Abstract
Dengue viruses and other members of the Flaviviridae family are emerging human pathogens. Dengue is transmitted to humans by Aedes aegypti female mosquitoes. Following infection through the bite, cells of the hematopoietic lineage, like dendritic cells, are the first targets of dengue virus infection. Dendritic cells (DCs) are key antigen presenting cells, sensing pathogens, processing and presenting the antigens to T lymphocytes, and triggering an adaptive immune response. Infection of DCs by dengue virus may induce apoptosis, impairing their ability to present antigens to T cells, and thereby contributing to dengue pathogenesis. This review focuses on general mechanisms by which dengue virus triggers apoptosis, and possible influence of DC-apoptosis on dengue disease severity.
Keywords: Dendritic cell, apoptosis, dengue virus
1. Dengue Virus
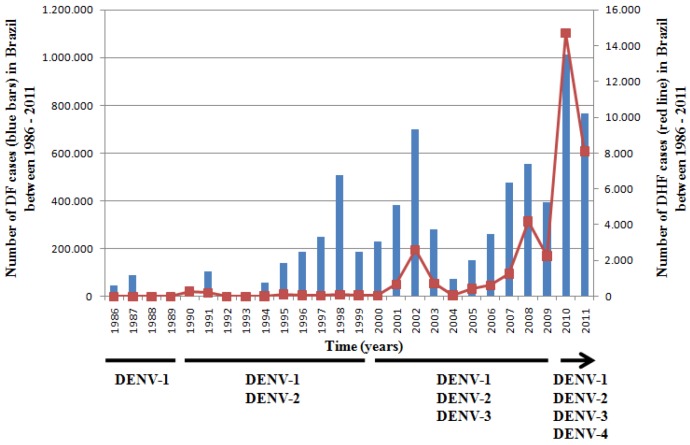
Dengue virus (DENV) is the most important arboviral infection in the tropical and sub-tropical regions of the world affecting more than 100 countries [1]. About two fifths of the world’s population lives in areas where there is a risk of dengue infection, and dengue affects 50 million people annually causing more than 25,000 deaths [2]. In Brazil, after a period of more than 30 years without DENV circulation, dengue virus serotype-1 (DENV-1) was detected in 1981 in the North region of the country [3] and caused a limited epidemic. In 1986, DENV-1 was re-introduced into the country through the Rio de Janeiro port, and since then more than 6 million cases have been confirmed with almost 2000 deaths up to December 2011 (Figure 1). All four dengue virus serotypes currently circulate in Brazil (hyperendemicity) and in the last 10 years the country has experienced an increase of more than 20-fold in DHF cases and deaths by dengue infection [4,5].
Figure 1.
Incidence of dengue fever (blue bars) and dengue hemorrhagic fever (red line) in Brazil since its re-introduction in 1986.
DENV belongs to the Flaviviridae familiy, genus flavivirus. The dengue virus is an icosaedrical particle, with approximately 50 nm, and contains a positive strand RNA genome of nearly 11Kb. The Dengue genome codes for three structural proteins (C, prM/M and E) and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) responsible for the structure and organization of replication complex, and of the virus particle, respectively [6,7].
Infection with any of the four DENV-serotypes can cause severe or non-severe Dengue. In severe clinical presentations can occur dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [8,9]. Non severe Dengue (Dengue fever) was further classified in “dengue with warning signs”. Patients that present Dengue with warning signs need to be hospitalized, since they will probably develop severe forms of disease. Patients that present Dengue without warning signs can be sent home [8]. Dengue fever (DF) usually involves high fever (≥40 °C for 2–7 days), generally accompanied by severe headache, retro-orbital pain, muscle/joint pains, nausea, vomiting, swollen glands and/or rash [9]. Cases of dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) present symptoms of DF and also severe abdominal pain, rapid breathing, fatigue, restlessness and low blood pressure in DSS [9]. The main pathological finding in DHF/DSS is plasma leakage due to endothelial damage during the infection (for a review see [10]).
There have been several hypotheses to explain why DHF/DSS occurs more frequently, but not exclusively, in heterologous secondary infections. Dengue hemorrhagic fever may be triggered due to antibody-dependent enhancement [11] and original antigenic sin of T cells [12]. Also, differences between viral strains [13,14] may also contribute to dengue pathogenesis, and indeed, it has been demonstrated that a DENV-strain isolated from a fatal dengue case induce higher apoptosis rates in dendritic cells than a strain isolated from a non-fatal case [15]. The host response to DENV [16,17] also accounts to DHF/DSS pathogenesis, as demonstrated for the type I IFN response after DENV infection [17]. Recently, host genetic polymorphisms of several genes, notably TNF-α [18], TAP [19], and the DC-SIGN promoter region [20] have been related to DHF/DSS. Finally, ethnic factors [21] and age [22] have been also correlated with DFH/DSS, albeit to a lesser extent. These various putative mechanisms are not mutually exclusive, and the combination of ADE / T-cell antigenic sin / viral strain / individual background / nutritional status may favor high viremia and the cytokine storm observed in DHF/DSS [11,12,13,14,18,19,20,21,22,23].
The host response to DENV infection starts with dendritic cells at the dermis, since resident dendritic cells and Langerhans cells in the dermis are the first cell targets of DENV infection [24]. Dendritic cells are specialized cells that can process and present antigens to T lymphocytes, and are therefore responsible for the induction of adaptive immune responses [25]. Interactions between DENV and DCs have a crucial role in the control of DENV infection, either directly or due to the stimulation of dengue-specific T lymphocytes, and may contribute to determining whether or not DHF/DSS develops.
2. Dendritic Cells
Dendritic cells are amongst the most important antigen presenting cells in humans and other mammals. Ralph M. Steinman and Zanvil Cohn first described these cells in 1973, as phagocytic cells with dendrite-like protrusions [26]. Monocytes, macrophages and dendritic cells have a common and exclusive precursor, the macrophage-DC progenitor (MDP), which differentiates into the common DC progenitor (CDP) generating precursor DCs (pre-DCs). Pre-DCs can migrate to lymph nodes, proliferate and differentiate into DCs [27]. Monocytes are derived from common myeloid progenitors (CMPs) and MDPs, and are known as classical macrophage precursors. Cells derived from pre-DCs were first classified as conventional DCs; they display classic DC form and function in steady state conditions. Conventional DCs comprise migratory DCs and lymphoid DCs. Migratory DCs are tissue-resident, and once they find an antigen, these cells migrate to lymph nodes, where they stimulate T-Lymphocytes. Lymphoid DCs are restricted to lymphoid tissues and are generally classified according to the expression of CD4 or CD8 (reviewed by [28]). Monocyte-derived DCs (CD11c+) and plasmocytoid DCs (CD123+) are referred as non-conventional DCs [28,29]. Particular DC subsets can be generated from monocytes during inflammation, although they have been reported even in steady-state conditions. Monocyte-derived DCs are classified as non-conventional mainly because of their origin and are commonly found in the periphery, migrating to draining lymph nodes whenever they find an antigen (reviewed by [28]). Monocyte differentiation into DCs can enhance antiviral immunity, and it was recently demonstrated that viral infection can induce monocyte differentiation into a CD16(–) CD83(+) DC subset with a strong potential to activate T cells [30]. Plasmocytoid DCs are found in lymphoid and non-lymphoid tissues, and express two types of Toll-like receptors (TLR7 and TLR9) that mediate the expression of interferon regulatory factor 7 (IRF7), a transcriptional activator that can modulate the production of type I interferon [31]. The secretion of IL-6 and IFN type I promotes the differentiation of activated B cells in plasma cells, highlighting the importance of this cell type in immune response modulation [32]. Plasmocytoid DCs also appear to be involved both in induction of tolerance and in modulation of autoimmune responses [33].
Dendritic cells have different activities and functions depending on their maturation status. Immature DCs (iDCs) are not only precursors, but also potent phagocytes that can capture and process antigens that may then be used to form MHC-peptide complexes (reviewed by [34]). Immature DCs express low levels of MHC-I, MHC-II and co-stimulatory molecules (CD80, CD83 and CD86) on their cell surface. As a consequence of this low expression, iDCs have a limited ability to present antigens. They can patrol peripheral areas and migrate to infection sites, coordinated by inflammatory chemokines. This explains the expression of chemokine receptors, like CCR1, CCR2, CCR5, CCR6, CXCR1, and CXCR2 on the surface of iDCs [35,36,37].
Maturation stimuli cause a decline on antigen uptake rates, as shown for some TLR ligands like Poly I:C and LPS [38]. An immunoproteasome is formed, and antigen processing in the now mature cells is facilitated by the acidification of endosomal compartments, proteosomal alterations and cathepsin activation [39,40]. Late events in the maturation process are the upregulation of genes related to antigen presentation [37] and down-regulation of receptors that recognize pro-inflammatory cytokines [35]. To interact with T-cells in the lymphoid tissues, the expression of several proteins in DCs is regulated. The half-life of MHC-I complexes on the cell surface increases, and activation molecules like MHC-I, MCH-II, and co-stimulatory factors like CD80, CD83 and CD86 are overexpressed (reviewed by [34]).
3. Dendritic Cells and Dengue Virus
Development of DHF or DSS may be related to the ability of DCs to counteract dengue replication [41]. Following DENV inoculation in the dermis the primary targets for infection are skin-resident Dendritic Cells (DCs) and Langerhan’s Cells (LCs) [24,42]. The specific receptor for DENV binding in DCs is the dendritic cell-specific intercellular adhesion molecule 3 grabbing non-integrin (DC-SIGN or CD209) [43]. DCs additionally sense pathogens by various pathways, involving Toll-like receptors (TLR), RIG-like receptors (RLR) and NOD-like receptors (NLR) (reviewed by [44]).
DENV-infected DCs acquire a maturation profile, expressing characteristic surface molecules like CD40, CD80, CD83, and CD86 [45,46]. These surface proteins facilitate a more efficient interaction between DENV-infected DCs and T-cells [47], and also contribute to B cell activation and differentiation [48,49,50]. Uninfected DENV-exposed DCs are also activated following the recognition of defective virus particles or proteins, the action of DC-secreted exosomes [51] and/or the effects of cytokines secreted by infected DCs (TNF-α, IFN type I and II ) [52]. DENV-infected DCs start the production of IL-6 [53], and upon activation, uninfected DENV-exposed DCs and infected DCs migrate to the lymph nodes and prime T cells through the major histocompatibility complex (MHC) type I and II, initiating the adaptive immune responses [54]. DENV infected DCs have increased expression of TNF-α, programmed death ligand 2 (PD-L2) and MHC-II, and a lower expression of IFN inducible protein 10 (IP-10), programmed death ligand 1 (PD-L1), CD80, CD86 and MHC-I, when compared with bystander DCs [55].
Pro-inflammatory cytokines, like TNF-α and IFN-α secreted by DENV-infected DCs enhance the activation of DCs and other immune cells, induce apoptosis of endothelial cells and the up-regulation of interferon-stimulated genes (ISGs) with antiviral activity [45,53,56]. Other important cytokines secreted by DENV-infected DCs are IL-6, IL-8 [15] and IL-12p70 [45]. Chemokine production by DENV-infected DC, including RANTES, CXCL9, MCP-1, IP-10, and IL-8, has also been demonstrated [23] and may contribute substantially to the plasma leakage and local inflammation, with cells overflowing into tissues. However, non-structural flavivirus proteins, notably NS5 [57], NS4B [58] and the NS2B-NS3 complex [59], are able to inhibit type I IFN responses compromising the innate immune response. This mechanism could be used to weaken the antiviral defense mechanisms, although it has not been demonstrated yet for DENV- infected dendritic cells.
Dendritic cells infected with dengue virus may also contribute to the development of vascular leakage through the secretion of metalloproteinases [60]. It has been shown that soluble gelatinolytic matrix metalloproteinase (MMP)-9 and MMP-2 enhance endothelial permeability by reducing the expression of the endothelial adhesion molecule 1 (PECAM-1) and vascular endothelium (VE)-cadherin cell adhesion molecules, and by causing a redistribution of F-actin fibers [60].
4. Apoptosis
Type I programmed cell death or apoptosis is a controlled mechanism that is triggered under normal physiological conditions, for example during development and aging, as well as in response to various stresses and pathologies, including viral infection [61,62]. There are two main apoptotic pathways that are interconnected: the extrinsic pathway and the intrinsic (autonomous) pathway. The extrinsic pathway is mediated by transmembrane receptor-mediated interactions, called death-receptors. The best characterized are the Tumor Necrosis Factor (TNF) and the Fas receptors [63,64]. Once these receptors are activated they lead to the formation of the death-inducing signaling complex (DISC), activation of caspases 8 and 10 and the beginning of the execution phase of apoptosis. Members of the Bcl-2 protein family, which can act either as pro-apoptotic or anti-apoptotic factors, regulate the intrinsic pathway. The pro-apoptotic Bax and Bak transmembrane proteins form pores in the mitochondrial membrane allowing the release of cytochrome C, and the formation of apoptosomes; this leads to the activation of caspase 9, that activates caspase 3 resulting in the start of the execution phase as well as triggering the extrinsic pathway [61,62]. During the execution phase, caspase 3 activates CAD protein, an endonuclease that degrades the chromosomal DNA and also induces cytoskeleton collapse leading to the formation of apoptotic bodies [61,62,65,66].
Infection by virus and other pathogens can induce DC apoptosis. During measles virus infection, apoptosis is induced by Fas- and TRAIL-mediated pathways [67]; in foot and mouth disease, apoptosis occurs through the binding of viral proteins to integrin receptors [68] and DENV-induced cytokines seems to trigger DC apoptosis [53]. DC homeostasis also has an important role in tolerance induction, as DC apoptosis induces the formation of antigen-specific Tregs through engulfment of apoptotic DCs by viable DCs [69]. Tregs in this microenvironment also have increased activity [70] and negatively modulate the activity of the remaining DCs [71].
5. Dengue Virus-Induced Apoptosis
Experimental and clinical data suggests that DENV has a wide range of cellular and tissue tropisms. DENV antigens have been found in mononuclear cells, B cells, Langerhans cells in the skin, mouse neurons, human endothelial cells, liver cells, heart and skeletal muscle ([24,25,72,73,74,75,76] for a review see [77]). Apoptosis induction by DENV in a broad of cell types can contribute to dengue pathogenesis. Autopsy examinations of fatal DHF/DSS cases have demonstrated apoptotic cells in liver, brain, intestinal and lung tissues [78]. Apoptotic microvascular endothelial cells in intestinal and pulmonary tissues may explain the plasma leakage manifestations observed in patients experiencing the severe forms of the disease [78].
The mechanisms by which DENV induces apoptosis are not completely understood and may differ between cell types or tissues [79]. In vitro studies have demonstrated that accumulation of DENV-proteins in the endoplasmic reticulum (ER) induces apoptosis of hepatoma cells (HepG2) [80]. Accumulation of viral proteins in the ER membranes, rather than virus release, may cause ER stress and thereby activate the apoptotic pathway, as occurs in mouse neuroblastoma cells [81]. Apoptosis of hepatic cells due to DENV infection may explain the high levels of transaminases and liver damage observed in some DENV patients [82,83,84]. Additionally, NF-κB is activated in DENV-infected hepatocytes [80] and induces the expression of CD137, a member of TNF-α receptor family [85]. Anti-CD137 antibody binding to CD137 may activate caspase cascades triggering apoptosis of hepatocytes [85]. Activation of NF-κB is controlled by its ligation to Daxx, a death domain-associated protein [86]. During DENV infection, capsid protein binds to Daxx [86] and free NF-κB is then available to regulate CD137 expression [87], what could trigger apoptosis of infected cells. Despite its role in the induction of apoptosis, DENV-capsid protein may also contribute to subvert apoptosis: DENV-C protein interacts with the calcium modulating cyclophilin-binding ligand (CMAL), a regulator of intracellular calcium levels [88]. DENV-infected cells express high levels of CAML and consequently have high cytosolic calcium concentration, and this may help DENV to subvert apoptosis since it protects cells against mitochondrial damage [89].
In neuroblastoma cells infected with DENV, apoptosis seems to be induced due to production of phospholipase A2 (PLA2). This enzyme converts membrane phospholipids to arachidonic acid (AA), a major lipid second mediator of several intracellular reactions [90]. PLA2 activation is essential for the induction of apoptosis, and occurs in response to diverse stress stimuli, such as TNF-α and FasL signaling [91]. DENV infection induces the activation of PLA2 and production of AA [92], and PLA2 is abundant in the serum of DENV-infected patients [93]. Arachidonic acid stimulates the synthesis of NADPH oxidase, producing superoxide anions and other reactive oxygen species (ROS), and these are detected in neural cells infected with DENV-2 [94]. ROS can function as signal transducers and activate molecules like NF-κB that regulates the expression of several genes involved in immune responses [95]. The well-studied tumor suppressor p53 has its expression regulated by NF-κB and is involved in apoptosis and cell cycle arrest. SK-N-SK cells infected with DENV-2 express both NF-κB and p53, a further evidence for the role of those molecules in DENV-induced apoptosis [94]. Other dengue virus proteins, like prM/M [96], envelope (E) and helicase NS3 [97], participate in the control of apoptosis (for a review see [79]).
The apoptosis of endothelial cells, one of the major findings in DHF/DSS, may be induced directly by viral replication in infected cells or by DENV-NS1 activating the complement system [98,99]. DENV-NS1 is secreted from infected mammalian cells as soluble hexamers (sNS1) [100] but can also remain associated with membranes of infected cells as dimers [101]; free sNS1 itself, or in the presence of anti-sNS1 antibodies, can directly activate the complement system. In the presence of specific antibodies, complement may be activated even by cell-associated NS1 [99]. However, it was recently demonstrated that the sNS1 from DENV, West Nile and Yellow Fever Virus interacts with C4 and attenuates complement activation [102].
The mechanism of the apoptosis induced by DENV also appears to depend on the viral strain and/or serotype. Receptor-interacting serine/threonine protein kinase 2 (RIPK2) is a mediator that activates caspases, NF-κB and other kinases. RIPK2 contributes to DENV-mediated apoptosis induced by capsid protein but only for infections involving DENV-2 and -4 serotypes [103]. Additionally, it was recently demonstrated that DENV-3 isolated from a fatal case induces higher rates of apoptosis in monocyte-derived DCs than a strain isolated from a non-fatal case of DF [15].
6. Dendritic Cells Apoptosis: Mechanisms and Dengue Pathogenesis
Dendritic cell apoptosis has a role on immune regulation, because it controls antigen availability to T cells and any alteration in DC cell death has a major effect on the antigen-specific immune response, inflammation and immune tolerance [104]. In the absence of feeding citokynes (GM-CSF and IL-4), DCs undergo spontaneous apoptosis [53]. IL-10 induces cell death by down-regulating anti-apoptotic proteins such Bcl-2 and TGF-β [105,106,107,108].
The mechanisms by which some members of the Flaviviridae family induce apoptosis in DCs have been established. HCV proteins NS3, NS4, polyprotein NS3-NS4, NS5 and core protein induce DCs apoptosis through FasL expression. As a consequence, important antiviral signaling, such as Toll-like pathway, NF-κB, and secretion of inflammatory mediators, like IL-12 are inhibited [109]. Type I IFN is important for antiviral responses, but high type I IFN secretion during infections with HBV and HCV can contribute to DC apoptosis via the intrinsic pathway [110]. Classical Swine Fever Virus (CSFW) infection induces activation of plasmocytoid and conventional DCs [111] and the virus can replicate inside DCs, causing apoptosis and controlling type I IFN responses [112]. The cell death mechanisms induced by DENV in other cell types, and particular features of DC apoptosis induced by members of Flaviviridae family, implicate Ca2+, PLA2, AA, ROS, cytokines and other mediators in the apoptotic process. However, further studies are necessary to establish the exact pathways involved in DENV-mediated DC apoptosis, once different DENV serotypes and strains may induce DC apoptosis through different mechanisms.
Despite the specific pathway used by DENV to induce DC apoptosis, this phenomenon certainly have consequences for dengue pathogenesis. It is therefore plausible that apoptosis of DCs (directly and indirectly) enhances Dengue viremia and cytokine production favoring the development of severe forms of Dengue disease (Figure 2). Also, apoptosis may facilitate viral escape, contributing to tissue damage and pathogenesis, as occurs during infection with some filovirus, picornavirus, and flavivirus [113].
Recently, it was demonstrated that the plasmacytoid and myeloid dendritic cell counts were reduced in the blood of Dengue patients. However, no differences in these counts were observed between mild and severe cases [114]. Furthermore, the numbers of myeloid and plasmacitoyd DCs were significantly lower in DENV patients with high viremia than in non-infected controls, suggesting a correlation between viremia and DC counts [114]. Several explanations can be proposed for the low DC counts in dengue-infected patients: (1) viral cytopathic effects; (2) death of DC induced by ADE; (3) DC migration to secondary lymphoid organs and 4) virus-driven impairment of bone marrow function leading to lower DC production [114].
In addition to its effects on infected cells, DENV can also kill uninfected neighbor cells (bystander effect), contributing to weakening the host immune response [15,115]. An example of indirect induction of apoptosis is provided by the Ebola virus (EBOV), which induces massive apoptosis of T CD4, T CD8 and NK lymphocytes, although the virus does not directly infect these cells [116]. It has been suggested that DENV-induced DC apoptosis is dependent on viral infection and replication [15,52]. However, it was recently demonstrated that bystander apoptosis of DENV-uninfected DCs is strain dependent and that TNF-α secreted during DENV infection protects DCs from apoptosis [15], possibly by inducting DCs maturation [117]. Some viruses, such as Foot and Mouth Disease Virus (FMDV) can even induce DC apoptosis and affect its functions before the infection, as shown for murine Bone Marrow Derived DCs (BMDCs). The apoptosis of DCs due to FMDV impairs the connection between host innate and adaptive immune responses [68]. Additionally, Measles virus (MV) induces apoptosis of DCs contributing to viral spreading, through the releasing of virus (that have an intense replication on this cells) or the contamination of other phagocytes that engulf apoptotic bodies released by dying cells. The contact of bystander DCs with apoptotic MV-infected DCs can induce cell maturation. DCs can also spread MV to secondary lymphoid organs, where they get in touch with CD40L signals from activated T cells. The contact with CD40L can generate cytotoxic DCs that are unable to prime naïve T cells, and moreover can lead activated T cells to death and inhibit their proliferation. It results in an immune suppression that persists even after MV clearance, since the organism needs a time to recover these cells [118].
Moreover, DC apoptosis induced by dengue virus infection would be a mechanism which contribute to dengue pathogenesis, as already demonstrated for other diseases, like Measles Virus infection [67,118], Streptococcus pneumoniae [119,120], Foot and Mouth Disease Virus [68], and Malaria [121]. Additionally, in 2005, Palmer et al., demonstrated that DENV-infected DCs secrete IL-10 and were apoptotic [52]. The authors suggest that those results are an immune evasion mechanism used by DENV to escape immune response.Therefore, we believe that DC-apoptosis contributes to pathogenesis of infectious disease due to impairment of innate immune response or as a viral escape from immune surveillance.
Figure 2.
Possible mechanisms by which dengue virus may induce apoptosis of Dendritic cells (DCs). Dengue virus (DENV) can directly induce DC-apoptosis through replication inside infected cells. Apoptosis may also be induced in infected and uninfected-DC via exosomes, cytokines and viral proteins secreted from infected DCs. It has been demonstrated that TNF-α protects DCs from apoptosis induced by DENV, possibly inducing DC maturation. Also, structural and non-structural viral proteins may either induce apoptosis or protect DC from apoptosis. Finally, induction of apoptosis by DENV in infected and uninfected DC presumably contributes to dengue pathogenesis by promoting high viremia, production of inflammatory cytokines, reduced antigen-presentation and low DENV-specific T-cell activation.
7. Concluding Remarks
DC-DENV interaction is the first point for the immune control after DENV infection. DCs may reduce DENV replication through secretion of type I IFN. However, some DENV strains are able to replicate more efficiently in these cells, due to mutations in non-structural genes, which consequently inhibit the type I IFN response [52,57,59]. In these cases, DENV replication may induce DC apoptosis directly and may even induce apoptosis of uninfected DCs through the action of cytokines, viral proteins or exosomes secreted by infected DCs. Apoptosis of DENV-infected and uninfected DCs may weaken the host immune response, increasing the viral load and cytokine storm observed in severe dengue cases (see Figure 2). Elucidating the mechanisms that control DC apoptosis after DENV infection would be a significant step forward in the understanding of dengue pathogenesis, and consequently for vaccine and antiviral development.
Acknowledgments
Authors are indebted to Daniel Mansur from the Federal University of Santa Catarina and Samuel Goldenberg from ICC/Fiocruz for critical reading of the manuscript. Authors also thank Itamar Crispim for figure 2 design. Financial support: Fundação Oswaldo Cruz, Fundação Araucária, CNPq and CAPES. CNDS and LRA hold CNPq fellowships, GFS holds a Fiocruz fellowship and STM holds a CAPES fellowship.
Conflict of Interest
The authors declare no conflict of interest.
References and Notes
- 1.OMS. Dengue and Dengue Haemorragic Fever. 2009. [(assessed on: 10 June 2012)]. Available online: http://www.who.int/mediacentre/factsheets/fs117/en/
- 2.Gubler D.J., Meltzer M. Impact of dengue/dengue hemorrhagic fever on the developing world. Adv. Virus Res. 1999;53:35–70. doi: 10.1016/S0065-3527(08)60342-5. [DOI] [PubMed] [Google Scholar]
- 3.Nogueria R.M., de Araújo J.M., Schatzmayr H.G. Dengue viruses in Brazil, 1986–2006. Rev. Panam. Salud Publica. 2007;22:358–363. doi: 10.1590/S1020-49892007001000009. [DOI] [PubMed] [Google Scholar]
- 4.Nogueira R.M.R., Schatzmayr H.G., Fillipis A.M.B. Dengue virus type 3, Brazil, 2002. Emerging Infect. Dis. 2005;11:1376–1381. doi: 10.3201/eid1109.041043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministério da Saúde MS. Balanço Dengue: Semana Epidemiológica 1 a 26 de 2011. [(assessed on: 10 June 2012)]. Available online: http://portal.saude.gov.br/portal/arquivos/pdf/informe_dengue_072011.pdf.
- 6.Chambers T.J., Hahn C.S., Galler R., Rice C.M. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990;44:649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- 7.Lindenbach D.B., Rice C.M. Flaviviridae: The Viruses and their Replication. In: Knipe D.M., Howley P.M., Fields B.N., editors. Fields Virology. 4th. Lippincott Williams and Wilkins; New York, NY, USA: 2001. pp. 991–1042. [Google Scholar]
- 8.Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. World Health Organization, 2009. [(assessed on: 9 October 2012)]. Available online: http://whqlibdoc.who.int/publications/2009/9789241547871_eng.pdf. [PubMed]
- 9.WHO. 2012. [(assessed on:10 June 2012)]. Available online: http://www.who.int/mediacentre/factsheets/fs117/en/
- 10.Srikiatkhachorn A. Plasma leakage in dengue haemorrhagic fever. Thromb. Haemost. 2009;102:1042–1049. doi: 10.1160/TH09-03-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halstead S.B., O’Rourke E.J. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J. Exp. Med. 1977;146:201–217. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mongkolsapaya J., Dejnirattisai W., Xu X.N., Vasanawathana S., Tangthawornchaikul N., Chairunsri A., Sawasdivorn S., Duangchinda T., Dong T., Rowland-Jones S., et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 2003;9:921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 13.Leitmeyer K.C., Vaughn D.W., Watts D.M., Salas R., Villalobos I., de Chacon, Ramos C., Rico-Hesse R. Dengue virus strucutural differences that correlate with pathogenesis. J. Virol. 1999;73:4738–4747. doi: 10.1128/jvi.73.6.4738-4747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cologna R., Rico-Hesse R. American genotype structures decrease dengue virus output from human monocytes and dendritic cells. J. Virol. 2003;77:3929–3938. doi: 10.1128/JVI.77.7.3929-3938.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silveira G.F., Meyer F., Delfraro A., Mosimann A.L.P., Coluchi N., Vasquez C., Probst C.M., Báfica A., Bordignon J., Duarte Dos Santos C.N. Dengue virus type-3 isolated from a fatal case with visceral complications induces enhanced pro-inflammatory responses and apoptosis of human dendritic cells. J. Virol. 2011;85:5374–5383. doi: 10.1128/JVI.01915-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long H.T., Hibberd M.L., Hien T.T., Dung N.M., Van Ngoc T., Farrar J., Wills B., Simmons C.P. Patterns of gene transcript abundance in the blood of children with severe or uncomplicated dengue highlight differences in disease evolution and host response to dengue virus infection. J. Infect Dis. 2009;19:537–546. doi: 10.1086/596507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmons C.P., Popper S., Dolocek C., Chau T.N., Griffiths M., Dung N.T., Long T.H., Hoang D.M., Chau N.V., le Thao T.T., et al. Patterns of host genome-wide gene transcript abundance in the peripheral blood of patients with acute dengue hemorrhagic fever. J. Infect. Dis. 2007;195:1097–1107. doi: 10.1086/512162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernández-Mestre M.T., Gendzekhadze K., Rivas-Vetencourt P., Layrisse Z. TNF-alpha-308A allele, a possible severity risk factor of hemorrhagic manifestation in dengue fever patients. Tissue Antigens. 2004;64:469–472. doi: 10.1111/j.1399-0039.2004.00304.x. [DOI] [PubMed] [Google Scholar]
- 19.Soundravally R., Hoti S.L. Polymorphisms of the TAP 1 and 2 gene may influence clinical outcome of primary dengue viral infection. Scand. J. Immunol. 2008;67:618–625. doi: 10.1111/j.1365-3083.2008.02109.x. [DOI] [PubMed] [Google Scholar]
- 20.Sakuntabhai A., Turbpaiboon C., Casadémont I., Chuansumrit A., Lowhnoo T., Kajaste-Rudnitski A., Kalayanarooj S.M., Tangnararatchakit K., Tangthawornchaikul N., Vasanawathana S., et al. A variant in the CD209 promoter is associated with severity of dengue disease. Nat. Gen. 2005;37:507–513. doi: 10.1038/ng1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De la Sierra B., García G., Pérez A.B., Morier L., Alvarez M., Kourí G., Guzmán M.G. Ethnicity and difference in dengue virus-specific memory T cell responses in Cuban individuals. Viral Immunol. 2006;19:662–668. doi: 10.1089/vim.2006.19.662. [DOI] [PubMed] [Google Scholar]
- 22.Egger J.R., Coleman P.G. Age and clinical dengue illness. Emerg. Infect. Dis. 2007;13:924–927. doi: 10.3201/eid1306.070008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pang T., Cardosa M.J., Guzman M.G. Of cascades and perfect storms: The immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome (DHF/DSS) Immunol. Cell Biol. 2007;85:43–45. doi: 10.1038/sj.icb.7100008. [DOI] [PubMed] [Google Scholar]
- 24.Wu S.J., Grouard-Vogel G., Mascola J.R., Brachtel E., Putvatana R., Louder M.K., Filgueira L., Marovich M.A., Wong H.K., Blauvelt A., et al. Human skin Langerhans cells are targets of dengue virus infection. Nat. Med. 2000;6:816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]
- 25.25. Salgado D.M., Eltit J.M., Mansfield K., Panqueba C., Castro D., Vega M.R., Xhaja K., Schmidt D., Martin K.J., Allen P.D., et al. Heart and skeletal muscle are targets of dengue virus infection. Pediatr. Infect. Dis. J. 2010;29:238–242. doi: 10.1097/INF.0b013e3181bc3c5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinman R.M., Cohn Z.A. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K., Victora G.D., Schwickert T.A., Guermonprez P., Meredith M.M., Yao K., Chu F.F., Randolph G.J., Rudensky A.Y., Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kushwah R., Hu J. Complexity of dendritic cell subsets and their function in the host immune system. Immunology. 2011;133:409–419. doi: 10.1111/j.1365-2567.2011.03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grouard G., Rissoan M.C., Filgueira L., Durand I., Banchereau J., Liu Y.J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou W., Gibbs J.S., Lu X., Brooke C.B., Roy D., Modlin R.L., Bennink J.R., Yewdell J.W. Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood. 2012;119:3128–3131. doi: 10.1182/blood-2011-09-379479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guiducci C., Ghirelli C., Marloie-Provost M.A., Matray T., Coffman R.L., Liu Y.J., Barrat F.J., Soumelis V. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN productionby human plasmacytoidpredendritic cells in response to TLR activation. J. Exp. Med. 2008;205:315. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jego G., Palucka A.K., Blanck J.P., Chalouni C., Pascual V., Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/S1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 33.Irla M., Kupfer N., Suter T., Lissilaa R., Benkhoucha M., Skupsky J., Lalive P.H., Fontana A., Reith W., Hugues S. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J. Exp. Med. 2010;207:1891–1905. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hopkins R.A., Connolly J.E. The specialized roles of immature and mature dendritic cells in antigen cross-presentation. Immunol. Res. 2012;53:91–107. doi: 10.1007/s12026-012-8300-z. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto F., Schaerli P., Loetscher P., Schaniel C., Lenig D., Mackay C.R., Qin S., Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 36.Sozzani S., Luini W., Borsatti A., Polentarutti N., Zhou D., Piemonti L., D’Amico G., Power C.A., Wells T.N., Gobbi M., et al. Receptor expression and responsiveness of human dendritic cells to a defined set of CC and CXC chemokines. J. Immunol. 1997;159:1993–2000. [PubMed] [Google Scholar]
- 37.Watarai H., Hinohara A., Nagafune J., Nakayama T., Taniguchi M., Yamaguchi Y. Plasma membrane-focused proteomics: dramatic changes in surface expression during the maturation of human dendritic cells. Proteomics. 2005;5:4001–4011. doi: 10.1002/pmic.200401258. [DOI] [PubMed] [Google Scholar]
- 38.Weck M.M., Grünebach F., Werth D., Sinzger C., Bringmann A., Brossart P. TLR ligands differentially affect uptake and presentation of cellular antigens. Blood. 2007;109:3890–3894. doi: 10.1182/blood-2006-04-015719. [DOI] [PubMed] [Google Scholar]
- 39.Groettrup M., Soza A., Kuckelkorn U., Kloetzel P.M. Peptide antigen production by the proteasome: Complexity provides efficiency. Immunol. Today. 1996;17:429–435. doi: 10.1016/0167-5699(96)10051-7. [DOI] [PubMed] [Google Scholar]
- 40.Kloetzel P.M., Ossendorp F. Proteasome and peptidase function in MHC-class-I-mediated antigen presentation. Curr. Opin. Immunol. 2004;16:76–81. doi: 10.1016/j.coi.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 41.Navarro-Sánchez E., Dèspres P., Cedillo-Barrón L. Innate immune response to dengue virus. Arch. Med. Res. 2005;36:273–277. doi: 10.1016/j.arcmed.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Taweechaisupapong S., Sriurairatana S., Angsubhakorn S., Yoksan S., Khin M.M., Sahaphong S., Bhamarapravati N. Langerhans cell density and serological changes following intradermal immunisation of mice with dengue 2 virus. J. Med. Microbiol. 1996;45:138–145. doi: 10.1099/00222615-45-2-138. [DOI] [PubMed] [Google Scholar]
- 43.Satthaporn S., Eremin O. Dendritic cells (I): Biological functions. J. Roy. Coll. Surg. Edinb. 2001;46:9–20. [PubMed] [Google Scholar]
- 44.Bowie A.G., Unterholzner L. Viral evasion and subversion of pattern-recognition receptor signaling. Nat. Rev. Immunol. 2008;8:911–922. doi: 10.1038/nri2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Libraty D.H., Pichyangkul S., Ajariyakhajorn C., Endy T.P., Ennis F.A. Human dendritic cells are activated by dengue virus infection: Enhancement by gamma interferon and implications for disease pathogenesis society. J. Virol. 2001;75:3501–3508. doi: 10.1128/JVI.75.8.3501-3508.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lechmann M., Berchtold S., Hauber J., Steinkasserer A. CD83 on dendritic cells: More than just a marker for maturation. Trends Immunol. 2002;23:273–275. doi: 10.1016/S1471-4906(02)02214-7. [DOI] [PubMed] [Google Scholar]
- 47.Inaba K., Metlay J.P., Crowley M.T., Steinman R.M. Dendritic cells pulsed with protein antigens in vitro can prime antigen-specific, MHC-restricted T cells in situ. J. Exp. Med. 1990;172:631–640. doi: 10.1084/jem.172.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dubois B., Vanbervliet B., Fayette J., Massacrier C., Van Kooten C., Brière F., Banchereau J., Caux C. Dendritic cells enhance growth and differentiation of CD40-activated B lymphocytes. J. Exp. Med. 1997;185:941–951. doi: 10.1084/jem.185.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubois B., Massacrier C., Vanbervliet B., Fayette J., Brière F., Banchereau J., Caux C. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J. Immunol. 1998;161:2223–2231. [PubMed] [Google Scholar]
- 50.Wykes M., Pombo A., Jenkins C., MacPherson G.G. Dendritic cells interact directly with naive B lymphocytes to transfer antigen and initiate class switching in a primary T-dependent response. J. Immunol. 1998;161:1313–1319. [PubMed] [Google Scholar]
- 51.Herrera O.B., Golshayan D., Tibbott R., SalcidoOchoa F., James M.J., Marelli-Berg F.M., Lechler R.I. A novel pathway of alloantigen presentation by dendritic cells. J. Immunol. 2004;173:4828–4837. doi: 10.4049/jimmunol.173.8.4828. [DOI] [PubMed] [Google Scholar]
- 52.Palmer D.R., Sun P., Celluzzi C., Bisbing J., Pang S., Sun W., Marovich M.A., Burgess T., Cross A.R., Foundation H.M.J. Differential effects of dengue virus on infected and bystander dendritic cells. J. Virol. 2005;79:2432–2439. doi: 10.1128/JVI.79.4.2432-2439.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho L.J., Wang J.J., Shaio M.F., Kao C.L., Chang D.M., Han S.W., Lai J.H. Infection of human dendritic cells by dengue virus causes cell maturation and cytokine production. J. Immunol. 2001;166:1499–1506. doi: 10.4049/jimmunol.166.3.1499. [DOI] [PubMed] [Google Scholar]
- 54.Dejnirattisai W., Duangchinda T., Lin C.S., Vasanawathana S., Jones M., Jacobs M., Malasit P., Xu X., Screaton G., Mongkolsapaya J. A complex interplay among virus, dendritic cells, T cells, and cytokines in dengue virus infections. J. Immunol. 2008;181:5865–5874. doi: 10.4049/jimmunol.181.9.5865. [DOI] [PubMed] [Google Scholar]
- 55.Nightingale Z.D., Patkar C., Rothman A.L. Viral replication and paracrine effects result in distinct, functional responses of dendritic cells following infection with dengue 2 virus. J. Leukoc. Biol. 2008;84:1028–1038. doi: 10.1189/jlb.0208105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cardier J.E., Maríno E., Romano E., Taylor P., Liprandi F., Bosch N., Rothman A.L., Cardier E., Marin E. Proinflammatory factors present in sera from patients with acute dengue infection induce activation and apoptosis of human microvascular endothelial cells: Possible role of TNF-alpha in endothelial cell damage in dengue. Cytokine. 2005;30:359–365. doi: 10.1016/j.cyto.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 57.Ashour J., Laurent-Rolle M., Shi P.Y., García-Sastre A. NS5 of dengue virus mediates STAT2 binding and degradation. J. Virol. 2009;83:5408–5418. doi: 10.1128/JVI.02188-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muñoz-Jordán J.L., Laurent-Rolle M., Ashour J., Martínez-Sobrido L., Ashok M., Lipkin WI., García-Sastre A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 2005;79:8004–8013. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rodriguez-Madoz J.R., Belicha-Villanueva A., Bernal-Rubio D., Ashour J., Ayllon J., Fernandez-Sesma A. Inhibition of the type I interferon response in human dendritic cells by dengue virus infection requires a catalytically active NS2B3 complex. J. Virol. 2010;84:9760–9774. doi: 10.1128/JVI.01051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luplerdlop N., Missé D., Bray D., Deleuze V., Gonzalez V.L., Yssel H., Veas F. Dengue virus infected dendritic cells trigger vascular leakage through metalloproteinase overproduction. EMBO Rep. 2006;7:1176–1181. doi: 10.1038/sj.embor.7400814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Portt L., Norman G., Clapp C., Greenwood M., Greenwood M.T. Anti-apoptosis and cell survival: A review. Biochim. Biophys. Acta. 2011;1813:238–259. doi: 10.1016/j.bbamcr.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 62.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chicheportiche Y., Bourdon P.R., Xu H., Hsu Y.M., Scott H., Hession C., Garcia I., Browning J.L. TWEAK, a new secreted ligand in the tumor necrosis factor family that weakly induces apoptosis. J. Biol. Chem. 1997;272:32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 64.Ashkenazi A., Dixit V.M. Death receptors: Signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 65.Sakahira H., Enari M., Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 66.Kothakota S., Azuma T., Reinhard C., Klippel A., Tang J., Chu K., McGarry T.J., Kirschner M.W., Koths K., Kwiatkowski D.J., et al. Caspase-3-generated fragment of gelsolin: Effector of morphological change in apoptosis. Science. 1997;278:294–298. doi: 10.1126/science.278.5336.294. [DOI] [PubMed] [Google Scholar]
- 67.Fugier-Vivier I., Servet-Delprat C., Rivailler P., Rissoan M.C., Liu Y.J., Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin H., Xiao C., Zhao G., Du X., Yu Y., Kang Y., Wang B. Induction of immature dendritic cell apoptosis by foot and mouth disease virus is an integrin receptor mediated event before viral infection. J. Cell. Biochem. 2007;102:980–991. doi: 10.1002/jcb.21332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kushwah R., Oliver J.R., Zhang J., Siminovitch K.A., Hu J. Apoptotic dendritic cells induce tolerance in mice through suppression of dendritic cell maturation and induction of antigen-specific regulatory T cells. J. Immunol. 2009;183:7104–7118. doi: 10.4049/jimmunol.0900824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kessel A., Bamberger E., Masalha M., Toubi E. The role of T regulatory cells in human sepsis. J. Autoimmun. 2009;32:211–215. doi: 10.1016/j.jaut.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 71.Kushwah R., Hu J. Dendritic cell apoptosis: regulation of tolerance versus immunity. J. Immun. 2010;185:795–802. doi: 10.4049/jimmunol.1000325. [DOI] [PubMed] [Google Scholar]
- 72.Halstead S.B., O’Rourke E.J., Allison A.C. Dengue viruses and mononuclear phagocytes. II. Identity of blood and tissue leukocytes supporting in vitro infection. J. Exp. Med. 1977;146:218–229. doi: 10.1084/jem.146.1.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.King A.D., Nisalak A., Kalayanrooj S., Myint K.S., Pattanapanyasat K., Nimmannitya S., Innis B.L. B cells are the principal circulating mononuclear cells infected by dengue virus. Southeast Asian J. Trop. Med. Public Health. 1999;30:718–728. [PubMed] [Google Scholar]
- 74.Imbert J.L., Guevara P., Ramos-Castañeda J., Ramos C., Sotelo J. Dengue virus infects mouse cultured neurons but not astrocytes. J. Med. Virol. 1994;42:228–233. doi: 10.1002/jmv.1890420304. [DOI] [PubMed] [Google Scholar]
- 75.Huang Y.H., Lei H.Y., Liu H.S., Lin Y.S., Liu C.C., Yeh T.M. Dengue virus infects human endothelial cells and induces IL-6 and IL-8 production. Am. J. Trop. Med. Hyg. 2000;63:71–75. doi: 10.4269/ajtmh.2000.63.71. [DOI] [PubMed] [Google Scholar]
- 76.Thepparit C., Smith D.R. Serotype-specific entry of dengue virus into liver cells: Identification of the 37-kilodalton/67-kilodalton high-affinity lamin receptor as a dengue virus serotype 1 receptor. J. Virol. 2004;78:12647–12656. doi: 10.1128/JVI.78.22.12647-12656.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anderson R. Manipulation of cell surface macromolecules by flaviviruses. Adv. Virus Res. 2003;59:229–274. doi: 10.1016/S0065-3527(03)59007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Limonta D., Capó V., Torres G., Pérez A.B., Guzmán M.G. Apoptosis in tissues from fatal dengue shock syndrome. J. Clin. Virol. 2007;40:50–54. doi: 10.1016/j.jcv.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 79.Courageot M.P., Catteau A., Desprès P. Mechanisms of dengue virus-induced cell death. Adv. Virus Res. 2003;60:157–186. doi: 10.1016/S0065-3527(03)60005-9. [DOI] [PubMed] [Google Scholar]
- 80.Marianneau P., Cardona A., Edelman L., Deubel V., Desprès P. Dengue virus replication in human hepatoma cells activates NF-kappaB which in turn induces apoptotic cell death. J. Virol. 1997;71:3244–3249. doi: 10.1128/jvi.71.4.3244-3249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Desprès P., Flamand M., Ceccaldi P.E., Deubel V. Human isolates of dengue type 1 virus induce apoptosis in mouse neuroblastoma cells. J. Virol. 1996;70:4090–4096. doi: 10.1128/jvi.70.6.4090-4096.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bhamarapravati N., Tuchinda P., Boonyapaknavik V. Pathology of Thailand haemorrhagic fever: A study of 100 autopsy cases. Ann. Trop. Med. Parasitol. 1967;61:500–510. doi: 10.1080/00034983.1967.11686519. [DOI] [PubMed] [Google Scholar]
- 83.Kuo C.H., Tai D.I., Chang-Chien C.S., Lan C.K., Chiou S.S., Liaw Y.F. Liver biochemical tests and dengue fever. Am. J. Trop. Med. Hyg. 1992;47:265–270. doi: 10.4269/ajtmh.1992.47.265. [DOI] [PubMed] [Google Scholar]
- 84.Nguyen T.L., Nguyen T.H., Tieu N.T. The impact of dengue haemorrhagic fever on liver function. Res Virol. 1997;148:273–277. doi: 10.1016/S0923-2516(97)88364-1. [DOI] [PubMed] [Google Scholar]
- 85.Nagila A., Netsawang J., Srisawat C., Noisakran S., Morchang A., Yasamut U., Puttikhunt C., Kasinrerk W., Malasit P., Yenchitsomanus P.T., et al. Role of CD137 signaling in dengue virus-mediated apoptosis. Biochem. Biophys. Res. Commun. 2011;410:428–433. doi: 10.1016/j.bbrc.2011.05.151. [DOI] [PubMed] [Google Scholar]
- 86.Netsawang J., Noisakran S., Puttikhunt C., Kasinrerk W., Wongwiwat W., Malasit P., Yenchitsomanus P.T., Limjindaporn T. Nuclear localization of dengue virus capsid protein is required for DAXX interaction and apoptosis. Virus Res. 2010;147:275–283. doi: 10.1016/j.virusres.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 87.Kim J.O., Kim H.W., Baek K.M., Kang C.Y. NF-kappaB and AP-1 regulate activation-dependent CD137 (4-1BB) expression in T cells. FEBS Lett. 2003;541:163–170. doi: 10.1016/S0014-5793(03)00326-0. [DOI] [PubMed] [Google Scholar]
- 88.Bram R.J., Crabtree G.R. Calcium signalling in T cells stimulated by a cyclophilin B-binding protein. Nature. 1994;371:355–358. doi: 10.1038/371355a0. [DOI] [PubMed] [Google Scholar]
- 89.Li J., Huang R., Liao W., Chen Z., Zhang S., Huang R. Dengue virus utilizes calcium modulating cyclophilin-binding ligand to subvert apoptosis. Biochem. Biophys. Res. Commun. 2012;418:622–627. doi: 10.1016/j.bbrc.2012.01.050. [DOI] [PubMed] [Google Scholar]
- 90.Axelroid J., Burch R.M., Jelsema C.L. Receptor-mediated activation of phospholipase A2 via GTP-binding proteins: Arachidonic acid and its metabolites as second messengers. Trends Neurosci. 1988;1:117–123. doi: 10.1016/0166-2236(88)90157-9. [DOI] [PubMed] [Google Scholar]
- 91.Jaattela M., Benedict M., Tewari M., Shayman J.A., Dixit V.W. Bcl-x and Bcl-2 inhibit TNF and Fas-induced apoptosis and activation of phospholipase A2 in breast carcinoma cells. Oncogene. 1995;10:2297–2305. [PubMed] [Google Scholar]
- 92.Malewicz B., Parthsarathy S., Jenkin H.M., Baumann W.J. Rapid phospholipase A2 stimulation and diacylglycerolcholinephosphotransferase inhibition in baby hamster kidney cells during initiation of dengue virus infection. Biochem. Biophys. Res. Commun. 1981;101:404–410. doi: 10.1016/0006-291X(81)91274-2. [DOI] [PubMed] [Google Scholar]
- 93.Nevalainen T.J., Losacker W. Serum phospholipase A2 in dengue. J. Infect. 1997;35:251–252. doi: 10.1016/S0163-4453(97)92966-2. [DOI] [PubMed] [Google Scholar]
- 94.Jan J.T., Chen B.H., Ma S.H., Liu C.I., Tsai H.P., Wu H.C., Jiang S.Y., Yang K.D., Shaio M.F. Potential dengue virus-triggered apoptotic pathway in human neuroblastoma cells: Arachidonic acid, superoxide anion, and NF-kappa B are sequentially involved. J. Virol. 2000;74:8680–8691. doi: 10.1128/JVI.74.18.8680-8691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xiao C., Ghosh S. NF-kappaB, an evolutionarily conserved mediator of immune and inflammatory responses. Adv. Exp. Med. Biol. 2005;560:41–45. doi: 10.1007/0-387-24180-9_5. [DOI] [PubMed] [Google Scholar]
- 96.Catteau A., Kalinina O., Wagner M., Deubel V., Courageot M., Philippe D. Dengue virus M protein contains a proapoptotic sequence referred to as ApoptoM. J. Gen. Virol. 2003;84:2781–2793. doi: 10.1099/vir.0.19163-0. [DOI] [PubMed] [Google Scholar]
- 97.Duarte dos Santos C.N., Frenkiel M.P., Courageot M.P., Rocha C.F., Vazeille-Falcoz M.C., Wien M.W., Rey F.A., Deubel V., Desprès P. Determinants in the envelope E protein and viral RNA helicase NS3 that influence the induction of apoptosis in response to infection with dengue virus type-1. Virology. 2000;274:292–308. doi: 10.1006/viro.2000.0457. [DOI] [PubMed] [Google Scholar]
- 98.Avirutnan P., Malasit P., Seliger B., Bhakdi S., Husmann M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J. Immunol. 1998;161:6338–6346. [PubMed] [Google Scholar]
- 99.Avirutnan P., Punyadee N., Noisakran S., Komoltri C., Thiemmeca S., Auethavornanan K., Jairungsri A., Kanlaya R., Tangthawornchaikul N., Puttikhunt C., et al. Vascular leakage in severe dengue virus infections: A potential role for the nonstructural viral protein NS1 and complement. J. Infect. Dis. 2006;193:1078–1088. doi: 10.1086/500949. [DOI] [PubMed] [Google Scholar]
- 100.Flamand M., Megret F., Mathieu M., Lepault J., Rey F.A., Deubel V. Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as soluble hexamer in a glycosylation-dependent fashion. J. Virol. 1999;73:6104–6110. doi: 10.1128/jvi.73.7.6104-6110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Winkler G., Maxwell S.E., Ruemmler C., Stollar V. Newly synthesized dengue-2 virus nonstructural ürotein NSl is a soluble protein but becomes partially hydrophobic and membrane-associated after dimerization pages. Virology. 1989;305:302–305. doi: 10.1016/0042-6822(89)90544-8. [DOI] [PubMed] [Google Scholar]
- 102.Avirutnan P., Fuchs A., Hauhart R.E., Somnuke P., Youn S., Diamond M.S., Atkinson J.P. Antagonism of the complement component C4 by flavivirus nonstructural protein NS1. J. Exp. Med. 2010;207:793–806. doi: 10.1084/jem.20092545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Morchang A., Yasamut U., Netsawang J., Noisakran S., Wongwiwat W., Songprakhon P., Srisawat C., Puttikhunt C., Kasinrerk W., Malasit P., et al. Cell death gene expression profile: Role of RIPK2 in dengue virus-mediated apoptosis. Virus Res. 2011;156:25–34. doi: 10.1016/j.virusres.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 104.Chen M., Wang J. Regulation of immune responses by spontaneous and T cell-mediated dendritic cell death. J. Clin. Cell Immunol. 2011;11 doi: 10.4172/2155-9899.S3-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kingston D., Schmid M.A., Onai N., Obata-Onai A., Baumjohann D., Manz M.G. The concerted action of GM-CSF and Flt3-ligand on in vivo dendritic cell homeostasis. Blood. 2009;114:835–843. doi: 10.1182/blood-2009-02-206318. [DOI] [PubMed] [Google Scholar]
- 106.Chang W.L., Baumgarth N., Eberhardt M.K., Lee C.Y., Baron C.A., Gregg J.P., Barry P.A. Exposure of myeloid dendritic cells to exogenous or endogenous IL-10 during maturation determines their longevity. J. Immunol. 2007;178:7794–7804. doi: 10.4049/jimmunol.178.12.7794. [DOI] [PubMed] [Google Scholar]
- 107.Borkowski T.A., Nelson A.J., Farr A.G., Udey M.C. Expression of gp40, the murine homologue of human epithelial cell adhesion molecule (Ep-CAM), by murine dendritic cells. Eur. J. Immunol. 1996;26:110–114. doi: 10.1002/eji.1830260117. [DOI] [PubMed] [Google Scholar]
- 108.Ito M., Minamiya Y., Kawai H., Saito S., Saito H., Nakagawa T., Imai K., Hirokawa M., Ogawa J. Tumor-derived TGFbeta-1 induces dendritic cell apoptosis in the sentinel lymph node. J. Immunol. 2006;176:5637–5643. doi: 10.4049/jimmunol.176.9.5637. [DOI] [PubMed] [Google Scholar]
- 109.Krishnadas D.K., Ahn J.S., Han J., Kumar R., Agrawal B. Immunomodulation by hepatitis C virus-derived proteins: targeting human dendritic cells by multiple mechanisms. Int. Immunol. 2010;22:491–502. doi: 10.1093/intimm/dxq033. [DOI] [PubMed] [Google Scholar]
- 110.Swiecki M., Wang Y., Vermi W., Gilfillan S., Schreiber R.D., Colonna M. Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J. Exp. Med. 2011;208:2367–2374. doi: 10.1084/jem.20110654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jamin A., Gorin S., Cariolet R., Le Potier M.F., Kuntz-Simon G. Classical swine fever virus induces activation of plasmacytoid and conventional dendritic cells in tonsil, blood, and spleen of infected pigs. Vet. Res. 2008;39:7. doi: 10.1051/vetres:2007045. [DOI] [PubMed] [Google Scholar]
- 112.Carrasco C.P., Rigden R.C., Vincent I.E., Balmelli C., Ceppi M., Bauhofer O., Tâche V., Hjertner B., McNeilly F., Van Gennip H.G., et al. Interaction of classical swine fever virus with dendritic cells. J. Gen. Virol. 2004;85:1633–1641. doi: 10.1099/vir.0.19716-0. [DOI] [PubMed] [Google Scholar]
- 113.Kaminskyy V., Zhivotovsky B. To kill or be killed: How viruses interact with the cell death machinery. J. Intern. Med. 2010;267:473–482. doi: 10.1111/j.1365-2796.2010.02222.x. [DOI] [PubMed] [Google Scholar]
- 114.De Carvalho Bittencourt M., Martial J., Cabié A., Thomas L., Césaire R. Decreased peripheral dendritic cell numbers in dengue virus infection. J. Clin. Immunol. 2012;32:161–172. doi: 10.1007/s10875-011-9592-9. [DOI] [PubMed] [Google Scholar]
- 115.Galluzzi L., Brenner C., Morselli E., Touat Z., Kroemer G. Viral control of mitochondrial apoptosis. PLoS Pathog. 2008;4:e1000018. doi: 10.1371/journal.ppat.1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Reed D.S., Hensley L.E., Geisbert J.B., Jahrling P.B., Geisbert T.W. Depletion of peripheral blood T lymphocytes and NK cells during the course of ebola hemorrhagic fever in cynomolgusmacaques. Viral Immunol. 2004;17:390–400. doi: 10.1089/vim.2004.17.390. [DOI] [PubMed] [Google Scholar]
- 117.Lundqvist A., Nagata T., Kiessling R., Pisa P. Mature dendritic cells are protected from Fas/CD95-mediated apoptosis by up-regulation of Bcl-Xl. Cancer Immunol. Immunother. 2002;51:139–144. doi: 10.1007/s00262-002-0265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Servet-Delprat C., Vidalain P.O., Azocar O., Le Deist F., Fischer A., Rabourdin-Combe C. Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus. J Virol. 2000;74:4387–4393. doi: 10.1128/JVI.74.9.4387-4393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marriott H.M., Dockrell D.H. Streptococcus pneumoniae: The role of apoptosis in host defense and pathogenesis. Int. J. Biochem. Cell Biol. 2006;38:1848–1854. doi: 10.1016/j.biocel.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 120.Colino J., Snapper C.M. Two distinct mechanisms for induction of dendritic cell apoptosis in response to intact Streptococcus pneumoniae. J. Immunol. 2003;171:2354–2365. doi: 10.4049/jimmunol.171.5.2354. [DOI] [PubMed] [Google Scholar]
- 121.Woodberry T., Minigo G., Piera K.A., Amante F.H., Pinzon-Charry A., Good M.F., Lopez J.A., Engwerda C.R., McCarthy J.S., Anstey N.M. Low-level Plasmodium falciparum blood-stage infection causes dendritic cell apoptosis and dysfunction in healthy volunteers. J. Infect. Dis. 2012;206:333–340. doi: 10.1093/infdis/jis366. [DOI] [PubMed] [Google Scholar]