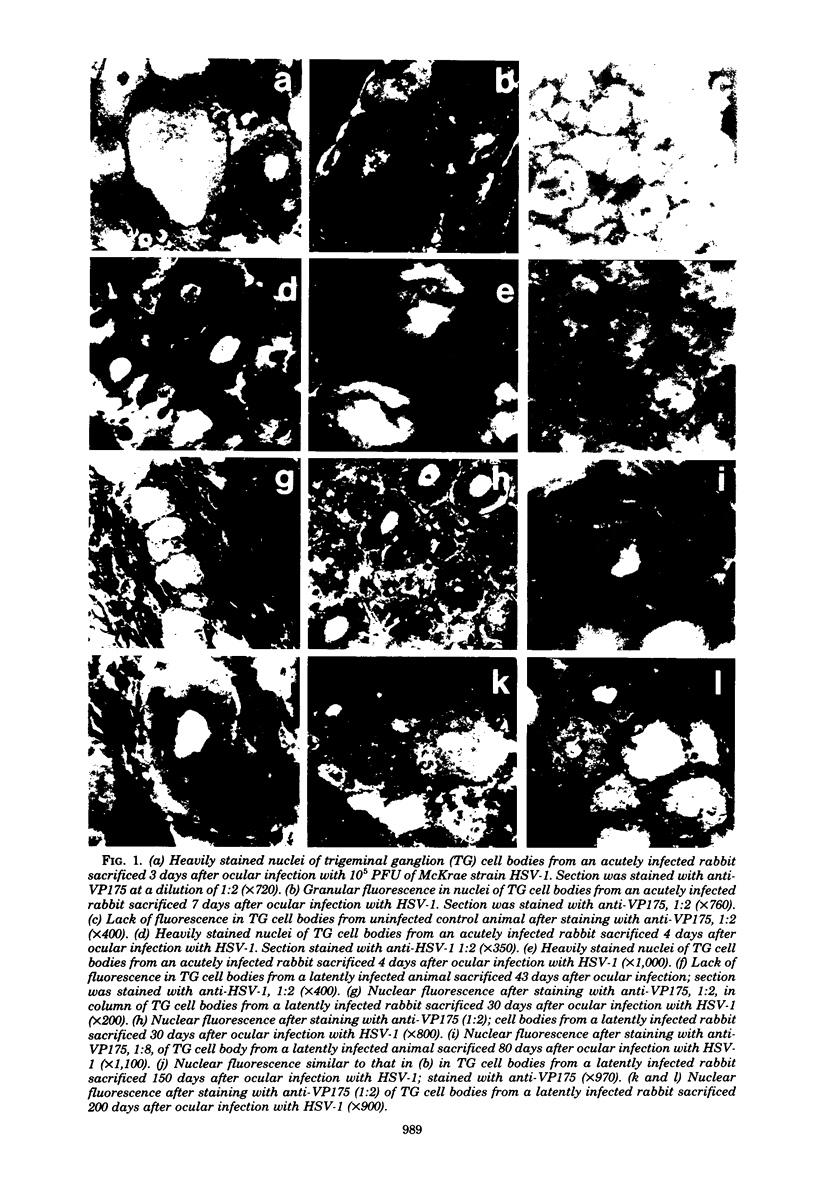
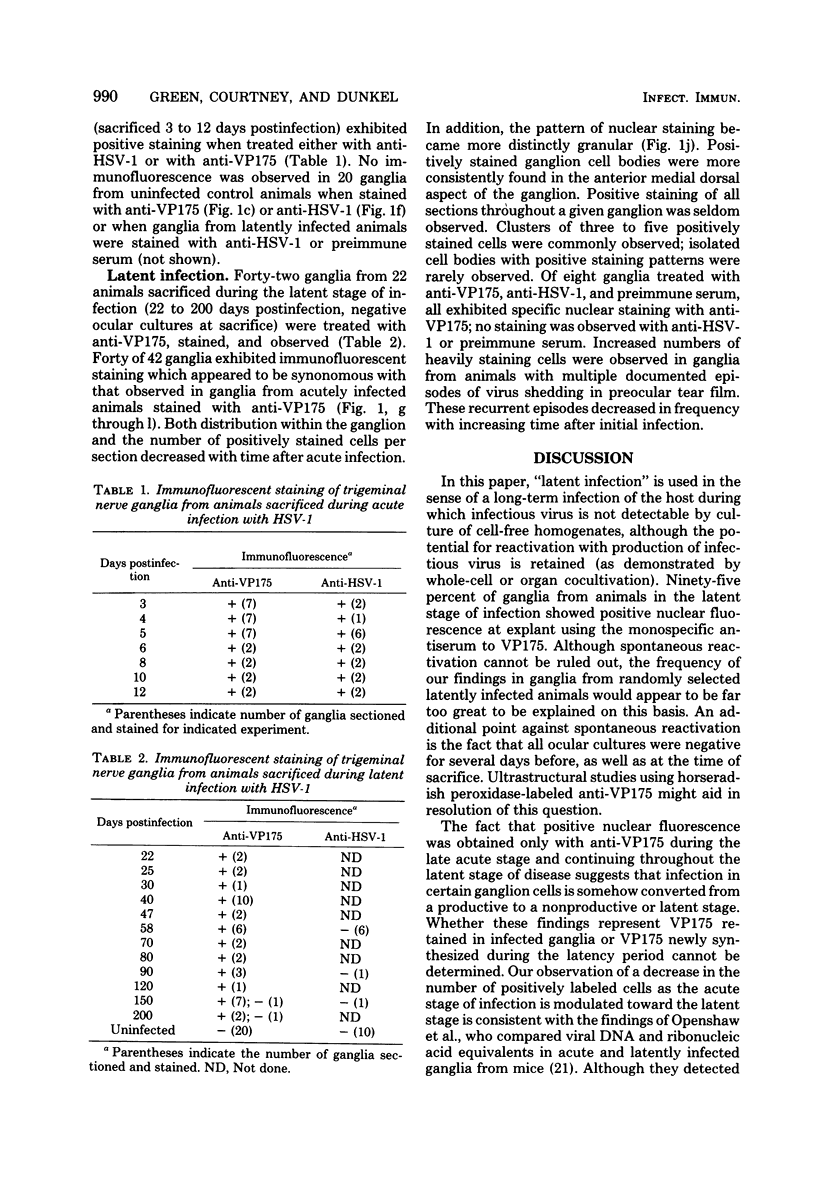
Abstract
In this study, trigeminal sensory ganglia from animals with acute herpes simplex virus, type 1 (HSV-1) infection were compared to those with a latent infection for the expression of HSV-specific antigens. By the indirect immunofluorescence assay, antisera to an immediate early polypeptide of molecular weight 175,000, designated VP175 or ICP4, and a hyperimmune antiserum to HSV-1 were used to determine whether early viral polypeptides were being expressed in neurons during the latent stage of infection. All 17 ganglia from animals with acute infection (sacrificed 3 to 12 days postinfection) exhibited positive staining when treated either with anti-HSV-1 or with anti-VP175. Forty of 42 ganglia from animals sacrificed during the latent stage of infection (22 to 200 days postinfection) exhibited immunofluorescent staining when treated with anti-VP175. The staining appeared to be similar to that observed in ganglia from acutely infected animals stained with anti-VP175, except that the number and distribution of stained cells were markedly reduced. No immunofluorescence was observed in ganglia from noninfected control animals when stained with anti-VP175 or anti-HSV-1, or when ganglia from latently infected animals were stained with anti-HSV-1 or preimmune serum.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baringer J. R., Swoveland P. Recovery of herpes-simplex virus from human trigeminal ganglions. N Engl J Med. 1973 Mar 29;288(13):648–650. doi: 10.1056/NEJM197303292881303. [DOI] [PubMed] [Google Scholar]
- Cabral G. A., Courtney R. J., Schaffer P. A., Marciano-Cabral F. Ultrastructural characterization of an early, nonstructural polypeptide of herpes simplex virus type 1. J Virol. 1980 Mar;33(3):1192–1198. doi: 10.1128/jvi.33.3.1192-1198.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. L., Bastone V. B., Stevens J. G. Evidence that neurons harbor latent herpes simplex virus. Infect Immun. 1974 May;9(5):946–951. doi: 10.1128/iai.9.5.946-951.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. L., Stevens J. G. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infect Immun. 1973 Feb;7(2):272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R. J., Benyesh-Melnick M. Isolation and characterization of a large molecular-weight polypeptide of herpes simplex virus type 1. Virology. 1974 Dec;62(2):539–551. doi: 10.1016/0042-6822(74)90414-0. [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Schaffer P. A. Fine-structure mapping and functional analysis of temperature-sensitive mutants in the gene encoding the herpes simplex virus type 1 immediate early protein VP175. J Virol. 1980 Oct;36(1):189–203. doi: 10.1128/jvi.36.1.189-203.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel N., Jacob R. J., Honess R. W., Hayward G. S., Locker H., Roizman B. Anatomy of herpes simplex virus DNA. III. Characterization of defective DNA molecules and biological properties of virus populations containing them. J Virol. 1975 Jul;16(1):153–167. doi: 10.1128/jvi.16.1.153-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Fenoglio C., Shevchuk M., McDougall J. K. Detection of herpes simplex RNA in human sensory ganglia. Virology. 1979 May;95(1):265–268. doi: 10.1016/0042-6822(79)90429-x. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knotts F. B., Cook M. L., Stevens J. G. Latent herpes simplex virus in the central nervous system of rabbits and mice. J Exp Med. 1973 Sep 1;138(3):740–744. doi: 10.1084/jem.138.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knotts F. B., Cook M. L., Stevens J. G. Pathogenesis of herpetic encephalitis in mice after ophthalmic inoculation. J Infect Dis. 1974 Jul;130(1):16–27. doi: 10.1093/infdis/130.1.16. [DOI] [PubMed] [Google Scholar]
- Lancz G. J., Zettlemoyer T. L. Restricted replication of herpes simplex virus in neural cells. Proc Soc Exp Biol Med. 1976 Jul;152(3):302–306. doi: 10.3181/00379727-152-39384. [DOI] [PubMed] [Google Scholar]
- Leiden J. M., Buttyan R., Spear P. G. Herpes simplex virus gene expression in transformed cells. I. Regulation of the viral thymidine kinase gene in transformed L cells by products of superinfecting virus. J Virol. 1976 Nov;20(2):413–424. doi: 10.1128/jvi.20.2.413-424.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung W. C. Evidence for a herpes simplex virus-specific factor controlling the transcription of deoxypyrimidine kinase. J Virol. 1978 Aug;27(2):269–274. doi: 10.1128/jvi.27.2.269-274.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Goldin A. L., Glorioso J. C. Persistence of herpes simplex virus genes in cells of neuronal origin. J Virol. 1980 Jul;35(1):203–210. doi: 10.1128/jvi.35.1.203-210.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofgren K. W., Stevens J. G., Marsden H. S., Subak-Sharpe J. H. Temperature-sensitive mutants of herpes simplex virus differ in the capacity to establish latent infections in mice. Virology. 1977 Jan;76(1):440–443. doi: 10.1016/0042-6822(77)90319-1. [DOI] [PubMed] [Google Scholar]
- Murray B. K., Biswal N., Bookout J. B., Lanford R. E., Courtney R. J., Melnick J. L. Cyclic appearance of defective interfering particles of herpes simplex virus and the concomitant accumulation of early polypeptide VP175. Intervirology. 1975;5(3-4):173–184. doi: 10.1159/000149894. [DOI] [PubMed] [Google Scholar]
- Nesburn A. B., Green M. T. Editorial: Recurrence in ocular herpes simplex infection. Invest Ophthalmol. 1976 Jul;15(7):515–518. [PubMed] [Google Scholar]
- Openshaw H., Puga A., Notkins A. L. Herpes simplex virus infection in sensory ganglia: immune control, latency, and reactivation. Fed Proc. 1979 Dec;38(13):2660–2664. [PubMed] [Google Scholar]
- Pereira L., Wolff M. H., Fenwick M., Roizman B. Regulation of herpesvirus macromolecular synthesis. V. Properties of alpha polypeptides made in HSV-1 and HSV-2 infected cells. Virology. 1977 Apr;77(2):733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Wimberly I., Benyesh-Melnick M. Prevalence of antibodies to EB virus and other herpesviruses. JAMA. 1969 Jun 2;208(9):1675–1679. [PubMed] [Google Scholar]
- Powell K. L., Purifoy D. J. DNA-binding proteins of cells infected by herpes simplex virus type 1 and type 2. Intervirology. 1976;7(4-5):225–239. doi: 10.1159/000149955. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Control of herpes simplex virus type 1 mRNA synthesis in cells infected with wild-type virus or the temperature-sensitive mutant tsK. J Virol. 1979 Jan;29(1):275–284. doi: 10.1128/jvi.29.1.275-284.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A., Rosenthal J. D., Openshaw H., Notkins A. L. Herpes simplex virus DNA and mRNA sequences in acutely and chronically infected trigeminal ganglia of mice. Virology. 1978 Aug;89(1):102–111. doi: 10.1016/0042-6822(78)90044-2. [DOI] [PubMed] [Google Scholar]
- Rajcáni J., Ciampor F. Experimental pathogenesis of non-lethal herpesvirus infection and the establishment of latency. Acta Virol. 1978 Jul;22(4):278–286. [PubMed] [Google Scholar]
- Rodda S., Jack I., White D. O. Herpes-simplex virus from trigeminal ganglion. Lancet. 1973 Jun 16;1(7816):1395–1396. doi: 10.1016/s0140-6736(73)91730-3. [DOI] [PubMed] [Google Scholar]
- Sim C., Watson D. H. The role of type specific and cross reacting structural antigens in the neutralization of herpes simplex virus types 1 and 2. J Gen Virol. 1973 May;19(2):217–233. doi: 10.1099/0022-1317-19-2-217. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Latent herpes simplex virus in spinal ganglia of mice. Science. 1971 Aug 27;173(3999):843–845. doi: 10.1126/science.173.3999.843. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Cook M. L. Maintenance of latent herpetic infection: an apparent role for anti-viral IgG. J Immunol. 1974 Dec;113(6):1685–1693. [PubMed] [Google Scholar]
- Stevens J. G. Latent characteristics of selected herpesviruses. Adv Cancer Res. 1978;26:227–256. doi: 10.1016/s0065-230x(08)60089-5. [DOI] [PubMed] [Google Scholar]
- Stevens J. G. Latent herpes simplex virus and the nervous system,. Curr Top Microbiol Immunol. 1975;70:31–50. doi: 10.1007/978-3-642-66101-3_2. [DOI] [PubMed] [Google Scholar]
- Stevens J. G., Nesburn A. B., Cook M. L. Latent herpes simplex virus from trigeminal ganglia of rabbits with recurrent eye infection. Nat New Biol. 1972 Feb 16;235(59):216–217. doi: 10.1038/newbio235216a0. [DOI] [PubMed] [Google Scholar]
- Vahlne A., Lycke E. Herpes simplex virus infection of in vitro cultured neuronal cells (mouse neuroblastoma C 1300 cells). J Gen Virol. 1978 May;39(2):321–332. doi: 10.1099/0022-1317-39-2-321. [DOI] [PubMed] [Google Scholar]
- Walz M. A., Price R. W., Notkins A. L. Latent ganglionic infection with herpes simplex virus types 1 and 2: viral reactivation in vivo after neurectomy. Science. 1974 Jun 14;184(4142):1185–1187. doi: 10.1126/science.184.4142.1185. [DOI] [PubMed] [Google Scholar]
- Wilcox K. W., Kohn A., Sklyanskaya E., Roizman B. Herpes simplex virus phosphoproteins. I. Phosphate cycles on and off some viral polypeptides and can alter their affinity for DNA. J Virol. 1980 Jan;33(1):167–182. doi: 10.1128/jvi.33.1.167-182.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., Schulte-Holthausen H. Persistence of herpesvirus nucleic acid in normal and transformed human cells - a review. IARC Sci Publ. 1975;(11 Pt 1):117–123. [PubMed] [Google Scholar]