Abstract
Most growth factor receptor tyrosine kinases (RTKs) signal through similar intracellular pathways, but they often have divergent biological effects. Therefore, elucidating the mechanism of channeling the intracellular effect of RTK stimulation to facilitate specific biological responses represents a fundamental biological challenge. Lens epithelial cells express numerous RTKs with the ability to initiate the phosphorylation (activation) of Erk1/2 and PI3-K/Akt signaling. However, only Fgfr stimulation leads to lens fiber cell differentiation in the developing mammalian embryo. Additionally, within the lens, only Fgfrs activate the signal transduction molecule Frs2α. Loss of Frs2α in the lens significantly increases apoptosis and decreases phosphorylation of both Erk1/2 and Akt. Also, Frs2α deficiency decreases the expression of several proteins characteristic of lens fiber cell differentiation, including Prox1, p57KIP2, aquaporin 0 and β-crystallins. Although not normally expressed in the lens, the RTK TrkC phosphorylates Frs2α in response to binding the ligand NT3. Transgenic lens epithelial cells expressing both TrkC and NT3 exhibit several features characteristic of lens fiber cells. These include elongation, increased Erk1/2 and Akt phosphorylation, and the expression of β-crystallins. All these characteristics of NT3-TrkC transgenic lens epithelial cells depend on Frs2α. Therefore, tyrosine phosphorylation of Frs2α mediates Fgfr-dependent lens cell survival and provides a mechanistic basis for the unique fiber-differentiating capacity of Fgfs on mammalian lens epithelial cells.
Keywords: FGF receptor, Lens differentiation, Frs2α, Mouse
INTRODUCTION
The lens serves as an important instrument for understanding cellular differentiation, in part, because it allows for visualization of all stages of lens development within a single sagittal section of the eye. The lens consists entirely of cells developing from the surface ectoderm-derived lens placode. These cells are divided into epithelial cells, forming a single layer of cuboidal epithelium immediately under the anterior lens capsule, and fiber cells, which make up the remainder of the lens. Primary fiber cells differentiate from cells comprising the posterior hemisphere of the lens vesicle and secondary fiber cells are continually generated from epithelial cells approaching the lens equator. Lens fiber cell differentiation follows a continuum beginning in the equatorial epithelial cells and continuing from periphery to the center where the oldest fiber cells are found. The vitreous humor, which surrounds the posterior hemisphere of the lens, exhibits potent fiber cell differentiating activity (Beebe et al., 1980).
The vitreous humor contains numerous growth factors that activate specific receptor tyrosine kinases (RTKs) present on lens epithelial cells. These growth factors include: insulin-like growth factor (IGF) (Beebe et al., 1987), platelet-derived growth factor (PDGF) (Cassidy et al., 1998), epidermal growth factor (EGF) (Majima, 1997), hepatocyte growth factor (HGF) (Katsura et al., 1998), vascular endothelial growth factor (VEGF) (Shui et al., 2003) and fibroblast growth factor (FGF) (McAvoy and Chamberlain, 1990). Binding of any of these growth factors to their specific RTKs induces the activation of Ras/Erk and PI3-K/Akt signaling pathways in lens epithelial cells (Iyengar et al., 2006). Inhibition of either Erk or PI3K-Akt phosphorylation prevents vitreous humor from differentiating lens epithelial cells into fiber cells (Wang et al., 2009). Lens fiber cell differentiation requires sustained Erk1/2 (Mapk3/Mapk1) phosphorylation and both vitreous humor and FGF have the ability to induce this sustained phosphorylation. Other growth factors, such as IGF, PDGF or EGF only induce transient phosphorylation of Erk1/2 in lens epithelial explants (Wang et al., 2010).
FGF signaling plays an essential role in lens fiber cell differentiation, because FGFs uniquely induce lens epithelial cells to differentiate both in vitro and in vivo (reviewed by Robinson, 2006). Transgenic mouse lenses expressing Fgf1, Fgf3, Fgf4, Fgf7, Fgf8 or Fgf9 undergo ectopic fiber cell differentiation of the lens epithelium (Lovicu and Overbeek, 1998; Robinson et al., 1998; Robinson et al., 1995). Likewise, normal lens development requires Fgf receptor (Fgfr) activity. Elevated apoptosis and eventual lens degeneration follows deletion of Fgfr2 in the lens placode (Garcia et al., 2011; Garcia et al., 2005). Furthermore, simultaneous deletion of Fgfr1 and Fgfr2 in the lens placode virtually ablates lens formation (Garcia et al., 2011). Similarly, simultaneous lens-specific deletion of Fgfr1, Fgfr2 and Fgfr3 in the lens vesicle leads to increased apoptosis and failure of primary and secondary fiber cell differentiation (Zhao et al., 2008).
In addition to Fgfrs, the lens expresses several other RTKs, including EGF receptor, PDGF receptor, IGF receptor, insulin receptor, VEGF receptors and Eph receptor A2 (Cooper et al., 2008; Faber et al., 2002; Ireland and Mrock, 2000; Reneker and Overbeek, 1996; Saint-Geniez et al., 2009; Xie et al., 2007). Ligand stimulation of most RTKs leads to receptor dimerization. This stimulation, in turn, produces trans/autophosphorylation of the receptor. The phosphorylated receptor then recruits molecular docking/signaling complexes that phosphorylate (activate) Erk1/2, Akt and PLCγ (Lemmon and Schlessinger, 2010). Although lens epithelial cells express multiple RTKs, only Fgf stimulation induces fiber cell differentiation. Furthermore, primary and secondary fiber cell differentiation occurs following deletion of non-Fgfr RTKs in the lens (reviewed by Robinson, 2006). Fgfr signaling plays a specific role in fiber cell differentiation, and in its absence other ligand/RTK combinations present in the lens epithelium fail to compensate for this role.
Among the RTKs expressed in the lens epithelium, only Fgfr stimulation directly leads to the phosphorylation of fibroblast growth factor receptor substrate 2 (Frs2). Two separate genes, Frs2 and Frs3, encode the Frs2 isoforms Frs2α and Frs2β, respectively. Frs2 proteins act as intermediary docking scaffolds for Fgfrs, but they also play an important role in Trk, Ret and Alk receptor signaling (Meakin et al., 1999; Degoutin et al., 2007; Kurokawa et al., 2001). Frs2α is widely expressed during development whereas Frs2β exhibits a more restricted expression pattern that includes neural, hepatic and nephritic tissues (McDougall et al., 2001). The amino terminal half of Frs2α contains a post-translationally added myristyl moiety that anchors the protein to the plasma membrane, and a phosphotyrosine-binding (PTB) domain that binds to specific receptors. The carboxyl terminal half of Frs2α contains multiple tyrosine phosphorylation sites, including four binding sites for the adaptor protein Grb2 and the two binding sites for the protein tyrosine phosphatase Shp2 (Ptpn11 – Mouse Genome Informatics) (Gotoh, 2008). Upon tyrosine phosphorylation (activation), Frs2α recruits multiple Shp2-Grb2-Sos complexes leading to the activation of both the Ras/Erk signaling pathway (Kouhara et al., 1997; Ong et al., 2000) and the PI3 kinase (PI3-K)/Akt pathway (Ong et al., 2001). Mutations in the Shp2-binding sites of Frs2α result in a variety of developmental defects in various organs, including the lens and retina, whereas mice with mutations in Grb2-binding sites of Frs2α have a milder phenotype and no overt ocular abnormalities (Gotoh et al., 2004; Yamamoto et al., 2005). Tyrosine phosphorylation of Frs2 might explain why FGFs induce fiber cell differentiation effectively whereas other growth factors present in the vitreous do not.
As the phosphorylation of Frs2α provides a unique function for Fgfr activity in the lens (Garcia et al., 2011), the exclusive function of Fgfrs during lens development might require Frs2α. The implementation of two complementary experimental approaches tested the function of Frs2α in lens cell survival and fiber cell differentiation. Assessment of cell proliferation, apoptosis and fiber differentiation in lenses lacking Frs2α demonstrated the requirement of this protein for lens cell survival and robust fiber differentiation. Furthermore, lens epithelial cells in transgenic mice ectopically expressing both NT3 (Ntf3 – Mouse Genome Informatics) and TrkC (Ntrk3 – Mouse Genome Informatics) in the lens underwent Frs2α-dependent morphological and biochemical features consistent with premature fiber cell differentiation, suggesting that Frs2α activation through TrkC is sufficient to initiate fiber cell differentiation.
MATERIALS AND METHODS
Mice
Mice were housed in a pathogen-free vivarium and used in accordance with the Miami University Institutional Animal Care and Use Committee policies and with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research.
Transgenic mice expressing Fgf1 (OVE 371) or NT3 (OVE 613) in the lens were previously described (Lavail et al., 2008; Robinson et al., 1995). Transgenic mice expressing Cre in the ocular surface ectoderm (Ashery-Padan et al., 2000) and mice with conditional mutations in Fgfr2 (Fgfr2L/L) (Yu et al., 2003) were obtained from Dr Ruth Ashery-Padan (Tel Aviv University, Ramat Aviv, Israel) and Dr David Ornitz (Washington University School of Medicine, St Louis, MO, USA), respectively. Mice engineered with a conditional mutation in Frs2α (Frs2αL/L) were obtained from Dr Fen Wang (Zhang et al., 2008).
To generate mice expressing TrkC in the lens, a rat TrkC cDNA clone (George D. Yancopoulos, Regeneron Pharmaceuticals, Tarrytown, NY, USA) was subcloned between the XmaI and XbaI sites of αAV3 (Reneker et al., 2004a). The αAV3 plasmid contained a chicken δ-crystallin enhancer fused to the mouse αA-crystallin promoter (αA-cry) to facilitate transgene expression in the entire lens. The resulting transgenic construct was injected into pronuclear stage FVB/N embryos to obtain transgenic founders. Three initial founder lines (MLR 65, 66 and 67) were identified by PCR. To obtain the appropriate genotypes for experimentation, transgenic and targeted animals were crossed and the resultant animals were genotyped using primers shown in supplementary material Table S1. In the case of the Le-Cre, TrkC and NT3 transgenic animals, only mice hemizygous for these transgenes were analyzed.
Histology and immunohistochemistry
The gestational age of experimental embryos was determined with the detection of a vaginal plug designated as 0.5 days gestation, i.e. embryonic day (E) 0.5. One hour prior to embryo collection, pregnant mice were injected with bromodeoxyuridine (BrdU; 100 mg/ml). The embryos were fixed in 10% neutral buffered formalin (NBF), processed and embedded in paraffin according to standard protocols (Zhao et al., 2008). For immunofluorescence labeling, sections were antigen retrieved as described (Zhao et al., 2008). Tissue sections were blocked with 10% normal goat serum prior to incubation with primary antibodies. Apoptosis was detected using the In Situ Cell Death Detection Kit (TMR Red, Roche Applied Science, 2156792). Sources and dilutions for all primary antibodies are listed in supplementary material Table S2. Binding of primary antibodies was detected using the appropriate secondary antibodies (Alexafluor 488 goat anti-rabbit IgG, Alexafluor 546 goat anti-rat IgG and Alexafluor 633 donkey anti-goat IgG; Molecular Probes, A11008, A11081, A21082, respectively). Negative controls consisted of sections incubated in the appropriate serum without primary antibodies. Sections were counterstained with Vectashield mounting medium containing DAPI (Vector Laboratories, H-1200). Photomicrographs were captured on the Olympus FV500 laser scanning confocal system or the Zeiss 710 laser scanning confocal system at The Center for Advanced Microscopy and Imaging at Miami University.
For area/volume analysis, photomicrographs of Hematoxylin and Eosin-stained sections were taken using a Nikon TI-80 microscope and the area of the lens or the epithelial length directly obtained using the Nikon NIS-Elements (version: 3.0) software. To estimate lens volume, lines were drawn from the anterior to the posterior part of the lens, and the radius value obtained from the software. Three to four sections for every sample and values from at least three embryos were obtained, and the volume of the lens (which is almost spherical) was calculated using the formula 4/3πr3. Statistical significance for all these assays was determined using Student’s t-test.
Transfection of lens epithelial explants
The full-length cDNA encoding TrkC (αAV3/rTrkC) was inserted into the multiple cloning site of pLXSG to generate pLXSG-TrkC. The pLXSG retroviral expression vector is derived from the MMLV-based recombinant vector pLXSN (Clontech, CA, USA) (Miller and Rosman, 1989). In this instance, the neomycin resistance gene of pLXSN was replaced by a cDNA-encoding enhanced green fluorescence protein (EGFP) to generate pLXSG. Lens epithelial explants were prepared as described previously using neonatal [postnatal day (P) 3] albino Wistar rats (Lovicu et al., 2008). Approximately 4 hours after explanting, lens epithelial cells from two explants in each culture dish were transfected with either pLXSG or pLXSG-TrkC. Plasmid DNA (2 μg) was diluted in 50 μl of Opti-MEM I Reduced Serum Medium (Invitrogen, CA, USA). Lipofectamine 2000 was diluted 1:10 with Opti-MEM I Reduced Serum Medium, and was incubated for 5 minutes at room temperature (RT). The Lipofectamine 2000 solution (50 μl) and 50 μl of the 2 μg plasmid DNA were combined and incubated for 20 minutes at RT. Explants were rinsed three times with M199 medium without antibiotics. The DNA-Lipofectamine 2000 complex (100 μl) was added to each dish containing 900 μl of M199 medium without antibiotics followed by incubated at 37°C, 5% CO2 for 48 hours. Following transfection, culture medium in each dish was replaced with fresh M199 medium with antibiotics, supplemented with either Fgf2 (100 ng/ml) or TrkC (100 ng/ml) and cultured for up to 5 days. The transfected lens epithelial cells expressing EGFP were observed using a fluorescent microscope with a UV source (Leica DMIL, Germany) and photographed (Leica Firecam, Germany). The cultures were treated with TRITC-conjugated lectin from Triticum vulgaris (L5266, Sigma-Aldrich, Australia) at 30 μg/ml for 20 minutes at RT to counter-label the cell membranes.
Immunofluorescence quantification
Indirect immunofluorescence labeling on tissue sections was quantified using previously described methods (Garcia et al., 2011; Plageman et al., 2011). Briefly, for any given immunofluorescent assay, all experimental slides were treated concurrently with identical exposure times. IMAGEJ v1.44 software (http://rsbweb.nih.gov/ij/) was used to plot standard fixed areas and to measure the signal intensity of the pixels (RGB) on the tissue being analyzed as well as on the neural retina (which was used as an internal standard). The values obtained for any given data point were from ∼128 measurements (64 lens and 64 retina) from each of three different embryos in which the ratio of the fluorescence intensity of the two tissues was computed. Differences in pixel intensity between control and experimental eyes were evaluated using Student’s t-test. Similar assays were also carried out using ZEN (Zeiss confocal software) for verification. The areas selected for analysis depended upon the antibody used; for example, only nuclear staining in the bow region was calculated for Prox1 whereas E-cadherin was only measured in the anterior layer of lens cells.
For the estimation of BrdU, p57KIP2 and the TUNEL assays, the ratio of the lens cell nuclei positive for the mentioned markers over the total DAPI-stained nuclei cells in the tissue compartment being analyzed was determined. Student’s t-test was performed to determine statistical significance.
Western blot analysis
Lenses and brain tissue (positive control) from 3-week-old mice were subjected to western blot analysis according to established procedures (Pan et al., 2010). The primary antibodies used for the analysis were anti-TrkC antibody (1:1000, Cell Signaling Technology, 3376) and anti-Gapdh antibody (1:2000, Cell Signaling Technology, 2118). After incubation with the horseradish peroxidase-conjugated secondary antibody for 2 hours (1:2000, Cell Signaling Technology, 7074), the proteins were analyzed on X-ray films after treatment with the chemiluminescent substrate Lumiglo (Cell Signaling Technology, 7003).
RESULTS
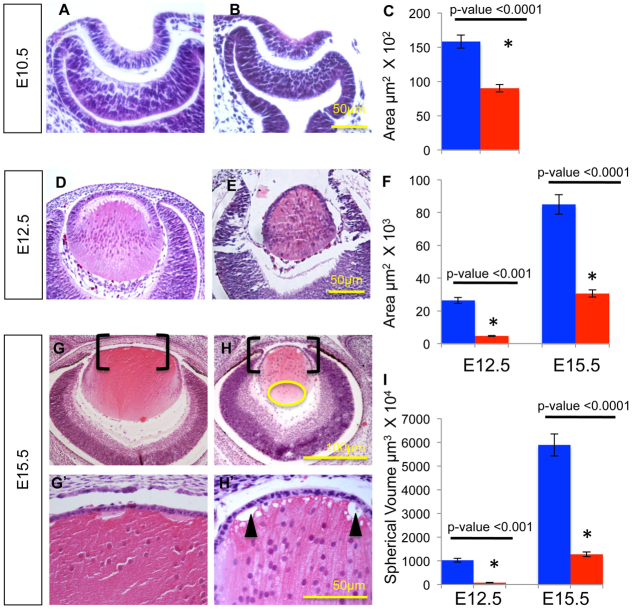
To determine the requirement of Frs2α for normal lens development, mice carrying loxP-flanked alleles of Frs2 and the Le-Cre transgene mediated the deletion of Frs2 in all surface ectoderm-derived eye structures. Littermate embryos, homozygous for the floxed Frs2 alleles (Frs2αL/L) but lacking the Le-Cre transgene, served as controls. Le-Cre; Frs2αL/L and Frs2αL/L denote mice expressing the Cre transgene in the lens-forming ectoderm, and those lacking the Cre transgene, respectively. Cre expression in Le-Cre mice initiates in the head surface ectoderm at ∼9 days post coitus (E9), so examinations of Le-Cre; Frs2αL/L and Frs2αL/L eyes commenced at E10.5 and continued throughout embryonic development. Le-Cre; Frs2αL/L embryos experienced delayed lens pit invagination resulting in a significantly smaller lens pit containing fewer cells that did not invaginate as deeply into the optic cup compared with control littermates (Fig. 1A-C). The lenses of Le-Cre; Frs2αL/L embryos remained smaller (both in planar surface area and in estimated spherical volume) than those of control embryos at E12.5 and E15.5 despite evidence of primary fiber cell differentiation (compare Fig. 1D-I). Numerous gaps between the epithelium and fiber cells as well as vacuoles near the apical tips of the fiber cells formed in the Le-Cre; Frs2αL/L lenses by E15.5 (compare Fig. 1G′,H′, arrowheads). In addition, Frs2α-deficient lenses retained nuclei abnormally in primary fiber cells (Fig. 1H, nuclei within the yellow circle).
Fig. 1.
Frs2α deficiency leads to decreased lens size. (A-I) Mouse lenses were analyzed at E10.5 (A,B), E12.5 (D,E) and E15.5 (G,H). G′ and H′ are higher magnifications of the bracketed areas in G and H, respectively. At E10.5, the Frs2α-deficient lens placodes (B) exhibited delayed lens pit invagination and reduced planar area (C) relative to Cre-negative controls (A). At E12.5 (compare E with D) and E15.5 (compare H,H′ with G,G′), Frs2α-deficient lenses exhibited reduced lens planar area (F) as well as reduced estimated lens spherical volume (I). Error bars on the graphs represent s.e.m. By E15.5, numerous gaps appear between the epithelium and fiber cells as well as vacuoles within the apical portion of the fiber cells (arrowheads in H′). Also, the Frs2α-deficient lens displays abnormal posterior placement of nuclei in the primary fiber cells (yellow circle in H) well behind the lens equator. Asterisks in C, F and I represent significant differences from the control values.
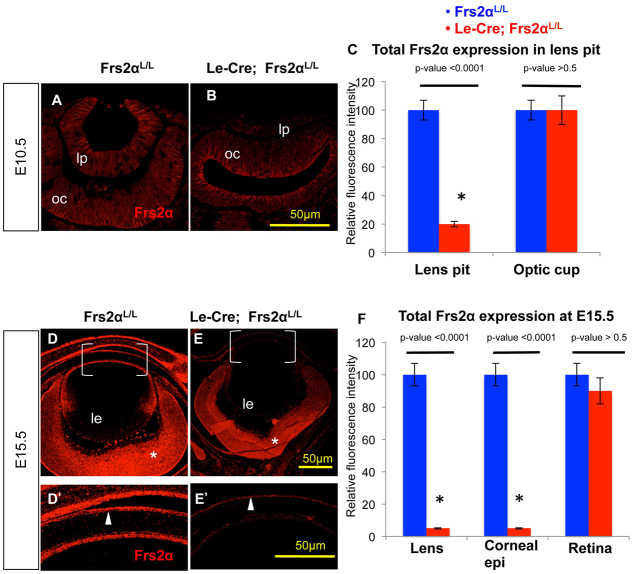
Assessment of the effectiveness of Frs2 deletion revealed an ∼80% decrease of total immunologically detectable Frs2α protein in the Le-Cre; Frs2αL/L lenses by E10.5 (Fig. 2A-C). Frs2α expression in the optic cup (where Cre is absent) remained unaffected. Total Frs2α expression in the lens and the corneal epithelium of the Le-Cre; Frs2αL/L embryos diminished by 97% relative to the Cre-negative littermates despite persistent Frs2α expression in the retina by E15.5 (Fig. 2D-F). RT-PCR of RNA from wild-type or Frs2α-deficient lenses failed to detect Frs2β expression despite the amplification of a robust band from kidney RNA (data not shown).
Fig. 2.
Loss of Frs2α in Le-Cre; Frs2αL/L surface ectoderm-derived eye structures is evident by E10.5. (A-F) Total Frs2α protein was measured by indirect immunofluorescence at E10.5 (A,B) and E15.5 (D,E). Frs2α protein was significantly reduced in the Le-Cre positive lens pit (at E10.5) in the corneal epithelium (arrowheads in D′,E′) and in the lens (at E15.5) relative to the Cre-negative control at both stages. Note that Frs2α levels appear similar in age-matched control and mutant optic cups (oc, compare A with B) and retinas (asterisks in D,E). D′ and E′ are higher magnifications of the bracketed areas in D and E, respectively. Quantitative relative expression levels at E10.5 (C) and E15.5 (F) were calculated using ImageJ. Error bars on the graphs represent s.e.m. Asterisks in C and F represent significant differences. le, lens; lp, lens pit.
Frs2α regulates known targets of FGFR signaling
The similarity between the Le-Cre; Frs2αL/L and the Le-Cre; Fgfr2L/L lenses described previously warranted the comparison of Frs2α phosphorylation and the expression of the ETS transcription factors ER81 (Etv1) and Erm (Etv5), which are direct downstream transcriptional targets of Fgfr signaling. Phosphorylated Frs2α appeared in both the lens pit and the posterior optic cup of control embryos (Fig. 3A). By contrast, phosphorylation of Frs2α remained virtually undetectable in the Le-Cre; Frs2αL/L and the Le-Cre; Fgfr2L/L lens pits despite abundant Frs2α phosphorylation in the optic cups of these mice (compare Fig. 3D-F,M). Similarly, the expression of ER81 (Fig. 3J-L,N) and Erm (data not shown) exhibited marked and specific reduction in the lens pits of both Le-Cre; Frs2αL/L and Le-Cre; Fgfr2L/L embryos.
Fig. 3.
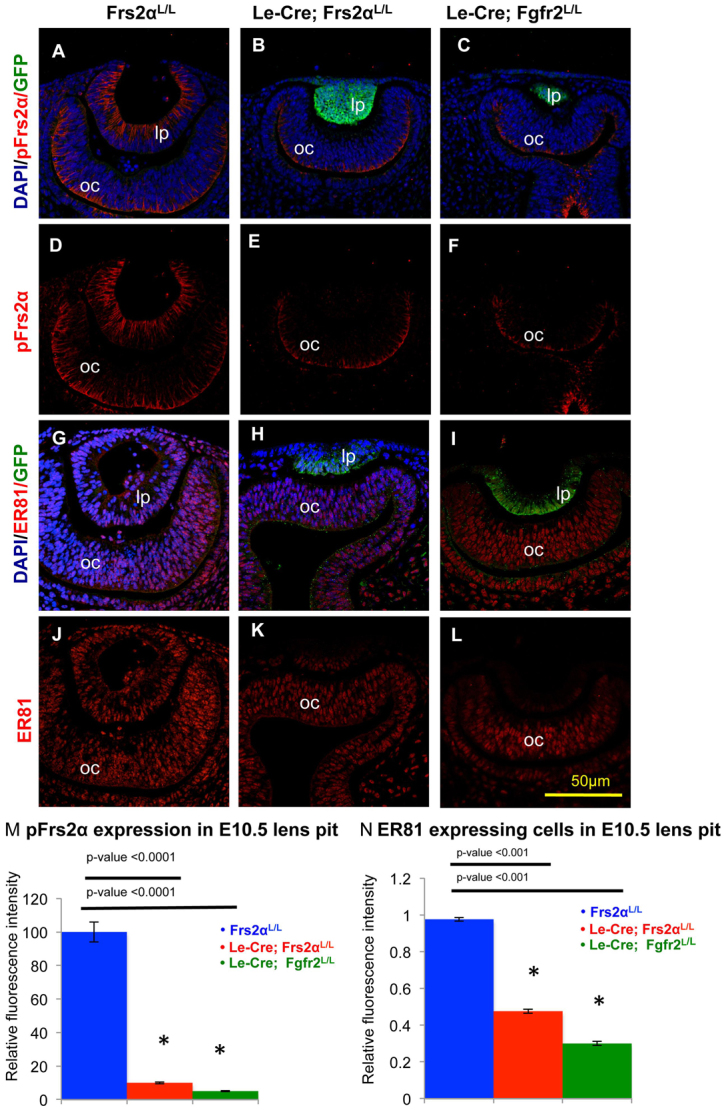
Loss of Frs2α is similar to Fgfr2 deficiency with respect to the lens. (A-N) Lens pits from Cre-negative control (A,D,G,J), Frs2α-deficient (B,E,H,K) and Fgfr2-deficient (C,F,I,L) mice were analyzed for phosphorylated Frs2α (pFrs2α) and ER81 proteins at E10.5. Lens pits were visualized simultaneously for DAPI (for nuclei), GFP (to visualize Cre expression pattern) and either pFrs2α (A-F) or ER81 (G-L). Frs2α phosphorylation was lost both in the Frs2α-deficient (E) and Fgfr2-deficient (F) lens pits (M). Similarly, ER81 expression was significantly reduced in both Frs2α-deficient (K) and Fgfr2-deficient (L) lens pits (N). lp, lens pit; oc, optic cup. Error bars on the graphs represent s.e.m. Asterisks in M and N represent significant differences from the control values.
Loss of Frs2α or Fgfr2 in the lens results in reduced lens size and increased apoptosis
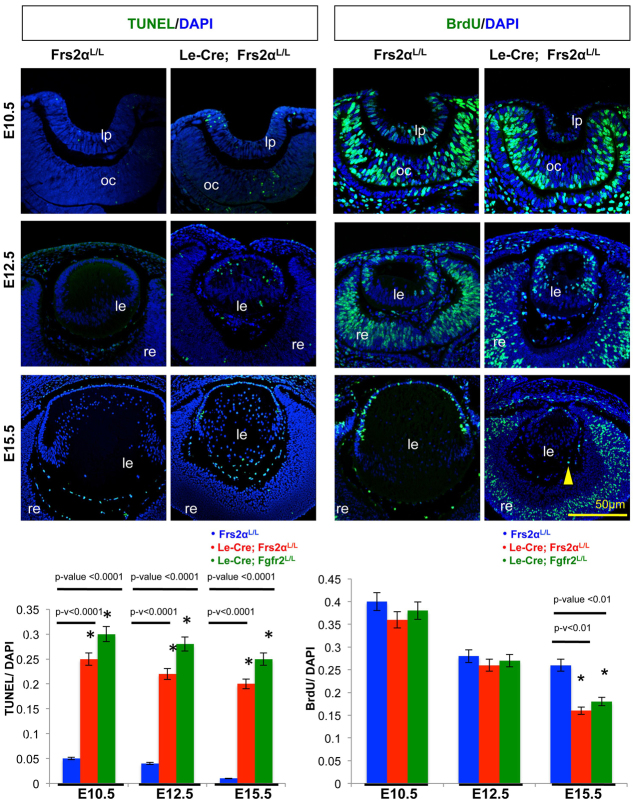
Cell death and proliferation assays conducted at E10.5, E12.5 and E15.5 facilitated mechanistic investigations to explain the size reduction in Le-Cre; Frs2αL/L lenses. Frs2α-deficient lenses presented significantly increased apoptosis at E10.5 and this trend continued at both of the later stages (E12.5 and E15.5) (Fig. 4). Nevertheless, proliferation rates in Le-Cre; Frs2aL/L and control lenses stayed similar at E10.5 and E12.5, but by E15.5, the proliferation rate decreased in the Frs2α-deficient lenses (Fig. 4). The significant decrease in cell proliferation at E15.5 in Frs2α-deficient lenses prompted a re-investigation of lens apoptosis and proliferation in Le-Cre; Fgfr2L/L embryos. These studies revealed no statistically significant differences in apoptosis or cell proliferation between the Le-Cre; Fgfr2L/L and the Le-Cre; Frs2αL/L lenses at any stage examined (Fig. 4, bar graphs). Both the Fgfr2- and Frs2α-deficient lenses exhibited a significant decrease in proliferation at E15.5 relative to controls. In contrast to control lenses (but like Fgfr2-deficient lenses), fiber cells in the Frs2α-deficient lenses occasionally incorporated BrdU at E15.5 (Fig. 4, arrowhead). Therefore, the loss of either Frs2α or Fgfr2 impairs efficient cell cycle withdrawal during lens fiber differentiation.
Fig. 4.
Frs2α-deficient lenses exhibit increased apoptosis followed by secondary reduction in proliferation. Cell death was analyzed at E10.5, E12.5 and E15.5 in Cre-negative control and Le-Cre-positive lens cells using the TUNEL assay. Likewise, proliferation rates were assayed by BrdU incorporation to determine the S-phase fraction of lens cells. Apoptosis was significantly increased in Frs2α-deficient and Fgfr2-deficient lens cells at all stages examined. Conversely, proliferation was similar in control and Frs2α-deficient and Fgfr2-deficient lenses until E15.5 when mutant lenses exhibited a significant decrease in BrdU incorporation. Error bars on the graphs represent s.e.m. Asterisks represent significant differences. le, lens; lp, lens pit; oc, optic cup; re, retina. The yellow arrowhead indicates a fiber cell that has incorporated BrdU.
Frs2α loss impairs fiber cell differentiation despite leaving lens epithelial identity intact
An investigation of E-cadherin (cadherin 1; a protein normally expressed in the lens epithelium) expression was undertaken to determine whether the Frs2α mutant lenses retained lens epithelial cell characteristics. In addition to being undersized, Frs2α-deficient lenses contain fewer lens epithelial cells that remain shorter than those of control lenses at E15.5. However, a normal pattern (Fig. 5A,B) and level (Fig. 5C) of E-cadherin expression in the Frs2α-deficient lens epithelium persisted. Similarly, Frs2α loss left the pattern and intensity of Pax6 expression in the lens unchanged at E15.5 (data not shown).
Fig. 5.
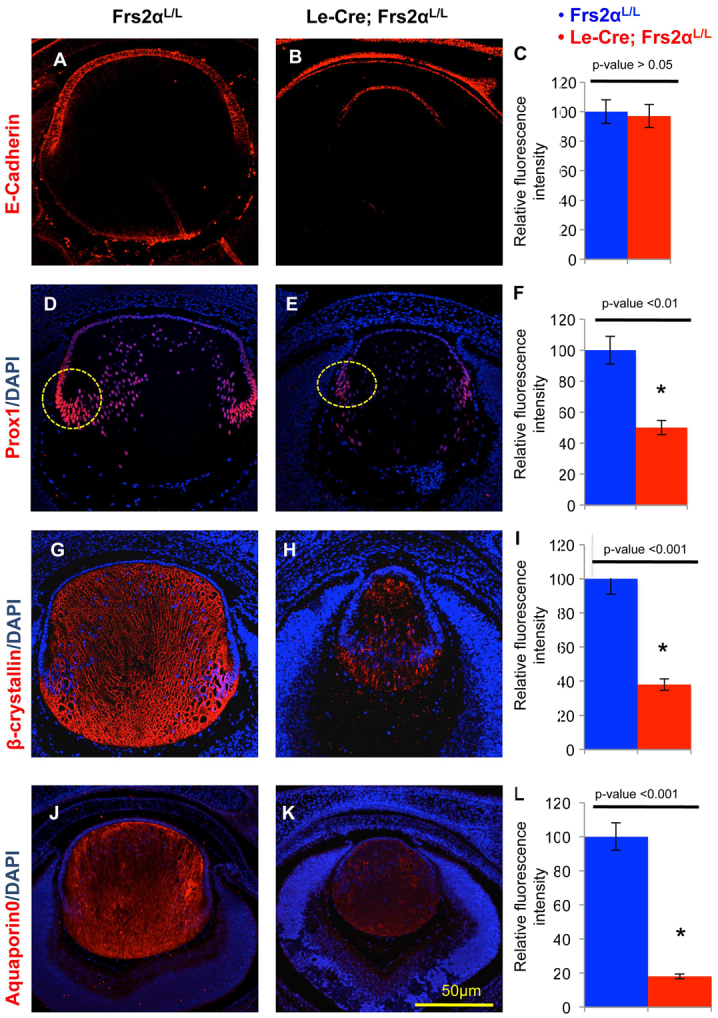
Frs2α-deficiency in the lens maintains lens identity but displays reduced levels of fiber differentiation-specific proteins. (A-L) Lenses from control (A,D,G,J) and Frs2α-deficient (B,E,H,K) E15.5 embryos were analyzed for E-cadherin (expressed in lens epithelial cells), Prox1, β-crystallin and aquaporin 0 (characteristic of lens fiber cells). Both the pattern (compare A with B) and expression level (C) of E-cadherin is similar in Frs2α-deficient and control lenses. Conversely, although the normal expression pattern of Prox1 (D,E), β-crystallin (G,H) and aquaporin 0 (J,K) were preserved, these proteins were significantly less abundant in the Frs2α-deficient lenses (F,I,L). Nuclear Prox1 fluorescence intensity was analyzed in the areas represented by the yellow circles in D and E. Error bars on the graphs represent s.e.m. Asterisks represent significant differences.
Reduced expression of several proteins associated with fiber cell differentiation, including Prox1, β-crystallins, and aquaporin 0 (Mip – Mouse Genome Informatics), demonstrated that Frs2α deficiency impaired differentiation. Le-Cre; Frs2αL/L lenses expressed Prox1 in fewer cells (Fig. 5E) and with significantly less intensity (Fig. 5F) in the bow region relative to the control lenses (Fig. 5D, circled in yellow). Likewise, the expression of β-crystallins (compare Fig. 5G and 5H,I) and aquaporin 0 (compare Fig. 5J and 5K,L) diminished in the Frs2α-deficient lenses.
Frs2α phosphorylation promotes differentiation of lens epithelial cells
If Frs2α functions as the main mediator in Fgf signaling with respect to fiber cell differentiation, then activation (phosphorylation) of Frs2α should result in a premature differentiation phenotype similar to that previously reported in Fgf-overexpressing lenses (Lovicu and Overbeek, 1998; Robinson et al., 1998; Robinson et al., 1995). A test of this hypothesis exploited the rat lens explant system to determine whether ectopic expression and activation of the TrkC RTK could induce lens epithelial cell differentiation upon binding the TrkC ligand NT3. As shown previously, exposure of lens explants transfected with a control (GFP expression) vector to Fgf2 leads to differentiation of the normally cuboidal epithelial cells of the explant (Fig. 6A) to elongating fiber cells (Fig. 6B). By contrast, explants transfected with the control vector and exposed to the TrkC ligand NT3 failed to demonstrate morphological signs of differentiation (Fig. 6C). By comparison, lens epithelial cells transfected with a TrkC expression vector elongated following exposure to either Fgf2 (Fig. 6E) or NT3 (Fig. 6F). The demonstration that NT3 activation of TrkC led to the elongation (consistent with differentiation) of lens epithelial cells in explants prompted further investigation of this phenomenon in vivo using transgenic mice.
Fig. 6.
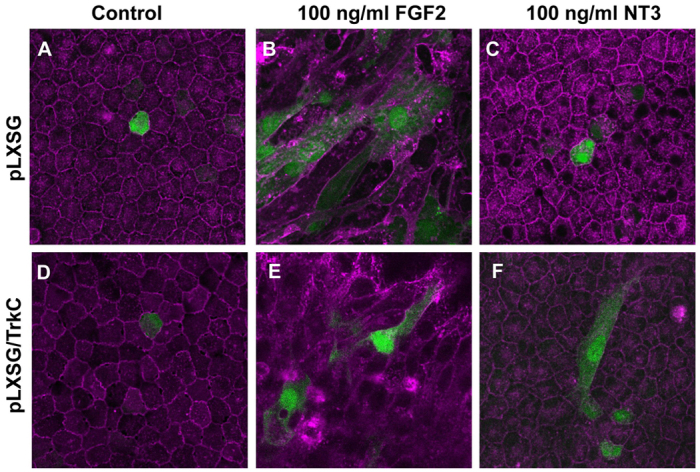
Transfection of rat lens epithelial explants with TrkC leads to NT3-dependent fiber differentiation. (A-F) Lens epithelial explants were transfected with PLXSG retroviral vector alone (A-C) or the PLXSG/TrkC expression construct (D-F). Transfected explants were treated with FGF2 (B,E) or NT3 (C,F). Whereas FGF2 induced lens epithelial cell differentiation (as measured by elongation; B,E) in both vector- and TrkC-transfected cells, NT3-induced differentiation only occurred in TrkC-transfected explants (F). The PLXSG vector transfected cells express GFP (green), and cells were counterstained with TRITC-conjugated lectin (purple).
Lens-specific TrkC overexpression
Transgenic mice overexpressing NT3 from lens fiber cells displayed clear and morphologically normal lenses up to at least 14 months of age (Lavail et al., 2008). The absence of TrkC expression in the lens explains the lack of lens phenotype in these NT3-overexpressing mice (supplementary material Fig. S1B,C). In fact, none of RTKs known to tyrosine phosphorylate Frs2α [TrkA (Ntrk1), TrkB (Ntrk2), TrkC, Ret or Alk] were amplified by RT-PCR of mouse lens RNA (data not shown). Implementation of a murine αA-crystallin promoter supplemented with the chicken δ1-crystallin enhancer facilitated the expression of TrkC in both the developing lens epithelium and fibers of transgenic mice (supplementary material Fig. S1A). Adult lenses from three established transgenic founder lines (MLR65, 66 and 67) exhibited evidence of correctly spliced transgenic transcripts in both epithelial cells and fiber cells by RT-PCR (supplementary material Fig. S1B). In addition, soluble protein isolated from adult lenses from each of these lines demonstrated evidence of TrkC expression (supplementary material Fig. S1C). Subsequent analyses focused on MLR66, the transgenic line expressing the highest level of TrkC protein. TrkC protein expression initiated by E12.5 and spread to both fiber cells and epithelial cells by E15.5 (supplementary material Fig. S1D).
NT3-TrkC downstream signaling in the lens is dependent on Frs2α activation
Mice carrying transgenes for both the receptor (TrkC) and the ligand (NT3) exhibited significantly increased lens epithelial cell elongation (100% increase) at E15.5, suggesting the induction of premature differentiation into fiber cells (Fig. 7A-B′,D). A subepithelium of epithelial cells beneath the elongated anterior epithelium consistently formed in these double transgenic (NT3-TrkC) lenses. Premature elongation of the lens epithelial cells in the NT3-TrkC transgenic embryos emulated that seen in transgenic mice overexpressing Fgf1, in which premature fiber cell differentiation is well documented (Robinson et al., 1995) (supplementary material Fig. S2A,B). The deletion of Frs2 confirmed the dependence of the NT3-TrkC induced epithelial elongation on Frs2α activation.
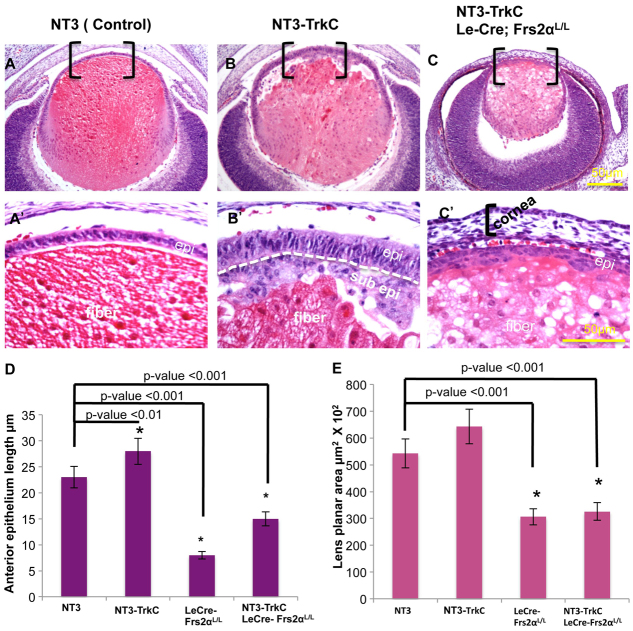
Fig. 7.
Activated NT3-TrkC signaling induces Frs2α-dependent lens epithelial cell elongation. (A-E) NT3 (control, A), NT3-TrkC (B) and NT3-TrkC/Frs2α-deficient (C) lenses were analyzed at E15.5 by Hematoxylin & Eosin staining with quantitative analysis of lens epithelial cell length and total lens planar area represented graphically (D,E). A′-C′ are higher magnifications of the bracketed areas of A-C. E15.5 NT3-transgenic embryos have normal lens morphology whereas the double transgenic NT3-TrkC lenses exhibit significantly elongated lens epithelial cells (epi), fiber cell (fiber) dysmorphology and epithelial multilayering compared with control (compare A with B), forming a sub-epithelial layer (sub epi). Lens epithelial cells fail to elongate in the NT3-TrkC transgenic lenses when Frs2α is deleted (compare A′,B′,C′) but fiber cells exhibit multiple vacuoles with significant reduction in the size of the lens (C,E). The cells immediately anterior to the lens in C′ are from the cornea. Error bars on the graphs represent s.e.m. Asterisks represent significant differences.
The loss of Frs2α in the NT3-TrkC double transgenic mice minimized the transgenic lens phenotype at E15.5. With respect to size, the NT3-TrkC lenses lacking Frs2α phenocopied the Le-Cre; Frs2αL/L lenses (50% decreased planar area compared with NT3 transgenic control) (Fig. 7A,C,E). The fiber cells in the NT3-TrkC transgenic lenses presented disorganization with numerous vacuoles and retained nuclei with or without Frs2α (Fig. 7B,C). Frs2-deficient, NT3-TrkC lens epithelial cells, though multilayered, failed to achieve the length of either the NT3-TrkC transgenic lens epithelium or the NT3 control lens epithelium (Fig. 7A′-C′,D). The NT3-TrkC eyes lacking Frs2α also lacked both the anterior and the posterior chambers, resulting in the close apposition of the lens and the cornea (Fig. 7C,C′). Thus, the elongated lens epithelial phenotype in NT3-TrkC transgenic mice depends on Frs2α.
Although present in the control lens epithelium (Fig. 8A), tyrosine phosphorylated (active) Frs2α increases during normal fiber cell differentiation (supplementary material Fig. S3A′). At E15.5, transgenic NT3-TrkC lenses, with Frs2α intact, revealed significant upregulation of Frs2α phosphorylation in the elongating lens epithelium, similar to that seen in Fgf1 transgenic lenses (compare Fig. 8A,B,M with supplementary material Fig. S2A). Therefore, transgenic overexpression of either Fgf1 or NT3, and TrkC increased Frs2α phosphorylation in the lens epithelium.
Fig. 8.
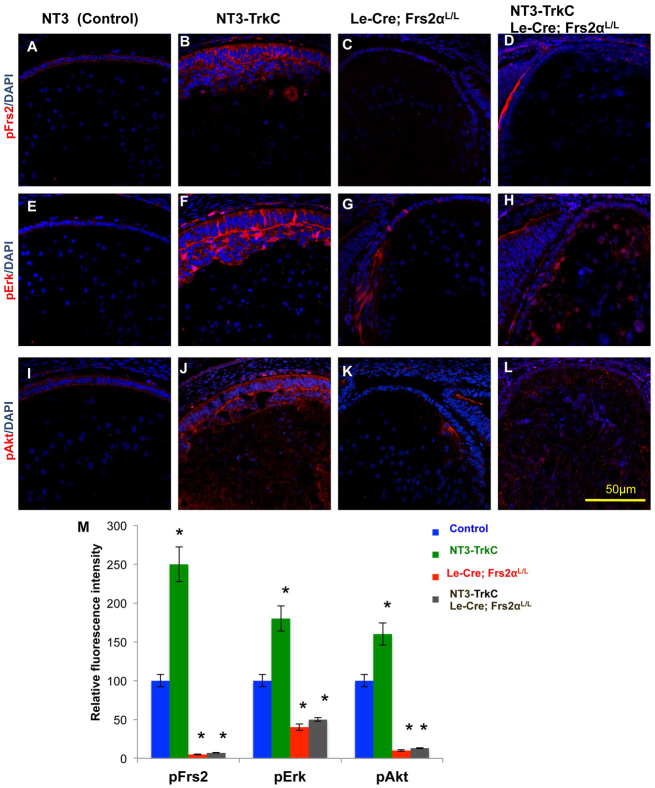
Activated NT3-TrkC signaling induces Frs2α-dependent phosphorylation of Frs2α, Erk and Akt in the lens epithelium. (A-L) Control (A,E,I), NT3-TrkC (B,F,J), Frs2α-deficient (C,G,K) and NT3-TrkC lenses lacking Frs2α (D,H,L) were analyzed at E15.5 to determine the activation (phosphorylation) state of Frs2α (A-D), Erk (E-H) and Akt (I-L) in the lens epithelium. The loss of Frs2α decreases phosphorylation of Erk and Akt in the lens epithelium and this effect is dominant because although NT3-TrkC induces increased phosphorylation of Frs2α, Erk and Akt in the transgenic lens epithelium, this response requires Frs2α (compare B,F,J with D,H,L). (M) Relative fluorescence intensity for immunofluorescent staining was analyzed with the NT3 control value being set at 100%. Error bars on the graphs represent s.e.m. Asterisks represent significant differences (all significant P-values were <0.001).
In the E15.5 control lens, endogenous Fgfr signaling promoted weaker activation (phosphorylation) of downstream targets Erk and Akt, in lens epithelial cells than in newly differentiated fiber cells of the bow region (compare Fig. 8E,I with supplementary material Fig. S3E′,I′). The elongating lens epithelial cells of NT3-TrkC E15.5 embryos elevated both Erk and Akt phosphorylation (compare Fig. 8E,I with 8F,J,M). Absence of Frs2α in these lenses diminished the phosphorylation of Erk and Akt below the level observed in control lenses (compare Fig. 8E,I, with 8G,K,M), but to a similar level as that observed in Frs2 knockout lenses. Therefore, both TrkC-mediated lens epithelial elongation and the activation of downstream TrkC signaling in the lens epithelium requires Frs2α (compare Fig. 8E,I with 8H,L,M). With respect to phosphorylation of Erk and Akt in the lens epithelium, the loss of Frs2α exhibits epistasis to the transgenic expression of NT3 and TrkC (compare Fig. 8G,K with 8H,L,M). Although efforts concentrated on the expression of phosphorylated Erk and Akt in the lens epithelium, NT3 and TrkC expression also increased the phosphorylation of these proteins in the fiber cells (supplementary material Fig. S3).
Induction of premature fiber cell differentiation by NT3-TrkC signaling is dependent on Frs2α
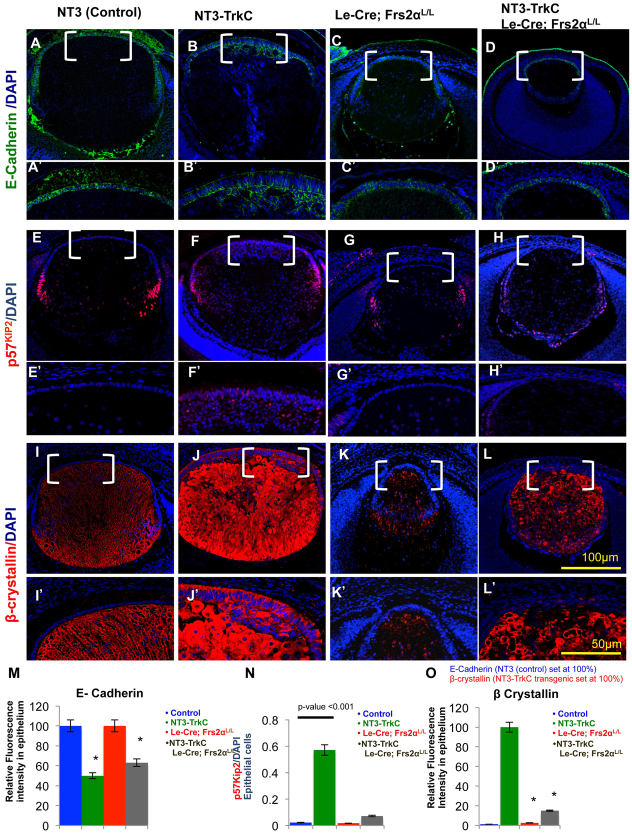
Investigations of E-cadherin expression were carried out to determine whether the lens epithelium retained epithelial characteristics in the NT3-TrkC transgenic lenses. At E15.5, the NT3-TrkC lens epithelium exhibited weaker E-cadherin expression relative to the control lens epithelium (compare Fig. 9A,A′ with 9B,B′,M). In particular, E-cadherin staining no longer appeared on the lateral cell membranes and diminished in intensity on the basal and apical surfaces of the NT3-TrkC lenses. Interestingly, the subepithelial cells in the NT3-TrkC lenses retained strong E-cadherin expression (Fig. 9B′). Loss of Frs2 failed to alter E-cadherin expression in the lens epithelium or to restore epithelial E-cadherin expression in the NT3-TrkC lenses (compare Fig. 9A,A′,C,C′ with 9D,D′,M).
Fig. 9.
Premature fiber cell differentiation induced by activated NT3-TrkC signaling requires Frs2α. (A-L′) Control (A,E,I), NT3-TrkC (B,F,J), Frs2α-deficient (C,G,K) and NT3-TrkC lenses lacking Frs2α (D,H,L) were analyzed at E15.5 to determine the expression of the E-cadherin (A-D), p57KIP2 (E-H) and β-crystallins (I-L). Expression of E-cadherin is markedly downregulated in NT3-TrkC lens (compare A with B), as well as the NT3-TrkC lens lacking Frs2α (compare A with D). The transcription factor p57KIP2 is induced in pockets of elongating epithelial cells in the NT3-TrkC transgenic lenses (compare E with F) indicating their premature differentiation status. Likewise, these prematurely differentiating epithelial cells express β-crystallin (compare I with J). Both the induction of p57KIP2 and β-crystallin in the anterior lens cells in NT3-TrkC lenses is entirely dependent on Frs2α (compare F with H and J with L). The bracketed areas in A-L are shown at higher magnifications in A′-L′. (M-O) The proportion of p57KIP2-positive lens epithelial nuclei in represented genotypes is depicted graphically (N). Relative fluorescence intensity for immunofluorescent staining was analyzed for β-crystallin with the NT3-TrkC value being set at 100% (O) and for E-cadherin, the NT3 (control) value was set at 100% (M). Asterisks represent significant differences (all significant P-values were <0.01).
Nuclear p57KIP2 and β-crystallin expression distinguishes differentiating fiber cells from anterior epithelial cells (Fig. 9E,E′,I,I′) but both of these proteins emerge in elongating NT3-TrkC lens epithelial cells. In NT3-TrkC lenses, nuclear staining of p57KIP2 appeared in ∼55% of elongating lens epithelial cells (compare Fig. 9E-F′,N). However, nuclear p57KIP2 was detected in almost all elongating epithelial cells in Fgf1 transgenic lenses (supplementary material Fig. S2C). The NT3-TrkC elongating lens epithelium exhibited robust β-crystallin expression (Fig. 9J,J′), which mimicked that seen in the Fgf1 transgenic lenses (supplementary material Fig. S2B). By contrast, NT3-TrkC lenses lacking Frs2 expressed fiber cell markers p57KIP2 (compare Fig. 9E,G,H,N) and β-crystallin (compare Fig. 9I,K,L,O) in a pattern resembling that seen in the Le-Cre;Frs2αL/L lens.
Although Fgf1 transgenic lens epithelial cells withdrew from the cycle, proliferation persisted in the NT3-TrkC transgenic lens epithelium. In fact, the expression of NT3 and TrkC rescued the reduction in epithelial cell proliferation seen at E15.5 in the Frs2α-deficient lens epithelium (supplementary material Fig. S4A). Whereas the expression of NT3 and TrkC alone induced significant lens cell apoptosis, the loss of Frs2 induced several fold increased apoptosis with or without NT3 and TrkC expression (supplementary material Fig. S4B).
Although we used NT3-TrkC compound transgenic mice for most analyses, we did see increased expression of phosphorylated Frs2α, Erk, Akt and nuclear p57KIP2 and β-crystallin expression in TrkC transgenic mice without the expression of transgenic NT3 ligand (supplementary material Fig. S5). Here, activation of TrkC might be the result of endogenous NT3 expression within the eye or from ligand-independent activation due to overexpression of the receptor. In TrkC transgenic mice, the increased expression of these fiber differentiation markers was abolished following loss of a single allele of Frs2α (supplementary material Fig. S5).
DISCUSSION
Cellular identity depends on differential gene expression. During development, cells communicate, in part, through diffusible growth factors that activate receptors and intracellular responses, ultimately changing the transcriptional landscape of the responding cell. Understanding the mechanisms that distinguish how different RTKs elicit differential gene activation, despite apparently activating similar intracellular pathways, will enhance the comprehension of normal development, and could provide new targets for therapeutic intervention for diseases, such as cancer, that involve aberrant cellular signaling.
Lens cell survival and fiber cell differentiation require Fgfr signaling (Garcia et al., 2011; Garcia et al., 2005; Zhao et al., 2008). Of the growth factors that activate RTKs on the mammalian lens epithelium, only Fgfs stimulate lens fiber differentiation. Although Fgfrs and other RTKs activate several common signaling cascades, the activation of Frs2α in the lens is unique to Fgfrs. Therefore, Frs2α represents an attractive candidate to explain the specific capability of Fgfrs to induce lens fiber differentiation.
Despite numerous experiments demonstrating the requirement of Fgfr signaling for fiber differentiation, the specific role of Frs2α in this process remained undefined. To address this role, Le-Cre transgenic mice, which express Cre recombinase in the lens placode, mediated the deletion of a LoxP-flanked Frs2 allele in the presumptive lens. The loss of Frs2α in the lens increased lens cell death, reduced the phosphorylation of both Erk1/2 and Akt, and reduced the expression of the Fgfr-induced ETS transcription factors Erm and ER81 as well as several fiber cell-specific proteins. As the phenotypic effects of Frs2α loss appeared at the lens pit stage, 24-36 hours after the initiation of Cre expression, the alterations in lens cell survival and gene expression are likely to be cell-autonomous. However, the possibility remains that the loss of Frs2α in the presumptive lens ectoderm alters the development of the underlying optic vesicle resulting in secondary, non-autonomous loss of lens cell survival and fiber differentiation. Supplying the lens with NT3 and TrkC increased tyrosine phosphorylation of Frs2α as well as phosphorylation of Erk1/2 and Akt in both epithelial cells and fiber cells. Although stimulation of other non-Fgfr RTKs in the lens fails to induce fiber differentiation, the NT3-TrkC transgenic lens epithelium exhibited elongation and expression of fiber cell-specific proteins, consistent with a premature fiber differentiation response. Notably, the absence of Frs2α abolished both elongation and fiber cell-specific protein expression in the lens epithelium of NT3-TrkC transgenic mice. Taken together, these observations demonstrate that tyrosine phosphorylation of Frs2α mediates lens cell survival and fiber cell differentiation.
Although previous investigations exposed mammalian lens epithelial cells to a number of RTK ligands, only Fgfs, and now NT3 in the TrkC-expressing transgenic lens, induce robust elongation and β-crystallin expression in the central lens epithelium. Fgf1-overexpressing lenses phosphorylated Frs2α, Erk and Akt in the prematurely differentiating fiber cells (supplementary material Fig. S1), but both p57KIP2 induction and lens epithelial cell cycle withdrawal was more efficient in Fgf1 than in NT3-TrkC transgenic mice. These differences might result from the constitutive association of Frs2α with Fgfrs, which contrasts with the activation-dependent recruitment of Frs2α to Trk and Ret receptors (Ong et al., 2000; Xu et al., 1998). Therefore, tyrosine phosphorylation of Frs2α by Fgfrs might be more efficient than by other RTKs, such as TrkC.
With respect to the lens, the loss of Frs2 in the ocular surface ectoderm resembled the Le-Cre-mediated loss of Fgfr2. Deficiency in either Frs2α or Fgfr2 led to increased apoptosis, inefficient cell cycle withdrawal, and reduction in lens size. Furthermore, Fgfr2 deletion in the lens inhibited tyrosine phosphorylation of Frs2α as early as E10.5 (Fig. 3). These observations suggest that Fgfr2 exclusively activates Frs2α or, perhaps, that Fgfr2 requires Frs2α for all of its relevant functions. Alternatively, survival and fiber differentiation in the lens might require a certain level of Fgfr activity and Fgfr2 might mediate the majority of this activity. Thus, the loss of Frs2α conceivably reduces the overall activity of the Fgfrs present in the lens (Fgfrs 1-4) (reviewed by Robinson, 2006) to nearly the same extent as the loss of Fgfr2 when Frs2 remains intact. Fgfr signaling in the lens, although attenuated, continues despite the loss of Frs2 as lenses from both the combined deletion of Fgfr1 and Fgfr2 in the ocular surface ectoderm (Garcia et al., 2011) and the lens-specific loss of Fgfr1, Fgfr2 and Fgfr3 (Zhao et al., 2008) display increased apoptosis and decreased fiber differentiation (relative to the Frs2α-deficient lenses).
Although the loss of either Frs2 or Fgfr2 results in similar lens phenotypes, homozygous mutations of the Frs2α Shp2-binding sites (Frs2α2F/2F) compromised eye development earlier and more severely, with ocular defects ranging from microphthalmia to anophthalmia due to arrested lens formation at the induction stage (Gotoh et al., 2004). However, in contrast to the tissue-specific deletion of Frs2 or Fgfr2, the Frs2α2F/2F mutation affected all the tissues in the developing embryo. Therefore, in the Frs2α2F/2F mice, the lens deficits might result from impaired Fgfr signaling in the surface ectoderm or in the optic vesicle or in any other tissues involved in early eye development. Hence, impaired Fgfr signaling in the surface ectoderm prior to E9.0 and/or compromised Fgfr signaling in the formation of the optic vesicle/cup provides an explanation for the anophthalmic phenotypes in the Frs2α2F/2F mice.
Although the Frs2α2F/2F mutation specifically disrupts the association of Shp2 with Frs2α, lens development in the Le-Cre; Frs2αL/L mice proceeded less efficiently than in the mice in which Shp2 was deleted from the ocular surface ectoderm (Pan et al., 2010). Le-Cre; Shp2 mice exhibited normal lens size at E12.5 and only demonstrated decreased lens size and increased apoptosis by E14.5. Furthermore, in contrast to the Frs2α-deficient lenses, the expression of Prox1 and β-crystallin remained unchanged in the Shp2-deficient lenses. This might be explained by the failure of Shp2 deficiency to lower the level of phosphorylated Akt (Pan et al., 2010). In contrast to the Shp2-deficient lenses, the Frs2α-deficient lenses exhibited a dramatic reduction in phosphorylated Akt, suggesting that, consistent with previous findings in fibroblasts (Hadari et al., 2001), the Grb2-binding sites in Frs2α mediate Akt activation.
These studies demonstrate the necessity of Frs2α for Fgfr-mediated lens cell survival and for endogenous Fgfr-induced, as well as ectopic TrkC-induced, fiber cell differentiation. However, the mechanism by which Frs2α facilitates these events remains obscure. The level and duration of Fgfr/Ras/Erk signaling depends on Frs2α in fibroblasts (Hadari et al., 2001) and it is likely that the same is true in lens cells. Despite this, Erk1/2 phosphorylation alone fails to induce a fiber cell differentiation response in explants (Wang et al., 2009). Also, transgenic mice expressing a constitutively active form of Ras in the lens exhibit increased Erk phosphorylation in the lens epithelium without the expression of either filensin (Bfsp1 – Mouse Genome Informatics) or β-crystallins (Burgess et al., 2010; Reneker et al., 2004b), again demonstrating the insufficiency of Erk activation alone to induce a robust fiber differentiation response.
Although similar lens phenotypes result from Fgfr2 or Frs2 loss at the lens placode stage, in several other tissues loss of either of these two molecules causes distinct abnormalities. For example, eyelids never close in Le-Cre; Fgfr2L/L embryos (Garcia et al., 2005), but eyelid formation completes in Le-Cre; Frs2αL/L mice, demonstrating that Frs2α-independent signaling of Fgfr2 is sufficient for eyelid formation. Likewise, mice with conditional deletion of Frs2 in the ureteric bud possess mild kidney defects whereas mutations in the Frs2α-binding site of Fgfr2 (Fgfr2LR/LR) retain normal kidneys. Mice with combined Fgfr2LR/LR mutation and Frs2 loss exhibited more severe ureteric branching deficits compared with the individual mutations, suggesting that although both Frs2α and Fgfr2 participate in the development of the ureteric bud, they act separately and additively (Sims-Lucas et al., 2011). However, whereas Fgfrs exclusively activate Frs2α in the lens, the ureteric bud expresses the Ret receptor and the metanephrogenic mesenchyme expresses the Ret ligand GDNF (Schuchardt et al., 1996), leading to Fgfr2-independent activation of Frs2α in the kidney.
Frs2α is likely to mediate the balance of Erk and Akt phosphorylation required for appropriate cell survival and fiber differentiation in the lens, explaining why Fgfr signaling leads to a fiber cell differentiation response, whereas the other RTKs normally expressed in the lens fail to do so. Although Fgfrs activate Frs2α, other RTKs have the potential to affect Frs2α signaling negatively through their ability to increase Erk phosphorylation transiently. Activated Erk phosphorylates Frs2α on several threonine residues, thereby inhibiting Frs2α tyrosine phosphorylation (Lax et al., 2002; Wu et al., 2003; Zhou et al., 2009). Therefore, although all RTKs expressed have the ability to indirectly suppress Frs2α activation through increased Erk activation, only Fgfrs can directly activate Frs2α by tyrosine phosphorylation in the lens. As a further illustration of this balance, during lens induction the attenuation of Ras signaling by Nf1 prevents the induction of Spry2. In homozygous Nf1 mutant embryos, despite the early increased Erk phosphorylation, Ras-induced Spry2 suppressed Erk phosphorylation during the lens vesicle stage, leading to aphakia (Carbe and Zhang, 2011).
Frs2α-mediated activation of Akt and Erk1/2 ultimately leads to a cascade of post-translational protein modifications, epigenetic changes in chromatin structure, and altered transcriptional activity. These changes are likely to involve members of the Pea3 subfamily of Ets transcription factors [Erm, Er81 and Pea3 (Etv4)]. Efficient induction of the Erm and Er81 by Fgfr signaling in the lens requires Frs2α. Knocking down the expression of the Pea3 and Erm orthologs in developing zebrafish mimicked the loss of Fgf8, and these Ets-subfamily members directly activate the transcription of the Fgf-responsive gene Dusp6 (Znosko et al., 2010). Precisely how induction of these Ets subfamily members activates the transcriptional network leading to cellular differentiation in the lens remains an important avenue of investigation for identifying targets of RTK signaling controlling differentiation in the lens and other tissues.
Supplementary Material
Acknowledgments
The authors acknowledge Dr Ruth Ashery-Padan for the Le-Cre transgenic mice; Dr David Ornitz for the Fgfr2L/L mice; Dr George Yancopoulos for the rat TrkC cDNA; and Dr Sam Zigler for antibodies to β-crystallins. We also thank Dr David C. Beebe, Blake R. Chaffee, Melissa R. Leonard, Benjamin D. Schwarz and Adam S. LeFever for critical reading of the manuscript; and Dr Richard Edelmann and the Miami University Center for Advanced Microscopy and Imaging (NSF-MRI award DBI-0821211) for their assistance.
Footnotes
Funding
This work was supported by a grant from the National Institutes of Health [R01EY012995 to M.L.R.]. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.081737/-/DC1
References
- Ashery-Padan R., Marquardt T., Zhou X., Gruss P. (2000). Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 14, 2701–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe D. C., Feagans D. E., Jebens H. A. (1980). Lentropin: a factor in vitreous humor which promotes lens fiber cell differentiation. Proc. Natl. Acad. Sci. USA 77, 490–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe D. C., Silver M. H., Belcher K. S., Van Wyk J. J., Svoboda M. E., Zelenka P. S. (1987). Lentropin, a protein that controls lens fiber formation, is related functionally and immunologically to the insulin-like growth factors. Proc. Natl. Acad. Sci. USA 84, 2327–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess D., Zhang Y., Siefker E., Vaca R., Kuracha M. R., Reneker L., Overbeek P. A., Govindarajan V. (2010). Activated Ras alters lens and corneal development through induction of distinct downstream targets. BMC Dev. Biol. 10, 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbe C., Zhang X. (2011). Lens induction requires attenuation of ERK signaling by Nf1. Hum. Mol. Genet. 20, 1315–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy L., Barry P., Shaw C., Duffy J., Kennedy S. (1998). Platelet derived growth factor and fibroblast growth factor basic levels in the vitreous of patients with vitreoretinal disorders. Br. J. Ophthalmol. 82, 181–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. A., Son A. I., Komlos D., Sun Y., Kleiman N. J., Zhou R. (2008). Loss of ephrin-A5 function disrupts lens fiber cell packing and leads to cataract. Proc. Natl. Acad. Sci. USA 105, 16620–16625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degoutin J., Vigny M., Gouzi J. Y. (2007). ALK activation induces Shc and FRS2 recruitment: Signaling and phenotypic outcomes in PC12 cells differentiation. FEBS Lett. 581, 727–734 [DOI] [PubMed] [Google Scholar]
- Faber S. C., Robinson M. L., Makarenkova H. P., Lang R. A. (2002). Bmp signaling is required for development of primary lens fiber cells. Development 129, 3727–3737 [DOI] [PubMed] [Google Scholar]
- Garcia C. M., Yu K., Zhao H., Ashery-Padan R., Ornitz D. M., Robinson M. L., Beebe D. C. (2005). Signaling through FGF receptor-2 is required for lens cell survival and for withdrawal from the cell cycle during lens fiber cell differentiation. Dev. Dyn. 233, 516–527 [DOI] [PubMed] [Google Scholar]
- Garcia C. M., Huang J., Madakashira B. P., Liu Y., Rajagopal R., Dattilo L., Robinson M. L., Beebe D. C. (2011). The function of FGF signaling in the lens placode. Dev. Biol. 351, 176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N. (2008). Regulation of growth factor signaling by FRS2 family docking/scaffold adaptor proteins. Cancer Sci. 99, 1319–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh N., Ito M., Yamamoto S., Yoshino I., Song N., Wang Y., Lax I., Schlessinger J., Shibuya M., Lang R. A. (2004). Tyrosine phosphorylation sites on FRS2alpha responsible for Shp2 recruitment are critical for induction of lens and retina. Proc. Natl. Acad. Sci. USA 101, 17144–17149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadari Y. R., Gotoh N., Kouhara H., Lax I., Schlessinger J. (2001). Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways. Proc. Natl. Acad. Sci. USA 98, 8578–8583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland M. E., Mrock L. K. (2000). Differentiation of chick lens epithelial cells: involvement of the epidermal growth factor receptor and endogenous ligand. Invest. Ophthalmol. Vis. Sci. 41, 183–190 [PubMed] [Google Scholar]
- Iyengar L., Patkunanathan B., Lynch O. T., McAvoy J. W., Rasko J. E., Lovicu F. J. (2006). Aqueous humour- and growth factor-induced lens cell proliferation is dependent on MAPK/ERK1/2 and Akt/PI3-K signalling. Exp. Eye Res. 83, 667–678 [DOI] [PubMed] [Google Scholar]
- Katsura Y., Okano T., Noritake M., Kosano H., Nishigori H., Kado S., Matsuoka T. (1998). Hepatocyte growth factor in vitreous fluid of patients with proliferative diabetic retinopathy and other retinal disorders. Diabetes Care 21, 1759–1763 [DOI] [PubMed] [Google Scholar]
- Kouhara H., Hadari Y. R., Spivak-Kroizman T., Schilling J., Bar-Sagi D., Lax I., Schlessinger J. (1997). A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89, 693–702 [DOI] [PubMed] [Google Scholar]
- Kurokawa K., Iwashita T., Murakami H., Hayashi H., Kawai K., Takahashi M. (2001). Identification of SNT/FRS2 docking site on RET receptor tyrosine kinase and its role for signal transduction. Oncogene 20, 1929–1938 [DOI] [PubMed] [Google Scholar]
- Lavail M. M., Nishikawa S., Duncan J. L., Yang H., Matthes M. T., Yasumura D., Vollrath D., Overbeek P. A., Ash J. D., Robinson M. L. (2008). Sustained delivery of NT-3 from lens fiber cells in transgenic mice reveals specificity of neuroprotection in retinal degenerations. J. Comp. Neurol. 511, 724–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lax I., Wong A., Lamothe B., Lee A., Frost A., Hawes J., Schlessinger J. (2002). The docking protein FRS2alpha controls a MAP kinase-mediated negative feedback mechanism for signaling by FGF receptors. Mol. Cell 10, 709–719 [DOI] [PubMed] [Google Scholar]
- Lemmon M. A., Schlessinger J. (2010). Cell signaling by receptor tyrosine kinases. Cell 141, 1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovicu F. J., Overbeek P. A. (1998). Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development 125, 3365–3377 [DOI] [PubMed] [Google Scholar]
- Lovicu F. J., McAvoy J. W. (2008) Epithelial explants and their application to study developmental processes in the lens. In Animal Models in Eye Research (ed. Tsonis P. A.), pp.134–147 San Diego: Academic Press; [Google Scholar]
- Majima K. (1997). Presence of growth factor in human vitreous. Ophthalmologica 211, 226–228 [DOI] [PubMed] [Google Scholar]
- McAvoy J. W., Chamberlain C. G. (1990). Growth factors in the eye. Prog. Growth Factor Res. 2, 29–43 [DOI] [PubMed] [Google Scholar]
- McDougall K., Kubu C., Verdi J. M., Meakin S. O. (2001). Developmental expression patterns of the signaling adapters FRS-2 and FRS-3 during early embryogenesis. Mech. Dev. 103, 145–148 [DOI] [PubMed] [Google Scholar]
- Meakin S. O., MacDonald J. I., Gryz E. A., Kubu C. J., Verdi J. M. (1999). The signaling adapter FRS-2 competes with Shc for binding to the nerve growth factor receptor TrkA. A model for discriminating proliferation and differentiation. J. Biol. Chem. 274, 9861–9870 [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. (1989). Improved retroviral vectors for gene transfer and expression. Biotechniques 7, 980-982, 984-986, 989-990 [PMC free article] [PubMed] [Google Scholar]
- Ong S. H., Guy G. R., Hadari Y. R., Laks S., Gotoh N., Schlessinger J., Lax I. (2000). FRS2 proteins recruit intracellular signaling pathways by binding to diverse targets on fibroblast growth factor and nerve growth factor receptors. Mol. Cell. Biol. 20, 979–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S. H., Hadari Y. R., Gotoh N., Guy G. R., Schlessinger J., Lax I. (2001). Stimulation of phosphatidylinositol 3-kinase by fibroblast growth factor receptors is mediated by coordinated recruitment of multiple docking proteins. Proc. Natl. Acad. Sci. USA 98, 6074–6079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Carbe C., Powers A., Feng G. S., Zhang X. (2010). Sprouty2-modulated Kras signaling rescues Shp2 deficiency during lens and lacrimal gland development. Development 137, 1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plageman T. F., Jr, Zacharias A. L., Gage P. J., Lang R. A. (2011). Shroom3 and a Pitx2-N-cadherin pathway function cooperatively to generate asymmetric cell shape changes during gut morphogenesis. Dev. Biol. 357, 227–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reneker L. W., Overbeek P. A. (1996). Lens-specific expression of PDGF-A alters lens growth and development. Dev. Biol. 180, 554–565 [DOI] [PubMed] [Google Scholar]
- Reneker L. W., Chen Q., Bloch A., Xie L., Schuster G., Overbeek P. A. (2004a). Chick delta1-crystallin enhancer influences mouse alphaA-crystallin promoter activity in transgenic mice. Invest. Ophthalmol. Vis. Sci. 45, 4083–4090 [DOI] [PubMed] [Google Scholar]
- Reneker L. W., Xie L., Xu L., Govindarajan V., Overbeek P. A. (2004b). Activated Ras induces lens epithelial cell hyperplasia but not premature differentiation. Int. J. Dev. Biol. 48, 879–888 [DOI] [PubMed] [Google Scholar]
- Robinson M. L. (2006). An essential role for FGF receptor signaling in lens development. Semin. Cell Dev. Biol. 17, 726–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. L., Overbeek P. A., Verran D. J., Grizzle W. E., Stockard C. R., Friesel R., Maciag T., Thompson J. A. (1995). Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development 121, 505–514 [DOI] [PubMed] [Google Scholar]
- Robinson M. L., Ohtaka-Maruyama C., Chan C. C., Jamieson S., Dickson C., Overbeek P. A., Chepelinsky A. B. (1998). Disregulation of ocular morphogenesis by lens-specific expression of FGF-3/int-2 in transgenic mice. Dev. Biol. 198, 13–31 [DOI] [PubMed] [Google Scholar]
- Saint-Geniez M., Kurihara T., D’Amore P. A. (2009). Role of cell and matrix-bound VEGF isoforms in lens development. Invest. Ophthalmol. Vis. Sci. 50, 311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuchardt A., D’Agati V., Pachnis V., Costantini F. (1996). Renal agenesis and hypodysplasia in ret-k– mutant mice result from defects in ureteric bud development. Development 122, 1919–1929 [DOI] [PubMed] [Google Scholar]
- Shui Y. B., Wang X., Hu J. S., Wang S. P., Garcia C. M., Potts J. D., Sharma Y., Beebe D. C. (2003). Vascular endothelial growth factor expression and signaling in the lens. Invest. Ophthalmol. Vis. Sci. 44, 3911–3919 [DOI] [PubMed] [Google Scholar]
- Sims-Lucas S., Cusack B., Eswarakumar V. P., Zhang J., Wang F., Bates C. M. (2011). Independent roles of Fgfr2 and Frs2alpha in ureteric epithelium. Development 138, 1275–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Stump R., McAvoy J. W., Lovicu F. J. (2009). MAPK/ERK1/2 and PI3-kinase signalling pathways are required for vitreous-induced lens fibre cell differentiation. Exp. Eye Res. 88, 293–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., McAvoy J. W., Lovicu F. J. (2010). Growth factor signaling in vitreous humor-induced lens fiber differentiation. Invest. Ophthalmol. Vis. Sci. 51, 3599–3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Chen Z., Ullrich A. (2003). EGFR and FGFR signaling through FRS2 is subject to negative feedback control by ERK1/2. Biol. Chem. 384, 1215–1226 [DOI] [PubMed] [Google Scholar]
- Xie L., Chen H., Overbeek P. A., Reneker L. W. (2007). Elevated insulin signaling disrupts the growth and differentiation pattern of the mouse lens. Mol. Vis. 13, 397–407 [PMC free article] [PubMed] [Google Scholar]
- Xu H., Lee K. W., Goldfarb M. (1998). Novel recognition motif on fibroblast growth factor receptor mediates direct association and activation of SNT adapter proteins. J. Biol. Chem. 273, 17987–17990 [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Yoshino I., Shimazaki T., Murohashi M., Hevner R. F., Lax I., Okano H., Shibuya M., Schlessinger J., Gotoh N. (2005). Essential role of Shp2-binding sites on FRS2alpha for corticogenesis and for FGF2-dependent proliferation of neural progenitor cells. Proc. Natl. Acad. Sci. USA 102, 15983–15988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Xu J., Liu Z., Sosic D., Shao J., Olson E. N., Towler D. A., Ornitz D. M. (2003). Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development 130, 3063–3074 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang J., Lin Y., Lan Y., Lin C., Xuan J. W., Shen M. M., McKeehan W. L., Greenberg N. M., Wang F. (2008). Role of epithelial cell fibroblast growth factor receptor substrate 2alpha in prostate development, regeneration and tumorigenesis. Development 135, 775–784 [DOI] [PubMed] [Google Scholar]
- Zhao H., Yang T., Madakashira B. P., Thiels C. A., Bechtle C. A., Garcia C. M., Zhang H., Yu K., Ornitz D. M., Beebe D. C., et al. (2008). Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev. Biol. 318, 276–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Feng X., Wu Y., Benge J., Zhang Z., Chen Z. (2009). FGF-receptor substrate 2 functions as a molecular sensor integrating external regulatory signals into the FGF pathway. Cell Res. 19, 1165–1177 [DOI] [PubMed] [Google Scholar]
- Znosko W. A., Yu S., Thomas K., Molina G. A., Li C., Tsang W., Dawid I. B., Moon A. M., Tsang M. (2010). Overlapping functions of Pea3 ETS transcription factors in FGF signaling during zebrafish development. Dev. Biol. 342, 11–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.