Abstract
The canonical Wnt/β-catenin signaling pathway is known to play crucial roles in organogenesis by regulating both proliferation and differentiation. In the inner ear, this pathway has been shown to regulate the size of the otic placode from which the cochlea will arise; however, direct activity of canonical Wnt signaling as well as its function during cochlear mechanosensory hair cell development had yet to be identified. Using TCF/Lef:H2B-GFP reporter mice and transfection of an independent TCF/Lef reporter construct, we describe the pattern of canonical Wnt activity in the developing mouse cochlea. We show that prior to terminal mitosis, canonical Wnt activity is high in early prosensory cells from which hair cells and support cells will differentiate, and activity becomes reduced as development progresses. Using an in vitro model we demonstrate that Wnt/β-catenin signaling regulates both proliferation and hair cell differentiation within the developing cochlear duct. Inhibition of Wnt/β-catenin signaling blocks proliferation during early mitotic phases of development and inhibits hair cell formation in the differentiating organ of Corti. Conversely, activation increases the number of hair cells that differentiate and induces proliferation in prosensory cells, causing an expansion of the Sox2-positive prosensory domain. We further demonstrate that the induced proliferation of Sox2-positive cells may be mediated by the cell cycle regulator cyclin D1. Lastly, we provide evidence that the mitotic Sox2-positive cells are competent to differentiate into hair cells. Combined, our data suggest that Wnt/β-catenin signaling has a dual function in cochlear development, regulating both proliferation and hair cell differentiation.
Keywords: Mouse, β-catenin, Hair cells, Proliferation, Sox2, Cyclin D1
INTRODUCTION
The mammalian organ of Corti (OC) comprises two types of mechanosensory hair cells (HCs) along with underlying support cells that together are responsible for transducing auditory signals within the sensory epithelium of the cochlea (supplementary material Fig. S1). The number of cells fated to each of these cell types, in addition to the size and shape of the cochlea, is crucial for auditory processing (Kelley et al., 2009). HCs and support cells originate from a prosensory pool of progenitors that can be identified by expression of the transcription factor Sox2 (Kiernan et al., 2005), the cyclin-dependent kinase inhibitor p27kip1 (Cdkn1b) (Chen and Segil, 1999) and the Notch ligand jagged 1 (Jag1) (Lewis et al., 1998). Following terminal mitosis around embryonic day (E) 13.5 in the mouse cochlea (Ruben, 1967), HC differentiation begins as a subset of cells within the prosensory domain upregulates Atoh1, a bHLH transcription factor shown to be necessary and sufficient for HC formation (Bermingham et al., 1999; Zheng and Gao, 2000), and downregulates Sox2 (Dabdoub et al., 2008). Once HC differentiation has begun, Notch signaling diverts the adjacent Sox2-positive progenitors toward a support cell fate (Lanford et al., 1999).
The canonical Wnt/β-catenin pathway is known to regulate both proliferation and differentiation in many tissues (MacDonald et al., 2009), and has been suggested to be upstream of both Atoh1 (Shi et al., 2010) and Sox2 (Agathocleous et al., 2009) during neural progenitor cell maintenance, and is also involved in cross-regulation with the Notch pathway (Jayasena et al., 2008; Rodilla et al., 2009). Recently, it has been demonstrated that ectopic activation of Wnt/β-catenin signaling can induce proliferation and HC formation in a limited subset of support cells isolated from the postnatal OC (Chai et al., 2012; Shi et al., 2012), yet its function in OC development and initial formation had not been determined. Wnts are secreted glycoproteins that act as morphogens in many developmental processes. In vertebrates, the biological effects of the large Wnt gene family are mediated through binding with members of the Frizzled receptor family (Huang and Klein, 2004; Malbon, 2004; Nusse, 2005). Activation of Wnt/β-catenin signaling causes the downregulation of glycogen synthase kinase 3β (Gsk3β), resulting in the stabilization and nuclear translocation of cytoplasmic β-catenin (Clevers, 2006). Within the nucleus, β-catenin associates with TCF/Lef transcription factors to effect transcription of target genes (Jin et al., 2008).
During early inner ear development, canonical Wnt/β-catenin signaling is necessary for the specification of the otic placode, as conditional deletion or activation of β-catenin in Pax2-positive ectodermal cells or Foxg1-positive placodal cells significantly reduces or expands the size of the otic placode, respectively (Freyer and Morrow, 2010; Ohyama et al., 2006). In the otocyst, both nuclear and membranous expression of β-catenin is observed (Takebayashi et al., 2005); following terminal mitosis, however, β-catenin expression becomes primarily membranous (see supplementary material Fig. S1) and this pattern is maintained during postnatal stages (Simonneau et al., 2003). Recent analysis of reporter mice for downstream targets of Wnt/β-catenin signaling (Chai et al., 2011) has suggested that this pathway might be active in the developing cochlea. In this study, we identify and characterize a dual role for Wnt/β-catenin signaling during proliferation of the cochlear prosensory domain and during mechanosensory HC differentiation.
MATERIALS AND METHODS
Animal care and use
Animals were maintained and euthanized in accordance with NIH, UCSD and Memorial Sloan-Kettering Cancer Center guidelines for the care and use of laboratory animals. Embryos from CD-1, ICR or heterozygous TCF/Lef:H2B-GFP reporter mice were obtained, developmental stages were determined (Kaufman, 1992) and cochleae were dissected.
Cochlear explant cultures
E12-16 cochlear explants were prepared as described previously (Jacques et al., 2007). Explants were grown in media containing 10% fetal bovine serum (FBS) along with Wnt activators at the following concentrations: LiCl (10 mM); Wnt Agonist (0.75 μM; Calbiochem); BIO, Gsk3 inhibitor IX (3 μM; Calbiochem). Control media contained 10 mM NaCl or vehicle control (DMSO). For Wnt inhibition, FH535 (3 μM; Calbiochem) was added to culture media containing 2.5% FBS, similar to controls, or IWR-1 (150 μM; Sigma) was added to media containing 10% FBS. Dose-response curves were generated to determine appropriate concentrations (n>6 explants per dose per treatment type). For Notch inhibition, γ-secretase inhibitor IX (DAPT, 25 μM; Calbiochem) was added to some explants. Some explants were cultured in BrdU (3.5 μg/ml; BD Biosciences). Explant experiments consisted of at least six cochleae per condition, from a minimum of three independent litters.
Electroporation
A square-wave electroporator (BTX 830, Harvard Instruments) was used to transfect E13.5 cochleae with either the TCF/Lef:H2B-GFP or TOP-dGFP/pCAG-mCherry reporter constructs as previously described (Jones et al., 2006).
Immunocytochemistry
Immunocytochemistry was performed as previously described (Dabdoub et al., 2008). Primary antibodies were used at the following concentrations: β-catenin (1:500; BD Biosciences and Sigma); Sox2 and Jag1 (1:200; Santa Cruz Biotechnology); myosin 6 (1:1000; Proteus BioSciences); myosin 7a (1:150; DSHB); cyclin D1 (1:250; Thermo Scientific); Ki-67 (1:500; AbCam); GFP-Alexa Fluor 488 (1:500; Invitrogen); p27kip1 (1:100; Thermo Scientific and NeoMarkers); nuclei were stained with DAPI (1:3000; Roche). For BrdU labeling (1:250; BD Biosciences), tissue was first incubated in 1 M HCl for 30 minutes. Some antibodies required antigen recovery at 95°C in citrate buffer for 15 minutes. Species-specific Alexa-conjugated secondary antibodies (1:1000; Invitrogen) were applied for 1 hour in PBST (PBS with 0.5% Tween 20). For p27kip1 immunohistochemistry on sections, standard alkaline phosphatase reactions (DAB Kit, Vector Laboratories) were performed following the manufacturer's instructions.
Cell quantification
Explants were established at E12 and maintained for 4 days in vitro (DIV) in control, FH535- or LiCl-treated media along with BrdU. For each explant we counted the number of Sox2-positive cells at the basal-apical midpoint within a 200×100 μm box positioned at the lateral edge of the Sox2-positive domain and extending 100 μm medially. The percentage of Sox2+ BrdU+ cells was determined (n=9 explants counted per condition). Similar counts were obtained for explants established at E13.5 from the region including the first row of outer HCs and extending laterally to the edge of the culture; the number of Ki-67+ BrdU+ cells was also determined (Sox2/BrdU: n=8 control, n=7 LiCl explants counted; Sox2/Ki-67: n=6 control, n=3 LiCl explants counted). Images were obtained with a Zeiss LSM510 confocal microscope.
Quantitative RT-PCR and affinity ligation protein assays
E13.5 explants were maintained in FH535, Wnt Agonist, LiCl or control media for 5 DIV. Explants were pooled by treatment type, placed into RNAlater (Ambion) and frozen. RNA and protein was purified from each sample (n=3-5 independent sets of paired control/treated cultures for each condition consisting of six explants per condition per sample set) using the PARIS Kit (Ambion). Protein was quantified using the RC/DC Lowry assay (BioRad). RNA was reverse transcribed using the High Capacity RNA-to-cDNA Kit (Applied Biosystems). For qRT-PCR gene expression assays, Applied Biosystems primers were used: Gapdh (Mm99999915_g1) as control, Sox2 (Mm00488369_s1) and Atoh1 (Mm00476035_s1), with an automatic threshold and baseline. For affinity ligation-based protein expression assays (Swartzman et al., 2010), TaqMan Protein Assay Kits were used (Applied Biosystems) according to the manufacturer's instructions with polyclonal biotinylated cathepsin B (as control) or Sox2 antibodies (R&D Systems) to generate probes to measure relative protein levels between control and Wnt/β-catenin-activated cultures.
RESULTS
Canonical Wnt signaling is active in the developing mammalian cochlea
We first sought to confirm that direct activation of the TCF/Lef complex occurs within the developing cochlea. We analyzed canonical Wnt activity in embryonic cochleae from TCF/Lef:H2B-GFP reporter mice possessing six copies of a TCF/Lef responsive element linked to an hsp68 promoter that drives expression of an H2B-GFP fusion protein (Ferrer-Vaquer et al., 2010). This transgenic mouse provides highly specific single-cell resolution readouts of canonical Wnt activity. We analyzed cochleae at the proliferative E12.5 prosensory stage, at postmitotic E13.5, which corresponds to the onset of differentiation, at E14.5 when HC differentiation begins, and at E17.5 when HC differentiation is complete (n>4 embryos analyzed for each stage); we also verified the specificity of this reporter in vitro by photobleaching the GFP signal then treating tissue with a Wnt/β-catenin-specific inhibitor and observed a failure of the GFP signal to recover (supplementary material Fig. S2).
Within E12.5 TCF/Lef:H2B-GFP cochleae we observed high levels of nuclear GFP activity throughout the floor of the cochlear duct where most of the GFP-positive cells expressed the prosensory marker Sox2 and many were also positive for Ki-67 (Mki67 – Mouse Genome Informatics) (Fig. 1A), a protein that is expressed when cells are actively mitotic (Scholzen and Gerdes, 2000). Similar levels of GFP were present in E13.5 cochlear ducts, where Wnt activity was observed in the p27kip1-positive presumptive OC (supplementary material Fig. S3A). At E14.5, high levels of GFP were still detected within the apex (Fig. 1B), whereas the basal region showed weak expression of GFP in the Sox2-positive presumptive OC (Fig. 1C), which did not express Ki-67 (data not shown). High levels of GFP were also detected in the developing stria vascularis. In the midbasal region, an intermediate level of GFP expression was observed (supplementary material Fig. S3B,C). Thus, reporter activity appeared highest in younger prosensory cells and progressively diminished in some regions coinciding with the onset of differentiation. At E17.5, when HC differentiation is mostly complete, low-level activity was detected primarily within the pillar cells, inner phalangeal cells and lateral support cells (Fig. 1D; supplementary material Fig. S4), comparable to the levels observed in the E14.5 base. This pattern of activity was similar to that in reporter mice for Lgr5, a target of Wnt/β-catenin signaling (Chai et al., 2011).
Fig. 1.
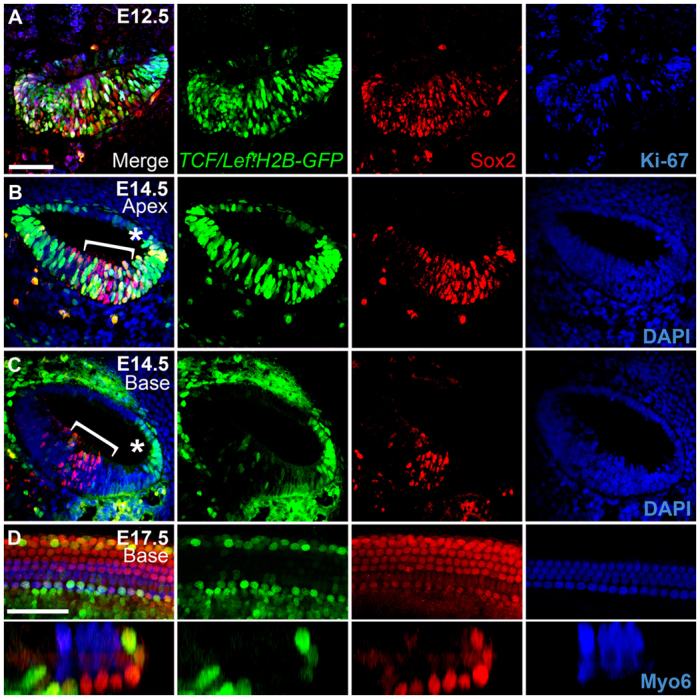
In vivo canonical Wnt/β-catenin reporter activity in the mouse developing cochlear duct. (A-C) Transverse sections through heterozygous E12.5 (A) and E14.5 (B,C) TCF/Lef:H2B-GFP reporter cochleae (medial is left, lateral is right). Merged views of GFP (green), Sox2 (red) and either Ki-67 or DAPI (blue) are shown on the left, with individual channels to the right. The apical (B) and basal (C) turns from the same E14.5 section are shown (a low-magnification image of this section is shown in supplementary material Fig. S3). Asterisk indicates the stria vascularis; brackets indicate the Sox2-positive domain. (D) High-magnification lumenal surface view (top) and z-section (bottom) of an E17.5 TCF/Lef:H2B-GFP reporter cochlea; colors are the same as above, except that hair cells (HCs) are labeled for myosin 6 (Myo6, blue). Note that for E14.5 confocal images, settings were calibrated to the brightest apical region in the sample and these settings were used for the high-magnification imaging of the basal region; thus, levels in the base appear much weaker than in the E17.5 sample, which was calibrated separately. Scale bars: 50 μm.
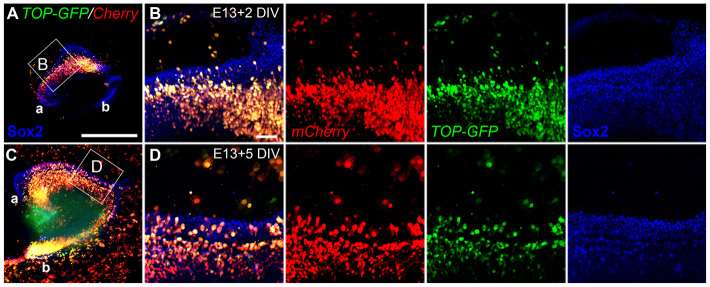
To confirm our in vivo results, we performed an independent assay for canonical Wnt activity in which organotypic embryonic cochlear cultures were transfected with a TOP-dGFP/pCAG-mCherry dual expression plasmid. The mCherry provides a transfection marker and TOP is the promoter/enhancer region of TOPFLASH containing four TCF binding sites that drive expression of a degradable GFP to report Wnt activity (Woodhead et al., 2006; Yokota et al., 2009). Explants electroporated at E13.5 and fixed after 2-5 DIV showed GFP expression in transfected cells both within and outside of the prosensory domain, as identified by co-expression with Sox2 (Fig. 2; n>6 explants examined per condition).
Fig. 2.
Wnt/β-catenin signaling is active in cultured mouse embryonic cochlear explants. (A-D) Low- (A,C) and high- (B,D) magnification images of E13.5 cochlear explant cultures electroporated with the TOP-dGFP/pCAG-mCherry reporter construct and maintained for either 2 (A,B) or 5 (C,D) DIV. The boxed regions in A and C are magnified in B and D, respectively; medial is towards the bottom and lateral towards the top. Red (mCherry) marks transfected cells; green (TOP-dGFP) indicates cells with active TCF signaling; Sox2 (blue) is shown to indicate the prosensory/organ of Corti (OC) domain. a, apical region; b, basal region. Scale bars: 500 μm in A,C; 50 μm in B,D.
Combined, our in vivo and in vitro reporters demonstrate that Wnt/β-catenin signaling is present and active in and surrounding the prosensory domain during cochlear development, and that the level of signaling is reduced with the onset of differentiation in the OC.
Wnt/β-catenin regulates proliferation within the mitotic E12 prosensory domain
Wnt/β-catenin signaling is known to regulate proliferation within developing neural progenitor cells and stem cells (MacDonald et al., 2009). Given the high levels of TCF/Lef reporter activity observed within the mitotic prosensory domain and the reduced reporter activity coinciding with terminal mitosis, we hypothesized that canonical Wnt signaling might regulate proliferation within the prosensory domain. At E12, when reporter activity is highest throughout the cochlear duct (Fig. 1A), the prosensory domain is still proliferating (Ruben, 1967). If Wnt/β-catenin activity is required for proliferation during this period, then inhibition or activation of the pathway prior to terminal mitosis should result in a reduction or enhancement in the number of proliferating cells, respectively. To test this hypothesis, we utilized an in vitro strategy in which cochlear explants were established at E12 and maintained in one of two different Wnt/β-catenin inhibitors: FH535, a specific TCF inhibitor (Handeli and Simon, 2008), or IWR-1, which acts by stabilizing axin and subsequently increasing β-catenin degradation (Chen et al., 2009). Conversely, some explants were maintained in LiCl, an agonist of Wnt/β-catenin signaling that blocks the kinase activity of Gsk3β, preventing β-catenin degradation (Klein and Melton, 1996). Controls were treated with either NaCl or DMSO. Cultures were also exposed to the DNA replication indicator 5-bromodeoxyuridine (BrdU) throughout the culture period and were fixed after 5 DIV.
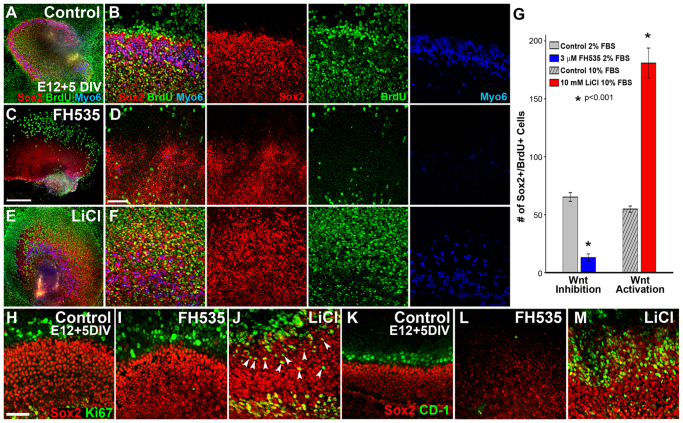
Immunocytochemistry and subsequent quantification revealed a moderate level of BrdU incorporation in the OC of control explants (Fig. 3A,B,G), whereas FH535-treated cultures showed a significant reduction in the number of BrdU-positive cells (Fig. 3C,D,G), as well as a reduction in Sox2 expression and an almost complete absence of HCs, as indicated by reduced expression of the HC marker myosin 6 (Fig. 3C,D). Similar effects on proliferation and HC differentiation were obtained with IWR-1 (supplementary material Fig. S5). By contrast, LiCl-treated explants showed a significant increase in the number of BrdU-positive cells within the OC (Fig. 3E-G; n=9 explants per condition; P≤0.001), a concurrent expansion in the size of the Sox2-positive domain and an increase in HCs (Fig. 3E,F). Thus, inhibition of Wnt/β-catenin signaling significantly decreased proliferation, whereas activation significantly increased proliferation.
Fig. 3.
Modulation of Wnt/β-catenin signaling in the mitotic E12 prosensory domain affects proliferation and HC differentiation. (A-F) Low- (A,C,E) and high- (B,D,F) magnification lumenal surface views of E12 sensory epithelial explants maintained for 5 DIV with BrdU in control media (A,B), the Wnt/β-catenin inhibitor FH535 (C,D) or the Wnt/β-catenin activator LiCl (E,F). (B,D,F) Merged views are shown on the left and individual channels to the right. In controls, immunocytochemistry for Sox2 (red), BrdU (green) and Myo6 (blue) indicated that HCs develop normally and there is a moderate level of BrdU incorporation by Sox2-positive cells (A,B). Wnt inhibition results in a substantial reduction in HCs, BrdU incorporation and the size of the prosensory field (C,D), whereas Wnt/β-catenin activation substantially increases the number of HCs and BrdU-positive cells and the size of the prosensory field (E,F). (G) Quantification of Sox2+ BrdU+ cells within a 200×100 μm box positioned over the lateral HC domain at the basal-apical midpoint in control or Wnt-modulated explants after 4 DIV revealed a significant reduction or expansion in the number of cells that had undergone mitosis following treatment with Wnt inhibitor, or Wnt activator, respectively, compared with controls. Control samples maintained in low (2.5%) or high (10%) fetal bovine serum (FBS) appeared similar by immunocytochemistry, but were quantified independently; n=9 explants counted per condition; *P≤0.001 for both treatments. Error bars indicate ±s.e.m. (H-J) To identify ongoing proliferation, immunocytochemistry for Ki-67 (green) was performed on E12 control (H), FH535-treated (I) and LiCl-treated (J) explants maintained for 5 DIV. Almost no Ki-67-positive cells were observed in the Sox2-positive OC domain in control (H) and FH535-treated (I) cultures, whereas many were observed in LiCl-treated explants (J, arrowheads). (K-M) Expression of the cell cycle regulator cyclin D1 (CD-1, green) was observed just outside of the Sox2-positive (red) sensory domain in control explants (K), whereas CD-1 was almost completely absent from FH535-treated cultures (L). In LiCl-treated samples, CD-1 expression was increased and overlapped with the expanded Sox2-positive domain (M). Scale bars: 300 μm in A,C,E; 100 μm in B,D,F; 50 μm in H-M.
To determine whether proliferation was ongoing in the prosensory domain of these explants or was restricted to the initial phase of treatment, we immunolabeled some explants for the active proliferation marker Ki-67. Whereas almost no cells within the Sox2-positive domain of control or FH535-treated explants established at E12 and maintained for 5 DIV were Ki-67 positive (Fig. 3H,I), many Sox2+ Ki-67+ cells were observed in LiCl-treated explants (Fig. 3J; n>6 explants per condition). Given that Sox2 has been shown to be downstream of Wnt/β-catenin signaling during retinal progenitor cell maintenance (Agathocleous et al., 2009) and within embryonic stem cells (MacDonald et al., 2009), our results suggested that sustained Wnt/β-catenin activity could maintain the proliferative state of Sox2-positive progenitor cells.
In addition to Sox2, cyclin D1 (Ccnd1; CD-1), a cell cycle regulator that drives cells into the proliferative phase of the cell cycle, has been shown to be downstream of Wnt/β-catenin (Shtutman et al., 1999) and Sox2 (Chen et al., 2008), and in the cochlea CD-1 regulates the proliferative state of prosensory and support cells (Laine et al., 2010). Therefore, we assayed for CD-1 expression following modulation of Wnt/β-catenin signaling in cultures established at E12. After 5 DIV, a few rows of cells on the lateral edge of the OC expressed CD-1 in control explants (Fig. 3K), whereas this expression was absent following FH535 treatment (Fig. 3L). Conversely, LiCl induced the upregulation of CD-1 immunoreactivity within most Sox2-positive cells (Fig. 3M; n>6 explants per condition), suggesting that CD-1 might mediate the Wnt/β-catenin-induced proliferation of Sox2-positive prosensory cells.
Canonical Wnt activation induces proliferation within the postmitotic cochlear prosensory domain
Although our results indicated that Wnt/β-catenin signaling regulates endogenous proliferation within the early E12 prosensory domain, we wanted to establish whether Wnt activation could induce proliferation after the period of terminal mitosis in cultured E13.5 cochleae, as Wnt/β-catenin has been shown to induce proliferation in typically quiescent cells in other organ systems, as well as promote oncogenesis. Activating Wnt/β-catenin signaling at E13.5 with LiCl increased the number of HCs compared with controls and also caused a robust lateral expansion of the Sox2-positive domain after 4 DIV (Fig. 4A,B). After 6 DIV, the lateral Sox2 domain was expanded even further (Fig. 4C). This continued expansion of the lateral prosensory domain suggested that these Sox2-positive cells were proliferating. To confirm this, cultures were established at E13.5 and maintained in LiCl or control conditions for 3 DIV, after which BrdU was applied (equivalent to stage E16.5); explants were then maintained for an additional 3 DIV in control/BrdU or LiCl/BrdU media. Following this treatment, almost no Sox2+ BrdU+ cells were observed in control explants, whereas many were observed in LiCl-treated samples (Fig. 4D,E; n>15 explants per condition). However, unlike treatment at E12 (Fig. 3), few Sox2+ BrdU+ cells were found in the medial OC region, and most were restricted to the lateral domain. This suggested that Wnt/β-catenin activation at E13.5 induces proliferation and expands the lateral domain of Sox2-positive cells, whereas under control conditions Sox2-positive cells do not proliferate at this stage. When treatment with LiCl and BrdU was delayed until E16, no change in HCs was apparent, although a limited increase in Sox2-positive cells and BrdU incorporation within the pillar cells, inner phalangeal cells and the greater epithelial ridge was observed after 4 DIV compared with controls (Fig. 4F,G). It is important to note that these are the same cells that showed high levels of canonical Wnt activity in both the E17.5 TCF/Lef:H2B-GFP (Fig. 1E; supplementary material Fig. S4) and Lgr5 reporter mice (Chai et al., 2011) and that can respond to ectopic Wnt activation in postnatal cochlear cells (Chai et al., 2012; Shi et al., 2012).
Fig. 4.
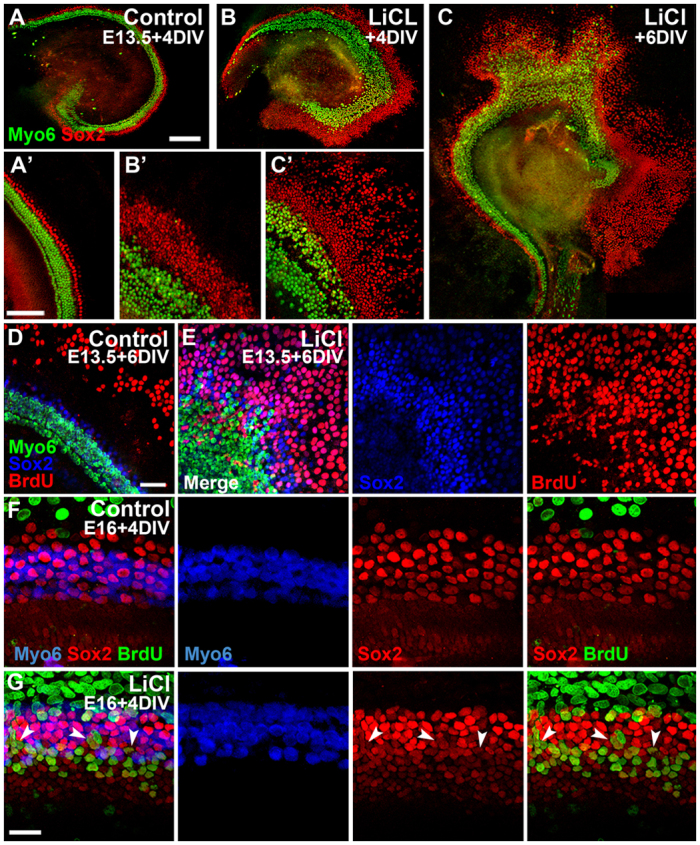
Wnt/β-catenin activation induces proliferation in typically quiescent Sox2-positive cells of the differentiating prosensory domain. (A-C′) Low- (A-C) and high- (A′-C′) magnification lumenal views of different E13.5 control and LiCl-treated explants maintained for 4 (A,B) or 6 (C) DIV and immunostained for Sox2 (red) and Myo6 (green). In controls (A,A′), Sox2 expression extends two rows beyond the lateralmost outer HCs. After 4 DIV in LiCl (B,B′), Sox2-positive cells extend more than 100 μm beyond the lateral HC domain and, after 6 DIV in LiCl, the Sox2 domain is expanded even further laterally (>300 μm; C,C′). (D,E) Surface views of control (D) and LiCl-treated (E) explants established at E13.5 and maintained for 6 DIV; BrdU was applied after 3 DIV. No Sox2+ BrdU+ cells are observed in controls (D), whereas LiCl-treated explants have many Sox2+ BrdU+ cells in the expanded lateral domain (E; individual channels shown to right). (F,G) Control cultures established at E16 and maintained for 5 DIV in BrdU have no Sox2+ BrdU+ cells in the OC (F). In LiCl-treated explants, a subset of Sox2-positive cells, including pillar cells (arrowheads), inner phalangeal cells and support cells at the lateralmost edge of the OC (G), are BrdU positive, although HCs are unchanged. Scale bars: 200 μm in A-C; 100 μm in A′-C′; 50 μm in D,E; 25 μm in F,G.
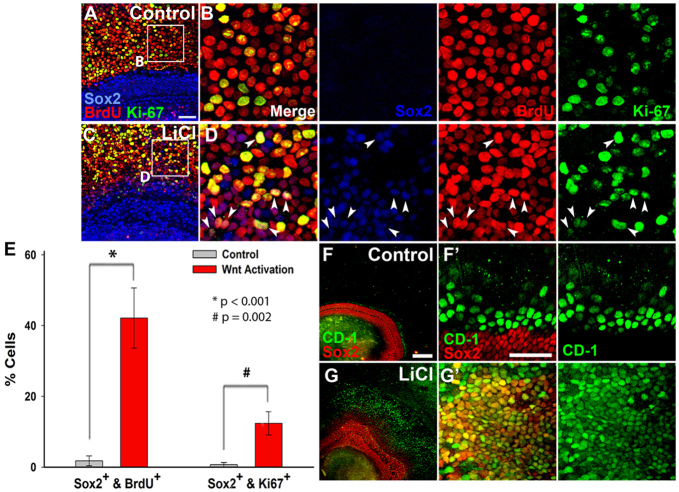
To further examine the effect of Wnt/β-catenin activation on typically postmitotic cells we performed a second mitotic assay to identify cells that were actively proliferating. In E13.5 explants maintained for 5 DIV, almost no cells were Ki-67+ BrdU+ as well as Sox2 positive (Fig. 5A,B). By contrast, numerous triple-labeled cells were observed following LiCl treatment (Fig. 5C,D). Quantification revealed a significant increase in the total number of Sox2-positive cells at the basal-apical midpoint, as well as a significant increase the number of Sox2+ BrdU+ and Sox2+ Ki-67+ cells in LiCl-treated explants compared with controls (Fig. 5E; n=4 control and n=3 LiCl-treated explants). Similar to activation at E12, almost all ectopic Sox2-positive cells in cultures treated with LiCl at E13.5 for 5 DIV also expressed CD-1, whereas its expression was limited in control explants (Fig. 5F,G). Moreover, CD-1 expression was never observed in mitotic lesser epithelial ridge (LER) cells of control explants, further suggesting that CD-1 might mediate the Wnt/β-catenin-induced proliferation of Sox2-positive prosensory cells.
Fig. 5.
Wnt/β-catenin-induced sustained proliferation within differentiating Sox2-positive prosensory cells is mediated by upregulation of cyclin D1. (A-D) Explants established at E13.5 and maintained for 5 DIV in BrdU show high levels of BrdU (red), Sox2 (blue) and Ki-67 (green) co-staining in LiCl-treated explants (C,D) compared with controls (A,B). The boxed regions in A and C are magnified in B and D, respectively; individual channels are shown to the right of merge. Arrowheads indicate Sox2+ Ki-67+ BrdU+ cells. (E) Quantification of the number of Sox2+ BrdU+ and Sox2+ Ki-67+ cells in control and LiCl-treated samples. Error bars indicate ±s.e.m.; *P<0.001 for Sox2+ BrdU+; #P=0.002 for Sox2+ Ki-67+. (F-G′) Immunocytochemistry for CD-1 (green) showing co-expression with Sox2 (red) in LiCl-treated explants (G,G′) but not in controls (F,F′) established at E13.5 and maintained for 5 DIV. (F′,G′) High-magnification views of F and G, showing Sox2 and CD-1 shown merged at left and CD-1 alone at right. Scale bars: 50 μm in A,C,F′,G′; 200 μm in F,G.
Combined, these experiments demonstrate that sustained canonical Wnt activation after E13.5 can promote continuous proliferation within a subset of Sox2-positive cells in the developing cochlear duct that would otherwise be quiescent after the period of terminal mitosis. By contrast, treatment during the mitotic phase at E12 induces enhanced proliferation in Sox2-positive cells throughout the entire prosensory domain and within the OC.
Wnt/β-catenin signaling is required for HC differentiation
The reduced proliferation following FH535 treatment at E12 was not sufficient to explain the nearly complete loss of HCs as most prosensory cells have formed by this stage, suggesting that Wnt/β-catenin signaling might have an additional function during HC differentiation. Recent studies have identified that high-level Wnt activity promotes proliferation, whereas low-level signals may be required for differentiation (Fossat et al., 2011). Thus, to further investigate the role of Wnt/β-catenin signaling during HC differentiation, and to characterize these functions independently of its proliferative role, we analyzed explants established at E13.5 after the period of terminal mitosis. Similar to treatment at E12, E13.5 explants maintained in FH535 for either 3 or 5 DIV showed a substantial reduction in the number of HCs compared with controls (Fig. 6A,B; n>10 explants per condition).
Fig. 6.
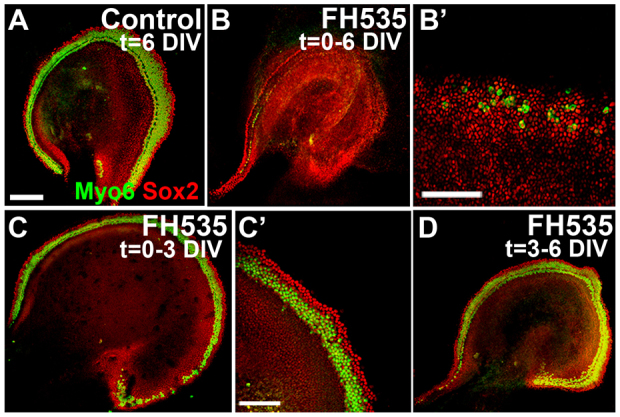
Canonical Wnt signaling is necessary for cochlear HC differentiation. (A-B′) Lumenal views of E13.5 explants maintained for 6 DIV in control medium (A) or with FH535 (B,B′) and immunostained for Sox2 (red) and Myo6 (green). (B′) High-magnification view of the midbasal region from an explant treated with FH535, similar to that in B. (C,C′) Explants treated with FH535 for the initial 3 DIV then maintained for an additional 3 DIV in control media developed a relatively normal complement of HCs and Sox2 expression compared with control (A). (D) When explants were maintained under control conditions for 3 DIV, then treated with FH535 for an additional 3 DIV beginning at a stage equivalent to E16.5, no change in Sox2 expression or HCs was observed compared with control (A). Scale bars: 200 μm in A,B,C,D; 100 μm in B′,C′.
Further analysis revealed that the effect of FH535 on HC formation was reversible. We performed a washout experiment in which cultures were established at E12 or E13.5 and maintained for 3 DIV in FH535 or control media, then all cultures were washed and maintained for an additional 3 DIV under control conditions. Following this treatment course a relatively normal pattern of HCs was observed in all explants (E13.5 shown in Fig. 6C,C′; E12 shown in supplementary material Fig. S6A,B) and there was no significant difference in the number of HCs between control cultures and those transiently exposed to FH535 (supplementary material Fig. S7). Explants fixed 1, 2 or 3 days after removing FH535 from the medium showed that HC differentiation followed the normal basal-to-apical and medial-to-lateral pattern (data not shown). Alternatively, if explants were established at E13.5 and maintained for the first 3 DIV in control media (equivalent to stage E16.5) and then were incubated in FH535 for an additional 3 DIV, no effect on HC formation was observed (Fig. 6D; n>6 explants per condition). These results demonstrate that Wnt/β-catenin signaling is required between E13.5 and E16 for HC differentiation, distinct from its function during early prosensory proliferation.
In contrast to inhibition, Wnt/β-catenin activation induced supernumerary HCs. To further investigate this effect we activated the signaling cascade in postmitotic E13.5 explants utilizing three different pharmacological agents (Liu et al., 2005; Meijer et al., 2003). Activating Wnt/β-catenin signaling with LiCl, Wnt Agonist or BIO at E13.5 (n>10 explants per condition) for 5 DIV increased the number of both inner and outer HCs compared with controls (LiCl results shown in Fig. 4; supplementary material Fig. S8A-D shows similar results from the three activators). Determination of inner versus outer HCs was based on their separation by p75ntr-positive pillar cells, and overall there was an increase in the number of nerve growth factor receptor (p75ntr)-positive cells following Wnt activation (supplementary material Fig. S8E,F). To quantify the change in OC size, we measured the overall width of the HC domain and found that, in explants that had been exposed to LiCl, it was almost double that in controls (P<0.001; n=6 explants per condition; supplementary material Fig. S8G,H shows examples of measured explants; supplementary material Fig. S9A shows the quantification).
To further quantify the effects of Wnt modulation, we also examined the relative expression levels of Sox2 and Atoh1, which are required for HC formation and are also downstream targets of Wnt/β-catenin signaling (Agathocleous et al., 2009; Leow et al., 2004; Shi et al., 2010). Exposure of E13.5 cultures to Wnt inhibitor for 5 DIV resulted in a significant decrease in both Sox2 and Atoh1 mRNA levels compared with controls (supplementary material Fig. S9B). Conversely, exposure to Wnt activators (LiCl or Wnt Agonist) for 5 DIV resulted in a significant doubling of both Sox2 and Atoh1 mRNA levels as well as Sox2 protein levels compared with controls (supplementary material Fig. S9C,D). These results suggest that both Sox2 and Atoh1 might be regulated by Wnt/β-catenin signaling during prosensory and HC formation.
Wnt/β-catenin signaling promotes prosensory identity
The increase in the HC domain following Wnt activation at E13.5 and the large lateral expansion of Sox2 suggested that Wnt/β-catenin was not only required for HC differentiation but might also promote prosensory cell identity. Within all inner ear sensory patches, early expression of the Notch ligand Jag1 marks the prosensory domains; HCs eventually downregulate Jag1, although it is maintained in support cells (Lewis et al., 1998; Morrison et al., 1999). To determine whether Wnt/β-catenin signaling induces prosensory cell identity, E12 and E13.5 explants were maintained for 5 DIV in control or LiCl-treated media then assayed for Jag1 expression. In control samples, Jag1 was highly expressed in support cells (Fig. 7A,B), but appeared even higher in LiCl-treated explants where it extended far beyond the HC domain and overlapped with the expanded pool of Sox2-positive cells (Fig. 7A′,B′). By contrast, E12 cultures treated with Wnt inhibitor for 5 DIV showed reduced levels of Jag1 (Fig. 7A″), similar to the reduction in Sox2. These results suggested that Wnt/β-catenin signaling might be required for prosensory identity.
Fig. 7.
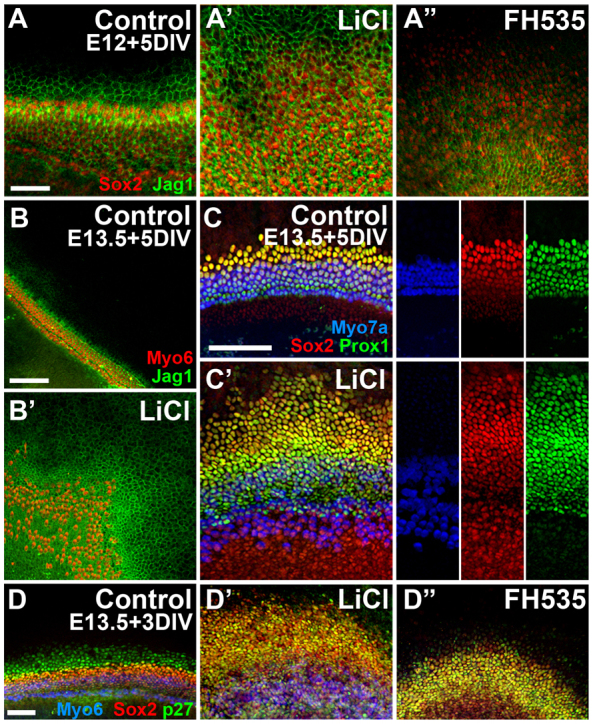
Canonical Wnt signaling induces ectopic prosensory identity. (A-B′) Lumenal surface views showing immunocytochemistry for jagged 1 (Jag1, green) and Sox2 (red, A) or Myo6 (red, B) in E12 (A) or E13.5 (B) explants maintained for 5 DIV. Compared with controls (A,B), Jag1 expression is increased following LiCl treatment (A′,B′) and reduced following Wnt inhibition with FH535 (A″). (C,C′) Prox1 expression is also increased in E13.5 cultures maintained for 5 DIV in LiCl (C′) compared with controls (C); counterstaining is for Sox2 (red) and Myo7a (blue), with merge at left and individual channels to right. (D-D″) Immunocytochemistry for the prosensory marker p27kip1 (green) in cultures established at E13.5 and maintained for only 3 DIV in control media (D), LiCl (D′) or FH535 (D″) and counterstained for Myo6 (blue) and Sox2 (red); n>10 explants per condition per antibody. Scale bars: 50 μm in A-A″,D-D″; 100 μm in B-C′.
In the cochlea, Sox2 has been shown, at least in part, to regulate the expression of the transcription factor Prox1 (Dabdoub et al., 2008), another prosensory marker that is eventually downregulated in HCs (Bermingham-McDonogh et al., 2006). We performed immunocytochemistry for Prox1 following 5 days of LiCl treatment beginning at E13.5, and further confirmed the increase in prosensory cells as it was also co-expressed in the laterally expanded Sox2 domain (Fig. 7C,C′). Like Jag1, Sox2 and Prox1, p27kip1 is also an early marker of the prosensory domain that eventually becomes restricted to support cells of the OC (Chen and Segil, 1999). In E13.5 control and LiCl-treated cultures, p27kip1 was co-expressed in all Sox2-positive cells after 3 DIV (Fig. 7D,D′), including the expanded domain in LiCl-treated cultures. In FH535-treated explants, p27kip1 was also observed in the Sox2-positive domain after 3 DIV (Fig. 7D″), although these explants lacked the population of Sox2− p27kip1+ cells at the lateral edge of the epithelium that was observed in controls. Given that HCs are able to differentiate once FH535 is removed from the medium, it is likely that the epithelium is held in developmental stasis preventing differentiation; thus, similar to the expression of p27kip1 in all prosensory cells early in development, in FH535-treated samples this pattern is likely to be maintained. These p27kip1-positive cells following FH535 treatment are therefore undifferentiated prosensory cells rather than supporting cells.
Sox2-positive cells induced by Wnt/β-catenin activation are competent to differentiate into HCs
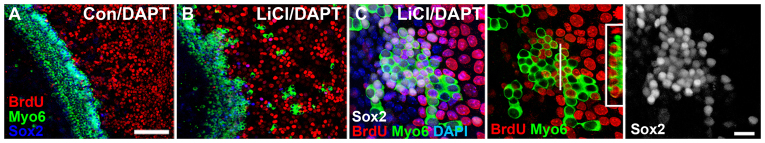
If the Wnt-induced Sox2-positive cells at the lateral edge of the OC in E13.5 explants represent an expanded prosensory domain, they should be competent to differentiate into HCs. Inhibition of the Notch pathway with the γ-secretase inhibitor DAPT has been shown to promote the transdifferentiation of Sox2-positive cells into HCs (Dabdoub et al., 2008; Takebayashi et al., 2007). We thus hypothesized that Notch inhibition within Wnt/β-catenin-induced Sox2-positive prosensory cells should result in the conversion of some of these ectopic Sox2-positive cells into HCs. E13.5 explants were incubated in LiCl for 3 DIV to induce an expansion of the Sox2 domain. Control and LiCl-treated media were then replaced with media containing DAPT and cultures were maintained for an additional 4 DIV (7 DIV total). Control explants maintained in DAPT for 4 DIV had supernumerary HCs in the OC region but no HCs were observed in the LER (Fig. 8A), as previously described (Takebayashi et al., 2007). DAPT treatment of cells pretreated with LiCl, however, resulted in the differentiation of many ectopic HCs within the far lateral domain, in addition to supernumerary HCs in the OC (Fig. 8B).
Fig. 8.
Wnt/β-catenin-induced Sox2-positive proliferative cells are competent to develop into HCs. (A,B) Surface views of explants established at E13 and maintained in control (A) or LiCl-treated (B) media with BrdU for 3 DIV followed by incubation for an additional 3 DIV in media containing DAPT (25 μM) without NaCl or LiCl. DAPT treatment increased HC numbers (Myo6, green) in control and LiCl-treated samples in the OC region. In LiCl/DAPT explants, many HCs are found in the lesser epithelial ridge (LER) but none are observed in control/DAPT explants. (C) High-magnification view of a cluster of HCs from the LER of a LiCl/DAPT-treated culture demonstrating that LiCl conditioning followed by DAPT exposure produces ectopic BrdU-positive HCs. Numerous HCs (Myo6, green) have BrdU-positive (red) and Sox2-positive (white) nuclei, suggesting recent mitotic origins. Inset in the middle panel is an optical transverse section through the HC cluster (position indicated by the white line) showing characteristic HC morphology. Scale bars: 100 μm in A,B; 20 μm in C.
Furthermore, if explants were exposed to BrdU during pretreatment with LiCl and then incubated in DAPT, many of the ectopic lateral HCs were also BrdU positive (Fig. 8C; n>20 explants per condition). If BrdU was added at the time of DAPT treatment (after the initial 3 DIV in LiCl; n≥6 explants per condition), no ectopic BrdU-positive HCs were observed (data not shown). This demonstrates that Wnt/β-catenin signaling, not DAPT treatment, is responsible for BrdU incorporation in the laterally expanded Sox2-positive cells that differentiated into HCs. Combined, these results suggest that the Wnt/β-catenin-mediated proliferative expansion of the prosensory domain produces cells that are competent to differentiate into HCs.
DISCUSSION
In the chicken basilar papilla it has been reported that overexpression of activated β-catenin induces ectopic HC formation (Stevens et al., 2003) and, more recently, it has been shown that canonical Wnt activation can induce proliferation within dissociated epithelial cells of the avian utricle (Alvarado et al., 2011). The role for this pathway during mammalian cochlear development, however, was unknown. Although multiple transgenic canonical Wnt reporter mice have been generated (listed on the Wnt homepage http://www.stanford.edu/group/nusselab/cgi-bin/wnt/), inconsistencies existed as to the exact spatiotemporal pattern of endogenous Wnt/β-catenin activity (Barolo, 2006). In the inner ear, Qian et al. (Qian et al., 2007) reported no Wnt/β-catenin activity in the otocyst and developing cochlea using the BAT-gal mouse (Maretto et al., 2003), whereas Laine et al. (Laine et al., 2010) identified low-level activity in cochleae of the same BAT-gal strain as well as in the TOP-gal reporter (DasGupta and Fuchs, 1999), and Ohyama et al. (Ohyama et al., 2006) observed endogenous Wnt activity in otocysts of the TCF/Lef-lacZ reporter line (Mohamed et al., 2004). A more recent study sought to identify the pattern of Wnt/β-catenin signaling by analyzing reporter mice of the Wnt targets Lgr5 and Axin2; although both were present, their expression was non-overlapping in the cochlea (Chai et al., 2011). Utilizing the TCF/Lef:H2B-GFP mouse (Ferrer-Vaquer et al., 2010), which provides high-resolution single-cell reporting, we describe the pattern of Wnt/β-catenin activity within the developing cochlear duct, which appeared similar to the reported pattern of Lgr5 (Chai et al., 2011). We further confirmed canonical Wnt activity in vitro by transfecting embryonic cochlear explants with an independent reporter construct. Based on in vivo reporter activity, we can conclude that Wnt/β-catenin signaling is highest in the early mitotic prosensory domain and is downregulated with the onset of HC differentiation, although activity is maintained at low levels. The canonical Wnt activity reported throughout the E9.5 otocyst (Ferrer-Vaquer et al., 2010; Ohyama et al., 2006), combined with the activity we observed with both of our reporters outside of the prosensory domain at later stages, suggest that Wnt/β-catenin signaling might also regulate the development of nonsensory cochlear regions.
That low-level TCF/Lef activity was still detected at later developmental stages suggested that it plays a role during both the early proliferative phase of prosensory development as well as during later OC differentiation, which was confirmed by our in vitro functional analysis. We demonstrate that Wnt/β-catenin activation in embryonic cochlear explants causes an increase in proliferation as well as an expansion in the number of HCs and in the expression domain of the prosensory transcription factor Sox2 (Kiernan et al., 2005). Conversely, inhibition of Wnt activity significantly reduces proliferation and HC differentiation. Following Wnt inhibition at postmitotic E13.5, the pattern of Sox2 expression was reminiscent of that at E13, suggesting that inhibiting Wnt/β-catenin signaling delays OC maturation. Recently, it has been shown that Sox2 regulates the level of Atoh1 expression in the prosensory domains of the chick inner ear (Neves et al., 2012), which might partly explain the effects that we observed on HC formation. That the extent of HC formation following Wnt/β-catenin modulation was dose dependent (data not shown) suggests that precise regulation of endogenous levels of Wnt activity is required to regulate normal pattern formation during development of the sensory epithelium. Similarly, a recent study has demonstrated that slight changes in the level of Wnt/β-catenin activity can have significant effects on developmental outcomes (Fossat et al., 2011). Consistent with our observations of TCF/Lef:H2B-GFP reporter mouse cochleae, it is likely that, during normal development, high levels of Wnt/β-catenin activity in the early prosensory domain promote proliferation and low-level activity is needed for differentiation to proceed.
Comparable to that reported for retinal progenitor cells (Agathocleous et al., 2009), continuous Wnt/β-catenin activation in the cochlea upregulates Sox2 and confers a more progenitor-like character. We observed differences, however, in the upregulation of proliferation between E12 and E13.5. Activation at E12 induced proliferation throughout the presumptive OC domain, whereas Wnt activation after E13.5 induced proliferation only in a limited subset of Sox2-positive cells, specifically those at the lateral edge of the OC domain and around the pillar cell region. The usually quiescent Sox2-positive cells, which mitotically respond to canonical Wnt activation at E13.5, are the same cells that maintain endogenous Wnt reporter activity during later developmental stages (including pillar cells, phalangeal cells and lateral edge support cells), suggesting that sustained Wnt/β-catenin activity confers a higher mitotic capacity. Thus, the lateral expansion of the Sox2 domain at E13.5 can be partly attributed to the continued proliferation of lateral Sox2-positive prosensory cells, although the possibility exists that some of these cells might represent LER cells that have been induced to express Sox2. Under normal conditions, however, no Sox2-positive cells are found beyond the lateral edge of the OC. Additionally, if LER cells were being induced to express Sox2, then Sox2-positive cells should be found throughout the LER, which was never the case, as ectopic Sox2-positive cells were only found in a contiguous pool extending from the lateral edge of the OC region. Future lineage-tracing experiments should confirm the source of these ectopic prosensory cells. Furthermore, experiments will also be needed to clarify whether the apparent Wnt/β-catenin induction in prosensory cells is due to direct effects of this pathway on gene expression or is indirectly attributable to the Wnt-induced proliferative expansion within the epithelium.
A recent study of heart development demonstrated that up- or downregulation of β-catenin signaling results in a concurrent increase or decrease in Sox2 expression, respectively, and suggested that the upregulation of Wnt/β-catenin signaling enhances the proliferation of cardiomyocytes (Heallen et al., 2011). In embryonic neural stem cells, Sox2 upregulation via Wnt/β-catenin activation also mediated the ability to proliferate (Cai et al., 2002; Pevny and Nicolis, 2010); thus, the increased mitosis we observed in both mitotic phase E12 prosensory cells as well as in normally quiescent E13.5 prosensory cells is likely to be the result of downstream regulation of Sox2. Although the specific mechanism responsible for inducing this proliferation is unknown, our data suggest that it might be meditated by upregulation of the cell cycle regulator CD-1 (Besson et al., 2008). In cancer cells, β-catenin signaling induces proliferation via TCF/Lef regulation of CD-1 expression (Shtutman et al., 1999), and Laine et al. (Laine et al., 2010) recently suggested that the downregulation of CD-1 is responsible for the reduced proliferative capacity of cochlear prosensory and support cells. Whereas that study did not identify a link between CD-1 and Wnt/β-catenin in postnatal cochleae, here we report that modulation of Wnt signaling regulates CD-1 expression in the embryonic cochlear epithelium, suggesting that CD-1 might be a target of the Wnt/β-catenin pathway during early stages of development. Moreover, it is possible that this upregulation of CD-1 is enhanced by synergistic interactions between β-catenin and Sox2, which have been shown to enhance transcription of CD-1 during oncogenesis (Chen et al., 2008). Although more experiments will be needed to confirm this mode of transcriptional regulation, our data suggest that Wnt/β-catenin-induced upregulation of Sox2 and CD-1 might act in a positive-feedback loop to mitotically produce more Sox2-positive cells. Within the typically quiescent zone of non-proliferation, ectopic Wnt/β-catenin activation selectively enhances the proliferation of Sox2-positive cells, effectively expanding the size of the prosensory domain and the number of cells that are competent to differentiate into mechanosensory HCs.
In addition to Sox2, Notch signaling is also known to function in OC development (Brooker et al., 2006; Hayashi et al., 2008; Kiernan et al., 2006; Pan et al., 2010; Tateya et al., 2011). The Notch ligand Jag1 has been shown to be required for cochlear prosensory formation (Pan et al., 2010), and, in other systems, Wnt/β-catenin signaling regulates the expression of Jag1 (Chen et al., 2010; Rodilla et al., 2009). In this study we observed an expansion of the Jag1 domain following Wnt/β-catenin activation. Moreover, the reported phenotype of Jag1 conditional mutants (Kiernan et al., 2006) is similar to the effects of Wnt/β-catenin inhibition in vitro, with both possessing very few or patchy HCs. Thus, it is likely that Wnt/β-catenin may be upstream of Jag1 and the Notch signaling pathway during OC development. Notch signaling, however, does not seem to be required for proliferation. Conditional deletion of Rbpj, a crucial transcription factor required for Notch signaling, as well as triple knockdowns of Hes1, Hes5 and Hey1, had no effect on prosensory proliferation or the number of prosensory cells that formed (Tateya et al., 2011; Yamamoto et al., 2011), suggesting that the proliferative effect of Wnt/β-catenin might be independent of Notch signaling.
In conclusion, our results suggest that Wnt/β-catenin signaling plays a dual function in the development of the cochlear sensory epithelium by regulating proliferation within the early prosensory domain and HC differentiation at later developmental stages. Our data also demonstrate that, within the postmitotic OC, activation of Wnt/β-catenin signaling induces proliferation, enabling the mitotic generation of HCs.
Supplementary Material
Acknowledgements
We thank Dr A. Chenn for the TOP-dGFP/pCAG-mCherry construct, Willy Sun for technical assistance and Drs E. Keithley, T. Ohyama and D. Fekete for commenting on the manuscript. The myosin 7a antibody was from the Developmental Studies Hybridoma Bank, which is maintained under the NICHD and the University of Iowa.
Footnotes
Funding
Images were generated at the UCSD Cancer Center Microscopy Facility funded by Specialized Support Grant [P30 CA23100]. This work was funded by a Deafness Research Foundation Grant and a grant from the National Institutes of Health [R01DC011104] to A.D. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.080358/-/DC1
References
- Agathocleous M., Iordanova I., Willardsen M. I., Xue X. Y., Vetter M. L., Harris W. A., Moore K. B. (2009). A directional Wnt/beta-catenin-Sox2-proneural pathway regulates the transition from proliferation to differentiation in the Xenopus retina. Development 136, 3289-3299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado D. M., Hawkins R. D., Bashiardes S., Veile R. A., Ku Y. C., Powder K. E., Spriggs M. K., Speck J. D., Warchol M. E., Lovett M. (2011). An RNA interference-based screen of transcription factor genes identifies pathways necessary for sensory regeneration in the avian inner ear. J. Neurosci. 31, 4535-4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barolo S. (2006). Transgenic Wnt/TCF pathway reporters: all you need is Lef? Oncogene 25, 7505-7511 [DOI] [PubMed] [Google Scholar]
- Bermingham N. A., Hassan B. A., Price S. D., Vollrath M. A., Ben-Arie N., Eatock R. A., Bellen H. J., Lysakowski A., Zoghbi H. Y. (1999). Math1: an essential gene for the generation of inner ear hair cells. Science 284, 1837-1841 [DOI] [PubMed] [Google Scholar]
- Bermingham-McDonogh O., Oesterle E. C., Stone J. S., Hume C. R., Huynh H. M., Hayashi T. (2006). Expression of Prox1 during mouse cochlear development. J. Comp. Neurol. 496, 172-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson A., Dowdy S. F., Roberts J. M. (2008). CDK inhibitors: cell cycle regulators and beyond. Dev. Cell 14, 159-169 [DOI] [PubMed] [Google Scholar]
- Brooker R., Hozumi K., Lewis J. (2006). Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development 133, 1277-1286 [DOI] [PubMed] [Google Scholar]
- Cai J., Wu Y., Mirua T., Pierce J. L., Lucero M. T., Albertine K. H., Spangrude G. J., Rao M. S. (2002). Properties of a fetal multipotent neural stem cell (NEP cell). Dev. Biol. 251, 221-240 [DOI] [PubMed] [Google Scholar]
- Chai R., Xia A., Wang T., Jan T. A., Hayashi T., Bermingham-McDonogh O., Cheng A. G. (2011). Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J. Assoc. Res. Otolaryngol. 12, 455-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai R., Kuo B., Wang T., Liaw E. J., Xia A., Jan T. A., Liu Z., Taketo M. M., Oghalai J. S., Nusse R., et al. (2012). Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc. Natl. Acad. Sci. USA 109, 8167-8172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Segil N. (1999). p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 126, 1581-1590 [DOI] [PubMed] [Google Scholar]
- Chen Y., Shi L., Zhang L., Li R., Liang J., Yu W., Sun L., Yang X., Wang Y., Zhang Y., et al. (2008). The molecular mechanism governing the oncogenic potential of SOX2 in breast cancer. J. Biol. Chem. 283, 17969-17978 [DOI] [PubMed] [Google Scholar]
- Chen B., Dodge M. E., Tang W., Lu J., Ma Z., Fan C. W., Wei S., Hao W., Kilgore J., Williams N. S., et al. (2009). Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat. Chem. Biol. 5, 100-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Stoeck A., Lee S. J., Shih le-M., Wang M. M., Wang T. L. (2010). Jagged1 expression regulated by Notch3 and Wnt/β-catenin signaling pathways in ovarian cancer. Oncotarget 1, 210-218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. (2006). Wnt/beta-catenin signaling in development and disease. Cell 127, 469-480 [DOI] [PubMed] [Google Scholar]
- Dabdoub A., Puligilla C., Jones J. M., Fritzsch B., Cheah K. S., Pevny L. H., Kelley M. W. (2008). Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc. Natl. Acad. Sci. USA 105, 18396-18401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R., Fuchs E. (1999). Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557-4568 [DOI] [PubMed] [Google Scholar]
- Ferrer-Vaquer A., Piliszek A., Tian G., Aho R. J., Dufort D., Hadjantonakis A. K. (2010). A sensitive and bright single-cell resolution live imaging reporter of Wnt/β-catenin signaling in the mouse. BMC Dev. Biol. 10, 121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossat N., Jones V., Khoo P. L., Bogani D., Hardy A., Steiner K., Mukhopadhyay M., Westphal H., Nolan P. M., Arkell R., et al. (2011). Stringent requirement of a proper level of canonical WNT signalling activity for head formation in mouse embryo. Development 138, 667-676 [DOI] [PubMed] [Google Scholar]
- Fredriksson S., Gullberg M., Jarvius J., Olsson C., Pietras K., Gustafsdottir S. M., Ostman A., Landegren U. (2002). Protein detection using proximity-dependent DNA ligation assays. Nat. Biotechnol. 20, 473-477 [DOI] [PubMed] [Google Scholar]
- Freyer L., Morrow B. E. (2010). Canonical Wnt signaling modulates Tbx1, Eya1, and Six1 expression, restricting neurogenesis in the otic vesicle. Dev. Dyn. 239, 1708-1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handeli S., Simon J. A. (2008). A small-molecule inhibitor of Tcf/beta-catenin signaling down-regulates PPARgamma and PPARdelta activities. Mol. Cancer Ther. 7, 521-529 [DOI] [PubMed] [Google Scholar]
- Hayashi T., Kokubo H., Hartman B. H., Ray C. A., Reh T. A., Bermingham-McDonogh O. (2008). Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Dev. Biol. 316, 87-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heallen T., Zhang M., Wang J., Bonilla-Claudio M., Klysik E., Johnson R. L., Martin J. F. (2011). Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science 332, 458-461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. C., Klein P. S. (2004). The Frizzled family: receptors for multiple signal transduction pathways. Genome Biol. 5, 234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques B. E., Montcouquiol M. E., Layman E. M., Lewandoski M., Kelley M. W. (2007). Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development 134, 3021-3029 [DOI] [PubMed] [Google Scholar]
- Jayasena C. S., Ohyama T., Segil N., Groves A. K. (2008). Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development 135, 2251-2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin T., George Fantus I., Sun J. (2008). Wnt and beyond Wnt: multiple mechanisms control the transcriptional property of beta-catenin. Cell. Signal. 20, 1697-1704 [DOI] [PubMed] [Google Scholar]
- Jones J. M., Montcouquiol M., Dabdoub A., Woods C., Kelley M. W. (2006). Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J. Neurosci. 26, 550-558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman M. H. (1992). The Atlas of Mouse Development. London: Academic Press; [Google Scholar]
- Kelley M. W., Driver E. C., Puligilla C. (2009). Regulation of cell fate and patterning in the developing mammalian cochlea. Curr. Opin. Otolaryngol. Head Neck Surg. 17, 381-387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan A. E., Pelling A. L., Leung K. K., Tang A. S., Bell D. M., Tease C., Lovell-Badge R., Steel K. P., Cheah K. S. (2005). Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434, 1031-1035 [DOI] [PubMed] [Google Scholar]
- Kiernan A. E., Xu J., Gridley T. (2006). The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2, e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P. S., Melton D. A. (1996). A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 93, 8455-8459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine H., Sulg M., Kirjavainen A., Pirvola U. (2010). Cell cycle regulation in the inner ear sensory epithelia: role of cyclin D1 and cyclin-dependent kinase inhibitors. Dev. Biol. 337, 134-146 [DOI] [PubMed] [Google Scholar]
- Lanford P. J., Lan Y., Jiang R., Lindsell C., Weinmaster G., Gridley T., Kelley M. W. (1999). Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat. Genet. 21, 289-292 [DOI] [PubMed] [Google Scholar]
- Leow C. C., Romero M. S., Ross S., Polakis P., Gao W. Q. (2004). Hath1, down-regulated in colon adenocarcinomas, inhibits proliferation and tumorigenesis of colon cancer cells. Cancer Res. 64, 6050-6057 [DOI] [PubMed] [Google Scholar]
- Lewis A. K., Frantz G. D., Carpenter D. A., de Sauvage F. J., Gao W. Q. (1998). Distinct expression patterns of notch family receptors and ligands during development of the mammalian inner ear. Mech. Dev. 78, 159-163 [DOI] [PubMed] [Google Scholar]
- Liu J., Wu X., Mitchell B., Kintner C., Ding S., Schultz P. G. (2005). A small-molecule agonist of the Wnt signaling pathway. Angew. Chem. Int. Ed. Engl. 44, 1987-1990 [DOI] [PubMed] [Google Scholar]
- MacDonald B. T., Tamai K., He X. (2009). Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbon C. C. (2004). Frizzleds: new members of the superfamily of G-protein-coupled receptors. Front. Biosci. 9, 1048-1058 [DOI] [PubMed] [Google Scholar]
- Maretto S., Cordenonsi M., Dupont S., Braghetta P., Broccoli V., Hassan A. B., Volpin D., Bressan G. M., Piccolo S. (2003). Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA 100, 3299-3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L., Skaltsounis A. L., Magiatis P., Polychronopoulos P., Knockaert M., Leost M., Ryan X. P., Vonica C. A., Brivanlou A., Dajani R., et al. (2003). GSK-3-selective inhibitors derived from Tyrian purple indirubins. Chem. Biol. 10, 1255-1266 [DOI] [PubMed] [Google Scholar]
- Mohamed O. A., Clarke H. J., Dufort D. (2004). Beta-catenin signaling marks the prospective site of primitive streak formation in the mouse embryo. Dev. Dyn. 231, 416-424 [DOI] [PubMed] [Google Scholar]
- Morrison A., Hodgetts C., Gossler A., Hrabé de Angelis M., Lewis J. (1999). Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mech. Dev. 84, 169-172 [DOI] [PubMed] [Google Scholar]
- Neves J., Uchikawa M., Bigas A., Giraldez F. (2012). The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1. PLoS ONE 7, e30871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. (2005). Wnt signaling in disease and in development. Cell Res. 15, 28-32 [DOI] [PubMed] [Google Scholar]
- Ohyama T., Mohamed O. A., Taketo M. M., Dufort D., Groves A. K. (2006). Wnt signals mediate a fate decision between otic placode and epidermis. Development 133, 865-875 [DOI] [PubMed] [Google Scholar]
- Pan W., Jin Y., Stanger B., Kiernan A. E. (2010). Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc. Natl. Acad. Sci. USA 107, 15798-15803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny L. H., Nicolis S. K. (2010). Sox2 roles in neural stem cells. Int. J. Biochem. Cell Biol. 42, 421-424 [DOI] [PubMed] [Google Scholar]
- Qian D., Jones C., Rzadzinska A., Mark S., Zhang X., Steel K. P., Dai X., Chen P. (2007). Wnt5a functions in planar cell polarity regulation in mice. Dev. Biol. 306, 121-133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodilla V., Villanueva A., Obrador-Hevia A., Robert-Moreno A., Fernández-Majada V., Grilli A., López-Bigas N., Bellora N., Albà M. M., Torres F., et al. (2009). Jagged1 is the pathological link between Wnt and Notch pathways in colorectal cancer. Proc. Natl. Acad. Sci. USA 106, 6315-6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruben R. J. (1967). Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. 220, 1-44 [PubMed] [Google Scholar]
- Scholzen T., Gerdes J. (2000). The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182, 311-322 [DOI] [PubMed] [Google Scholar]
- Shi F., Cheng Y. F., Wang X. L., Edge A. S. (2010). Beta-catenin up-regulates Atoh1 expression in neural progenitor cells by interaction with an Atoh1 3′ enhancer. J. Biol. Chem. 285, 392-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F., Kempfle J. S., Edge A. S. (2012). Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J. Neurosci. 32, 9639-9648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shtutman M., Zhurinsky J., Simcha I., Albanese C., D'Amico M., Pestell R., Ben-Ze'ev A. (1999). The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 96, 5522-5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneau L., Gallego M., Pujol R. (2003). Comparative expression patterns of T-, N-, E-cadherins, beta-catenin, and polysialic acid neural cell adhesion molecule in rat cochlea during development: implications for the nature of Kölliker's organ. J. Comp. Neurol. 459, 113-126 [DOI] [PubMed] [Google Scholar]
- Stevens C. B., Davies A. L., Battista S., Lewis J. H., Fekete D. M. (2003). Forced activation of Wnt signaling alters morphogenesis and sensory organ identity in the chicken inner ear. Dev. Biol. 261, 149-164 [DOI] [PubMed] [Google Scholar]
- Swartzman E., Shannon M., Lieu P., Chen S. M., Mooney C., Wei E., Kuykendall J., Tan R., Settineri T., Egry L., et al. (2010). Expanding applications of protein analysis using proximity ligation and qPCR. Methods 50, S23-S26 [DOI] [PubMed] [Google Scholar]
- Takebayashi S., Nakagawa T., Kojima K., Kim T. S., Endo T., Iguchi F., Kita T., Yamamoto N., Ito J. (2005). Nuclear translocation of beta-catenin in developing auditory epithelia of mice. Neuroreport 16, 431-434 [DOI] [PubMed] [Google Scholar]
- Takebayashi S., Yamamoto N., Yabe D., Fukuda H., Kojima K., Ito J., Honjo T. (2007). Multiple roles of Notch signaling in cochlear development. Dev. Biol. 307, 165-178 [DOI] [PubMed] [Google Scholar]
- Tateya T., Imayoshi I., Tateya I., Ito J., Kageyama R. (2011). Cooperative functions of Hes/Hey genes in auditory hair cell and supporting cell development. Dev. Biol. 352, 329-340 [DOI] [PubMed] [Google Scholar]
- Woodhead G. J., Mutch C. A., Olson E. C., Chenn A. (2006). Cell-autonomous beta-catenin signaling regulates cortical precursor proliferation. J. Neurosci. 26, 12620-12630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Chang W., Kelley M. W. (2011). Rbpj regulates development of prosensory cells in the mammalian inner ear. Dev. Biol. 353, 367-379 [DOI] [PubMed] [Google Scholar]
- Yokota Y., Kim W. Y., Chen Y., Wang X., Stanco A., Komuro Y., Snider W., Anton E. S. (2009). The adenomatous polyposis coli protein is an essential regulator of radial glial polarity and construction of the cerebral cortex. Neuron 61, 42-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J. L., Gao W. Q. (2000). Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci. 3, 580-586 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.