Abstract
Interneuronal subtype diversity lies at the heart of the distinct molecular properties and synaptic connections that shape the formation of the neuronal circuits that are necessary for the complex spatial and temporal processing of sensory information. Here, we investigate the role of Irx6, a member of the Iroquois homeodomain transcription factor family, in regulating the development of retinal bipolar interneurons. Using a knock-in reporter approach, we show that, in the mouse retina, Irx6 is expressed in type 2 and 3a OFF bipolar interneurons and is required for the expression of cell type-specific markers in these cells, likely through direct transcriptional regulation. In Irx6 mutant mice, presumptive type 3a bipolar cells exhibit an expansion of their axonal projection domain to the entire OFF region of the inner plexiform layer, and adopt molecular features of both type 2 and 3a bipolar cells, highlighted by the ectopic upregulation of neurokinin 3 receptor (Nk3r) and Vsx1. These findings reveal Irx6 as a key regulator of type 3a bipolar cell identity that prevents these cells from adopting characteristic features of type 2 bipolar cells. Analysis of the Irx6;Vsx1 double null retina suggests that the terminal differentiation of type 2 bipolar cells is dependent on the combined expression of the transcription factors Irx6 and Vsx1, but also points to the existence of Irx6;Vsx1-independent mechanisms in regulating OFF bipolar subtype-specific gene expression. This work provides insight into the generation of neuronal subtypes by revealing a mechanism in which opposing, yet interdependent, transcription factors regulate subtype identity.
Keywords: Retina, Transcription factor, Bipolar interneuron, Cell type diversity, Mouse
INTRODUCTION
The simple organization of the retina into five major neuronal classes makes it an excellent model for studying the developmental mechanisms that underlie cell type diversity (Ohsawa and Kageyama, 2008). The simple neuronal cell class organization of the retina, however, is accompanied by high degree of cell type heterogeneity. In mammals, the five major retinal neuronal classes give rise to 55 morphological cell types (Masland, 2001), which comprise functionally diverse circuits within the retina (Gollisch and Meister, 2010). The current literature defines 11 bipolar cell types based on morphology, gene expression and axonal tiling properties (Wässle et al., 2009) (see Fig. 1).
Fig. 1.
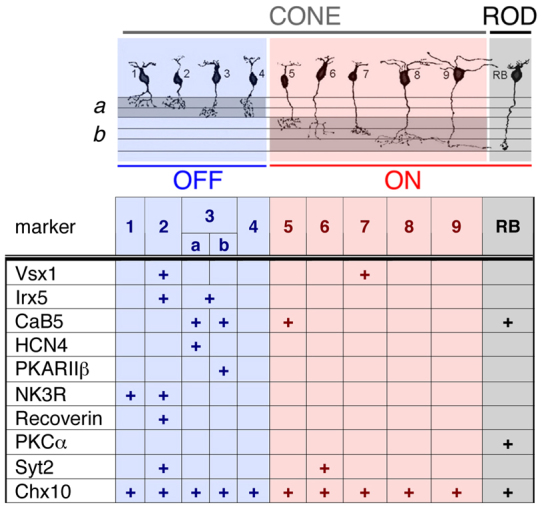
Bipolar cell type-specific markers used in this study. Morphological cell types [adapted with permission from Ghosh et al. (Ghosh et al., 2004)]. Markers used are: Vsx1 (Chow et al., 2001; Chow et al., 2004; Shi et al., 2011); Irx5 (Cheng et al., 2005); CaB5 (Haverkamp et al., 2003; Ghosh et al., 2004); Hcn4 and PKARII-β (Matargua et al 2007); Nk3r (Haverkamp et al., 2003; Ghosh et al., 2004); recoverin (Haverkamp et al., 2003); PKCα (Negishi et al., 1988; Haverkamp and Wassle, 2000); Syt2 (Wassle, 2009); Chx10 (Burmeister et al., 1996).
Combinatorial transcription factor coding based on the differential expression of homeodomain and basic helix-loop-helix transcription factors has been identified as a common mechanism underlying neuronal cell type diversity (Fode et al., 2000; Guillemot, 2007; Ma, 2006; Ohsawa and Kageyama, 2008; Shirasaki and Pfaff, 2002). Several homeodomain and basic helix-loop-helix transcription factors are important for the development and homeostasis of distinct retinal bipolar cell subtypes. The development of type 2 and 3 OFF bipolar cells is regulated by the overlapping requirements of several transcription factors (Cheng et al., 2005; Chow et al., 2004; Feng et al., 2006; Kerschensteiner et al., 2008; Ohtoshi et al., 2004). In mice lacking Vsx1 or Irx5 homeobox gene function, type 2 and 3 bipolar cells are specified but have defects in their terminal differentiation marked by reduced expression of type 2 and 3 cell-specific markers (Chow et al., 2004; Ohtoshi et al., 2004; Shi et al., 2012; Cheng et al., 2005). In Vsx1- mutant mice, expression of the type 2 cell markers recoverin (Rcvrn), neurokinin 3 receptor (Nk3r; Tacr3 – Mouse Genome Informatics) and Neto1 is reduced (Chow et al., 2004; Ohtoshi et al., 2004), type 3 cells have reduced Cabp5 immunolabeling in the axon terminal region (Chow et al., 2004), and there is a reduction in the total number of Hcn4-positive type 3a bipolar cells (Shi et al., 2012). In Irx5 mutants, type 2 bipolar cells have reduced levels of recoverin immunolabeling, but normal levels of Nk3r immunolabeling, and type 3 cells exhibit reduced levels of Cabp5 within their axon terminals (Cheng et al., 2005). Mice lacking the basic helix-loop-helix transcription factor Bhlhb5 (Bhlhb22 – Mouse Genome Informatics) have a reduction of recoverin and Nk3r immunolabeled type 2 cells, and also have a reduction in the total number of Vsx1-labeled bipolar cells (Feng et al., 2006). Although Vsx1 is not necessary for Irx5 bipolar cell expression (Cheng et al., 2005), it negatively regulates its own expression (Chow et al., 2004) and is a positive regulator of Bhlhb5 (M.Z. and R.L.C., unpublished). Conversely, Bhlhb5 functions as a positive regulator of Vsx1 in putative type 2 cells (Feng et al., 2006).
In the present study, we investigate the role of the Iroquois family member Irx6 in retinal development. The Iroquois (Irx) gene family encodes transcription factors that harbor a 63 amino acid TALE family homeodomain and a 9 amino acid conserved motif outside of the homeodomain known as the Iro box (Bilioni et al., 2005). Mammals have six Irx genes that exist in two clusters: the IrxA cluster (Irx1, Irx2 and Irx4) and IrxB cluster (Irx3, Irx5 and Irx6) (Gómez-Skarmeta and Modolell, 2002). Studies in both Drosophila and vertebrate models have implicated Iroquois genes in axon targeting events (Grillenzoni et al., 1998; Jin et al., 2003; Sato et al., 2006).
We show that Irx6 is expressed in type 2 and 3a bipolar cells where it is required for the expression of cell type-specific markers, including Bhlhb5. Presumptive type 3a cells in the Irx6 mutant stratify correctly to the OFF sublamina of the inner plexiform layer, but instead of the normal restricted segregation of their axon termini to sublamina 2, they can stratify within both OFF sublamina 1 and 2. Furthermore, these cells adopt molecular features of both type 2 and 3a cells, highlighted by the ectopic expression of Vsx1 and Nk3r. These defects in type 3a cell development in the Irx6 mutant appear to be independent of Vsx1 function. Our findings reveal Irx6 as a core regulator of type 3a bipolar cell identity that prevents them from adopting features characteristic of type 2 bipolar cells, and suggest that terminal differentiation of type 2 bipolar cells is dependent on the combined activity of Vsx1 and Irx6.
MATERIALS AND METHODS
Generation of Irx6lacZ knock-in mice
Gene targeting was performed in R1 (Svj129) embryonic stem (ES) cells. The targeting construct was generated by screening an M13 mouse R1 genomic library (Stratagene) with probes for Irx6 (supplementary material Fig. S1). Chimeric mice were generated by blastocyst injection of homologous recombinant ES cells (Scripps Research Institute Transgenic Core Facility), and the resulting mice were bred with the 129S1/SvImJ mouse strain (Jackson Laboratory). All experiments on mice were conducted with the approval of the University of Victoria Animal Care Committee.
Immunohistochemistry and microscopy
Unless otherwise noted, all retinas were taken from 2- to 3-month-old mice, and central sections of the retina were used for imaging. Eyes were fixed in 4% paraformaldehyde in PBS for 1 hour (on ice) or for 20 minutes (room temperature), cryoprotected in 30% sucrose, embedded in Tissue-Tek OCT (Sakura Finetek), and cryosectioned at 14 μm. Primary antibody information is given in supplementary material Table S1. Secondary antibodies were conjugated to Alexa dyes (Invitrogen) or Cy3 (anti-guinea pig; Jackson ImmunoResearch). Draq5 (Alexis Biochemicals) was used for nuclear labeling. For detection of β-galactosidase activity, sections were incubated in 1 mg/ml bromo-chloro-indolyl-galactopyranoside (X-gal) overnight at 37°C, as previously described (Mombaerts et al., 1996). Images were taken on a confocal microscope (Nikon Eclipse TE2000-U C1) and processed using Adobe Photoshop CS3. Cell counting and measurement of the fluorescence intensity within a 100 × 10 μm region of interest was carried out using EZ-C1 Version 3.60 software (Nikon Instruments). Data are presented as mean±s.e.m. Statistical analysis was performed using Student’s t-test.
PCR genotyping
The genomic DNA was prepared from mice ear clips. The following primers were used for Irx6 genotyping: IRX6-PF (GGCGGCCTGCTCCTGCAGCCC), IRX6-IR (GGATGTGCTGCCATACGGGTGT) and LacZR (AGATGAAACGCCGAGTTAACGC). The Vsx1 mutant mouse (AltB5 or Δ1-4 line) has been previously described (Chow et al., 2004).
Luciferase assay
HEK cells were transfected and assayed 24 hours later for luciferase activity with Dual-Glo luciferase assay system (Promega) according to the manufacturer’s protocol. Luciferase activity was measured by a Wallac 1420 Multilabel Counter (PerkinElmer). The vectors used in the assay are described in supplementary material Table S2.
Electroretinography
Retinal function of mice from four experimental groups (Irx6+/lacZ;Vsx1+/AltB5, Vsx1AltB5/AltB5, Irx6+/lacZ and Irx6lacZ/lacZ) was studied using the full field electroretinogram (ERG) as previously described (Alvarez et al., 2007). Light stimulation (10 μsecond duration flashes), signal amplification (0.3-300 Hz bandpass) and data acquisition were provided by the Espion E2 system (Diagnosys). Scotopic intensity responses consisted of single flash presentations at 19 increasing strengths from –5.22 to 2.86 log cds/m2. Ten minutes after transition from dark to light (30 cd/m2 white light background) adaptation, photopic intensity responses (30 cd/m2 background light) were recorded at 11 increasing flash strengths ranging from –1.6 to 2.9 log cds/m2, followed by OFF responses obtained with a square wave stimulus of 562.5 cd/m2, lasting 800 mseconds.
RESULTS
Irx6 reporter expression in the developing and mature mouse retina
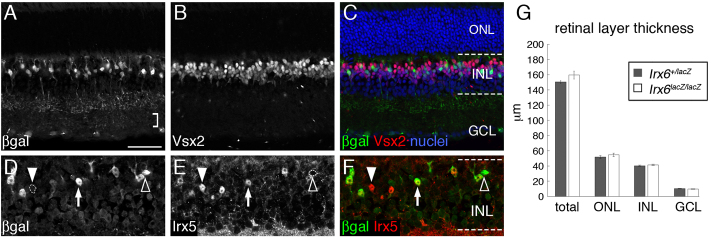
To investigate the role of Irx6 in mouse retinal development, we generated a knock-in lacZ reporter allele (Irx6lacZ) in which Irx6 exon 1 and part of exon 2 was replaced by the lacZ gene (supplementary material Fig. S1). Whole-mount X-gal detection of the lacZ gene product, β-galactosidase in Irx6+/lacZ mice at embryonic day 12.5 (E12.5) revealed scattered expression of the reporter throughout the embryo (Fig. 2B). X-gal staining was first detected in the central ganglion cell region of the retina in Irx6+/lacZ mice beginning at embryonic day 13.5 (E13.5) (Fig. 2D). Owing to the lack of a suitable antibody, we were unable to confirm reporter expression using Irx6 immunohistology. However, the onset and localization of the reporter expression is consistent with the previously described expression of Irx6 mRNA in the retina (Cohen et al., 2000; Erkman et al., 2000) (supplementary material Fig. S1), suggesting that the knock-in lacZ reporter faithfully recapitulates the normal Irx6 expression pattern. By E16.5, there was strong expression of the Irx6lacZ reporter in the developing retinal ganglion cell layer and optic nerve region (Fig. 2E). At postnatal day zero (P0), X-gal staining persisted in the ganglion cell layer, and was also observed in the apical margin region of the retina (Fig. 2G). By P7, reporter expression was detected in a number of cells within the outer half of the inner nuclear layer and in the ganglion cell layer (Fig. 2H).
Fig. 2.
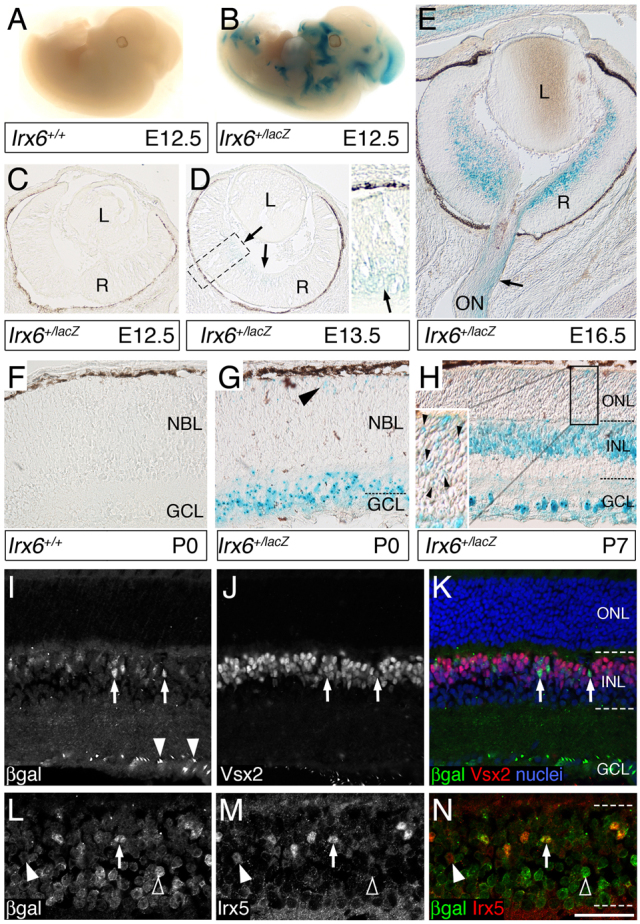
Expression of the Irx6:βgal reporter during development of the mouse. (A-H) X-gal staining results. (A,B) E12.5 wild-type (A) and Irx6+/lacZ (B) mouse, showing expression of Irx6:βgal in the midbrain. (C,D) Retinal section from an Irx6+/lacZ mouse at E12.5 (C) and E13.5 (D); arrows in D indicate faint X-gal precipitate. (E) E16.5 retinal section from an Irx6+/lacZ mouse showing staining down the optic nerve. (F-H) Wild type (F) and Irx6+/lacZ (G,H) retina at P0 (F,G) and P7 (H), with arrowheads (G and H, inset) indicating staining in the newly formed ONL. (I,J) Immunohistochemistry of an Irx6+/lacZ retina showing Irx6:βgal expression (I, arrows) and Vsx2/Chx10 expression (J, arrows). Arrowheads in I indicate Irx6:βgal expression in the GCL. (K) Colocalization of Irx6:βgal and Vsx2/Chx10 (arrows). The nuclear stain is Draq5. (L,M) Irx6:βgal (L, arrow) and Irx5 (M, closed arrowhead) expression in the Irx6+/lacZ retina. (N) An Irx5-positive, Irx6:βgal-negative cell (closed arrowhead); an Irx5-positive, Irx6:βgal-positive cell (arrow); and an Irx5-negative, Irx6:βgal-positive cell (open arrowhead). L, lens; R, retina; ON, optic nerve; NBL, neuroblast layer; GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. Scale bar: 3 mm in A,B; 60 μm in C,D; 100 μm in E; 50 μm in F-K; 25 μm in L-N.
In the mature Irx6+/lacZ retina, immunolabeling of the β-galactosidase reporter (Irx6:βgal) together with Vsx2/Chx10, a pan-bipolar transcription factor (Burmeister et al., 1996), indicated Irx6 expression in a subset of bipolar cells (Fig. 2I-K). The Irx6 homologue Irx5, was co-expressed in a subset of Irx6:βgal- immunolabeled bipolar cells (Fig. 2L-N). Not all Irx5-positive cells in the inner nuclear layer co-labeled with Irx6:βgal, and not all Irx6:βgal positive cells co-labeled with Irx5 (Fig. 2L-N). Additionally, Irx6:βgal co-immunolabeled with a subset of cells expressing the ganglion cell marker Brn3b (Pou4f2 – Mouse, Genome Informatics) (supplementary material Fig. S2A). These results indicate that Irx6 is expressed in a subset of retinal ganglion cells and bipolar cells. Irx6 also appeared to be transiently expressed in newly born photoreceptor cells (Fig. 2H, inset), but not in adult photoreceptor cells (Fig. 2I).
Irx6lacZ is expressed in a subset of OFF bipolar cells
To determine the identity of the bipolar cell types that express the Irx6lacZ reporter, retinal sections of Irx6+/lacZ mice were co- immunolabeled for Irx6:βgal and a series of bipolar cell type specific markers (Figs 1, 3). Irx6:βgal co-immunolabeled in a subset of cells expressing two type 2 cell markers, Nk3r (Fig. 3A-C) and recoverin (Fig. 3D-F) (Ghosh et al., 2004; Haverkamp and Wässle, 2000; Milam et al., 1993). Irx6:βgal labeling was also detected in a subset of cells immunolabeled for the type 3 and 5, and the rod bipolar cell marker Cabp5 (Fig. 3G-I) (Ghosh et al., 2004; Haverkamp et al., 2003). Type 3 cells are defined by their morphology (Ghosh et al., 2004), but can be further categorized into two distinct cell types (type 3a and type 3b) based on their differential immunolabeling for Hcn4 and PKARIIβ (Prkar2b – Mouse Genome Informatics), respectively (Mataruga et al., 2007). Irx6:βgal was detected in type 3a cells co-immunolabeled with Hcn4 (Fig. 3J-L), but not in PKARIIβ-positive type 3b cells (Fig. 3M-O). Furthermore, Irx6:βgal expression was not detected in type 4 cells immunolabeled for calsenilin (Haverkamp et al., 2008) (supplementary material Fig. S2B), or in rod bipolar cells identified by PKCα immunolabeling (Haverkamp and Wässle, 2000; Negishi et al., 1988) (Fig. 3P-R). In summary, within the bipolar cell population of the mature retina, Irx6 knock-in reporter gene expression is observed in type 2 and 3a bipolar cells.
Fig. 3.
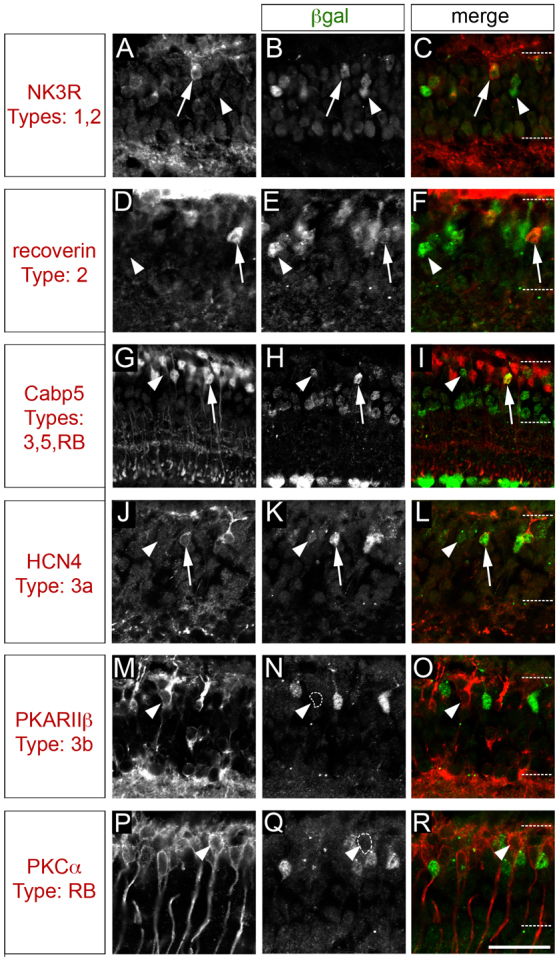
The Irx6:βgal reporter is expressed in a subset of retinal bipolar cells. (A-F) In the mature Irx6+/lacZ retina, the Irx6:βgal reporter is expressed in a subset of Nk3r- (A-C, arrow) and recoverin- (D-F, arrow) positive cells. (G-I) Irx6:βgal is also expressed in a subset of Cabp5 cells (arrows). (J-O) Irx6:βgal colocalizes with the type 3a marker Hcn4 (J-L, arrows), but not with the type 3b marker PKARIIβ (M-O). Arrowheads in A-O indicate βgal-labeled cells that are not co-labeled with the respective bipolar marker. (P-R) There is no colocalization between PKCα expression and Irx6:βgal expression. Arrowhead indicates a PKCα-positive cell that does not colabel with Irx6:βgal. Scale bar: 25 μm.
Loss of Irx6 does not disrupt retinal morphology or number of retinal ganglion cells
To examine the role of Irx6 during retinal development, we next examined the phenotype of Irx6lacZ/lacZ mice. Loss of Irx6 did not disrupt the gross morphology and layering of the retina (Fig. 4C,G). Irx6:βgal immunolabeling was evident in a subset of Vsx2-positive bipolar cells and ganglion cells (Fig. 4A-C) and was visibly more robust than in the Irx6+/lacZ retina (Fig. 2I). In addition to the presence of Irx6:βgal in the OFF sublamina of the inner plexiform layer, immunolabeling was also detected in the ON sublamina of the Irx6lacZ/lacZ retina (Fig. 4A; see below). Similar to the Irx6+/lacZ retina, Irx5 and Irx6:βgal immunolabeling exhibited overlapping and non-overlapping expression patterns in the Irx6lacZ/lacZ retina (Fig. 4D-F).
Fig. 4.
Irx6lacZ/lacZ mice have normal retinal morphology. (A) Immunolabeling for βgal in the Irx6lacZ/lacZ mouse. Bracket indicates immunolabeling in the ON sublamina of the retina. (B) Immunolabeling for Vsx2. (C) Merged image of A and B, including Draq5 labeling of nuclei in the Irx6lacZ/lacZ mouse. (D-F) Irx6:βgal (D) and Irx5 (E) expression in the Irx6lacZ/lacZ mouse, showing Irx6:βgal expression in a subset of Irx5-positive bipolar cells (F); an Irx6:βgal-negative, Irx5-positive (closed arrowhead) bipolar cell; Irx6:βgal-positive, Irx5-positive (arrow) bipolar cell; and Irx6:βgal-positive, Irx5-negative (open arrowhead) bipolar cell. INL, inner nuclear layer; ONL, outer nuclear layer; GCL, ganglion cell layer. Scale bar: 50 μm in A-C; 25 μm in D-F. (G) Retinal thickness in Irx6+/lacZ and Irx6lacZ/lacZ mice (n=6 retinas, each genotype). Data are mean±s.e.m.
Since reporter expression indicated that Irx6 is expressed in retinal ganglion cells (Fig. 2E,G,H; supplementary material Fig. S2A), we investigated whether ganglion cell number was affected in the Irx6lacZ/lacZ retina. We did not observe a difference in the number of Brn3b positive ganglion cells between the Irx6+/lacZ (10.3 cells/100 μm ±0.6) and Irx6lacZ/lacZ (10.6 cells/100 μm ±0.4) retina (n=3 mice).
Irx6 is required for the terminal differentiation of OFF bipolar cells
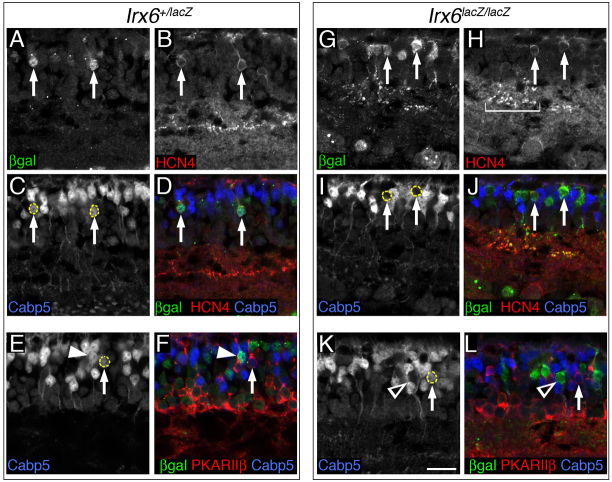
Given that the Irx6:βgal reporter is expressed in a subset of OFF bipolar cells, we next examined whether the formation of these cell types was affected in Irx6lacZ/lacZ mice. Loss of Irx6 led to the complete loss of recoverin in type 2 cells (Fig. 5A,B). Synaptotagmin II (Syt2) expression, which labels type 2 and 6 bipolar cells (Fox and Sanes, 2007; Wässle et al., 2009), was greatly reduced in the Irx6lacZ/lacZ retina (Fig. 5C,D). Reduced Syt2 immunolabeling of axon termini in the inner plexiform layer was observed for both putative type 2 and 6 bipolar cells (Fig. 5C,D). Axon terminals of Syt2-labeled putative type 6 cells in Irx6lacZ/lacZ mice co-immunolabeled with Irx6:βgal (supplementary material Fig. S3), suggesting that the defects in this cell type are cell-autonomous. In contrast to the reduction in recoverin and Syt2 expression, Nk3r immunolabeling of type 1 and 2 cells was still present in the Irx6lacZ/lacZ retina (Fig. 5E,F).
Fig. 5.
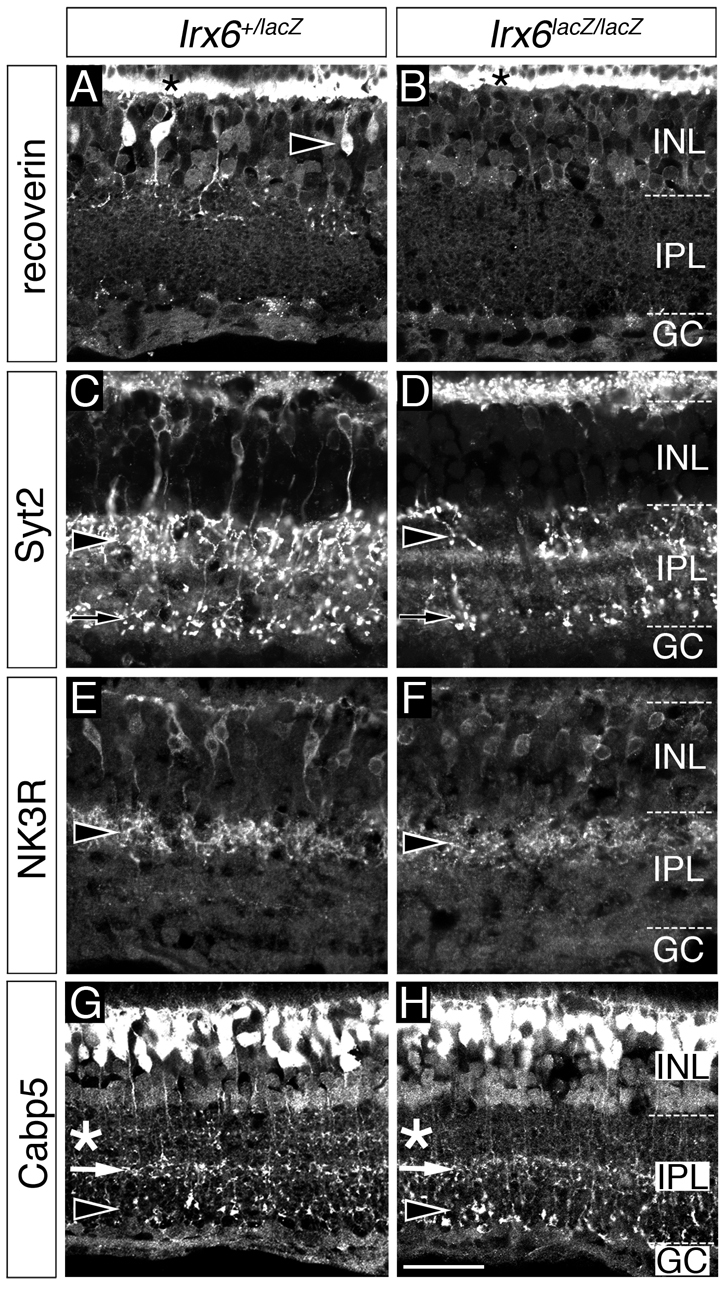
OFF cone bipolar cell gene expression defects in Irx6:βgal mice. (A-D) Recoverin (A,B) and synaptotagmin 2 (C,D) immunolabeling of type 2 cells (arrowheads in A,C,D) is reduced in Irx6lacZ/lacZ mice. The asterisks in A,B indicate recoverin immunolabeling of photoreceptor cells. Synaptotagmin 2 immunolabeling of the axon terminals of type 6 cells is also reduced in Irx6lacZ/lacZ mice (arrows in C,D). (E,F) Nk3r immunolabeling is not reduced in Irx6lacZ/lacZ mice, as judged by labeling of soma and axon terminals (arrowheads). (G,H) Cabp5 expression is reduced in the region of the inner plexiform layer normally occupied by the axon terminals of type 3 cells (compare asterisks in G and H), but is unaffected in the axon terminal of type 5 (arrows in G and H) and rod bipolar cells (arrowheads in G and H) in the Irx6lacZ/lacZ mouse. INL, inner nuclear layer; IPL, inner plexiform layer; GC, ganglion cell layer. Scale bar: 25 μm.
As Irx6:βgal is also expressed in type 3a bipolar cells, we next examined whether this cell type was affected by the loss of Irx6. In Irx6lacZ/lacZ mice, we observed a specific reduction in the level of Cabp5 immunolabeling within the OFF region corresponding to the position where type 3 axon terminals are located (Fig. 5G,H). Cabp5 immunolabeling of putative type 5 and rod bipolar cell axon terminals was unaffected (Fig. 5G,H). The identification of a Cabp5 immunolabeling defect in type 3 cells was correlated with a slight, but statistically significant, decrease in the number of cells expressing the type 3a cell marker Hcn4 (Fig. 6B,H; Fig. 8I). Furthermore, within the cell bodies of the Hcn4-expressing type 3a cells in the Irx6lacZ/lacZ retina, a loss of Cabp5 immunolabeling was observed (Fig. 6C,D,I,J). The reduction of Cabp5 immunolabeling was specific to type 3a cells and was not observed in PKARIIβ- positive type 3b cells (Fig. 6E,F,K,L; data not shown). Together, these results identify a specific defect in type 3a bipolar cells of the Irx6lacZ/lacZ retina marked by a loss of Cabp5 immunolabeling and a reduction in the total number of Hcn4-expressing bipolar cells.
Fig. 6.
Irx6lacZ/lacZ mice have a reduction in Cabp5 staining in type 3a bipolar cells. (A-L) Irx6+/lacZ (A-F) and Irx6lacZ/lacZ mice (G-L) were immunolabeled for Irx6:βgal (A,G), Hcn4 (B,H) and Cabp5 (C,I). Arrows indicate cells in the heterozygous retina (A-D) that are positive for Irx6:βgal, Hcn4 and Cabp5, and cells in the Irx6lacZ/lacZ retina (G-J) that are Irx6:βgal and Hcn4 positive, but have lost Cabp5 expression. Bracket in H indicates Hcn4 staining in the inner plexiform layer of the mutant mouse. Staining for Irx6:βgal (E,K) and PKARIIβ (F,L) shows that there is no change in the colocalization of these markers with Cabp5 in the heterozygous and Irx6lacZ/lacZ mouse. Arrows in E,F,K,L indicate cells that are positive for Cabp5 and PKARIIβ expression. Arrowheads in E,F,K,L indicate Irx6:βgal-expressing cells that are positive (E,F) or negative (K,L) for Cabp5. Scale bar: 12 μm.
Fig. 8.
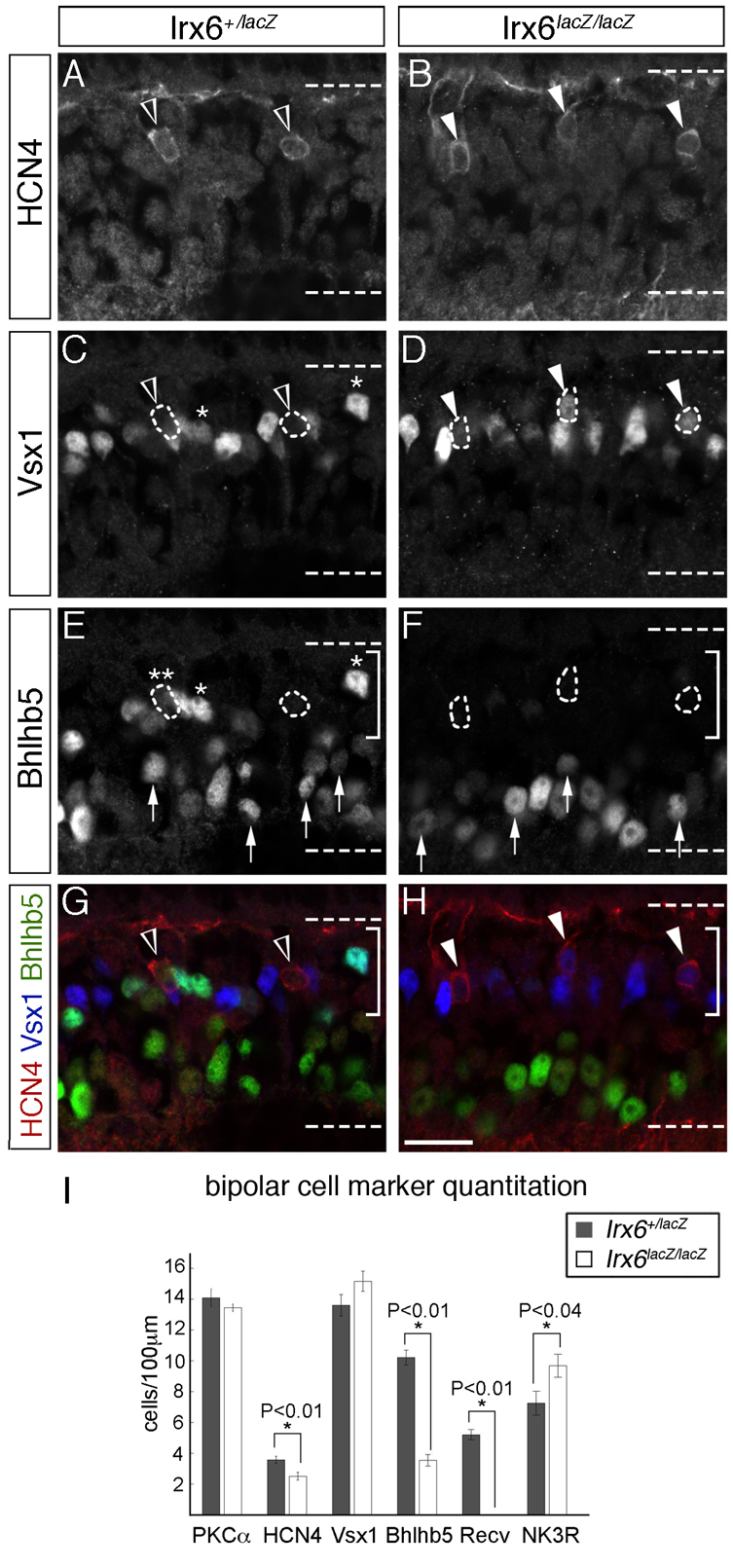
Irx6 regulates the expression of transcription factors required for OFF bipolar cell development. (A-H)Vsx1 immunolabeling is ectopically upregulated in Hcn4-expressing cells (A,B, arrowheads) in the Irx6lacZ/lacZ retina (compare C,D, arrowheads). The single asterisks in C and E indicate a putative type 2 cell, based on its co-labeling of Vsx1 and Bhlhb5 and on the lack of Hcn4 immunolabeling. In the Irx6lacZ/lacZ retina, Bhlhb5 immunolabeling of the bipolar cell region of the inner nuclear layer (indicated by the bracketed regions in E-H) is greatly reduced compared with the Irx6+/lacZ retina (E,F). Amacrine cell labeling of Bhlhb5 (E,F, arrows) is retained in the Irx6lacZ/lacZ retina. An example of a putative type 3a cell expressing low levels of Bhlhb5 is indicated by the double asterisk in E. Scale bar in H: 15 μm. (I) Quantitation of bipolar cell marker expression in the retina of Irx6+/lacZ (n=5 retinas) and Irx6lacZ/lacZ (n=6 retinas) mice. Recv, recoverin. Data are mean±s.e.m.
Hcn4-expressing OFF bipolar cells exhibit stratification defects in Irx6 mutant mice
In addition to a reduction in the number of Hcn4-immunolabeled putative type 3a bipolar cells in the Irx6lacZ/lacZ retina, we observed defects in the stratification of the remaining Hcn4-immunolabeled axon terminals (Fig. 6H; Fig. 7). These defects were characterized by the expansion of Hcn4-immunolabeled axon terminals into the entire OFF region of the inner plexiform layer (Fig. 7B,C). To determine more precisely the nature of this defect, we performed co-immunolabeling experiments with calretinin, a marker used to distinguish specific inner plexiform layer sublaminae (Ghosh et al., 2004; Haverkamp and Wässle, 2000). The five distinct sublaminae defined by calretinin immunolabeling were unaltered in the Irx6lacZ/lacZ retina (Fig. 7H,I). The axon terminals of type 3 bipolar cells are normally positioned in sublamina 2 (between the two outermost calretinin-labeled bands), whereas the axon terminals of type 1 and 2 bipolar cells are positioned within sublamina 1, located above the outermost calretinin-labeled band (Ghosh et al., 2004). In contrast to the invariable positioning of Hcn4-immunolabeled axon terminals in sublamina 2 in the wild-type (data not shown) and Irx6+/lacZ retina (Fig. 7D-F), we observed Hcn4 immunolabeling spanning both sublamina 1 and 2 in Irx6lacZ/lacZ mice (Fig. 7G-I). This expansion of Hcn4-labeled termini in Irx6lacZ/lacZ mice was observed across the entire retina. Hcn4-labeled axon terminals were not observed in sublaminae 3-5, where ON bipolar cell axon terminals reside (Fig. 7G-I). In support of the observation of expanded Hcn4-immunolabeled axon termini, we measured a slight but statistically significant increase in the ratio of fluorescence intensity between a region of interest located directly below the inner nuclear layer (sublamina 1) and region of equal size located immediately below and corresponding to sublamina 2 (0.84±0.02 for Irx6+/lacZ retina; 0.90±0.02 for Irx6lacZ/lacZ retina; n=5, P<0.03), suggesting that there is a shift in the axon stratification of putative type 3a bipolar cells in the Irx6lacZ/lacZ mouse. These results suggest that Irx6 is required for the correct axon stratification of Hcn4-expressing cells within the OFF sublamina of the inner plexiform layer.
Fig. 7.
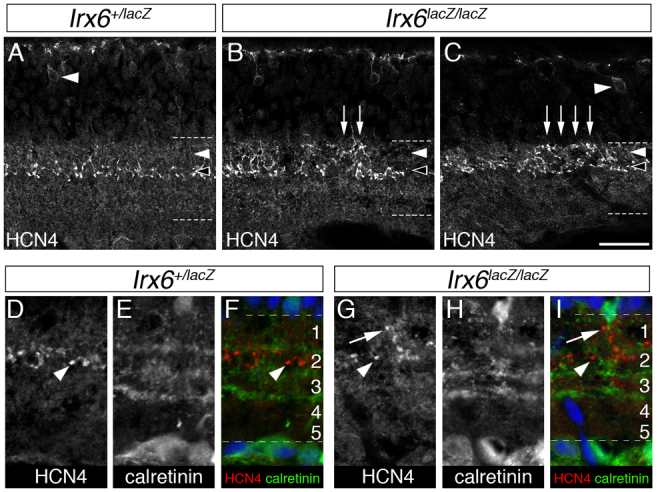
Abnormal distribution of Hcn4 immunolabeling in the inner plexiform layer of the Irx6lacZ/lacZ retina. (A-C) Immunolabeling for Hcn4 in the Irx6+/lacZ (A) and Irx6lacZ/lacZ (B,C) retina. Arrowheads indicate Hcn4-positive cell bodies in the inner nuclear layer of the retina. Arrows indicate increased expression of Hcn4 in the upper zone of the inner plexiform region. Broken lines define the boundary of the inner plexiform layer (A-C,F,I). (D-I) Immunolabeling using calretinin to further define the projection zones of the inner plexiform region of the retina. In the Irx6+/lacZ retina, Hcn4 immunolabeled axon terminals of putative type 3a cells (D) are restricted to sublamina 2 in the inner plexiform layer, as defined by calretinin immunolabeling (E). Arrowheads (D,F) indicate Hcn4-positive axon termini in sublamina 2. By contrast, in the Irx6lacZ/lacZ retina (G-I), Hcn4 immunolabeling (G) is detected in both sublamina 1 and 2, as defined by calretinin immunolabeling (H). Hcn4-positive axon termini in sublamina 1 (G,I, arrows) and sublamina 2 (G,I, arrowheads). Scale bar: 25 μm in A-C; 18 μm in D-I.
Dysregulation of Vsx1 and Bhlhb5 in Irx6 mutant mice
The OFF bipolar cell axon stratification defects observed in putative type 3a bipolar cells of Irx6lacZ/lacZ mice raises the possibility that OFF bipolar cell subtype identity is affected in the Irx6 mutant. We therefore asked whether the expression of Vsx1 and Bhlhb5, two transcription factors required for OFF bipolar cell differentiation, was affected in Irx6lacZ/lacZ mice. Vsx1 expression is undetectable in Hcn4-positive type 3a cells in the wild-type retina (Shi et al., 2012). In the Irx6+/lacZ retina, Vsx1 was normally not detected in Hcn4-expressing type 3a cells (Fig. 8C), but in rare instances, Hcn4-positive bipolar cells exhibited faint levels of Vsx1 immunolabeling (data not shown). By contrast, in the Irx6lacZ/lacZ retina, ectopic Vsx1 immunolabeling was prominent in Hcn4-expressing bipolar cells (Fig. 8B,D,H). Despite the presence of ectopic Vsx1 immunolabeling, there was no significant change in the number of Vsx1-labeled cells in Irx6lacZ/lacZ mice (Fig. 8I). Unlike Vsx1, a significant reduction in the level of Bhlhb5 immunolabeling of type 2 cells was observed in the bipolar cell region of the inner nuclear layer in Irx6lacZ/lacZ mice (Fig. 8E,F,I). A previously uncharacterized subset of Hcn4-positive type 3a cells in wild-type and Irx6+/lacZ mice that weakly co-immunolabeled for Bhlhb5 was also reduced in size in the Irx6lacZ/lacZ retina (Fig. 8E; data not shown). The population of Bhlhb5-positive amacrine cells, identified by both position and co-immunolabeling with the amacrine cell marker syntaxin, remained unchanged in the absence of Irx6 (Fig. 8E,F). These data demonstrate a differential requirement for Irx6 in the regulation of Bhlhb5 and Vsx1 such that Irx6 is required for the repression of Vsx1 in type 3a cells and for the activation of Bhlhb5 in type 2 and 3a bipolar cells.
Mixed subtype identity in Hcn4-expressing OFF bipolar cells in the Irx6-mutant retina
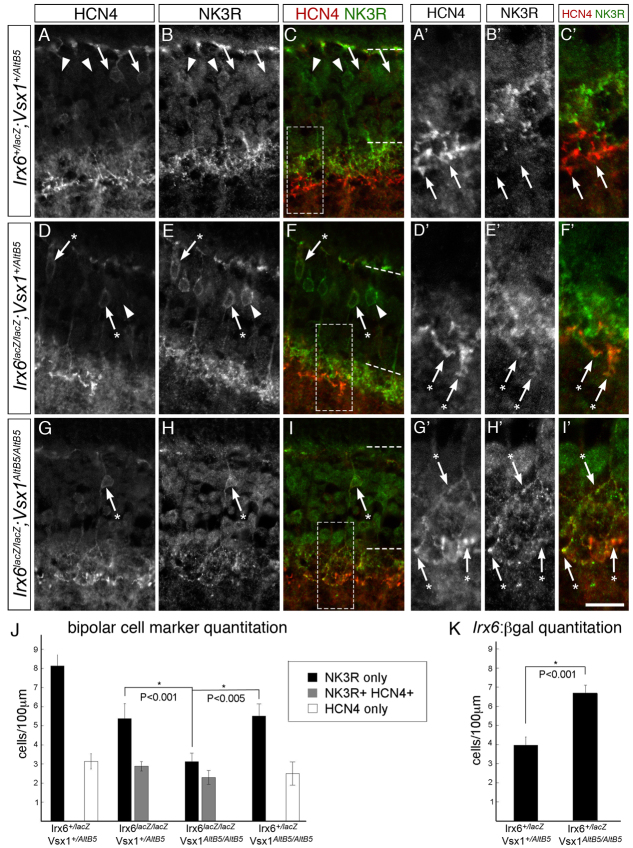
As Vsx1 is necessary for the expression of several type 2 bipolar cell-specific markers (Chow et al., 2004; Ohtoshi et al., 2004), we next examined the possibility that the presence of ectopic Vsx1 in Hcn4-expressing cells in the Irx6-mutant was correlated with the ectopic expression of type 2 markers regulated by Vsx1. We focused our attention on the type 1 and 2 bipolar cell marker Nk3r, because, unlike recoverin, its expression is not lost in the Irx6 mutant (Fig. 5E,F). In the wild type, Irx6+/lacZ and Irx6+/lacZ;Vsx1+/AltB5 double heterozygous retina, Nk3r and Hcn4 are expressed in a mutually exclusive manner in presumptive type 1, 2 and 3a cells, respectively (Fig. 9A-C′,J; data not shown). In both the Irx6lacZ/lacZ and Irx6lacZ/lacZ;Vsx1+/AltB5 retina, ectopic Nk3r immunolabeling was observed in Hcn4-immunolabled cell somas (Fig. 9D-F,J; data not shown) and in the axon terminals of putative type 3a cells (Fig. 9D′-I′). A population of Nk3r-positive putative type 1 or 2 cells was not labeled with Hcn4 (Fig. 9D-F). In agreement with our observation of co-immunolabeling between Hcn4 and Nk3r in the Irx6lacZ/lacZ retina, we measured a significant increase in the number of Nk3r-positive bipolar cells in the Irx6lacZ/lacZ retina when compared with the Irx6+/lacZ retina (Fig. 8I). We also observed a decrease in the fluorescence intensity ratio between sublamina 1 and 2 for Nk3r immunolabeling in the Irx6lacZ/lacZ retina (1.2±0.07) when compared with the Irx6+/lacZ retina (1.5±0.1; P<0.03, n=3 mice), providing further support for the finding that there is an increase in Nk3r immunolabeling in sublamina 2. Together, these findings suggest that NKR3 is ectopically expressed in putative type 3a bipolar cells in both the Irx6lacZ/lacZ and Irx6lacZ/lacZ;Vsx1+/AltB5 retina.
Fig. 9.
Co-labeling of Nk3r in putative type 3a bipolar cells in the Irx6lacZ/lacZ retina. (A-C′) In the Irx6+/lacZ;Vsx1+/AltB5 retina (A-C), Nk3r and Hcn4 immunolabeling of putative types 1 or 2 (arrowheads) and type 3a (arrows) cells, respectively, does not overlap in cell somas or axon terminals (A′-C′). (D-F′) By contrast, in the Irx6lacZ/lacZ;Vsx1+/AltB5 retina, Hcn4 immunolabeling in putative type 3a cells that exhibit type 3a axon stratification are co-immunolabeled with Nk3r (indicated by cells with asterisked arrows). Putative type 1 or 2 cells expressing only Nk3r and not Hcn4 are labeled with an arrowhead. (G-I′) In the Irx6lacZ/lacZ;Vsx1AltB5/AltB5 retina, co-labeling of Hcn4 and Nk3r is observed in the cell soma (indicated by asterisked arrows) and in the inner plexiform layer. Scale bar: 15 μm in A-I; 10 μm in A′-I′. (J) Quantitation of bipolar cells immunolabeled with Nk3r alone (black), Nk3r and Hcn4 (gray), and Hcn4 alone (white) in the retina of Irx6+/lacZ;Vsx1+/AltB5, Irx6lacZ/lacZ;Vsx1+/AltB5, Irx6lacZ/lacZ;Vsx1AltB5/AltB5 and Irx6+/lacZ;Vsx1AltB5/AltB5 mice (n=3, 3, 4, 2 mice, respectively). (K) Quantitation of Irx6:βgal expression in the retina of Irx6+/lacZ;Vsx1+/AltB5 and Irx6+/lacZ;Vsx1AltB5/AltB5 mice (n=3 mice for both genotypes). Data are mean±s.e.m.
Given that both Nk3r and Hcn4 are downregulated in the Vsx1-null retina (Chow et al., 2004; Shi et al., 2012), we next asked whether their co-expression in putative type 3a bipolar cells of the Irx6 mutant retina would be affected in mice deficient for both Irx6 and Vsx1. Unexpectedly, despite the predicted reduction in overall immunolabeling of the two markers Hcn4 and Nk3r, co-immunolabeled bipolar cells were still observed in the Irx6lacZ/lacZ;Vsx1AltB5/AltB5 double homozygous mutant retina (Fig. 9G-J). In the Irx6lacZ/lacZ;Vsx1AltB5/AltB5 mutant retina, all of the Hcn4-expressing bipolar cells co-immunolabeled with Nk3r (Fig. 9J). These data indicate that the expression of Nk3r in Hcn4-expressing cells appears to be independent of Vsx1. We also observed a population of Nk3r-positive cells that did not label with Hcn4 in the Irx6lacZ/lacZ;Vsx1AltB5/AltB5 double homozygous retina. In agreement with previous findings in the Vsx1-null retina (Chow et al., 2004), we observed a decrease in the number of Nk3r immunolabeled cells in the Irx6+/lacZ;Vsx1AltB5/AltB5 retina when compared with the Irx6+/lacZ;Vsx1+/AltB5 retina (Fig. 9J). In the Irx6lacZ/lacZ;Vsx1AltB5/AltB5 double homozygous retina we observed a further decrease in the number of Nk3r-only immunolabeled cells (Fig. 9J), suggesting that Irx6 and Vsx1 can both induce Nk3r expression.
To determine whether Vsx1 expression has an effect on the expression of the Irx6, we examined the expression of the Irx6:βgal reporter in the presence and absence of Vsx1. There was a significant increase in the number of Irx6:βgal positive cells in the Irx6+/lacZ;Vsx1AltB5/AltB5 retina when compared with the Irx6+/lacZ;Vsx1+/AltB5 retina (Fig. 9K). These results suggest that Vsx1 has a partial inhibitory effect on the expression of Irx6.
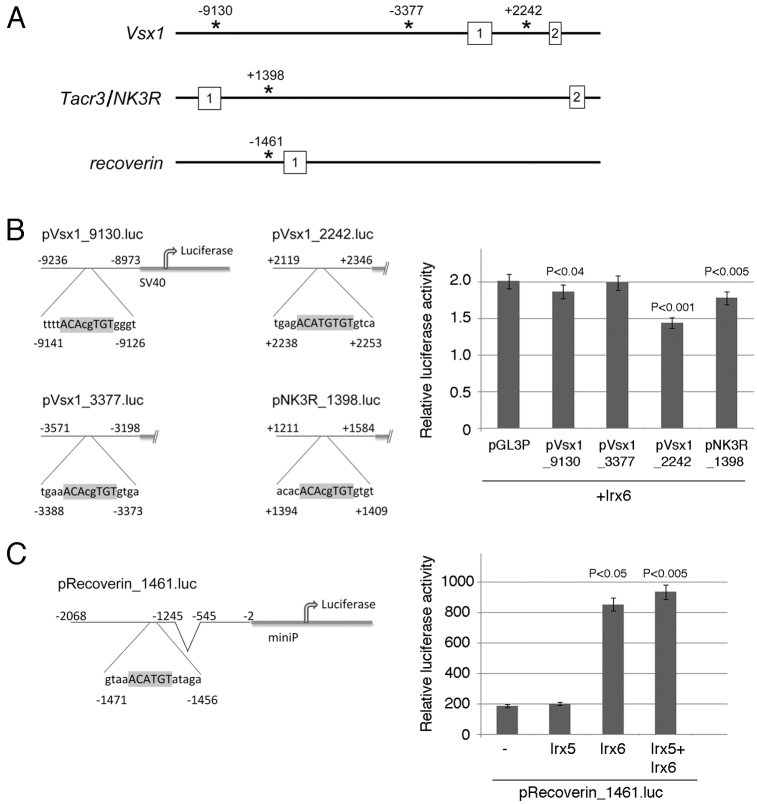
Irx6 can activate or repress transcription through Iroquois-binding sites found proximal to recoverin, Vsx1 and Nk3r
Members of the Iroquois family are known to bind in vitro to a DNA sequence known as the Iroquois-binding site (IBS) and function as transcriptional repressors (Bilioni et al., 2005; Berger et al., 2008). To gain insight into the molecular mechanism by which the expression of the OFF bipolar cell markers recoverin, Vsx1 and Nk3r is disrupted in the Irx6lacZ/lacZ mouse, we searched for candidate sequences that match the IBSs in the regions proximal to these genes. Candidate IBSs in Vsx1, Tacr3 (the gene encoding Nk3r) and Rcvrn were identified (Fig. 10A; supplementary material Table S2). We then performed a series of transcriptional reporter assays to determine whether Irx6 might directly regulate the expression of these factors. Regions of ∼200 bp of Vsx1 or Tacr3 surrounding the predicted IBSs were cloned upstream of an SV40 promoter-driven luciferase reporter plasmid. In three of the four IBS sequences examined, we observed repression of luciferase expression in the presence of co-transfected Irx6 (Fig. 10B). Upstream of Rcvrn we identified a fragment containing a potential IBS (Fig. 10A), and positioned a 1.3 kb region containing this site in front of a mini-promoter-driven luciferase reporter construct (supplementary material Table S2). Unexpectedly, in contrast to the well-characterized repressor activity of Irx transcription factors, reporter assays showed that the addition of Irx6 had an activating effect on transcription of the Rcvrn reporter (Fig. 10C). Interestingly, addition of Irx5 had no effect on transcription and did not alter Irx6-mediated activation (Fig. 10C). Together, these findings suggest that Irx6 can function in a context-dependent manner as either a transcriptional repressor or activator, and may explain the increased expression of Vsx1/Nk3r and decreased expression of recoverin in the Irx6lacZ/lacZ mouse.
Fig. 10.
Irx6 can act as a transcriptional repressor or activator on sites found proximal to OFF bipolar cell markers. (A) Potential IBS found proximal to Vsx1, Tacr3 (Nk3r) and Rcvrn. Numbered boxes indicate exons. (B,C) Irx6 represses both Vsx1-luciferase (B) and Nk3r-luciferase and activates Rcvrn-luciferase (C) expression in HEK cells. Data are mean±s.e.m.
Visual signaling defects in Irx6 mutant mice
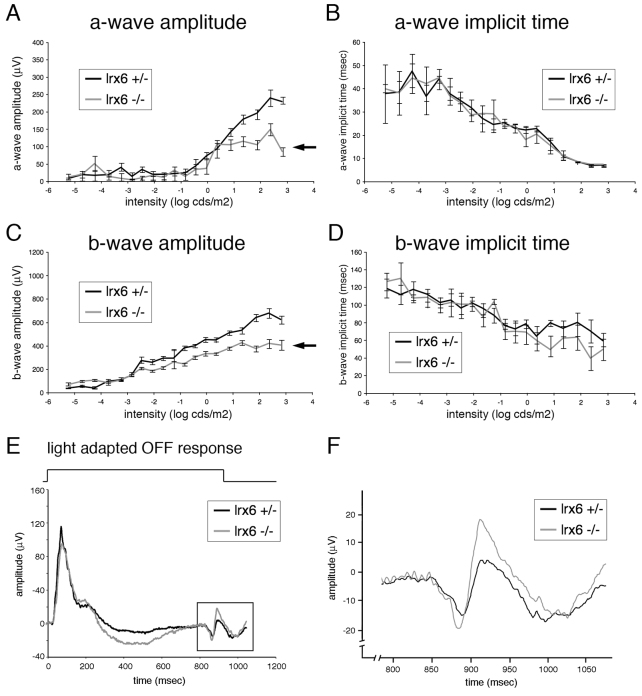
To determine whether the retinal defects in Irx6 mutant mice were accompanied by defects in visual signaling, electroretinography (ERG) was performed on mice at 3-4 months of age. In contrast to wild-type and Irx6+/lacZ mice, Irx6lacZ/lacZ mice exhibited a reduction of dark-adapted and light-adapted a-wave amplitude (Fig. 11A; data not shown), which reflects photoreceptor activity. Consistent with the reduced a-wave amplitude, a decreased ERG b-wave amplitude, which is indicative of post photoreceptor retinal activity, was also observed (Fig. 11C). No changes were observed in a-wave or b-wave kinetics (Fig. 11B,D), suggesting that the biochemical integrity of the photoreceptors and inner retina was not lost. Despite the presence of OFF bipolar differentiation defects in Irx6lacZ/lacZ mice, light-adapted OFF responses were intact in Irx6lacZ/lacZ mice compared with controls (Fig. 11E,F; data not shown). In summary, Irx6lacZ/lacZ mice exhibit a reduced ERG a-wave and b-wave, but have intact light-adapted OFF visual signaling responses.
Fig. 11.
Visual signaling defects in Irx6lacZ/lacZ mice. (A-D) Dark-adapted ERG a-wave (A) and b-wave (C) amplitudes were reduced in Irx6lacZ/lacZ (n=5) compared with Irx6+/lacZ mice (n=4). Both the a-wave (B) and b-wave (D) implicit time values were unaffected in Irx6lacZ/lacZ mice. Data are mean±s.e.m. (E) An example of the light-adapted OFF response in Irx6lacZ/lacZ and Irx6+/lacZ. (F) The inset in E revealing an intact d-wave that is generated in response to light OFF-set.
DISCUSSION
Here, we show that Irx6 is required downstream of bipolar cell specification for the terminal differentiation of type 2, type 3a and possibly type 6 bipolar cells (Fig. 12A).
Fig. 12.
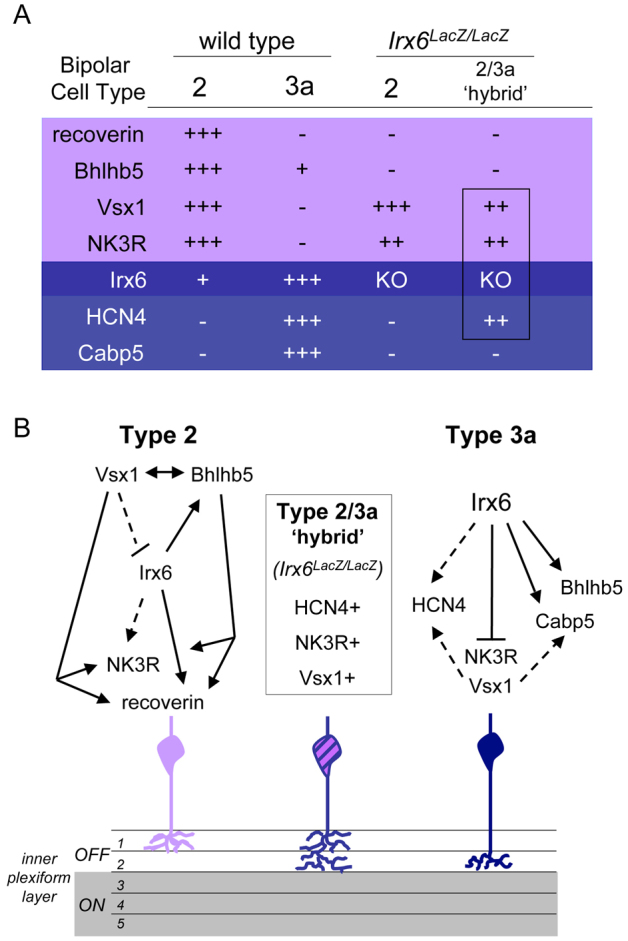
Regulatory network model of Irx6, Vsx1 and Bhlhb5 in directing terminal gene expression in OFF bipolar cells. (A) Expression of type 2 and 3a cell markers in the Irx6+/lacZ (wild-type retina), and the resulting changes in marker expression in the Irx6lacZ/lacZ retina. (B) We propose that Vsx1 and Bhlhb5 are key players in regulating the terminal differentiation and maturation of type 2 cells, while Irx6 has a leading role in regulating the formation of type 3a cells. Irx6 is necessary for preventing type 3a cells from adopting type 2 cell characteristics in a mechanism that is independent of Vsx1 activity. Dashed lines indicate partial repression or activation.
Our results suggest there is a complex regulatory network of transcription factors (i.e. Irx6, Vsx1 and Bhlhb5) that function to regulate the development of type 2 and 3a bipolar cells (Fig. 12B). In the Irx6lacZ/lacZ mutant mouse, type 2 cells have reduced recoverin and Bhlhb5 immunolabeling, but retain Vsx1 and some Nk3r immunolabeling. In the Irx6;Vsx1 homozygous mutant mouse, we see a decrease in the number of Nk3r alone-positive cells compared with either the Irx6+/lacZ;Vsx1AltB5/AltB5 mouse or Irx6lacZ/lacZ;Vsx1+/AltB5 mouse. This genetic interaction reveals that both Irx6 and Vsx1 contribute to the expression of Nk3r in type 2 cells in the mouse retina. Based on our observations and published work (Chow et al., 2004; Feng et al., 2006; Kerschensteiner et al., 2008; Ohtoshi et al., 2004), we suggest that both Vsx1 and Bhlhb5 have a central role in directing the terminal differentiation and maturation of type 2 bipolar cells, with Irx6 taking on a more minor role in regulating the formation of this cell type (Fig. 12B).
In type 3a bipolar cells, our findings reveal that Irx6 has a key role in defining cell type specific gene expression. Results from the transcriptional reporter assays suggest that Irx6 could directly repress the expression of both Vsx1 and Nk3r, and therefore is likely to be responsible in part for repressing the expression of these genes in type 3a cells. Members of the Irx family have been shown to act as transcriptional repressors (Bilioni et al., 2005; Costantini et al., 2005). Although the measured increase in Vsx1 immunolabeling in the Irx6lacZ/lacZ retina was not statistically significant, we think this is due to Vsx1 also being expressed in type 7 bipolar cells, and that any increase in Vsx1-positive cell number in the Irx6lacZ/lacZ retina is masked by the additional expression of Vsx1 in the ON bipolar cells.
Irx6 also promotes the expression of Bhlhb5 and Cabp5, and partially promotes the expression of Hcn4 in type 3a cells. In the Irx6lacZ/lacZ mouse retina, we observe a ‘hybrid’ cell that shows characteristics of both type 2 and 3a bipolar cells, and extends axon terminal projections throughout the OFF sublamina, suggesting that Irx6 expression is necessary to generate a type 3a bipolar cell with a separate identity from a type 2 cell (Fig. 12B). We propose that, in the Irx6lacZ/lacZ mouse, the type 3a bipolar cells adopt molecular features of type 2 cells, as we observe a large increase in the total number of Nk3r immunolabeled cells in the Irx6lacZ/lacZ retina. This increase in Nk3r immunolabeled cells is consistent with the ectopic expression of Nk3r in Hcn4-positive type 3a cells, but would not be expected if Nk3r immunolabeled type 1 or 2 cells ectopically expressed Hcn4. Additionally, in both the Irx6lacZ/lacZ and Irx6;Vsx1 double homozygous mutant retinas, we observe Irx6:βgal cells that are either positive for Hcn4 (and Nk3r) or negative for Hcn4, suggesting that there are two types of Irx6:βgal reporter cells in the Irx6lacZ/lacZ mouse (supplementary material Fig. S4). The putative type 2/3a hybrid cell is still present in the retina of the Irx6;Vsx1 homozygous mutant mouse, even in the absence of robust Bhlhb5 bipolar cell expression (supplementary material Fig. S4), suggesting that Irx6 is a primary regulator in controlling the formation of type 3a cells in a mechanism that is independent of Vsx1 function (Fig. 12B), but that other factors must also be functioning to regulate OFF bipolar cell specific gene expression. Together, these observations support a model in which the identities of type 2 and 3a bipolar cells are defined, in part by opposing, yet interdependent, transcription factor gene expression established in these cell types.
In Irx6 mutant retinas, recoverin immunolabeling in type 2 bipolar cells was lost. Results from the transcriptional reporter assay suggest that the putative IBS upstream of Rcvrn can induce Irx6-dependent transcriptional activation, and this is consistent with our genetic data showing loss of recoverin in the Irx6lacZ/lacZ mouse. In Irx5 mutant retinas, recoverin immunolabeling in type 2 cells is also downregulated, demonstrating that Irx5 and Irx6 are not functionally redundant. As we did not observe any effect in our transcriptional assay with the addition of Irx5, the Rcvrn transcriptional activation site for Irx5 remains unclear, and our data suggest that the function of putative Iroquois-binding sites is highly dependent on the surrounding sequence.
Although no ganglion cell defects were observed in Irx6lacZ/lacZ mice (supplementary material Fig. S5), nor in the individual Irx2, Irx4 or Irx5 gene knockout mice (Bruneau et al., 2001; Cheng et al., 2005; Lebel et al., 2003) previous studies in chick suggest a role for Iroquois genes in these cells (Jin et al., 2003). Although in situ hybridization studies have shown that ganglion cells express both IrxA and IrxB cluster genes, only the Iroquois cluster B genes are expressed in the bipolar cell region of the inner nuclear layer (Cohen et al., 2000) (R.L.C., unpublished). This difference in expression could explain why defects are observed in bipolar cells and not in ganglion cells of both Irx6 and Irx5 mutants, and suggests that the absence of a ganglion cell phenotype in individual Irx loss-of-function mutants is due to functional redundancy of the Irx proteins.
Iroquois genes appear to have a conserved function in subdividing neuronal projection domains. In the Drosophila notum, the Iro complex appears to control axonal projections of medial versus lateral mechanosensory bristles (Grillenzoni et al., 1998). In addition, the Iro complex prevents dorsal photoreceptor axons from misprojecting to the ventral lamina by inhibiting Drosophila Wnt4 (Sato et al., 2006). Similar to the fly, our results reveal that Irx6 functions in subdividing neuronal projection domains in the mammalian retinal inner plexiform layer. In Irx6lacZ/lacZ mice, axonal projections of presumptive type 3a bipolar cells, which are normally restricted to sublamina 2, are also observed in sublamina 1. Interestingly, the presumptive type 3a cells did not misproject to sublamina 3-5, where the axon terminals of ON bipolar cells reside. This observed axonal termini misprojection phenotype is reminiscent of the Sema5a–/–;Sema5b–/– mouse retinal phenotype, where Cabp5 immunolabeled type 3 bipolar cells also had ectopic projections to sublamina 1 (Matsuoka et al., 2011). These findings suggest that at least two mechanisms are required for bipolar cell type axonal development: one that defines the division of ON versus OFF boundaries; and one (involving Irx6) that is required for proper positioning within the OFF region. Parallels between the axon guidance defects in the mouse retina and in Drosophila suggest a conserved role for Irx genes in regulating axon projection domain specificity.
How the loss of Irx6 affects visual signaling is unclear at this point. In Irx6 mutants, the ERG b-wave amplitude, an indicator of bipolar cell activity was intact, but reduced. However, as the amplitude of the a-wave (a measure of photoreceptor activity) was also reduced, it was not possible to determine whether the reduced b-wave was due to defects in bipolar cell function. The reduced a-wave amplitude in Irx6lacZ/lacZ mice was unexpected. Although a reduced a-wave amplitude was previously observed in Irx5 mutant mice, this was attributed to the overall reduced size of Irx5 mice compared with controls (Cheng et al., 2005). Irx6lacZ/lacZ mice, however, do not exhibit any significant reduction in overall size (data not shown) or photoreceptor layer thickness, raising the possibility that the reduced a-wave amplitude is due to a defect in photoreceptor function. How this defect could be manifest is presently unclear given that the a-wave implicit time kinetics were unaffected in the Irx6 mutants. Interestingly, transient expression of the Irx6:βgal reporter was observed in photoreceptor cells, suggesting that there may be a requirement for Irx6 during photoreceptor development. The light adapted d-wave, a specific indicator of OFF bipolar cell function, was intact in Irx6 mutants; this result contrasts the OFF visual signaling defects previously observed in Vsx1 mutant mice (Chow et al., 2004; Kerschensteiner et al., 2008) and with our observation that Vsx1-null mice have a reduced d-wave (data not shown). This difference in visual signaling phenotype is surprising given that the Vsx1 and Irx6 mutants have overlapping gene expression defects in OFF bipolar cells. However, the Vsx1 and Irx6 mutant phenotypes are not identical. In particular, Vsx1 and Nk3r expression are upregulated in type 3a bipolar cells in the Irx6 mutant. Future work examining the signaling properties of Irx6 and Vsx1 mutant OFF bipolar cells in isolation and slice preparations will be important to begin to understand how these genes regulate functional differences in OFF bipolar subtypes.
One question that remains unanswered is to what extent the specification of diverse retinal bipolar cell subtypes is driven by intrinsic (i.e. transcription factor coding) and extrinsic mechanisms. Although dissociation studies have suggested that intrinsic factors play a dominant role in regulating E16-17 retinal progenitor cell cycle exit and cell fate determination, these studies have focused on retinal cell classes and not on subtype determination (Cayouette et al., 2003). In the developing neural tube, specification of ventral interneurons is determined in part by a dorsal-ventral Hedgehog signaling gradient that regulates the expression of homeodomain and basic helix-loop-helix transcription factors (Briscoe et al., 2000). As is the case in the retina, subtypes exist within different ventral interneuronal classes of the neural tube that possess distinct axonal targeting properties and neuronal activity (Shirasaki and Pfaff, 2002). Retinal type 7 bipolar cell dendrite stratification is not affected by the absence of photoreceptors (Keeley and Reese, 2010), supporting the idea that intrinsic mechanisms specify some aspects of bipolar cell type development that is independent of extrinsic cues. Future experiments aimed at understanding how Irx6 and its downstream targets are regulated by intrinsic and extrinsic cues will provide insight into the control of retinal bipolar cell type diversity.
Supplementary Material
Acknowledgments
We thank F. Haeseleer, R. R. McInnes, F. Müller, A. Hirano and C. C. Hui for reagents and members of the R.L.C. lab for comments and suggestions.
Footnotes
Funding
This work was supported by operating grants from the Canadian Institutes for Health Research, The Foundation Fighting Blindness – Canada (R.L.C.) and National Institutes of Health/National Heart, Lung, and Blood Institute (NIH/NHLBI) [R01 HL93414 ARRA to B.G.B.]. Y.S. is an Alberta Innovates-Health Solutions (AIHS) Senior Scholar [200800242]. E.N.S. is supported by a Michael Smith Foundation for Health Research Postdoctoral Fellowship. R.L.C. is supported by a Tier 2 Canada Research Chair. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.081729/-/DC1
References
- Alvarez B. V., Gilmour G. S., Mema S. C., Martin B. T., Shull G. E., Casey J. R., Sauvé Y. (2007). Blindness caused by deficiency in AE3 chloride/bicarbonate exchanger. PLoS ONE 2, e839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M. F., Badis G., Gehrke A. R., Talukder S., Philippakis A. A., Peña-Castillo L., Alleyne T. M., Mnaimneh S., Botvinnik O. B., Chan E. T., et al. (2008). Variation in homeodomain DNA binding revealed by high-resolution analysis of sequence preferences. Cell 133, 1266–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilioni A., Craig G., Hill C., McNeill H. (2005). Iroquois transcription factors recognize a unique motif to mediate transcriptional repression in vivo. Proc. Natl. Acad. Sci. USA 102, 14671–14676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe J., Pierani A., Jessell T. M., Ericson J. (2000). A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell 101, 435–445 [DOI] [PubMed] [Google Scholar]
- Bruneau B. G., Bao Z. Z., Fatkin D., Xavier-Neto J., Georgakopoulos D., Maguire C. T., Berul C. I., Kass D. A., Kuroski-de Bold M. L., de Bold A. J., et al. (2001). Cardiomyopathy in Irx4-deficient mice is preceded by abnormal ventricular gene expression. Mol. Cell. Biol. 21, 1730–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister M., Novak J., Liang M. Y., Basu S., Ploder L., Hawes N. L., Vidgen D., Hoover F., Goldman D., Kalnins V. I., et al. (1996). Ocular retardation mouse caused by Chx10 homeobox null allele: impaired retinal progenitor proliferation and bipolar cell differentiation. Nat. Genet. 12, 376–384 [DOI] [PubMed] [Google Scholar]
- Cayouette M., Barres B. A., Raff M. (2003). Importance of intrinsic mechanisms in cell fate decisions in the developing rat retina. Neuron 40, 897–904 [DOI] [PubMed] [Google Scholar]
- Cheng C. W., Chow R. L., Lebel M., Sakuma R., Cheung H. O., Thanabalasingham V., Zhang X., Bruneau B. G., Birch D. G., Hui C. C., et al. (2005). The Iroquois homeobox gene, Irx5, is required for retinal cone bipolar cell development. Dev. Biol. 287, 48–60 [DOI] [PubMed] [Google Scholar]
- Chow R. L., Snow B., Novak J., Looser J., Freund C., Vidgen D., Ploder L., McInnes R. R. (2001). Vsx1, a rapidly evolving paired-like homeobox gene expressed in cone bipolar cells. Mech. Dev. 109, 315–322 [DOI] [PubMed] [Google Scholar]
- Chow R. L., Volgyi B., Szilard R. K., Ng D., McKerlie C., Bloomfield S. A., Birch D. G., McInnes R. R. (2004). Control of late off-center cone bipolar cell differentiation and visual signaling by the homeobox gene Vsx1. Proc. Natl. Acad. Sci. USA 101, 1754–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D. R., Cheng C. W., Cheng S. H., Hui C. C. (2000). Expression of two novel mouse Iroquois homeobox genes during neurogenesis. Mech. Dev. 91, 317–321 [DOI] [PubMed] [Google Scholar]
- Costantini D. L., Arruda E. P., Agarwal P., Kim K. H., Zhu Y., Zhu W., Lebel M., Cheng C. W., Park C. Y., Pierce S. A., et al. (2005). The homeodomain transcription factor Irx5 establishes the mouse cardiac ventricular repolarization gradient. Cell 123, 347–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erkman L., Yates P. A., McLaughlin T., McEvilly R. J., Whisenhunt T., O’Connell S. M., Krones A. I., Kirby M. A., Rapaport D. H., Bermingham J. R., et al. (2000). A POU domain transcription factor-dependent program regulates axon pathfinding in the vertebrate visual system. Neuron 28, 779–792 [DOI] [PubMed] [Google Scholar]
- Feng L., Xie X., Joshi P. S., Yang Z., Shibasaki K., Chow R. L., Gan L. (2006). Requirement for Bhlhb5 in the specification of amacrine and cone bipolar subtypes in mouse retina. Development 133, 4815–4825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fode C., Ma Q., Casarosa S., Ang S. L., Anderson D. J., Guillemot F. (2000). A role for neural determination genes in specifying the dorsoventral identity of telencephalic neurons. Genes Dev. 14, 67–80 [PMC free article] [PubMed] [Google Scholar]
- Fox M. A., Sanes J. R. (2007). Synaptotagmin I and II are present in distinct subsets of central synapses. J. Comp. Neurol. 503, 280–296 [DOI] [PubMed] [Google Scholar]
- Ghosh K. K., Bujan S., Haverkamp S., Feigenspan A., Wässle H. (2004). Types of bipolar cells in the mouse retina. J. Comp. Neurol. 469, 70–82 [DOI] [PubMed] [Google Scholar]
- Gollisch T., Meister M. (2010). Eye smarter than scientists believed: neural computations in circuits of the retina. Neuron 65, 150–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Skarmeta J. L., Modolell J. (2002). Iroquois genes: genomic organization and function in vertebrate neural development. Curr. Opin. Genet. Dev. 12, 403–408 [DOI] [PubMed] [Google Scholar]
- Grillenzoni N., van Helden J., Dambly-Chaudière C., Ghysen A. (1998). The iroquois complex controls the somatotopy of Drosophila notum mechanosensory projections. Development 125, 3563–3569 [DOI] [PubMed] [Google Scholar]
- Guillemot F. (2007). Cell fate specification in the mammalian telencephalon. Prog. Neurobiol. 83, 37–52 [DOI] [PubMed] [Google Scholar]
- Haverkamp S., Wässle H. (2000). Immunocytochemical analysis of the mouse retina. J. Comp. Neurol. 424, 1–23 [PubMed] [Google Scholar]
- Haverkamp S., Ghosh K. K., Hirano A. A., Wässle H. (2003). Immunocytochemical description of five bipolar cell types of the mouse retina. J. Comp. Neurol. 455, 463–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverkamp S., Specht D., Majumdar S., Zaidi N. F., Brandstätter J. H., Wasco W., Wässle H., Tom Dieck S. (2008). Type 4 OFF cone bipolar cells of the mouse retina express calsenilin and contact cones as well as rods. J. Comp. Neurol. 507, 1087–1101 [DOI] [PubMed] [Google Scholar]
- Jin Z., Zhang J., Klar A., Chédotal A., Rao Y., Cepko C. L., Bao Z. Z. (2003). Irx4-mediated regulation of Slit1 expression contributes to the definition of early axonal paths inside the retina. Development 130, 1037–1048 [DOI] [PubMed] [Google Scholar]
- Keeley P. W., Reese B. E. (2010). Role of afferents in the differentiation of bipolar cells in the mouse retina. J. Neurosci. 30, 1677–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D., Liu H., Cheng C. W., Demas J., Cheng S. H., Hui C. C., Chow R. L., Wong R. O. (2008). Genetic control of circuit function: Vsx1 and Irx5 transcription factors regulate contrast adaptation in the mouse retina. J. Neurosci. 28, 2342–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel M., Agarwal P., Cheng C. W., Kabir M. G., Chan T. Y., Thanabalasingham V., Zhang X., Cohen D. R., Husain M., Cheng S. H., et al. (2003). The Iroquois homeobox gene Irx2 is not essential for normal development of the heart and midbrain-hindbrain boundary in mice. Mol. Cell. Biol. 23, 8216–8225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q. (2006). Transcriptional regulation of neuronal phenotype in mammals. J. Physiol. 575, 379–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masland R. H. (2001). The fundamental plan of the retina. Nat. Neurosci. 4, 877–886 [DOI] [PubMed] [Google Scholar]
- Mataruga A., Kremmer E., Müller F. (2007). Type 3a and type 3b OFF cone bipolar cells provide for the alternative rod pathway in the mouse retina. J. Comp. Neurol. 502, 1123–1137 [DOI] [PubMed] [Google Scholar]
- Matsuoka R. L., Chivatakarn O., Badea T. C., Samuels I. S., Cahill H., Katayama K., Kumar S. R., Suto F., Chédotal A., Peachey N. S., et al. (2011). Class 5 transmembrane semaphorins control selective Mammalian retinal lamination and function. Neuron 71, 460–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milam A. H., Dacey D. M., Dizhoor A. M. (1993). Recoverin immunoreactivity in mammalian cone bipolar cells. Vis. Neurosci. 10, 1–12 [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Wang F., Dulac C., Chao S. K., Nemes A., Mendelsohn M., Edmondson J., Axel R. (1996). Visualizing an olfactory sensory map. Cell 87, 675–686 [DOI] [PubMed] [Google Scholar]
- Negishi K., Kato S., Teranishi T. (1988). Dopamine cells and rod bipolar cells contain protein kinase C-like immunoreactivity in some vertebrate retinas. Neurosci. Lett. 94, 247–252 [DOI] [PubMed] [Google Scholar]
- Ohsawa R., Kageyama R. (2008). Regulation of retinal cell fate specification by multiple transcription factors. Brain Res. 1192, 90–98 [DOI] [PubMed] [Google Scholar]
- Ohtoshi A., Wang S. W., Maeda H., Saszik S. M., Frishman L. J., Klein W. H., Behringer R. R. (2004). Regulation of retinal cone bipolar cell differentiation and photopic vision by the CVC homeobox gene Vsx1. Curr. Biol. 14, 530–536 [DOI] [PubMed] [Google Scholar]
- Sato M., Umetsu D., Murakami S., Yasugi T., Tabata T. (2006). DWnt4 regulates the dorsoventral specificity of retinal projections in the Drosophila melanogaster visual system. Nat. Neurosci. 9, 67–75 [DOI] [PubMed] [Google Scholar]
- Shi Z., Trenholm S., Zhu M., Buddingh S., Star E. N., Awatramani G. B., Chow R. L. (2011). Vsx1 regulates terminal differentiation of type 7 ON bipolar cells. J. Neurosci. 31, 13118–13127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Jervis D., Nickerson P. E., Chow R. L. (2012). Requirement for the paired-like homeodomain transcription factor VSX1 in type 3a mouse retinal bipolar cell terminal differentiation. J. Comp. Neurol. 520, 117–129 [DOI] [PubMed] [Google Scholar]
- Shirasaki R., Pfaff S. L. (2002). Transcriptional codes and the control of neuronal identity. Annu. Rev. Neurosci. 25, 251–281 [DOI] [PubMed] [Google Scholar]
- Wässle H., Puller C., Müller F., Haverkamp S. (2009). Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J. Neurosci. 29, 106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.