;10(6):1383–1390. doi: 10.1128/iai.10.6.1383-1390.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr S. E., Allison A. C. Role of T lymphocytes in recovery from murine cytomegalovirus infection. Infect Immun. 1977 Aug;17(2):458–462. doi: 10.1128/iai.17.2.458-462.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutman O., Paige C. J., Figarella E. F. Natural cytotoxic cells against solid tumors in mice. I. Strain and age distribution and target cell susceptibility. J Immunol. 1978 Nov;121(5):1819–1826. [PubMed] [Google Scholar]
- Tracey D. E. The requirement for macrophages in the augmentation of natural killer cell activity by BCG. J Immunol. 1979 Aug;123(2):840–845. [PubMed] [Google Scholar]
- Welsh R. M., Jr, Kiessling R. W. Natural killer cell response to lymphocytic choriomeningitis virus in beige mice. Scand J Immunol. 1980;11(4):363–367. doi: 10.1111/j.1365-3083.1980.tb00001.x. [DOI] [PubMed] [Google Scholar]
- Welsh R. M., Jr, Zinkernagel R. M. Heterospecific cytotoxic cell activity induced during the first three days of acute lymphocytic choriomeningitis virus infection in mice. Nature. 1977 Aug 18;268(5621):646–648. doi: 10.1038/268646a0. [DOI] [PubMed] [Google Scholar]




