Abstract
Inflammation plays a key role in the development and progression of diabetic kidney disease; however, the role of the anti-inflammatory molecule netrin-1 in diabetic kidney disease is unknown. We examined the role of netrin-1 in diabetes-induced kidney inflammation and injury using tubule-specific netrin-1 transgenic mice. Diabetes was induced using streptozotocin in wild-type and netrin-1 transgenic animals. Kidney function, fibrosis, glucose excretion, albuminuria, and inflammation were evaluated. The mechanism of netrin-1-induced suppression of inflammation was studied in vitro using a proximal tubular epithelial cell line. Diabetes was associated with increased infiltration of neutrophils and macrophages, chemokine expression, and tubular epithelial cell apoptosis in kidney. These changes were minimal in kidney of netrin-1 transgenic mice. In addition, diabetes induced a large increase in the excretion of prostaglandin E2 (PGE2) in urine, which was suppressed in netrin-1 transgenic mice. Netrin-1-induced suppression of PGE2 production was mediated through suppression of NFκB-mediated cyclooxygenase-2 (COX-2) in renal tubular epithelial cells. Furthermore, netrin-1 also increased albumin uptake by proximal tubular epithelial cells through the PI3K and ERK pathways without increasing glucose uptake. These findings suggest that netrin-1 is a major regulator of inflammation and apoptosis in diabetic nephropathy and may be a useful therapeutic molecule for treating chronic kidney diseases such as diabetic nephropathy.
Diabetic nephropathy is the largest single cause of end-stage renal failure worldwide.1 Despite the available modern therapies of glycemic and blood pressure control for diabetes, many patients continue to experience progressive renal damage.2,3 It is extremely important, therefore, to identify novel interventions for halting the progression of diabetic nephropathy. Diabetic nephropathy has traditionally been considered a nonimmune disease; however, an increased presence of glomerular and interstitial immune cell infiltrates and increased expression of inflammatory cytokines in diabetic kidney have been reported in both human biopsies and animal models.4–7 Moreover, recent studies suggest that diabetic nephropathy is also a tubular disease, and that early changes in tubular epithelial cells may be a critical factor in development of progressive kidney diseases.8–12 Inflammation from tubular epithelial cells can damage other areas of the kidney, including the vasculature and glomerular mesangial cells, via inflammatory mediators such as prostanoid metabolites, cytokines, and chemokines. These mediators will induce hyperfiltration, matrix expansion, apoptosis, and vasodilation, and further increase the production of their own and other mediators of cell injury. Suppression of local inflammation in the tubular epithelium may therefore provide a more effective prevention strategy against the development of diabetic nephropathy, compared with treatments such as glycemic and blood pressure control. Recent studies from our laboratory have shown that netrin-1 effectively suppresses inflammation in an acute model of kidney disease. However, the role of netrin-1 in chronic kidney diseases is unknown. Moreover, the mechanisms as to how netrin-1 suppresses inflammation are unknown.
Netrin-1 is a laminin-related secreted molecule that has been identified as a neuronal guidance cue, directing neurons and their axons to targets during development of the nervous system. However, guidance is unlikely to be the only function of netrin-1, netrins are widely expressed outside the nervous system, including in vascular endothelial13,14 and kidney tubular epithelial cells. Vascular endothelial cells form a critical barrier for leukocyte migration into organs by producing repellent factors to leukocytes, such as netrin-1. Down-regulation of netrin-1 during organ injury is reported to exacerbate inflammation.13,14 We have reported that administration or overexpression of netrin-1 protects the kidney against ischemia-reperfusion injury.13 However, nothing was known about the involvement of netrin-1 in diabetic nephropathy, warranting further investigation. The purpose of the present study was to determine the effect of tubular-specific overexpression of netrin-1 on diabetes-induced inflammation and nephropathy in mice.
Materials and Methods
Induction of Diabetes
The low-dose streptozotocin (STZ) induction protocol we followed was as described by the Animal Models of Diabetic Complication Consortium (AMDCC), outlined below. The Institutional Animal Care and Use Committee of the Georgia Health Sciences University approved all protocols and procedures using animals (approval no. 2011–0348). Netrin-1 transgenic mice were characterized for transgene expression and phenotype.15 Eight-week-old netrin-1 transgenic mice, which express chicken netrin-1 in proximal tubular epithelial cells under control of the fatty acid binding protein promoter, and their wild-type (WT) littermates were given STZ (50 mg/kg per day in citrate buffer) for 5 days. At 3 weeks after injection, blood glucose was measured. Animals with a blood glucose level of <350 mg/dL were given one additional dose of STZ (50 mg/kg). Blood glucose was measured again 3 weeks after injection and every 4 weeks thereafter. Renal function and body weight were monitored every 4 weeks by measuring serum creatinine. At 36 weeks after induction of diabetes, 24-hour urine was collected and animals were sacrificed to collect kidney tissue and blood. Citrate buffer was administered to control animals, which were subjected to the same treatment as the diabetic animals.
Laboratory Assessments
Renal function was assessed by measurement of serum creatinine (DZ072B; Diazyme Laboratories, Poway, CA).
Chicken netrin-1 was quantified in plasma and urine using an enzyme-linked immunosorbent assay (ELISA) kit (Hölzel Diagnostika, Cologne, Germany). Cytokines and chemokines in plasma were measured using an ELISA array kit from SABiosciences (Qiagen, Frederick, MD) and an ELISA kit from eBioscience (San Diego, CA).
For urine microalbumin quantification by ELISA, 24-hour urine was collected using a metabolic cage. Urine volume was measured and the urine was centrifuged at 11,000 × g for 10 minutes. The supernatant was aliquoted and stored at −80°C until used. Urine albumin was measured using an ELISA kit (KT-343; Kamiya Biomedical, Seattle, WA).
Quantification of mRNA by Real-Time RT-PCR
RNA was isolated using TRIzol reagent (Life Technologies, Grand Island, NY). Real-time RT-PCR was performed in an ABI 7700 sequence detection system (Applied Biosystems; Life Technologies, Foster City, CA). Total RNA (3 μg) was reverse transcribed in a reaction volume of 40 μL using an Omniscript RT kit (Qiagen, Valencia, CA) and random primers. The product was diluted to a volume of 150 μL, and 5-μL aliquots were used as templates for amplification using SYBR Green PCR amplification reagent (Qiagen) and a mouse inflammatory cytokines and receptors PCR array (SABiosciences PAMM-011E-4; Qiagen). Data were analyzed using SABiosciences Web-based analysis tools (Qiagen).
Cell Culture
Mouse proximal tubular epithelial cells of the TKPT cell line (a gift from E. Bello-Reuss) were cultured in a low-glucose (1 g/L) Dulbecco's modified Eagle's medium with 5% serum, which was replaced by serum-free low-glucose medium 24 hours before the start of the experiment. On the day of experiments, cells were treated with different concentrations of d-glucose or d-mannitol. Cells and supernatants were harvested at different times after glucose addition. The cell pellets were lysed with radioimmunoprecipitation assay buffer containing protease and phosphatase inhibitors and used for Western blot analysis. Some of the cells were used for RNA isolation and RT-PCR analysis. The supernatants were used for quantification by ELISA of netrin-1, chemokine, and prostaglandin E2 (PGE2).
Prostaglandin and Leukotriene Quantification
PGE2 and leukotrienes B4, D4, and E4 were quantified in mouse urine and cell culture supernatant using an enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI).
Western Blot Analysis
Protein extraction from kidney and TKPTS cells and Western blot analysis were performed as described previously.13,16 The membrane was probed with goat anti-chicken netrin-1 antibody (R&D Systems, Minneapolis, MN), goat anti-mouse netrin-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA), fibronectin, α-smooth muscle actin (α-SMA), rabbit anti-cyclooxygenase-2 (COX-2) (Abcam, Cambridge, MA), and rabbit anti-IkBα antibodies (Cell Signaling Technology, Danvers, MA). Proteins were detected using enhanced chemiluminescence detection reagents (Amersham Pharmacia; GE Healthcare, Chalfont St Giles, UK). Protein loading was normalized to β-actin expression using anti-mouse actin antibody (Sigma-Aldrich, St. Louis, MO).
Histology and Immunostaining
Kidney tissue was fixed in buffered 10% formalin for 12 hours and then embedded in paraffin wax. For assessment of injury, 5-μm sections were stained with PAS, followed by hematoxylin. Acute tubular necrosis and apoptosis were quantified by determining the percentage of the tubules that showed epithelial cell necrosis, brush-border loss, cast formation, and apoptotic bodies in the cortex. Ten 40× fields were examined and the scores were averaged. Slides were scored by an individual (G.R.) masked to the genotype of the animal. To quantify leukocyte infiltration, sections were stained with rat anti-mouse neutrophil antibody or anti-mouse macrophage antibody (1:200 dilution; Abcam), followed by goat anti-rat biotin conjugate. Color was developed after incubation with ABC reagent (Vector Laboratories, Burlingame, CA). Stained sections were photographed, and five 40× fields of neutrophils were examined for quantification of leukocytes. To determine COX-2 and chicken netrin-1 (transgene) expression, sections were stained with rabbit anti-COX-2 antibody (1:100 dilution; eBioscience) or goat anti-chicken netrin-1 antibody (R&D Systems), followed by goat anti-rabbit biotin or rabbit anti-goat biotin conjugate. Color was developed after incubation with ABC reagent (Vector Laboratories). Stained sections were photographed using an Olympus inverted microscope with a color charge-coupled device (CCD) camera (Olympus USA, Pittsburgh, PA).
To determine the effect of netrin-1 on glucose-induced NFκB activation, TKPTS cells grown on chamber slides were treated with 600 mg/dL of glucose with and without 100 ng/mL of netrin-1. Cells were washed with PBS at the end of 3 hours and then were fixed with 10% buffered formalin for 15 minutes at 4°C, followed by fixation with methanol for 10 minutes at −20°C. Cells were washed and blocked with goat serum, followed by incubation with rabbit anti-p65 antibody for 1 hour. Cells were then washed and incubated with goat anti-rabbit fluorescein isothiocyanate conjugate and washed and mounted with DAPI-containing aqueous mounting medium. Cells were photographed using an Olympus inverted microscope with a CCD camera.
TACS TdT Apoptosis Detection in Situ
To identify apoptotic cells, tissue sections were stained using a TACS TdT-Blue Label in Situ Apoptosis Detection kit (R&D Systems) according to the manufacturer's instructions. Briefly, tissue sections were deparaffinized, hydrated, and washed with PBS. Sections were digested with proteinase K for 15 minutes at 24°C. Slides were then washed, and endogenous peroxidase activity was quenched with 3% H2O2 in methanol. Slides were washed and incubated with TdT labeling reaction mix at 37°C for 1 hour and then with streptavidin-horseradish peroxidase. Color was developed using TACS TdT-Blue Label substrate solution. Slides were washed, counterstained, and mounted with Permount medium. Sections were photographed, and labeled cells were counted and quantified.
Morphometry
For each kidney, digital images of 15 randomly chosen consecutive glomeruli were captured with an Olympus DP72 color CCD camera mounted on an Olympus light microscope by moving from cortex to medulla in a serpentine fashion. The glomerular tuft was traced, and the enclosed area was calculated with Olympus CellSens Standard software.
Glucose Uptake by Proximal Tubular Epithelial Cells
TKPTS cells were cultured in 24-well plates to confluency. On the day of uptake measurements, the culture medium was removed from the wells by aspiration, and a NaCl-containing medium (140 mmol/L NaCl, 5.4 mmol/L KCl, 1.8 mmol/L CaCl2, 0.8 mmol/L MgSO4, and 5 mmol/L d-glucose, buffered with 25 mmol/L HEPES/Tris, pH 7.4) with or without recombinant netrin-1 (200 ng/mL, final concentration) was added to the cells. The incubation time with netrin-1 was varied (10 minutes, 30 minutes, 1 hour, and 3 hours); control cells were treated for corresponding time periods in the absence of netrin-1. At the end of treatment, the medium was removed by aspiration and 0.5 mL of uptake buffer containing 0.5 μCi [3H]2-deoxy-d-glucose as a tracer and 50 μmol/L unlabeled 2-deoxy-d-glucose was added to cells, to initiate uptake. The composition of the uptake buffer was same as that of the treatment buffer, except that d-glucose was omitted. The incubation time for uptake measurements was 15 minutes. At the end of this incubation period, uptake was terminated by aspiration of the medium from the wells, followed by washing of the cells with ice-cold uptake buffer without any radiolabeled or unlabeled 2-deoxy-d-glucose three times. Cells were then dissolved in 1% sodium dodecylsulfate/0.2 mol/L NaOH, and the radioactivity associated with the cell lysates was measured. Uptake values were normalized to protein. The experiment was performed in triplicate.
Albumin Uptake by Proximal Tubular Epithelial Cells
Albumin uptake by TKPTS cells was assessed in vitro. TKPTS cells were grown to 80% confluency and serum-free medium was added. Bovine serum albumin conjugated with fluorescein isothiocyanate (Sigma-Aldrich) was added to the medium at 1 mg/mL concentration. Netrin-1 was added at different concentrations 30 minutes before albumin addition. At 2 hours after addition of albumin, the medium was aspirated and cells were washed thoroughly with PBS. Cells were then harvested and lysed with PBS-0.1% Triton X-100. Fluorescence was quantified using a fluorescence plate reader (Synergy HT; Biotek Instruments, Winooski, VT). Fluorescence was normalized to mg of total protein. To determine the role of the ERK and Akt pathways, specific inhibitors of MEK2 (U0126, 10 μmol/L) (Cell Signaling Technology, Inc., Danvers, MA) and PI3K (LY294002, 10 μmol/L) were added along with netrin-1.
Statistical Analysis
All assays were performed in duplicate or triplicate. Data are expressed as means ± SEM. Statistical significance was assessed by an unpaired, two-tailed Student's t-test for single comparisons or by analysis of variance for multiple comparisons.
Results
Kidney Transgene Expression and Regulation of Endogenous Netrin-1 during Diabetes
The chicken netrin-1 transgene is highly expressed in transgenic mouse kidney proximal tubular epithelial cells. The expression pattern was not altered in the diabetic state (Figure 1, C and D). High levels of chicken netrin-1 were found in urine from control transgenic mice, and levels were even higher after induction of diabetes (Figure 1, E and F). Chicken netrin-1 was undetectable in plasma from control and diabetic transgenic mice, which is consistent with absence of transgene expression in liver (Figure 1G) and blood.17 In addition, netrin-1 receptor UNC5B was localized in the brush border of proximal tubular epithelial cells in both WT and netrin-1 transgenic animal kidney, and the localization pattern was not altered in response to diabetes (see Supplemental Figure S1 at http://ajp.amjpathol.org). Endogenous netrin-1 expression was minimal in WT control animals and netrin-1 transgenic animal kidney. At 36 weeks after induction of diabetes, endogenous netrin-1 was up-regulated in WT mice, but not in transgenic mice (Figure 2A). Our recent in vitro studies demonstrated that hyperglycemia down-regulated netrin-1 production in TKPTS cells; however, high levels of protein (20 mg/mL of endotoxin-free bovine serum albumin) in the medium induced netrin-1 production, suggesting that tubular injury and proteinuria are a signal for induction of netrin-1.18 It is possible, therefore, that hyperglycemia-induced down-regulation of endogenous netrin-1 may predispose proximal tubular epithelial cells to increased inflammation and cell death.
Figure 1.
Characterization of transgene chicken netrin-1 expression in normal and diabetic kidney and plasma. A–D: Localization of chicken netrin-1 (brown) by immunostaining in cortical proximal tubular epithelial cells from control mouse kidney (A and B) and diabetic mouse kidney (C and D). E and F: Quantification by ELISA of chicken netrin-1 in plasma (pg/mL or ng/mL) and urine (pg/mg or ng/mg of urine creatinine) in control (E) and diabetic (F) netrin-1 transgenic animals; chicken netrin-1 was undetectable in plasma. G: Expression of transgene chicken netrin-1 in different tissues was determined by RT-PCR. Transgene expression was seen lung, small intestine, colon, spleen, and kidney; expression was absent in liver, heart, and brain. Original magnification: ×400 (A and C); ×660 (B and D).
Figure 2.

STZ-induced type 1 diabetes in WT and netrin-1 transgenic animals. A: Western blot analysis of the expression of endogenous netrin-1 and transgene netrin-1 in control and diabetic animal kidney. Endogenous netrin-1 is up-regulated in WT diabetic mouse, but not in netrin-1 transgenic mouse kidney. Expression of the transgene is not altered with diabetes. B: Body weight of control and diabetic mice at the start of the experiment and at week 36. Control animals exhibited a significant increase in body weight, but diabetic animals did not. ***P < 0.001 versus control animals. C: Blood glucose level was measured at various times after STZ administration. There was no increase in blood glucose levels in control animals. A significant rise in blood glucose was seen in STZ-administered animals. There was no significant difference in blood glucose levels between WT and netrin-1 transgenic diabetic animals. †P < 0.0001 versus control animals. D: Albumin excretion rate in WT and netrin-1 transgenic diabetic and control mice. The 24-hour urine was collected and urine albumin was measured by ELISA. Diabetes induced a significant rise in the excretion rate of albumin in urine in WT, which was completely suppressed in netrin-1 transgenic animals. ***P < 0.001 versus all other groups. E: Diabetes induced renal dysfunction in WT and netrin-1 transgenic animals. Kidney function was assessed by measuring serum creatinine at different times after STZ administration. Diabetes induced a significant rise in serum creatinine in WT diabetic animals, compared with control animals. ***P < 0.001 versus control animals. n = 8 to 10. N 1, netrin-1.
Effects of Netrin-1 Overexpression
Blood Glucose and Body Weight
There was no significant increase in body weight in diabetic mice, whether WT or netrin-1 transgenic (Figure 2B). Control mice gained weight, as expected. Blood glucose levels were increased significantly in diabetic mice after STZ injection and continued to be elevated throughout the study period, but were not elevated in citrate buffer-treated control groups (Figure 2C).
Urinary Albumin Excretion
The urinary albumin excretion rate was 28 ± 3 μg/24 hours (17 ± 3 μg/mg of creatinine) in the WT control group and increased to 48 ± 5 μg/24 hours (91 ± 12 μg/mg of creatinine) (P < 0.05) at week 36 in the WT diabetic group (Figure 2D). This effect was significantly reduced in netrin-1 transgenic mice, to 26 ± 8 μg/24 hours (42 ± 18 μg/mg of creatinine) (P < 0.05 versus WT diabetic mice), with no significant difference seen between netrin-1 transgenic control and diabetic mice.
Renal Function
Renal function deteriorated modestly but significantly, as shown by the increasing levels of serum creatinine in diabetic WT mice at 36 weeks (Figure 2E), compared with control mice. Although netrin-1 transgenic mice showed a rise in serum creatinine, compared with control mice, but the difference did not reach statistical significance. Control mice, both WT and transgenic, did not show any renal dysfunction at 36 weeks.
Renal Fibrosis
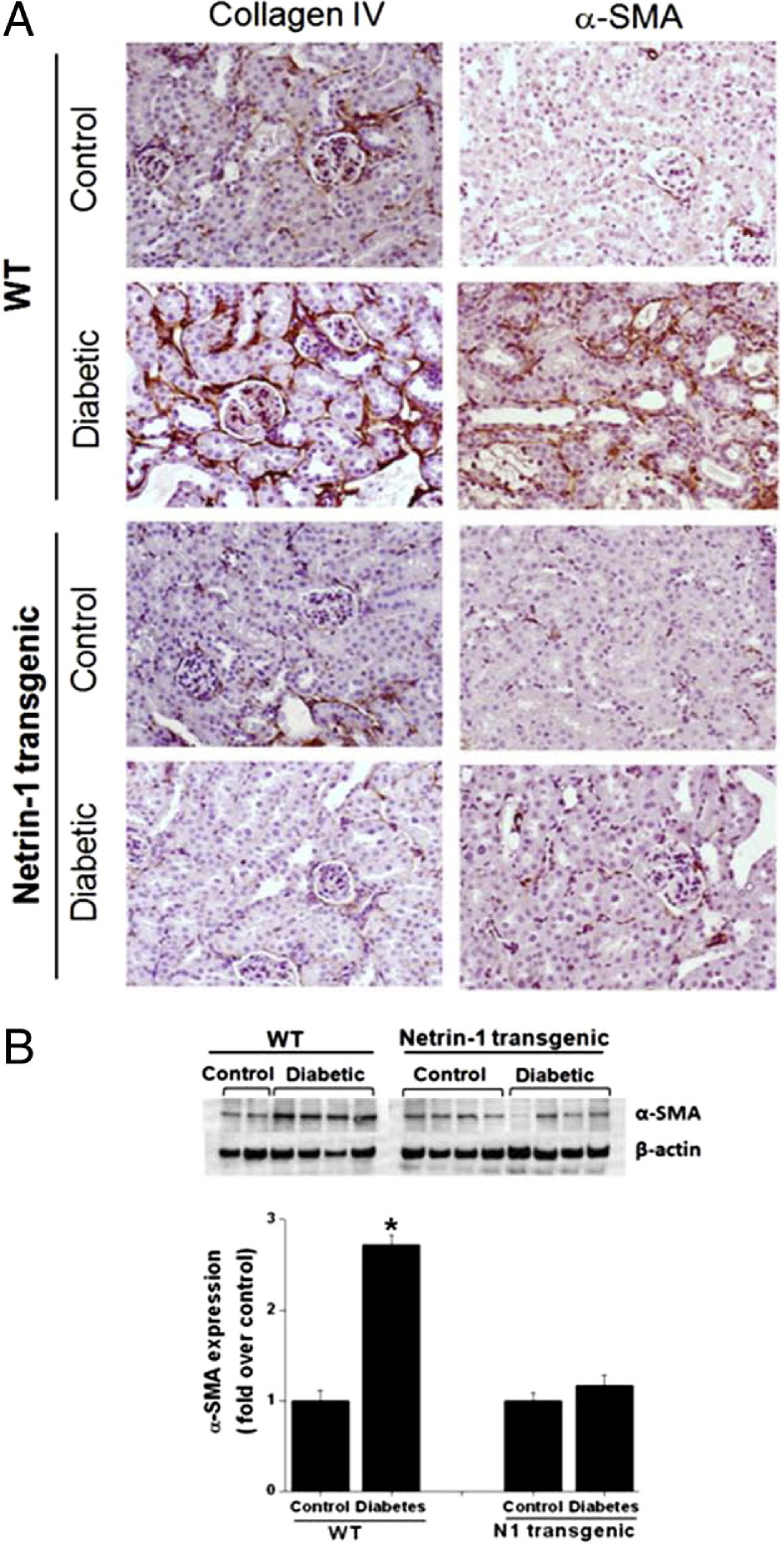
Very low or negligible levels of staining were seen in nondiabetic control WT and netrin-1 transgenic animal kidney, whereas a large increase in immunostaining for both collagen IV and α-SMA was seen in WT diabetic kidney. Diabetes-induced increased expression of collagen and SMA was abolished in netrin-1 transgenic mice, as shown by low levels of staining (Figure 3). Quantitative data for α-SMA expression in kidney by Western blot analysis are presented in Figure 3B. Diabetes significantly increased (P < 0.05) α-SMA expression in WT mice, compared with control, but α-SMA expression was suppressed in the netrin-1 transgenic diabetic mouse kidney.
Figure 3.
Diabetes induces renal fibrosis. A: Fibrosis was determined by immunostaining for collagen IV and α-SMA in kidney tissue sections from control and diabetic animals. Minimal or negligible staining was seen for both collagen IV and α-SMA in WT control kidney, but staining was significantly increased in WT diabetic kidney. Both control and diabetic kidney from netrin-1 transgenic mice showed low or negligible levels of staining for collagen IV and α-SMA. B: Quantification of α-SMA expression in kidney by Western blot analysis. Diabetes significantly increased α-SMA expression in WT mice, compared with control, but not in netrin-1 transgenic diabetic mouse kidney. *P < 0.05 versus all other groups.
Netrin-1 Suppresses Diabetes-Induced Tubular Epithelial Cell Apoptosis and Necrosis in Kidney
There was no increase in apoptosis and necrosis of the tubules in control animal kidney, both WT and netrin-1 transgenic (Figure 4); however, diabetes induced a large increase in necrosis, atrophy, and apoptosis in tubular epithelial cells. Overexpression of netrin-1 in tubular epithelial cells attenuated this diabetes-induced necrosis and apoptosis (Figure 4). Consistent with observations in PAS-stained tissue section, TUNEL staining showed a similar result. Apoptotic nuclei were seen in both tubular epithelium and glomerulus of WT diabetic kidney, but apoptosis was significantly suppressed in netrin-1 transgenic mouse kidney (see Supplemental Figure S2 at http://ajp.amjpathol.org). To determine whether netrin-1 inhibits caspase-3 activation and thereby suppresses apoptosis, activated caspase-3 staining was performed. No increase in active caspase-3 staining was seen in tubules of control animal kidney, both WT and netrin-1 transgenic (Figure 4); however, diabetes induced a large increase in the activation of caspase-3 in tubular epithelial cells. Overexpression of netrin-1 in tubular epithelial cells suppressed diabetes-induced activation of caspase-3. Furthermore, expression of caspase-3 expression was also increased significantly in diabetic WT kidney, compared with either control or diabetic netrin-1 transgenic mouse kidney.
Figure 4.
Netrin-1 overexpression reduces diabetes-induced necrosis and apoptosis of tubular epithelial cells in kidney. Top: Necrosis and apoptosis were assessed in kidney cortex using PAS-stained tissue sections. Control nondiabetic kidney from WT and netrin-1 transgenic mice shows normal tubular structure, but WT diabetic kidney shows high levels of tubular atrophy, apoptosis, and necrosis, which is significantly attenuated in diabetic netrin-1 transgenic mouse kidney. Bottom left panel: Quantification of apoptosis, necrosis, and tubular atrophy expressed as tubular injury score in five 40× fields from kidney cortex. Bottom right panel: Netrin-1 overexpression suppresses caspase-3 expression and activation in diabetic kidney. Control nondiabetic kidney from WT and netrin-1 transgenic mice shows no staining for active caspase-3, but staining was increased in WT diabetic kidney; this increase was significantly attenuated in diabetic netrin-1 transgenic mouse kidney. Caspase-3 mRNA expression in nondiabetic and diabetic WT and transgenic mouse kidney was analyzed by real-time RT-PCR. Red arrows indicate the fragmented apoptotic nuclei. Yellow outline indicates active caspase-3 staining in the proximal tubular epithelial cells. ***P < 0.001 versus all other groups. n = 6 to 8.
Netrin-1 Overexpression Regulates Inflammatory Cytokine Expression and Excretion
Chronic diabetes increased the expression of several cytokines and chemokines and their receptors in the kidney (Figure 5A). Netrin-1 overexpression in tubular epithelial cells significantly suppressed the expression of these cytokines and chemokines and their receptors.
Figure 5.
Netrin-1 suppresses diabetes-induced inflammation in kidney. A: Kidney cytokine and chemokine gene expression was determined by real-time RT-PCR. Diabetic WT mouse kidney showed a significant increase in expression of many cytokine and chemokine genes, which was suppressed in netrin-1 transgenic diabetic mice. B: Cytokine and chemokine excretion in 24-hour urine was determined by ELISA. Excretion of several cytokines and chemokines in urine is increased in WT diabetic mice, compared with netrin-1 transgenic mice. C–F: Neutrophil infiltration into kidney was determined by immunostaining with anti-neutrophil antibody. C: WT control kidney shows no neutrophils. D: WT diabetic kidney shows increased neutrophil infiltration (arrows). E: Netrin-1 transgenic control kidney shows no neutrophil staining. F: Netrin-1 transgenic diabetic mouse kidney shows few neutrophils. G: Neutrophil infiltration was quantified by counting cells in five 40× fields. H–K: Macrophage infiltration (indicated by arrows) into kidney was determined by immunostaining with anti-macrophage antibody. H: WT control kidney shows macrophage staining in the interstitium but not the glomerulus. I: WT diabetic kidney shows increased macrophage infiltration in glomerulus. J: Netrin-1 transgenic control kidney shows macrophage staining in the interstitium but not the glomerulus. K: Netrin-1 transgenic diabetic mouse kidney shows little macrophage staining in the glomerulus. *P < 0.05 versus control animals, ***P < 0.001 versus all other groups. n = 4.
Consistent with reduced expression of cytokines and chemokines in kidney, the excretion of cytokines and chemokines was reduced in urine of netrin-1 transgenic animals, compared with WT diabetic animals (Figure 5B). Most of the cytokines and chemokines analyzed were undetectable or present in very low levels in the urine of control mice. Chronic diabetes in WT mice showed increased excretion of a cytokine (IL-1β) and chemokines (MCP-1, SDF-1, and IP-10). However, the excretion of these cytokines is significantly suppressed in diabetic netrin-1 transgenic mice.
Netrin-1 Overexpression Suppresses Diabetes-Induced Leukocyte Infiltration into Kidney
Consistent with the improvement in renal function and reduced cytokine and chemokine expression in netrin-1 transgenic diabetic mouse kidney, neutrophil (Figure 5, C–G) and macrophage (Figure 5, H–K) infiltration was reduced significantly, compared with WT diabetic mouse kidney.
Netrin-1 Overexpression Suppresses Diabetes-Induced Prostaglandin Production in Kidney
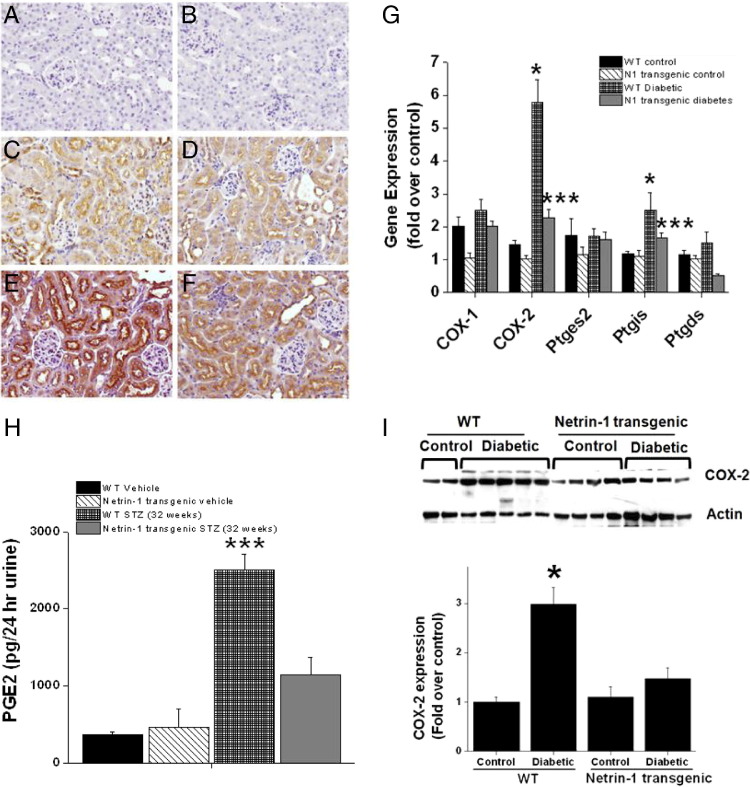
The mechanism responsible for netrin-1-mediated suppression of inflammation is unknown. We hypothesized that netrin-1 regulates inflammation through suppression of COX-2-mediated PGE2 production. Hyperglycemia caused a large increase in COX-2 enzyme expression in WT mouse kidney, compared with nondiabetic control (Figure 6G). Netrin-1 overexpression completely suppressed this diabetes-induced COX-2 expression. COX-1 expression was not altered significantly. Consistent with the increase in mRNA expression, expression of COX-2 protein increased significantly (Figure 6I), but expression was completely suppressed in netrin-1 transgenic mouse kidney. Consistent with the increase in COX-2 expression, the levels of PGE2 in urine were increased in WT diabetic mice, compared with control mice. The diabetes-induced increase in PGE2 excretion was robustly suppressed in netrin-1 transgenic animals with diabetes (Figure 6H).
Figure 6.
Regulation of COX-2 and PGE2 production by netrin-1 in vivo. A–F: COX-2 localization in control and diabetic kidney. Secondary antibody control (A and B) shows no staining; nondiabetic WT kidney (C) shows diffuse low level of staining in the proximal tubular epithelial cells; nondiabetic netrin-1 transgenic mouse kidney (D) shows diffuse low level of staining in the proximal tubular epithelial cells; diabetic WT mouse kidney (E) shows intense staining in the proximal tubular epithelial cells; and diabetic netrin-1 transgenic mouse kidney (F) shows diffuse low level of staining in the proximal tubular epithelial cells. G: Hyperglycemia significantly up-regulated COX-2 expression in WT diabetic mouse kidney, compared with WT control mouse kidney, as determined by real-time RT-PCR. Netrin-1 overexpression completely suppressed hyperglycemia-induced COX-2 expression. Prostaglandin I2 synthase (Ptgis) expression also increased moderately but significantly in WT diabetic kidney, but expression was down-regulated in netrin-1 transgenic mouse kidney. Expression of COX-1, prostaglandin E synthase 2 (Ptges2), and prostaglandin-D2 synthase (Ptgds) was unchanged. H: PGE2 in urine was quantified by enzyme immunoassay. PGE2 excretion was increased in WT diabetic mouse, compared with control and diabetic transgenic mice. I: Western blot analysis and quantification of COX-2 protein expression in control and diabetic mouse kidney revealed significant upregulation in WT diabetic kidney, but not in the other groups. *P < 0.05 versus nondiabetic control kidney (G) or versus all other groups (I); ***P < 0.001 versus diabetic WT mouse kidney (G) or versus all other groups (H). n = 6.
To determine the cell type that overexpresses COX-2, immunolocalization was performed. COX-2 staining was minimal in nondiabetic control mouse kidney, both WT and netrin-1 transgenic (Figure 6, C and D). Diabetes induced a large increase in immunostaining for COX-2 in the cortical tubules (proximal tubular epithelial cells) (Figure 6E), but netrin-1 transgenic diabetic animal kidney did not show any increase in COX-2 staining (Figure 6F). These results suggest that netrin-1 may suppress inflammation by inhibiting the production of COX-2-generated metabolites.
Hyperglycemia-Induced Increase in PGE2 Is Suppressed by Netrin-1 in TKPTS Cells
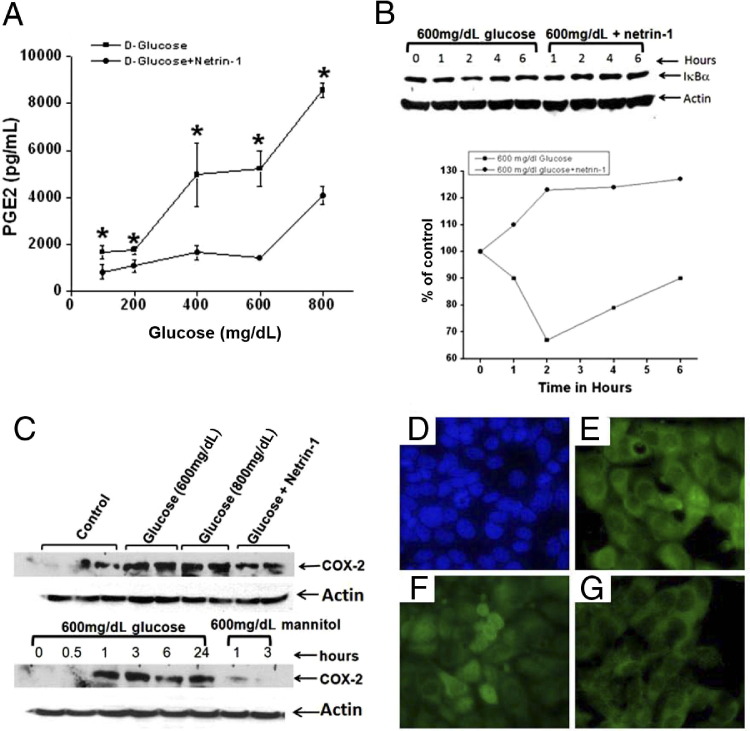
To determine directly the effect of netrin-1 on hyperglycemia-induced production of PGE2, TKPTS cells were treated with increasing doses of d-glucose with and without netrin-1. Glucose induced PGE2 synthesis in a dose-dependent manner, but synthesis was suppressed in the presence of netrin-1 (Figure 7). An increasing dose of d-mannitol (an osmotic agent) did not increase PGE2 synthesis, suggesting that increased PGE2 production in diabetes is specific to glucose (Figure 7A). To determine whether hyperglycemia induces COX-2 enzyme expression, thereby increasing PGE2 production, COX-2 expression in TKPTS cells was quantified by Western blot. Glucose increased COX-2 enzyme expression in a dose- and time-dependent manner, but expression was suppressed by netrin-1 (Figure 7B). These results suggest that netrin-1 reduces inflammation by suppressing COX-2 expression and PGE2 production in tubular epithelial cells.
Figure 7.
Hyperglycemia-induced COX-2 expression and NFκB activation are suppressed by netrin-1 in proximal tubular epithelial cells. A: TKPTS cells treated with increasing concentration of glucose with or without netrin-1 (100 ng/mL). Supernatant was harvested at 24 hours after addition of glucose and PGE2 was quantified by enzyme immunoassay. Glucose induced PGE2 production, but this effect was significantly attenuated by netrin-1. B: Western blot analysis of IkBα degradation in TKPTS cells in response to high levels of glucose. High levels of glucose induced a rapid degradation of IkBα, which was suppressed in the presence of netrin-1. Glucose is normal at hour 0. C: TKPTS cells were incubated with different concentrations of d-glucose or d-mannitol in the presence of netrin-1. Cells were harvested at 0 to 24 hours (glucose) or at 1 or 3 hours (mannitol), and Western blot analysis was performed for COX-2 expression. Glucose increased COX-2 expression in a dose- and time-dependent manner; the effect was suppressed by addition of netrin-1. The osmotic agent d-mannitol alone did not increase COX-2 expression. D–G: Immunocytochemical analysis of glucose-induced NFκB activation in TKPTS cells. Secondary antibody control (D) shows no staining for NFκB (green; blue staining is DAPI); untreated control (E) shows cytoplasmic localization of p65 subunit of NFκB. At 3 hours after addition of 600 mg/dL glucose (F), nuclear localization of p65 subunit of NFκB is observed. Addition of netrin-1 (G) suppressed activation of NFκB, as indicated by cytoplasmic localization of p65 subunit. *P < 0.05 versus glucose + netrin-1. n = 6.
Netrin-1 Mediates Suppression of Glucose-Induced NFκB Activation
The signaling pathway through which netrin-1 suppresses COX-2 expression is unknown. The COX-2 promoter, however, has an NFκB-binding site and is up-regulated in response to NFκB activation. Hyperglycemia is known to activate NFκB. We therefore investigated whether glucose-induced activation of NFκB is suppressed by netrin-1 in TKPTS cells. d-Glucose induced COX-2 expression in a dose- and time-dependent manner, but addition of netrin-1 significantly suppressed COX-2 expression (Figure 7C). The induction of COX-2 was associated with an increase in NFκB activation, as seen by nuclear translocation of the p65 subunit, which was suppressed in netrin-1-treated cells (Figure 7, D–G). Moreover, glucose induced degradation of IkBα, which is suppressed by netrin-1, suggesting that netrin-1-mediated suppression of COX-2 may occur through IkBα-mediated inhibition of NFκB.
Glucose and PGE2 Induce Inflammatory Cytokine and Chemokine Production in Renal Tubular Epithelial Cells
Hyperglycemia induced PGE2 production by increasing the expression of COX-2 (Figure 7). To determine whether high glucose levels or PGE2 increases inflammatory responses in the tubular epithelial cells, we measured cytokine and chemokine expression in response to glucose and PGE2. Incubation of TKPTS cells in high levels of glucose, but not mannitol, induced a significant increase in MCP-1 production, which was attenuated by netrin-1 (Figure 8A). Moreover, addition of PGE2 induced a dose-dependent increase in IP-10 production (Figure 8, C and D) and induced IL-1β expression as well (Figure 8B).
Figure 8.

Glucose and PGE2 induced cytokine and chemokine production in mouse proximal tubular epithelial cells. A: Cells were incubated with increasing concentration of d-glucose with and without netrin-1. MCP-1 production was quantified in the cell supernatant by ELISA. High levels of glucose (800 mg/dL) induced MCP-1 production in TKPTS cells; this effect was significantly attenuated in the presence of netrin-1. d-mannitol alone did not increase MCP-1 production, suggesting that the glucose effect is not due to osmotic effect. The 100 mg/dL glucose level served as control. B: PGE2-induced IL-1β expression in TKPTS cells. PGE2-induced IL-1β expression was analyzed by real-time RT-PCR. C: PGE2 induced IP-10 production in a dose-dependent manner. TKPTS cells were treated with increasing concentration of PGE2 and supernatant was harvested after 24 hours. IP-10 was quantified by ELISA. D: PGE2 (250 ng/mL) induced IP-10 expression significantly, compared with control, in TKPTS cells. *P < 0.05 versus high glucose (A) or versus control (D); ***P < 0.001 versus control (A–C). n = 4.
Netrin-1 Overexpression Suppresses Hyperglycemia-Induced Glomerular Mesangial Expansion
COX-2 has been shown to exacerbate mesangial expansion, a hallmark of diabetic nephropathy.19 Because netrin-1 suppressed hyperglycemia-induced COX-2 expression and PGE2 production, we quantified the glomerular area using cell scanning software (Olympus cellSens). No significant difference in mesangial area was seen in WT control and netrin-1 transgenic control mice (Figure 9, A and B). Diabetes induced a significant increase in mesangial expansion as seen by an increase in glomerular area (Figure 9C), which was completely suppressed in netrin-1 transgenic mice (Figure 9D). Quantitative data for glomerular area are shown in Figure 9E.
Figure 9.
Diabetes-induced increase in glomerular area is suppressed by netrin-1 overexpression in tubular epithelial cells. A–D: Glomerular area was measured in nondiabetic WT mouse kidney (A), nondiabetic netrin-1 transgenic mouse kidney (B), diabetic WT mouse kidney (C), and diabetic netrin-1 transgenic mouse kidney (D). E: Quantification of glomerular area revealed significant increase in area in WT diabetic mouse kidney. F: Netrin-1 increases albumin uptake through ERK and PI3 kinase pathways. Albumin uptake was measured. Netrin-1 significantly increased albumin uptake, which was suppressed in the presence of PI3 kinase and ERK MAP kinase inhibitors. G: Glucose excretion in urine was increased significantly in WT diabetic mice, compared with control mice; this effect was not altered in netrin-1 transgenic diabetic mice. H: Glucose uptake by TKPTS cells in vitro. There was no significant increase in 2-deoxy-d-glucose uptake by netrin-1, compared with vehicle-treated control, irrespective of the treatment period. *P < 0.05 versus control and †P < 0.05 versus netrin-1-treated group (F); ***P < 0.001 versus all other groups (E); ‡P < 0.0001 versus control (G). n = 5.
Netrin-1 Regulates Albumin Uptake in Renal Proximal Tubular Epithelial Cells
Netrin-1 increased the uptake of albumin by proximal tubular epithelial cells (TKPTS) in a dose-dependent manner (Figure 9F). Based on our earlier study,20 the role of PI3 kinase and ERK pathways on netrin-1-mediated albumin uptake was determined using pathway-specific inhibitors. Both pathway inhibitors suppressed netrin-1-induced albumin uptake (Figure 9F), suggesting that netrin-1 increases albumin uptake through the ERK and Akt pathways. To determine whether the netrin-1 effect on increased albumin uptake is due to effective reabsorption of glucose by tubular epithelial cells, urine glucose and glucose uptake by TKPTS cells were measured. Diabetes caused a large increase in glucose excretion, which was not reduced in netrin-1 transgenic diabetic mice (Figure 9G). Moreover, glucose uptake by TKPTS cells was also not significantly altered in the presence of netrin-1 (Figure 9H).
Discussion
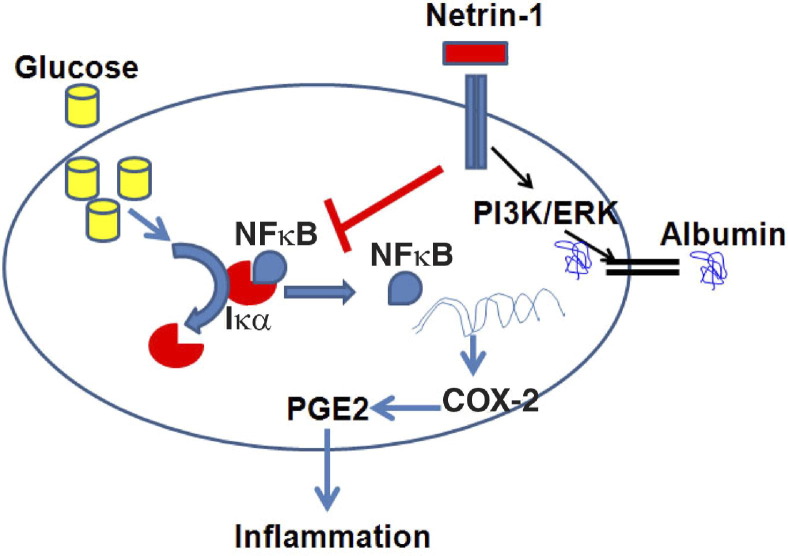
Inflammation is a major contributing factor in deterioration of kidney function due to chronic diabetes.21,22 Increasing evidence suggests that individuals who progress to diabetes mellitus display features of inflammation years before disease onset.6,23 The axon-guidance molecule netrin-1 is known to regulate inflammation and apoptosis in tubular epithelial cells in an acute kidney injury model in mice.15,24 However, whether netrin-1 has a similar role in chronic kidney disease, such as diabetic nephropathy, is unknown. In the present study, at 36 weeks after induction of diabetes in WT mice (C57BL/6), a marked increase in albuminuria, reduction of renal function, and histological changes characteristic of diabetic nephropathy were established. Furthermore, these changes were associated with evidence of inflammation, which was suppressed by netrin-1 overexpression in proximal tubular epithelial cells. We show here, for the first time, that netrin-1 suppresses inflammation through suppression of diabetes-induced COX-2 expression and PGE2 production. The suppressive effect on COX-2 was mediated through inhibition of NFκB activation. In addition, we show that part of the netrin-1-mediated reduction in albuminuria occurs through increased uptake of albumin by proximal tubular epithelial cells via activation of PI3k and ERK pathways. The overall mechanism of netrin-1-mediated renal protection is summarized in Figure 10.
Figure 10.
Model summarizing the role of netrin-1 in the regulation of inflammation and albumin uptake in proximal tubular epithelial cells of the diabetic kidney. Hyperglycemia and oxidative stress activate NFκB by inducing degradation of IκBα. Netrin-1 binding to UNC5B receptor will activate downstream signaling pathways like PI3K, ERK, and other unknown pathways. Activation of PI3K and ERK increases albumin uptake by tubular epithelial cells, thereby reducing albuminuria. Through an unknown pathway, netrin-1 also suppresses IκBα degradation and probably may increase the expression of IκBα as well, thereby suppressing NFκB release and translocation into nucleus and consequently suppressing COX-2 gene transcription. Suppression of COX-2 expression reduces the production of PGE2 and PGE2-mediated inflammation, and suppression of inflammation protects the kidney from damage and from development of nephropathy.
Netrin-1 is overexpressed in proximal tubular epithelial cells, is secreted into tubule lumen, and is excreted in urine.15 The large size of the netrin-1 molecule and the directional flow of urine make it harder for netrin-1 to act on the glomerulus. Thus, the observed reduction in albuminuria is unlikely due to netrin-1 effects on the glomerulus. This view was further supported by immunostaining data for chicken netrin-1 and the total absence of chicken netrin-1 in plasma. Recent studies suggest that albumin is filtered through the glomerulus, and that renal tubular epithelial cells actively reabsorb albumin from the lumen. Any defect in tubular epithelial cells will increase excretion of albumin.9,25,26 Our in vitro studies suggest that part of the netrin-1-mediated protective effect may occur through increased albumin uptake by proximal tubular epithelial cells.
An increase in the number of kidney neutrophils was seen in diabetic nephropathy, but the role of neutrophils in the progression of diabetic nephropathy is not clear.27 In the kidney, MCP-1 is produced by mesangial and tubular epithelial cells28,29 and mediates renal interstitial inflammation, tubular atrophy, and interstitial fibrosis.30 In the present study, we found significant elevation of urinary MCP-1 excretion and kidney expression with the progression of diabetes, which was associated with increased macrophage infiltration, suggesting that MCP-1 may play a role in increased urinary albumin excretion. In diabetes, urinary excretion of MCP-1 is due to MCP-1 production by the kidney.31 These data suggest the possibility that renal MCP-1 may contribute to the glomerular and tubulointerstitial lesions in diabetic nephropathy. Netrin-1-mediated suppression of urinary albumin may be mediated through suppression of MCP-1 production from renal epithelial cells. Macrophage infiltration into interstitium and into glomerulus was suppressed by tubule epithelial netrin-1. Although direct action of netrin-1 on glomerular macrophages is not possible, plausible indirect mechanisms include suppression of MCP-1 chemokines, reduction in glomerular hyperfiltration, and damage through suppression of PGE2 production and suppression of overall inflammation in the kidney.
The mechanism through which netrin-1 suppresses inflammation has been unknown. Here, we report for the first time that netrin-1 regulates prostanoid metabolism through COX-2 expression. Our in vitro results confirm the in vivo findings that netrin-1 suppresses hyperglycemia-induced PGE2 production and COX-2 expression. In addition, netrin-1 inhibited hyperglycemia-induced NFκB activation. Moreover, the addition of PGE2 induced chemokine and cytokine production from renal tubular epithelial cells. COX-2 and PGE2 have been linked to diabetic nephropathy in animal models.19,32–34 In addition to suppression of PGE2, netrin-1 also suppresses the production of leukotrienes (LTE4) in vivo (data not shown). Leukotrienes are known mediators of inflammation and have been implicated in diabetic nephropathy.35,36 The expression of prostaglandin E synthase 2 was not increased. Our results therefore suggest that netrin-1 suppresses inflammation through suppression of COX-2-mediated PGE2 production. The contribution of leukotriene suppression in netrin-1-mediated down-regulation of inflammation is not clear and needs further study.
In development of microalbuminuria, impaired tubular reabsorption of albumin plays a major role, in addition to glomerular hyperfiltration of albumin.25 The importance of tubular epithelial cells in development of proteinuria was reinforced by the recent observation that a human cubilin mutation caused proteinuria37 and that proximal tubular epithelial cell-specific cubilin knockout mice also developed albuminuria.38 Here, we show for the first time that proximal tubular epithelial cell-specific overexpression suppresses albuminuria, which reconfirms the importance of proximal tubular epithelial cells in controlling proteinuria. These results suggest that the reduction in albuminuria seen in vivo in transgenic animals may be mediated in part through increased uptake of albumin by proximal tubular epithelial cells. Netrin-1 is induced in diabetic kidney from WT mice, which may indicate tubular injury.39 Our recent in vitro studies suggest that increased albumin can induce the expression of netrin-1, whereas a high glucose level suppresses netrin-1 expression.18 Nonetheless, the involvement of netrin-1 up-regulation in diabetes is not clear.
In summary, the present study demonstrates that chronic diabetes induces renal dysfunction, necrosis, tubular atrophy, apoptosis, increased expression of cytokines and chemokines, and increased excretion of albumin in urine, characteristics of diabetic nephropathy that are seen in WT mice. Netrin-1 overexpression in tubular epithelial cells suppressed both structural and functional abnormalities, which may be mediated through suppression of inflammation and apoptosis. We conclude that netrin-1 administration represents a novel therapeutic option for the treatment of diabetic kidney disease and potentially for other diabetic complications.
Footnotes
Supported in part by the NIH (NIH-NIDDK grant 1-R01-DK083379-01A3 to G.R.).
Supplemental material for this article can be found at http://ajp.amjpathol.org or at http://dx.doi.org/10.1016/j.ajpath.2012.08.014.
Supplementary data
UNC5B localization in WT and netrin-1 transgenic mouse kidney. Immunostaining for UNC5B was performed in paraffin-embedded sections using goat anti-UNC5B antibody (R&D Systems) and donkey anti-goat antibody Fc fragment conjugated with horseradish peroxidase (Jackson ImmunoResearch Laboratories). Immunostaining for UNC5B was seen in the proximal tubular epithelial cells in control animal kidney, both WT and netrin-1 transgenic, with no change in response to diabetes. Intense immunostaining for UNC5B was seen in the brush border of proximal tubular epithelial cells of both WT and netrin-1 transgenic animals.
Effect of netrin-1 overexpression on diabetes-induced apoptosis. Top: Apoptotic cells were identified using TUNEL staining. WT and transgenic mouse control kidney did not show any apoptotic cells. Diabetes increased apoptosis in both tubular epithelial cells and glomerulus (blue staining, black arrows) in the WT animals, but few cells were positive for apoptosis in kidney of transgenic diabetic animals. Bottom: Quantification of apoptotic cells. Apoptotic positive cells were counted in five random 40× fields. *P < 0.001 versus all other groups. n = 4–6.
References
- 1.Hostetter T.H. Prevention of end-stage renal disease due to type 2 diabetes. N Engl J Med. 2001;345:910–912. doi: 10.1056/NEJM200109203451209. [DOI] [PubMed] [Google Scholar]
- 2.Rossing P., de Zeeuw D. Need for better diabetes treatment for improved renal outcome. Kidney Int Suppl. 2011;(120):S28–S32. doi: 10.1038/ki.2010.513. [DOI] [PubMed] [Google Scholar]
- 3.Rosolowsky E.T., Skupien J., Smiles A.M., Niewczas M., Roshan B., Stanton R., Eckfeldt J.H., Warram J.H., Krolewski A.S. Risk for ESRD in type 1 diabetes remains high despite renoprotection. J Am Soc Nephrol. 2011;22:545–553. doi: 10.1681/ASN.2010040354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cantor J., Haskins K. Recruitment and activation of macrophages by pathogenic CD4 T Cells in type 1 diabetes: evidence for involvement of CCR8 and CCL1. J Immunol. 2007;179:5760–5767. doi: 10.4049/jimmunol.179.9.5760. [DOI] [PubMed] [Google Scholar]
- 5.Chow F., Ozols E., Nikolic-Paterson D.J., Atkins R.C., Tesch G.H. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–128. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 6.Dandona P., Aljada A., Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 7.Moriwaki Y., Inokuchi T., Yamamoto A., Ka T., Tsutsumi Z., Takahashi S., Yamamoto T. Effect of TNF-alpha inhibition on urinary albumin excretion in experimental diabetic rats. Acta Diabetol. 2007;44:215–218. doi: 10.1007/s00592-007-0007-6. [DOI] [PubMed] [Google Scholar]
- 8.Baines R.J., Brunskill N.J. Tubular toxicity of proteinuria. Nat Rev Nephrol. 2011;7:177–180. doi: 10.1038/nrneph.2010.174. [DOI] [PubMed] [Google Scholar]
- 9.Gekle M. Renal proximal tubular albumin reabsorption: daily prevention of albuminuria. News Physiol Sci. 1998;13:5–11. doi: 10.1152/physiologyonline.1998.13.1.5. [DOI] [PubMed] [Google Scholar]
- 10.Magri C.J., Fava S. The role of tubular injury in diabetic nephropathy. Eur J Intern Med. 2009;20:551–555. doi: 10.1016/j.ejim.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Marcussen N. Atubular glomeruli and the structural basis for chronic renal failure. Lab Invest. 1992;66:265–284. [PubMed] [Google Scholar]
- 12.Morcos M., Sayed A.A.R., Bierhaus A., Yard B., Waldherr R., Merz W., Kloeting I., Schleicher E., Mentz S., Abd el Baki R.F., Tritschler H., Kasper M., Schwenger V., Hamann A., Dugi K.A., Schmidt A.M., Stern D., Ziegler R., Haering H.U., Andrassy M., van der Woude F., Nawroth P.P. Activation of tubular epithelial cells in diabetic nephropathy. Diabetes. 2002;51:3532–3544. doi: 10.2337/diabetes.51.12.3532. [DOI] [PubMed] [Google Scholar]
- 13.Wang W., Brian R.W., Ramesh G. Netrin-1 and kidney injury: I. Netrin-1 protects against ischemia-reperfusion injury of the kidney. Am J Physiol Renal Physiol. 2008;294:F739–F747. doi: 10.1152/ajprenal.00508.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ly N.P., Komatsuzaki K., Fraser I.P., Tseng A.A., Prodhan P., Moore K.J., Kinane T.B. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci USA. 2005;102:14729–14734. doi: 10.1073/pnas.0506233102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W., Reeves W.B., Pays L., Mehlen P., Ramesh G. Netrin-1 overexpression protects kidney from ischemia reperfusion injury by suppressing apoptosis. Am J Pathol. 2009;175:1010–1018. doi: 10.2353/ajpath.2009.090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramesh G., Reeves W.B. p38 MAP kinase inhibition ameliorates cisplatin nephrotoxicity in mice. Am J Physiol Renal Physiol. 2005;289:F166–F174. doi: 10.1152/ajprenal.00401.2004. [DOI] [PubMed] [Google Scholar]
- 17.Mazelin L., Bernet A., Bonod-Bidaud C., Pays L., Arnaud S., Gespach C., Bredesen D.E., Scoazec J.Y., Mehlen P. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431:80–84. doi: 10.1038/nature02788. [DOI] [PubMed] [Google Scholar]
- 18.Jayakumar C., Mohamed R., Ranganathan P.V., Ramesh G. Intracellular kinases mediate increased translation and secretion of netrin-1 from renal tubular epithelial cells. PLoS One. 2011;6:e26776. doi: 10.1371/journal.pone.0026776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng H., Fan X., Moeckel G.W., Harris R.C. Podocyte COX-2 exacerbates diabetic nephropathy by increasing podocyte (pro)renin receptor expression. J Am Soc Nephrol. 2011;22:1240–1251. doi: 10.1681/ASN.2010111149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W., Reeves W.B., Ramesh G. Netrin-1 increases proliferation and migration of renal proximal tubular epithelial cells via the UNC5B receptor. Am J Physiol Renal Physiol. 2009;296:F723–F729. doi: 10.1152/ajprenal.90686.2008. [DOI] [PubMed] [Google Scholar]
- 21.Navarro-González J.F., Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 22.Rivero A., Mora C., Muros M., García J., Herrera H., Navarro-González J.F. Pathogenic perspectives for the role of inflammation in diabetic nephropathy. Clin Sci. 2009;116:479–492. doi: 10.1042/CS20080394. [DOI] [PubMed] [Google Scholar]
- 23.Bloomgarden Z.T. Inflammation and insulin resistance. Diabetes Care. 2003;26:1922–1926. doi: 10.2337/diacare.26.6.1922. [DOI] [PubMed] [Google Scholar]
- 24.Tadagavadi R.K., Wang W., Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J Immunol. 2010;185:3750–3758. doi: 10.4049/jimmunol.1000435. [DOI] [PubMed] [Google Scholar]
- 25.Ishibashi F. Microalbuminuria in NIDDM is caused by increased excretion of unmodified albumin. Diabetes. 1996;45:731–735. doi: 10.2337/diab.45.6.731. [DOI] [PubMed] [Google Scholar]
- 26.Russo L.M., Sandoval R.M., Campos S.B., Molitoris B.A., Comper W.D., Brown D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol. 2009;20:489–494. doi: 10.1681/ASN.2008050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galkina E., Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol. 2006;17:368–377. doi: 10.1681/ASN.2005080859. [DOI] [PubMed] [Google Scholar]
- 28.Rollins B.J., Yoshimura T., Leonard E.J., Pober J.S. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant. MCP-1/JE Am J Pathol. 1990;136:1229–1233. [PMC free article] [PubMed] [Google Scholar]
- 29.Prodjosudjadi W., Gerritsma J.S., Klar-Mohamad N., Gerritsen A.F., Bruijn J.A., Daha M.R., van Es L.A. Production and cytokine-mediated regulation of monocyte chemoattractant protein-1 by human proximal tubular epithelial cells. Kidney Int. 1995;48:1477–1486. doi: 10.1038/ki.1995.437. [DOI] [PubMed] [Google Scholar]
- 30.Lloyd C.M., Minto A.W., Dorf M.E., Proudfoot A., Wells T.N.C., Salant D.J., Gutierrez-Ramos J.C. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–1380. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awad A.S., Huang L., Ye H., Duong E.T., Bolton W.K., Linden J., Okusa M.D. Adenosine A2A receptor activation attenuates inflammation and injury in diabetic nephropathy. Am J Physiol Renal Physiol. 2006;290:F828–F837. doi: 10.1152/ajprenal.00310.2005. [DOI] [PubMed] [Google Scholar]
- 32.Nasrallah R., Robertson S.J., Hébert R.L. Chronic COX inhibition reduces diabetes-induced hyperfiltration, proteinuria, and renal pathological markers in 36-week B6-Ins2(Akita) mice. Am J Nephrol. 2009;30:346–353. doi: 10.1159/000229304. [DOI] [PubMed] [Google Scholar]
- 33.Yabuki A., Taniguchi K., Yamato O. Immunohistochemical examination of cyclooxygenase-2 and renin in a KK-A(y) mouse model of diabetic nephropathy. Exp Anim. 2010;59:479–486. doi: 10.1538/expanim.59.479. [DOI] [PubMed] [Google Scholar]
- 34.Yao B., Xu J., Qi Z., Harris R.C., Zhang M.Z. Role of renal cortical cyclooxygenase-2 expression in hyperfiltration in rats with high-protein intake. Am J Physiol Renal Physiol. 2006;291:F368–F374. doi: 10.1152/ajprenal.00500.2005. [DOI] [PubMed] [Google Scholar]
- 35.Katoh T., Lianos E.A., Fukunaga M., Takahashi K., Badr K.F. Leukotriene D4 is a mediator of proteinuria and glomerular hemodynamic abnormalities in passive Heymann nephritis. J Clin Invest. 1993;91:1507–1515. doi: 10.1172/JCI116356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye Y., Lin Y., Perez-Polo J.R., Uretsky B.F., Ye Z., Tieu B.C., Birnbaum Y. Phosphorylation of 5-lipoxygenase at Ser523 by protein kinase A determines whether pioglitazone and atorvastatin induce proinflammatory leukotriene B4 or anti-inflammatory 15-epi-lipoxin A4 production. J Immunol. 2008;181:3515–3523. doi: 10.4049/jimmunol.181.5.3515. [DOI] [PubMed] [Google Scholar]
- 37.Ovunc B., Otto E.A., Vega-Warner V., Saisawat P., Ashraf S., Ramaswami G., Fathy H.M., Schoeb D., Chernin G., Lyons R.H., Yilmaz E., Hildebrandt F. Exome sequencing reveals cubilin mutation as a single-gene cause of proteinuria. J Am Soc Nephrol. 2011;22:1815–1820. doi: 10.1681/ASN.2011040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amsellem S., Gburek J., Hamard G., Nielsen R., Willnow T.E., Devuyst O., Nexo E., Verroust P.J., Christensen E.I., Kozyraki R. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol. 2010;21:1859–1867. doi: 10.1681/ASN.2010050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reeves W.B., Kwon O., Ramesh G. Netrin-1 and kidney injury: II. Netrin-1 is an early biomarker of acute kidney injury. Am J Physiol Renal Physiol. 2008;294:F731–F738. doi: 10.1152/ajprenal.00507.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
UNC5B localization in WT and netrin-1 transgenic mouse kidney. Immunostaining for UNC5B was performed in paraffin-embedded sections using goat anti-UNC5B antibody (R&D Systems) and donkey anti-goat antibody Fc fragment conjugated with horseradish peroxidase (Jackson ImmunoResearch Laboratories). Immunostaining for UNC5B was seen in the proximal tubular epithelial cells in control animal kidney, both WT and netrin-1 transgenic, with no change in response to diabetes. Intense immunostaining for UNC5B was seen in the brush border of proximal tubular epithelial cells of both WT and netrin-1 transgenic animals.
Effect of netrin-1 overexpression on diabetes-induced apoptosis. Top: Apoptotic cells were identified using TUNEL staining. WT and transgenic mouse control kidney did not show any apoptotic cells. Diabetes increased apoptosis in both tubular epithelial cells and glomerulus (blue staining, black arrows) in the WT animals, but few cells were positive for apoptosis in kidney of transgenic diabetic animals. Bottom: Quantification of apoptotic cells. Apoptotic positive cells were counted in five random 40× fields. *P < 0.001 versus all other groups. n = 4–6.