Abstract
BACKGROUND
Cardiac allograft vasculopathy (CAV) remains the main cause of long-term transplant rejection. It is characterized by hyperproliferation of vascular smooth muscle cells (VSMCs). Canonical β-catenin signaling is a critical regulator of VSMC proliferation in development; however, the role of this pathway and its regulation in CAV progression are obscure. We investigated the activity of β-catenin signaling and the role for a putative activating ligand, transglutaminase 2 (TG2), in chronic cardiac rejection.
METHODS
Hearts from Bm12 mice were transplanted into C57BL/6 mice (Class II mismatch), and allografts were harvested 8 weeks after transplantation. Accumulation and sub-cellular distribution of β-catenin protein and expression of several components of β-catenin signaling were analyzed as hallmarks of pathway activation. In vitro, PDGF treatment was used to mimic the inflammatory milieu in VSMC and organotypic heart slice cultures.
RESULTS
Activation of β-catenin in allografts compared to isografts or naïve hearts was evidenced by the augmented expression of β-catenin target genes, as well as the accumulation and nuclear localization of the β-catenin protein in VSMCs of the occluded allograft vessels. Expression of TG2, an activator of β-catenin signaling in VSMCs, was dramatically increased in allografts. Further, our ex vivo data demonstrate that TG2 is required for VSMC proliferation and for β-catenin activation by PDGF in cardiac tissue.
CONCLUSIONS
β-catenin signaling is activated in occluded vessels in murine cardiac allografts. TG2 is implicated as an endogenous activator of this signaling pathway and may therefore have a role in the pathogenesis of CAV during chronic allograft rejection.
Cardiac allograft vasculopathy (CAV) is one of the leading causes of chronic rejection for heart transplant recipients. CAV is characterized by neointimal growth and is described as a healing response to immune injury of the vessels, which leads to progressive thickening of the arterial wall by accumulation of vascular smooth muscle cells (VSMCs) and expansion of the extracellular matrix.1 During alloimmune responses a variety of cytokines and growth factors are up-regulated, including platelet-derived growth factor (PDGF),2,3 which is a potent regulator of proliferation in VSMCs and has been associated with CAV progression.4 Although the current regimens of anti-rejection therapies are aimed at suppressing the immune response, many of these drugs also owe part of their efficacy to the attenuation of VSMC proliferation 5 as has been shown for example for immunosuppressive inhibitors of mammalian target of rapamycin (mTOR).6 However, immunosuppressant drugs often confer high risk of infection and malignancy 5, 6 and there has been only a modest 2–4% increase in long-term graft survival,7 accentuating a clinical need for novel therapeutic approaches to chronic rejection.
The canonical β-catenin signal transduction cascade (described in detail previously in several excellent reviews 8–11) has been implicated in the regulation of VSMC proliferation. This signaling pathway can be activated by Wnt ligands as well as numerous non-Wnt proteins.8 Pathway activation inhibits glycogen synthase kinase 3 beta (GSK-3b)-mediated phosphorylation of β-catenin, leading to accumulation and translocation of the β-catenin protein into the nucleus, where it interacts with TCF/LEF transcription factors to promote the synthesis of β-catenin/TCF/LEF-responsive genes, such as axin 2,12 cyclin D1, 13 and NUMB,14 that regulate cell proliferation. Inhibition of β-catenin signaling in vitro prevents cytokine-induced proliferation,13 while excessively active β-catenin induces proliferation in VSMCs.15 Similarly, in vivo, knock down β-catenin in embryonic VSMCs results in a significant reduction of tunica media.16 Despite a growing appreciation of the β-catenin signaling cascade in VSMC hyperproliferation, evidence for the activation of this pathway in vivo in neointimal hyperplasia is scant.17
We have recently demonstrated that enzyme transglutaminase 2 (TG2) binds to the key receptor of the β-catenin signaling 18 and plays a critical role in its activation in VSMCs.19 TG2 is a ubiquitous calcium-dependent enzyme that catalyzes protein cross-linking, polyamination or deamidation, 20 and regulates various signaling cascades including β-catenin 18, 18 and PDGF.21 In the vasculature, TG2 is expressed by VSMCs, endothelial cells and macrophages,22 and has been associated with various vascular pathologies,23 including the progression of atherosclerotic lesions,24 nitric oxide inhibition-related hypertension,25 and in injury response in carotid artery.21
Here, we examined activation status of the canonical β-catenin signaling pathway in vivo during neointimal growth in cardiac vessels and addressed the potential role for TG2 in activation of this pathway induced by inflammatory cytokine PDGF, emulating pathology of CAV. We demonstrate activation of the β-catenin signaling pathway in the graft tissue from the well-characterized MHC class II-mismatched model of chronic cardiac rejection with limited inflammatory component 26 and localize it to VSMCs in the areas of neointimal thickening, presenting, to our knowledge, the first direct evidence of a role for active β-catenin signaling in the hyperproliferation of VSMCs in cardiac transplant tissue. In addition, we show a critical role for TG2 in β-catenin-induced VSMC proliferation and in the PDGF-mediated activation of β-catenin signaling in heart tissue.
METHODS
Cardiac allografts
The mouse strains used were C57BL/6 (H-2b) (BL/6) and B6.C-H-2bm12 (bm12), which differ only at the A beta gene of the I region of the mouse H-2 complex. In the allograft group, bm12 donor hearts were heterotopically transplanted into BL/6 recipient mice (N=6). In the isograft group, BALB/c (H-2d) mice were used as donors and recipients (N=3). Hearts were transplanted into the abdomen of the recipients as primary vascularized grafts by the microvascular technique previously described by Isobe et al.27 Graft survival was monitored by daily palpation. All of the cardiac allografts functioned up to 8 weeks (confirmed visually at the time of harvest). Animals received humane care in compliance with ‘Principles of Laboratory Animal Care’. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Maryland Medical School.
Graft histology
At the time of explant, cardiac grafts were trisected. The basal part was fixed in 10% formalin and embedded in paraffin for Verhoeff’s elastin staining according to standard protocol.28 The middle part was stored at −70°C in OCT (Tissue Tek) for immunohistochemistry. The apex was immediately snap-frozen for molecular analysis. Elastin-stained sections were used to assess transplant arteriosclerosis. CAV severity was graded by: 0, normal; 1, 1–20% occlusion; 2, 21–40% occlusion; 3, 41–60% occlusion; 4, 61–80% occlusion; 5, >80% occlusion. The CAV severity score for each explant was calculated using the following equation: (no. grade X vessels) × N [number at each grade]/total number of arterial vessels scored.
Immunohistochemistry
Frozen sections (10 μm) from cardiac grafts and naïve hearts were fixed in 4% paraformaldehyde for 10 minutes at 4°C and free aldehydes were quenched with 100 mM glycine. Non-specific staining was blocked with 5% normal goat serum (Thermo Scientific). Sections were incubated in primary antibodies [rabbit anti-β-catenin 1:80 (Santa Cruz biotechnology), rabbit anti-TG2 1:250 (Abcam), rabbit anti-PDGFRa (Abcam) 1:250, or fluorescein isothiocyanate (FITC)-conjugated mouse anti-smooth muscle actin 1:100 (1A4; Abcam)] overnight at 4°C and in secondary antibody [Dylight 555-conjugated goat anti-rabbit 1:400 (Jackson Immunoresearch)] for 90 minutes at room temperature. Sections were stained with DAPI (Sigma) to visualize nuclei. Images were taken using a Leica DMIL fluorescence microscope equipped with a SPOT-RT3 real-time CCD camera (Diagnostic Instruments).
Organotypic heart slice cultures
The organotypic heart slice cultures were performed as originally described.29 Briefly, 1 mm-thick transverse sections of hearts from 3-day old wild type and TG2−/− mice (a kind gift from Robert Graham, Victor Chang Cardiovascular Institute, New South Wales, Australia)30 were sliced using a rodent heart matrix (Harvard Apparatus). Heart slices were immediately transferred to a Millicell-CM 0.4 μm membrane (Millipore), and the insert was placed into a well containing 1 mL of medium in 12-well plates. Heart slices were maintained for 5 days at 37°C in a humidified atmosphere containing 5% CO2 in Dulbecco’s modified Eagle’s:F12 medium (DMEM:F12) supplemented with 20% knockout serum replacement (KSR), 1% non-essential amino acids, 2 mM L-glutamine, 0.1% β-mercaptoethanol, and 0.1% penicillin/streptomycin.. Recombinant mouse PDGF-BB (ProSpec, East Brunswick, NJ) was used at 10 ng/mL.
Quantitative real-time PCR
mRNA was isolated using the Qiagen RNeasy kit and cDNA was synthesized with iScript reverse transcriptase (Bio-Rad) using a DYAD thermocycler (MJ Research). Real-time PCR was performed with EVAgreen in a CFX96 (Bio-Rad) using specific primers (Table 1), designed using PrimerQuest software (Integrated DNA Technologies), or with SYBRgreen in a Roche Lightcycler 480 using the preformatted mouse Wnt/β-catenin signaling PCR array (SA Biosciences). mRNA levels were expressed as relative fold expression (using the ΔΔCq method) in grafts compared to naïve BL/6 heart tissue (all mouse strains used showed similar baseline expression of genes assayed in this study), or in PDGF-treated heart slices compared to untreated control slices.
Table 1.
Primer sequences for mouse genes analyzed by real-time PCR.
| Gene targets | Accession | Forward primer | Reverse primer |
|---|---|---|---|
| Housekeeping | |||
| Ribosomal protein | |||
| L19 (Rpl19) | NM_009078 | aagaggaagggtactgccaatgct | tgaccttcaggtacaggctgtgat |
| Beta-actin | NM_007393 | taatttctgaatggcccaggtct | ctggctgcctcaacacctcaa |
|
| |||
| Transglutaminase | |||
| TG2 | NM_009373 | aggtgtccctgaagaacccacttt | ttccacagacttctgctccttggt |
|
| |||
| β-catenin targets | |||
| Axin2 | NM_015732 | tgactctccttccagatccca | tgcccacactaggctgaca |
| NUMB | NM_010949 | cttcccagttaagtacctcggc | cccgtttttccaaagaagcct |
| Tcf4 | NM_013685 | cgaaaagttcctccgggtttg | cgtagccgggctgattcat |
| cyclinD1 (cycD1) | NM_007631 | gcgtaccctgacaccaatctc | ctcctcttcgcacttctgctc |
|
| |||
| Wnt Ligands | |||
| Wnt3a | NM_009522 | ctcctctcggatacctcttagtg | gcatgatctccacgtagttcctg |
| Wnt7a | NM_009527 | ccttgttgcgcttgttctcc | ggcggggcaatccacatag |
|
| |||
| Platelet-derived growth factor | |||
| PDGF-A | NM_008808 | gttgtaacaccagcagcgtcaag | tgacatactccactttggccacct |
| PDGF-B | NM_011057 | caccgaaagtttaagcacaccca | aaataaccctgcccacactcttg |
| PDGF-C | NM_019971 | atgccacaagtcacagaaaccacg | aaggcagtcacagcattgttgagc |
| PDGF-D | NM_027924 | ggcaggtcataccatgatcgg | acagagtgattcctgggagtgc |
| PDGFRα | NM_011058 | cttcatgagccaacacccaga | tgtgctctcgtctgcaggatt |
| PDGFRβ | NM_008809 | acggaatactgccgatacggtgat | acaatgcttgttggagtgtcgctg |
Cell culture, luciferase activity, and proliferation
The rat aortic smooth muscle cell line (A10 VSMC; ATCC) was maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; Invitrogen) with 10% fetal bovine serum (FBS; Hyclone) and 100 ng/mL penicillin and streptomycin (Invitrogen). A stable A10 VSMC β-catenin luciferase reporter cell line (A10-bcat) was established using the Cignal Lenti TCF/LEF Luciferase Reporter (SA Biosciences) according to manufacturer’s protocol. For the analysis of β-catenin activity and cell proliferation, A10-bcat cells were seeded at 1×104 cells/cm2.
At time 0, cells were transferred to DMEM containing 1% FBS and 0.0075 U/mL (0.005 mg/mL) purified TG2 (Sigma) for 48 hours. Medium was supplemented with quercetin (100 μM; Quercegen, Newton, MA),31 or ERW-1069 (quinolin-3-ylmethyl(S)-1-(((S)-3-bromo-4,5-dihydroisoxazol-5-yl)methylamino)-3-(5-fluoro-1H-indol-3-yl)-1-oxopropan-2-ylcarbamate) (30 μM), kindly provided by Dr. C. Khosla (Stanford University),32 as indicated. Luciferase activity in total cell lysates, normalized to constitutively-active Renilla, was measured in a 96-well plate luminometer (Harta Instruments, Bethesda, MD) using the Promega Dual Luciferase Assay Kit. Total lactate dehydrogenase (LDH) present in cell lysates was measured with LDH kit (Biovision, CA) using Polarstar Optima plate reader. Relative cell proliferation was determined by comparing the total amount of LDH present in cell lysates after 48 hours in culture to LDH present in cell lysates at time 0. Experiments were performed in triplicate.
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). One-way analysis of variance (ANOVA) was used for comparisons between three or more groups (STATVIEW; SAS Institute, Inc.). Unpaired Student t-test was used for comparison between two groups. Data were considered statistically significant at p < 0.05.
RESULTS
β-catenin expression in neointimal VSMCs during chronic allograft rejection
The class II mismatch bm12-to-BL/6 cardiac allograft model employed is characterized by limited acute cellular rejection.26 It supports allograft function for at least 8 weeks in the absence of immunosuppressive therapy, and results in the development of severe cardiac allograft vasculopathy (CAV, severity score 2.9±0.3, N=6) visualized as extensive occlusion of coronary vessels surrounded by multifocal patchy parenchymal and perivascular inflammatory infiltrates (Fig. 1A and Supplemental Fig. 1). Immunohistochemical staining for smooth muscle actin (sma) and β-catenin revealed that the neointimal lesions were largely comprised of VSMCs and that β-catenin protein was increased in the neointimal VSMCs of allograft vessels (Fig. 1B and Supplemental Fig. 2A). Moreover, in many neointimal VSMCs of allograft hearts an accumulation of nuclear β-catenin (arrows in Fig. 1C) was observed, implying activation of canonical β-catenin signaling. Of note, elevated β-catenin is detected only in the hyperproliferating neointimal cells and not in the contractile VSMCs of the vessel wall (outlined with dotted lines in Fig. 1B). In contrast, there was little if any intimal change in the isografts harvested at 100 days after transplantation (CAV score 0.1 ± 0.1, N=3) (Fig. 1A).
Figure 1.
Nuclear localization of β-catenin in CAV. (A) Frozen sections (10 μm) from naïve, isograft, and allograft murine hearts were histologically stained for elastin using Verhoeff-Van Gieson method to visualize coronary vessel occlusion. (B) Adjacent sections were immunohistochemically probed for smooth muscle actin (sma, green) – a marker of VSMCs – and β-catenin (red), and counterstained with DAPI (blue) to label nuclei. Dotted lines denoted tunica media. (C) Higher magnification of boxed area in (B) showing nuclear localization of β-catenin (arrowheads). Scale bar in A = 25 μm. Scale bar in C = 10 μm.
Activation of β-catenin signaling in allografts by TG2
To further assess whether β-catenin signaling is active during CAV progression, we analyzed the expression of downstream β-catenin targets using real-time PCR. Consistent with the increase in nuclear β-catenin protein, expression of the β-catenin target genes axin2, cyclin D1, and numb was also significantly increased in allografts with severe CAV compared to isografts and naïve hearts which show no CAV (Fig. 2A). Likewise, a semi-comprehensive PCR-based microarray analysis of 84 genes related to β-catenin-mediated signal transduction corroborate activation of β-catenin signaling in the allograft tissue by revealing the induction of several additional β-catenin target genes, including the transcription factors Lef1, Tcf7, Pitx2, and Wisp-1, accompanied by the repression of negative regulators of the β-catenin pathway, including Dkk1 and Gsk3 (Table 2). However, expression of Wnt3a and Wnt7a, previously reported as putative activators of β-catenin in vascular tissue 33, was not altered in the allografts (Fig. 2B). The microarray analysis further confirmed a limited role for canonical Wnt ligands in regulation of β-catenin, with four Wnt proteins being down-regulated, eleven family members showing no significant change in expression and only Wnt2 being moderately up-regulated although its role in the canonical β-catenin pathway is uncertain (Table 2).
Figure 2.
Activation of β-catenin signaling in allograft hearts. (A–C) Real-time PCR for downstream β-catenin target genes, axin 2, cyclin D1 (cycD1), and numb (A), putative canonical β-catenin activators Wnt3a and Wnt7a (B), and transglutaminase 2 (TG2) (C) in isograft and allograft murine hearts compared to naïve non-transplanted controls. Expression was normalized to ribosomal protein L19. Real-time PCR reactions were run in duplicate. 3–4 animals per group were analyzed. *, p<0.05; **, p<0.01; NS, not significant. (D) Frozen sections (10 μm) from naïve and allograft murine hearts were immunohistochemically probed for smooth muscle actin (sma, green) and TG2 (red), and counterstained with DAPI (blue) to label nuclei. Scale bar = 15 μm.
Table 2.
Analysis of the canonical β-catenin signaling profile in allografts compared to naïve hearts.
|
|
||
|---|---|---|
| Up-regulated (fold) | Down-regulated (fold) | |
| Wnt (canonical) | Wnt2 (3.55) | Wnt1,8b (2.52,2.68) |
| Wnt (non-canonical) | Wnt2 (3.55) | Wnt1,5b,9a (2.52,2.57,3.75) |
| Wnt binding antagonists | Sfrp1,2,4 (6.33,9.06,3.73) | |
| Negative regulators of β-catenin signaling |
Dkk1 (17.29) Gsk3b (2.68) Sox17 (3.52) |
|
| β-catenin target genes (related to proliferation) |
Slc9a3r1 (2.68) Wisp-1 (10.79) |
|
| β-catenin target genes (transcription factors) |
Lef1 (13.68) Pitx2 (3.52) Tcf7 (10.49) |
|
| Other β-catenin targets | Fshb (5.68) | Aes (3.08) |
These results of the PCR analyses suggest that activation of the β-catenin signaling may be mediated by a non-Wnt protein. Elaborating on our recent finding that TG2 can activate canonical β-catenin signaling in VSMCs in vitro,19 we investigated the expression of TG2 in allograft neointima. Expression of TG2 mRNA is dramatically increased 21.8±4.6-fold (p < 0.05) in allografts with severe CAV compared to isograft and naïve controls (Fig. 2C) in contrast to all known Wnt ligands, as shown by the real-time PCR analysis, and TG2 protein is increased in VSMCs in the occluded vessels in the rejected allograft (Fig. 2D and Supplemental Fig. 2B).
Regulation of VSMC proliferation and β-catenin signaling by TG2
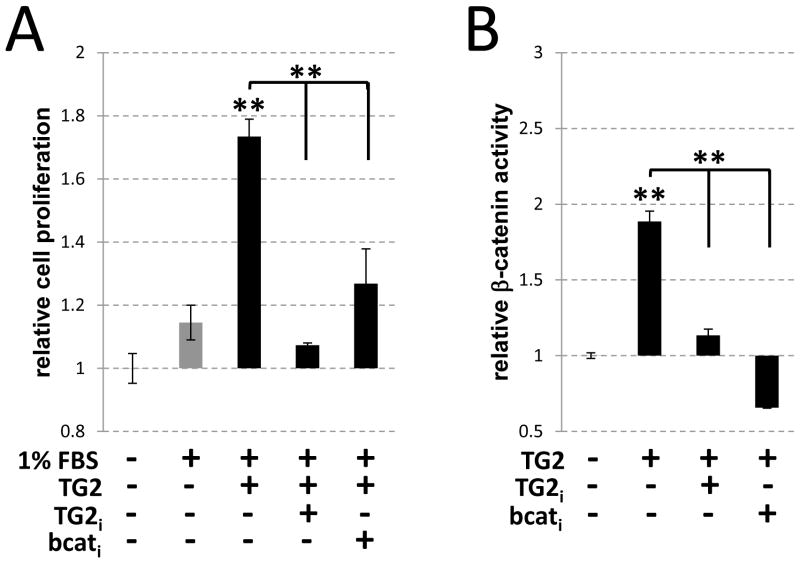
To examine the potential role for elevated TG2 in the regulation of β-catenin-dependent VSMC hyperproliferation we employed the A10 cell line of aortic smooth muscle cells. Purified exogenous TG2 (0.0075 U/ml) induced a 52% increase over serum-stimulated proliferation in VSMCs (p < 0.01) (Fig. 3A, TG2), and this effect was associated with a 1.9±0.1 fold (p < 0.01) increase in the β-catenin-dependent transcription of a luciferase reporter in A10 VSMCs (Fig. 3B, TG2) indicating TG2-induced activation of β-catenin signaling. The TG2-specific inhibitor ERW-1069 (Fig. 3, TG2i) significantly hampered both the activation of the β-catenin-dependent reporter gene and VSMC proliferation, similar to the effects of the β-catenin inhibitor quercetin 31 (Fig. 3, bcati). These results demonstrate that in vitro VSMC hyperproliferation is dependent on the TG2-induced activation of canonical β-catenin signaling.
Figure 3.
Catalytic activity of TG2 is required for β-catenin-dependent VSMC proliferation in vitro. (A–B) A10 VSMCs containing a β-catenin-responsive luciferase reporter (A10-bcat) were cultured in medium containing 1% FBS supplemented with purified TG2 (0.0075 U/mL), TG2 inhibitor ERW-1069 (30 μm; TG2i) or β-catenin inhibitor quercetin (100 μm; bcati) as indicated. (A) Proliferation of A10-bcat cells after 48 hours was determined by comparing lactate dehydrogenase (LDH) released by total cell lysis of treated cells to LDH released by cells at time zero. Cells cultured in the absence of purified TG2 served as a control for normal proliferation in culture. **, p<0.01. n=3–4 per treatment group. (B) Luciferase activity, normalized to constitutively-expressed renilla, was compared in all groups to untreated A10-bcat cells after 48 hours.
TG2 is critical for PDGF-induced activation of β-catenin in cardiac tissue
Increased PDGF levels have been observed in cardiac allograft tissue.2, 3, 34 To obtain a more comprehensive mechanistic understanding on how PDGF signaling can contribute to CAV in the class II mismatch mouse model, in which β-catenin signaling is activated, we examined the expression of PDGF isoforms and receptors in the cardiac grafts. Of the 4 PDGF isoforms, only PDGF-C mRNA was upregulated (3.8-fold±0.8, p < 0.05) in allograft tissue compared to naïve and isograft (Fig. 4A). In addition, PDGF receptor alpha (PDGFRa), was significantly upregulated in both allograft and isograft tissues (4.4-fold±1.4 and 4.2-fold±0.1, respectively) (Fig. 4A and Supplemental Fig. 3A); however, only vessels in the allograft hearts showed punctate staining for PDGFRa indicative of receptor clustering and activation (Fig. 4B and Supplemental Fig. 3B).
Figure 4.
Transglutaminase 2 (TG2) is critical for PDGF-induced activation of β-catenin. (A) Real-time PCR for PDGF-C and PDGF receptor alpha (PDGFRa) in isograft and allograft hearts compared to naïve non-transplanted controls. Expression was normalized to ribosomal protein L19. Real-time PCR reactions were run in duplicate. 3–4 animals per group were analyzed. *, p<0.05. (B) Frozen sections (10 μm) from naïve, isograft, and allograft murine hearts were immunohistochemically probed for smooth muscle actin (sma, green) and PDGFRa (red), and counterstained with DAPI (blue) to label nuclei. Scale bar = 15 μm. (C–E) Real-time PCR for TG2 and PDGFRa in cultured heart slices from wild-type mice treated for 5 days with PDGF (10 ng/mL; black bars) (C), or β-catenin target genes axin2, tcf4, and numb and PDGFRa in heart slices from wild-type (black bars) or TG2-null (dark grey bars) mice treated for 5 days with PDGF (10 ng/mL) (D–E). Expression was normalized to beta-actin, and data is shown as increase in expression compared to untreated control cultures from wild-type or TG2-null heart slices. Heart slices from three to four animals were combined for analysis. *, p<0.05; **, p<0.01.
We next analyzed whether PDGF activates β-catenin signaling in cardiac tissue and whether TG2 plays a role in this activation. For this, we employed a recently established organotypic model in which neonatal heart slices are cultured at the membrane-air interface. Similar to allograft tissue, both TG2 and PDGFRa mRNAs were significantly increased in wild-type heart slices treated with PDGF (3.0-fold±0.3 and 4.8-fold±0.1, respectively) (Fig. 4C). In addition, PDGF treatment induced significant up-regulation of the β-catenin target genes axin2, TCF4, and NUMB (56-fold±6.9, 55-fold±3.3, and 12-fold±0.4, respectively; Fig 4D, WT), indicating activation of the β-catenin signaling pathway in cardiac tissue. Furthermore, genetic ablation of TG2 attenuated the PDGF-induced expression of the β-catenin target genes (Fig. 4D, TG-KO) but not of PDGFRa (Fig. 4E). These results demonstrate a critical and specific role for TG2 in the PDGF-induced activation of β-catenin signaling in heart tissue.
DISCUSSION
In this study we report a novel observation on the activation of β-catenin signaling in neointimal VSMCs following cardiac allograft. In addition, we show increased transcriptional activity of β-catenin in proliferating VSMCs in vitro, and demonstrate coordinate regulation of both processes by TG2 specific inhibitors. These findings suggest that hyperproliferation in neointimal VSMCs may be regulated by the β-catenin pathway. While in healthy vascular tissue the canonical β-catenin signaling is usually inactive,35 it is activated in various cardiovascular disorders, such as cardiac wound healing and cardiac hypertrophy,35, 36 vascular remodeling induced by injury,37 and vascular calcification.33, 38 In the present study, activation of β-catenin in the vascularized rejected cardiac allograft tissue has been demonstrated by direct immunohistochemistry and molecular analysis. We report the in vivo accumulation of nuclear β-catenin protein in neointimal VSMCs of occluded vessels, suggesting activation of β-catenin signaling. Elevated expression of β-catenin target genes such as cyclinD1 (a key protein in cell cycle progression 13), Wisp-1 (a regulator of VSMC proliferation in restenosis 39), Numb (a regulator of Notch degradation in response to β-catenin activation 14 and phenotypic instability in VSMC 40) and the β-catenin transcriptional cofactors TCF and LEF,8 further confirm activation of this signaling pathway in the allograft tissue. A similar role for β-catenin activation has been proposed in the model of severe neointimal thickening in stent-induced endovascular injury.15 However, no direct evidence for elevated β-catenin activity in the graft tissue has been obtained in the latter study due to technical reasons with stent embedding, preventing an unequivocal conclusion on the potential role of this signaling pathway in neointimal growth. Thus, our results provide the first direct evidence, to the best of our knowledge, that canonical β-catenin signaling is activated in vessels with cardiac allograft vasculopathy (CAV),
Furthermore, our data suggest a non-Wnt protein may mediate β-catenin activation in severe CAV. In contrast to moderate changes in the expression of common canonical Wnt ligands, expression of TG2 is dramatically increased over 20-fold in in allograft tissue. These findings suggest that TG2 may contribute to the activation of canonical β-catenin signaling in proliferating neointimal VSMCs during CAV, while Wnt2 – which is also increased 2-fold in the allograft tissue – has previously been shown to have no effect on VSMC proliferation.17 In agreement with a proposed role for TG2 in CAV, elevated levels of this enzyme induce, while its inhibition attenuates, both the activation of β-catenin signaling and VSMC proliferation in vitro. Importantly, the β-catenin inhibitor quercetin 31 also attenuates TG2-induced proliferation suggesting that canonical β-catenin signaling mediates TG2-induced VSMC hyperproliferation, and therefore both TG2 itself and the downstream activation of β-catenin may be potential therapeutic targets to prevent neointimal hyperplasia in vivo.
Moreover, we demonstrate that genetic ablation of TG2 attenuates activation of β-catenin signaling in response to PDGF. This finding adds to the mechanistic understanding of PDGF-induced VSMC proliferation in vitro,4, 13, 17 and β-catenin regulation by PDGF in a Wnt-independent manner 15, 41. Although the precise mechanism of this regulation has not been determined, our data identifies the induction of TG2 as a key step in the regulation of β-catenin by PDGF in cardiac tissue. Considering the observed increase in PDGF in cardiac allografts in both patients 2 and animal models,3 and the fact that blockade of the PDGFRa attenuates coronary myointimal hyperplasia in rat allografts,34 a better understanding of the molecular mechanisms of PDGF activity in CAV may benefit the development of novel therapeutics. In addition to mediating the effects of PDGF on cell proliferation via the β-catenin pathway, as demonstrated by our data, TG2 has been also shown to augment PDGF effects on cell migration, acting as a scaffolding protein through fibronectin adhesive complexes to cluster the PDGF receptors.42 Taking into account a role of activated β-catenin in fibronectin synthesis in VSMCs,15 a tiered regulation of these two processes can be postulated with PDGF-dependent induction of TG2 being a primary (central) step. Furthermore, accumulating evidence suggests a role for TG2 as a common regulator of neointimal growth in various diseases including atherosclerotic lesions24, carotid artery balloon injury,21 and nitric oxide inhibition-related hypertension. 25 Therefore, our data imply that inhibitors specific for the TG2 enzyme, which already show promise in the treatment of neurodegenerative disease and cancer,43 may protect from CAV and thus provide a new approach to the treatment of this condition which remains a significant barrier to long-term graft survival 7 for cardiac transplant recipients.
Because CAV is a chronic condition requiring long-term treatment, effective therapies may require a combinatorial approach, and additional therapeutic options targeted at VSMC hyperproliferation in CAV should be considered to supplement common immunosuppressive therapies. Interestingly, in several cell types activation of β-catenin can be accompanied by activation of mTOR 44 via a GSK-3b-dependent mechanism different from the PI3 kinase/Akt signaling known to regulate mTOR in immune response. This suggests that therapeutic strategies targeting β-catenin activation in allografts may also attenuate mTOR activation; however, further studies will be required to assess whether a similar GSK-dependent activation of mTOR occurs in VSMCs. Regardless, our work has highlighted one pathway, the TG2-mediated activation of β-catenin signaling, that is significantly upregulated during cardiac allograft rejection, responds to the inflammation-induced cytokine PDGF in cardiac tissue, and induces VSMC hyperproliferation in vitro, suggesting that this pathway may provide novel therapeutic targets for the prevention of CAV associated with cardiac allograft chronic rejection.
Supplementary Material
Elastin-stained paraffin sections (5 μm) of heart grafts. A representative artery from an allograft heart harvested at 56 days after transplantation (left) shows extensive neointimal proliferation with multifocal patchy parenchymal infiltrates. In contrast, cardiac isograft harvested at 100 days after transplantation (right) shows an absence of neointimal thickening. Scale bar = 50 μm.
Frozen sections (10 μm) from allograft murine hearts were immunohistochemically probed for smooth muscle actin (sma, green) – a marker of VSMCs – and β-catenin (red) (A) or transglutaminase 2 (TG2, red) (B). Occluded vessels from each of the four allografts examined (A1–A4) show enhanced β-catenin and TG2 expression throughout VSMCs in the vessel neointima. Scale bars = 20 μm.
(A) Real-time PCR for PDGF-A, B, and D isoforms and PDGF receptor beta (PDGFRb) in isograft and allograft hearts compared to naïve non-transplanted controls. Expression was normalized to ribosomal protein L19. Real-time PCR reactions were run in duplicate. 3–4 animals per group were analyzed. (B) Frozen sections (10 μm) from allograft murine hearts were immunohistochemically probed for smooth muscle actin (sma, green) – a marker of VSMCs – and PDGF receptor alpha (PDGFRa, red). Occluded vessels from each of the four allografts examined (A1–A4) show clustered PDGFRa expression in VSMCs.
Abbreviations
- CAV
cardiac allograft vasculopathy
- VSMC
vascular smooth muscle cell
- PDGF
platelet-derived growth factor
- GSK-3b
glycogen synthase kinase 3 beta
- TG2
transglutaminase 2
- sma
smooth muscle actin
- PGDFRa
platelet-derived growth factor receptor alpha
- mTOR
mammalian target of rapamycin
Footnotes
Disclosure Statement
This work was supported by grants from the National Institutes of Health: R01HL093305 awarded to M.N., T32AR007592 fellowship to K.B., 5UO1-AI066719 awarded to Richard N. Pierson, and a University of Maryland Greenebaum Cancer Center Award to A.A. The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Mitchell RN. Graft vascular disease: immune response meets the vessel wall. Annu Rev Pathol. 2009;4:19–47. doi: 10.1146/annurev.pathol.3.121806.151449. [DOI] [PubMed] [Google Scholar]
- 2.Zhao XM, Yeoh TK, Frist WH, Porterfield DL, Miller GG. Induction of acidic fibroblast growth factor and full-length platelet-derived growth factor expression in human cardiac allografts. Analysis by PCR, in situ hybridization, and immunohistochemistry. Circulation. 1994 Aug;90(2):677–85. doi: 10.1161/01.cir.90.2.677. [DOI] [PubMed] [Google Scholar]
- 3.Lemstrom K, Sihvola R, Koskinen P. Expression of platelet-derived growth factor in the development of cardiac allograft vasculopathy in the rat. Transplant Proc. 1997 Feb;29(1–2):1045–6. doi: 10.1016/s0041-1345(96)00362-4. [DOI] [PubMed] [Google Scholar]
- 4.Abid MR, Yano K, Guo S, et al. Forkhead transcription factors inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia. J Biol Chem. 2005 Aug 19;280(33):29864–73. doi: 10.1074/jbc.M502149200. [DOI] [PubMed] [Google Scholar]
- 5.Autieri MV. Antiproliferative effects of immunosuppressant drugs on vascular smooth muscle cells: an additional advantage for attenuating transplant vasculopathy. Drug News Perspect. 2004 Mar;17(2):110–6. doi: 10.1358/dnp.2004.17.2.829044. [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Lazaro IJ, Almenar L, Martinez-Dolz L, et al. Proliferation signal inhibitors in heart transplantation: a 5-year experience. Transplant Proc. 2010 Oct;42(8):2992–3. doi: 10.1016/j.transproceed.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Taylor DO, Stehlik J, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Heart Transplant Report-2009. J Heart Lung Transplant. 2009 Oct;28(10):1007–22. doi: 10.1016/j.healun.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Hendrickx M, Leyns L. Non-conventional Frizzled ligands and Wnt receptors. Dev Growth Differ. 2008 May;50(4):229–43. doi: 10.1111/j.1440-169X.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- 9.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 10.Johnson ML, Rajamannan N. Diseases of Wnt signaling. Rev Endocr Metab Disord. 2006 Jun;7(1–2):41–9. doi: 10.1007/s11154-006-9003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009 Jul;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002 Feb;22(4):1172–83. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quasnichka H, Slater SC, Beeching CA, Boehm M, Sala-Newby GB, George SJ. Regulation of smooth muscle cell proliferation by beta-catenin/T-cell factor signaling involves modulation of cyclin D1 and p21 expression. Circ Res. 2006 Dec 8;99(12):1329–37. doi: 10.1161/01.RES.0000253533.65446.33. [DOI] [PubMed] [Google Scholar]
- 14.Katoh M, Katoh M. NUMB is a break of WNT-Notch signaling cycle. Int J Mol Med. 2006 Sep;18(3):517–21. [PubMed] [Google Scholar]
- 15.Perez VA, Ali Z, Alastalo TP, et al. BMP promotes motility and represses growth of smooth muscle cells by activation of tandem Wnt pathways. J Cell Biol. 2011 Jan 10;192(1):171–88. doi: 10.1083/jcb.201008060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest. 2009 Sep;119(9):2538–49. doi: 10.1172/JCI38079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsaousi A, Williams H, Lyon CA, et al. Wnt4/beta-catenin signaling induces VSMC proliferation and is associated with intimal thickening. Circ Res. 2011 Feb 18;108(4):427–36. doi: 10.1161/CIRCRESAHA.110.233999. [DOI] [PubMed] [Google Scholar]
- 18.Faverman L, Mikhaylova L, Malmquist J, Nurminskaya M. Extracellular transglutaminase 2 activates beta-catenin signaling in calcifying vascular smooth muscle cells. FEBS Lett. 2008 Apr 30;582(10):1552–7. doi: 10.1016/j.febslet.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 19.Beazley KE, Deasey S, Lima F, Nurminskaya MV. Transglutaminase 2-Mediated Activation of beta-Catenin Signaling Has a Critical Role in Warfarin-Induced Vascular Calcification. Arterioscler Thromb Vasc Biol. 2012 Jan;32(1):123–30. doi: 10.1161/ATVBAHA.111.237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003 Feb;4(2):140–56. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 21.Zemskov EA, Mikhailenko I, Smith EP, Belkin AM. Tissue transglutaminase promotes PDGF/PDGFR-mediated signaling and responses in vascular smooth muscle cells. J Cell Physiol. 2011 Jul;18:10. doi: 10.1002/jcp.22938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai TS, Liu Y, Li W, Greenberg CS. Identification of two GTP-independent alternatively spliced forms of tissue transglutaminase in human leukocytes, vascular smooth muscle, and endothelial cells. FASEB J. 2007 Dec;21(14):4131–43. doi: 10.1096/fj.06-7598com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sane DC, Kontos JL, Greenberg CS. Roles of transglutaminases in cardiac and vascular diseases. Front Biosci. 2007 Jan 1;12:2530–45. doi: 10.2741/2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho BR, Kim MK, Suh DH, et al. Increased tissue transglutaminase expression in human atherosclerotic coronary arteries. Coron Artery Dis. 2008 Nov;19(7):459–68. doi: 10.1097/MCA.0b013e3283108fc3. [DOI] [PubMed] [Google Scholar]
- 25.Pistea A, Bakker EN, Spaan JA, Hardeman MR, van RN, VanBavel E. Small artery remodeling and erythrocyte deformability in L-NAME-induced hypertension: role of transglutaminases. J Vasc Res. 2008;45(1):10–8. doi: 10.1159/000109073. [DOI] [PubMed] [Google Scholar]
- 26.Schenk S, Kish DD, He C, et al. Alloreactive T cell responses and acute rejection of single class II MHC-disparate heart allografts are under strict regulation by CD4+ CD25+ T cells. J Immunol. 2005 Mar 15;174(6):3741–8. doi: 10.4049/jimmunol.174.6.3741. [DOI] [PubMed] [Google Scholar]
- 27.Isobe M, Haber E, Khaw BA. Early detection of rejection and assessment of cyclosporine therapy by 111In antimyosin imaging in mouse heart allografts. Circulation. 1991 Sep;84(3):1246–55. doi: 10.1161/01.cir.84.3.1246. [DOI] [PubMed] [Google Scholar]
- 28.Sho M, Sandner SE, Najafian N, et al. New insights into the interactions between T-cell costimulatory blockade and conventional immunosuppressive drugs. Ann Surg. 2002 Nov;236(5):667–75. doi: 10.1097/00000658-200211000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habeler W, Pouillot S, Plancheron A, Puceat M, Peschanski M, Monville C. An in vitro beating heart model for long-term assessment of experimental therapeutics. Cardiovasc Res. 2009 Feb 1;81(2):253–9. doi: 10.1093/cvr/cvn299. [DOI] [PubMed] [Google Scholar]
- 30.Nanda N, Iismaa SE, Owens WA, Husain A, Mackay F, Graham RM. Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem. 2001 Jun 8;276(23):20673–8. doi: 10.1074/jbc.M010846200. [DOI] [PubMed] [Google Scholar]
- 31.Park CH, Chang JY, Hahm ER, Park S, Kim HK, Yang CH. Quercetin, a potent inhibitor against beta-catenin/Tcf signaling in SW480 colon cancer cells. Biochem Biophys Res Commun. 2005 Mar 4;328(1):227–34. doi: 10.1016/j.bbrc.2004.12.151. [DOI] [PubMed] [Google Scholar]
- 32.Watts RE, Siegel M, Khosla C. Structure-activity relationship analysis of the selective inhibition of transglutaminase 2 by dihydroisoxazoles. J Med Chem. 2006 Dec 14;49(25):7493–501. doi: 10.1021/jm060839a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005 May;115(5):1210–20. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mancini MC, Evans JT. Role of platelet-derived growth factor in allograft vasculopathy. Ann Surg. 2000 May;231(5):682–8. doi: 10.1097/00000658-200005000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goodwin AM, D’Amore PA. Wnt signaling in the vasculature. Angiogenesis. 2002;5(1–2):1–9. doi: 10.1023/a:1021563510866. [DOI] [PubMed] [Google Scholar]
- 36.van Gijn ME, Daemen MJ, Smits JF, Blankesteijn WM. The wnt-frizzled cascade in cardiovascular disease. Cardiovasc Res. 2002 Jul;55(1):16–24. doi: 10.1016/s0008-6363(02)00221-3. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Adhikari N, Li Q, Hall JL. LDL receptor-related protein LRP6 regulates proliferation and survival through the Wnt cascade in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2004 Dec;287(6):H2376–H2383. doi: 10.1152/ajpheart.01173.2003. [DOI] [PubMed] [Google Scholar]
- 38.Caira FC, Stock SR, Gleason TG, et al. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006 Apr 18;47(8):1707–12. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grzeszkiewicz TM, Lindner V, Chen N, Lam SC, Lau LF. The angiogenic factor cysteine-rich 61 (CYR61, CCN1) supports vascular smooth muscle cell adhesion and stimulates chemotaxis through integrin alpha(6)beta(1) and cell surface heparan sulfate proteoglycans. Endocrinology. 2002 Apr;143(4):1441–50. doi: 10.1210/endo.143.4.8731. [DOI] [PubMed] [Google Scholar]
- 40.Morrow D, Guha S, Sweeney C, et al. Notch and vascular smooth muscle cell phenotype. Circ Res. 2008 Dec 5;103(12):1370–82. doi: 10.1161/CIRCRESAHA.108.187534. [DOI] [PubMed] [Google Scholar]
- 41.Yang L, Lin C, Zhao S, Wang H, Liu ZR. Phosphorylation of p68 RNA helicase plays a role in platelet-derived growth factor-induced cell proliferation by up-regulating cyclin D1 and c-Myc expression. J Biol Chem. 2007 Jun 8;282(23):16811–9. doi: 10.1074/jbc.M610488200. [DOI] [PubMed] [Google Scholar]
- 42.Zemskov EA, Loukinova E, Mikhailenko I, Coleman RA, Strickland DK, Belkin AM. Regulation of platelet-derived growth factor receptor function by integrin-associated cell surface transglutaminase. J Biol Chem. 2009 Jun 12;284(24):16693–703. doi: 10.1074/jbc.M109.010769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siegel M, Khosla C. Transglutaminase 2 inhibitors and their therapeutic role in disease states. Pharmacol Ther. 2007 Aug;115(2):232–45. doi: 10.1016/j.pharmthera.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inoki K, Ouyang H, Zhu T, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006 Sep 8;126(5):955–68. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Elastin-stained paraffin sections (5 μm) of heart grafts. A representative artery from an allograft heart harvested at 56 days after transplantation (left) shows extensive neointimal proliferation with multifocal patchy parenchymal infiltrates. In contrast, cardiac isograft harvested at 100 days after transplantation (right) shows an absence of neointimal thickening. Scale bar = 50 μm.
Frozen sections (10 μm) from allograft murine hearts were immunohistochemically probed for smooth muscle actin (sma, green) – a marker of VSMCs – and β-catenin (red) (A) or transglutaminase 2 (TG2, red) (B). Occluded vessels from each of the four allografts examined (A1–A4) show enhanced β-catenin and TG2 expression throughout VSMCs in the vessel neointima. Scale bars = 20 μm.
(A) Real-time PCR for PDGF-A, B, and D isoforms and PDGF receptor beta (PDGFRb) in isograft and allograft hearts compared to naïve non-transplanted controls. Expression was normalized to ribosomal protein L19. Real-time PCR reactions were run in duplicate. 3–4 animals per group were analyzed. (B) Frozen sections (10 μm) from allograft murine hearts were immunohistochemically probed for smooth muscle actin (sma, green) – a marker of VSMCs – and PDGF receptor alpha (PDGFRa, red). Occluded vessels from each of the four allografts examined (A1–A4) show clustered PDGFRa expression in VSMCs.