Abstract
An intramolecular Pauson-Khand type cycloaddition reaction of ene-vinylidenecyclopropanes with carbon monoxide has been established by using [Rh(COD)Cl]2 as the catalyst. The reaction was found to be highly efficient in solvents of 1,2-dichloroethane and 1,1,2,2-tetrachloroethane to give excellent yields of 90 – 99%. The reaction provides easy access to a series of fused 6,5-ring structures containing spiro-cyclopropane units that are useful for drug design and development. A mechanism of this cycloaddition process has been proposed accounting for structures of resulting products that were unambiguously assigned by X-ray diffractional analysis.
The Pauson-Khand reaction has been actively investigated for several decades because it can provide easy access to cyclopentanone derivatives to serve as important structural motifs that exist in a huge number of pharmaceutical substances and biologically active natural products.1–3 This reaction occurs through a [2+2+1] cycloaddition mechanism involving alkyne, alkene and CO as reactants. Besides the use of alkyne and alkene as starting materials, allene derivatives including allenynes have also been employed for the Pauson-Khand reaction.2 In 1994, Narasaka and coworkers reported the first intramolecular version of the Pauson-Khand reaction by using allenynes as substrates in the presence of an iron complex.3 Later, Brummond's group extensively studied the allene-based Pauson-Khand reaction by using transition metals, molybdenum or rhodium, as the catalysts for highly efficient control of regioselectivity of this cycloaddition reaction.4 During our ongoing project on vinylidenecyclopropanes' (VDCPs) chemistry,5 we have developed a Rh(I)-catalyzed Pauson-Khand-type [3+2+1] cycloaddition reaction of ene-vinylidenecyclopropanes (ene-VDCPs) with carbon monoxide to generate a series of aza- or oxa-bicyclic products in moderate to good yields with a highly regioand diastereoselective fashions (Scheme 1).6 In this communication, we would like to report a highly efficient Rh(I)-catalyzed intramolecular Pauson-Khand type cycloaddition reaction of ene-vinylidenecyclopropanes with carbon monoxide for the synthesis of a series of fused 6,5-ring structures containing spiro-cyclopropane motifs, which afforded up to 99% chemical yields (Scheme 1).
Scheme 1.
Rh(I)-catalyzed Pauson-Khand reaction of Ene-VDCPs
Our initial study on this catalytic reaction started with ene-vinylidenecyclopropane 1a as the substrate. In this substrate alkene and vinylidenecyclopropane moieties are connected by an anchor of "TsN" (Ts = 4-toluenesulfonyl). As shown in Table 1, we found that when the reaction was carried out at 80 °C in toluene under argon atmosphere protection (without the use of CO) in the presence of 5 mol % or 20 mol % of [Rh(CO)2Cl]2, the corresponding cycloaddition adduct 2a was obtained in 9% and 30% yields, respectively (Table 1, entries 1 and 2). The reaction was then conducted under CO atmosphere and in the presence of 10 mol % [Rh(CO)2Cl]2 as the catalyst, adduct 2a was produced in a chemical yield of 54% (Table 1, entry 3). However, a similar catalyst, Rh(CO)Cl(PPh3)2, was found to be catalytically inactive under this condition. Pleasingly, [Rh(COD)Cl]2 was confirmed as more efficient catalyst for this reaction to afford 2a in 66% yield (Table 1, entries 4 and 5). Our examination of solvent effects revealed that DCE (1,2-dichloroethane) and TCE (1,1,2,2-tetrachloroethane) are suitable solvents together with 10 mol % of [Rh(CO)2Cl]2 as the catalyst, giving 2a in 91% and 99% yields, respectively (Table 1, entries 7 and 8). Decreasing the loading of [Rh(COD)Cl]2 from 10 mol % to less than 5 mol % can also give product 2a in 94 – 99% yields under the above conditions (Table 1, entries 9–11). Using Pd(PPh3)2Cl2 (4 mol %) as the catalyst, no reaction occurred (Table 1, entry 12).
Table 1.
Optimization of Rh(I)-catalyzed Pauson-Khand-type cycloaddition reaction of ene-vinylidenecyclopropane 1a with CO
 | ||||
|---|---|---|---|---|
| entrya | catalyst | xb | solvent | yield (%)c |
| 2a | ||||
| 1 | [Rh(CO)2Cl]2 | 5 | toluene | 9d |
| 2 | [Rh(CO)2Cl]2 | 20 | toluene | 30d |
| 3 | [Rh(CO)2Cl]2 | 10 | toluene | 54 |
| 4 | Rh(CO)Cl(PPh3)2 | 10 | toluene | - |
| 5 | [Rh(COD)Cl]2 | 10 | toluene | 66 |
| 6 | [Rh(CO)2Cl]2 | 10 | DCE | 73 |
| 7 | [Rh(COD)Cl]2 | 10 | DCE | 91 |
| 8 | [Rh(COD)Cl]2 | 10 | TCE | 99 |
| 9 | [Rh(COD)Cl]2 | 5 | TCE | 99 |
| 10 | [Rh(COD)Cl]2 | 2 | TCE | 99 |
| 11 | [Rh(COD)Cl]2 | 1 | TCE | 94 |
| 12 | Pd(PPh3)2Cl2 | 4 | TCE | - |
Ene-VDCP 1a (0.2 mmol), catalyst (x mol %) and the solvent (2.0 mL) were added into a reaction tube under argon. Then, the reaction was carried out under CO atmosphere at 80 °C with in 1 h.
The loading of the catalyst.
Isolated yields.
The reaction was carried out under argon.
Having identified the optimal conditions, we next examined the scope and limitations of this Rh(I)-catalyzed cycloaddition reaction; the results are summarized in Table 2. Using ene-VDCPs 1b–1k as the substrates in which R can be various primary or secondary alkyl groups with X as TsN or BsN anchor (Bs = 4-bromobenzenesulfonyl), the desired products 2b–2k were obtained in excellent yields ranging from 92% to 97% (Table 2, entries 2–11). Moreover, as for substrates 1l and 1m, in which alkene and vinylidenecyclopropane moieties are connected by oxygen and carbon anchors, the reactions was performed smoothly to give the corresponding products 2l and 2m in 91% and 90% yields, respectively (Table 2, entries 12 and 13). Interestingly, when ene-vinylidenecyclopropane 1n which contains an additional CH2 extension was used as the substrate, no reaction was observed under the above conditions (Table 2, entry 14). The substrates with other different R groups are difficult to be prepared.
Table 2.
Substrate scope of Rh(I)-catalyzed Pauson-Khand-type cycloaddition reaction of ene-vinylidenecyclopropanes and CO
 | ||||
|---|---|---|---|---|
| entrya | substrate | yield (%)b | ||
| X | R | no. | 2 | |
| 1 | TsN | Me | 1a | 2a, 99 |
| 2 | TsN | Et | 1b | 2b, 95 |
| 3 | TsN | c-Hex | 1c | 2c, 94 |
| 4 | TsN | Bn | 1d | 2d, 92 |
| 5 | BsN | Me | 1e | 2e, 95 |
| 6 | BsN | Et | 1f | 2f, 94 |
| 7 | BsN | c-Hex | 1g | 2g, 92 |
| 8 | BsN | Bn | 1h | 2h, 92 |
| 9 | BsN | 1i | 2i, 97 | |
| 10 | BsN | 1j | 2j, 95 | |
| 11 | BsN | n-Bu | 1k | 2k, 97 |
| 12 | O | Bn | 1l | 2l, 91 |
| 13 | CH2 | Bn | 1m | 2m, 90 |
| 14 |  |
1n | NR | |
1 (0.2 mmol), [Rh(COD)Cl]2 (2 mol %), and TCE (2.0 mL) were added into a schlaker reaction tube under CO (1 atm). The reactions were carried out at 80 °C within 1 h.
Isolated yields.
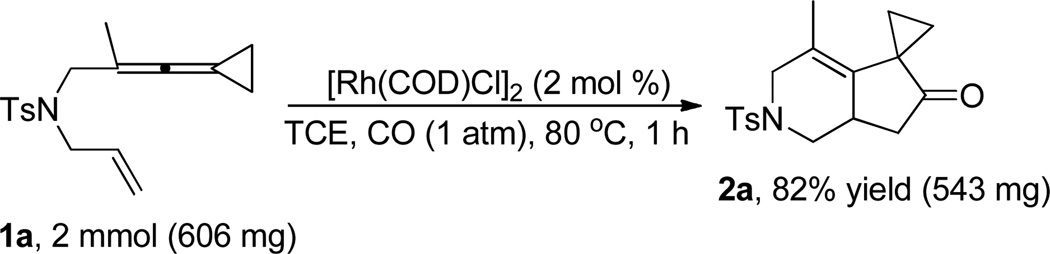
To our delight, on enlarging the reaction scale to 2.0 mmol by employing 1a as the substrate, similar results could be obtained, affording 2a in 82% yield (Scheme 2).
Scheme 2.
Rh(I)-catalyzed Pauson-Khand-type cycloaddition reaction of ene-vinylidenecyclopropane 1a (2 mmol) and CO
The resulting products have been determined by NMR spectroscopic and HRMS analysis (see SI supplement); and the product of 2a has been unambiguously assigned by X-ray diffractional analysis. The CIF data of this X-ray analysis are summarized in SI supplement materials and corresponding ORTEP drawing is presented in Figure 1.7
Figure 1.
X-ray Crystal Structure of Product 2a
A plausible reaction mechanism has been shown in Scheme 3 using 1a for the model reaction. At first, the coordination of Rh(I) with alkene and allene moieties in VDCP leads to formation of intermediate A; this intermediate then undergoes oxidative cyclization to give the rhodacycle intermediate B. Insertion of CO into B generates two regioisomers C and D which is subjected to reductive elimination followed by the formation of cycloadduct 2a. At this last step, the species Rh(I) is regenerated for catalytic cycles.
Scheme 3.
A Plausible Reaction Mechanism
In conclusion, we have developed a highly efficient Rh(I)-catalyzed intramolecular Pauson-Khand type cycloaddition reaction of ene-vinylidenecyclopropanes with CO, which can efficiently result in fused 6,5-ring structures containing spiro-cyclopropane units with excellent yields (> 90% for all cases). Further work will be devoted toward applications of this new methodology to the synthesis of biologically important targets.
Supplementary Material
Acknowledgements
We thank the Shanghai Municipal Committee of Science and Technology (11JC1402600), National Basic Research Program of China (973)-2009CB825300, and the National Natural Science Foundation of China (21072206, 20472096, 20872162, 20672127, 21121062, 20732008), and Robert A. Welch Foundation (D-1361) and NIH (R21DA031860-01) for their generous support.
Footnotes
Supporting Information Available: The reaction procedure for preparation of ene-vinylidenecyclopropanes and spectroscopic data of the compounds shown in Tables 1–2 along with the detailed descriptions of experimental procedures as well as the crystal structures of 2a. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Min Shi, Email: mshi@mail.sioc.ac.cn.
Guigen Li, Email: guigen.li@ttu.edu.
References
- 1.Narasaka K, Shibata T. Chem. Lett. 1994:315. [Google Scholar]
- 2.Alcaide B, Almendros P. Eur. J. Org. Chem. 2004:3377. [Google Scholar]
- 3.Narasaka K, Shibata T. Chem. Lett. 1994:315. [Google Scholar]
- 4. Brummond KM, Mitasev B. Org. Lett. 2004;6:2245. doi: 10.1021/ol0492391. Kent JL, Wan H, Brummond KM. Tetrahedron Lett. 1995;36:2407. Brummond KM, Chen H, Fisher KD, Kerekes AD, Rickards B, Sill PC, Geib S. J. Org. Lett. 2002;4:1931. doi: 10.1021/ol025955w. Brummond KM, Wan H, Kent JL. J. Org. Chem. 1998;63:6535. Brummond KM, Gao D. Org. Lett. 2003;5:3491. doi: 10.1021/ol035322x. Brummond KM, Curran D-P, Mitasev B, Fisher S. J. Org. Chem. 2005;70:1745. doi: 10.1021/jo0481607. Bayden AS, Brummond KM, Jordan KD. Organometallics. 2006;25:5204. doi: 10.1021/om0607503. For selected recent reports on the Pauson-Khand-type reaction of allenes, see: Grillet F, Huang C, Brummond KM. Org. Lett. 2011;13:6304. doi: 10.1021/ol2028515. Inagaki F, Itoh N, Hayashi Y, Matsui Y, Mukai C. Beilstein J. Org. Chem. 2011;7:404. doi: 10.3762/bjoc.7.52. Antras F, Laurent S, Ahmar M, Chermette H, Cazes B. Eur. J. Org. Chem. 2010:3312. Kawamura T, Inagaki F, Narita S, Takahashi Y, Hirata S, Kitagaki S, Mukai C. Chem. Eur. J. 2010;16:5173. doi: 10.1002/chem.200903568. Inagaki F, Mukai C. Org. Lett. 2006;8:1217. doi: 10.1021/ol0600990. Alcaide B, Almendros P. Eur. J. Org. Chem. 2004:3377. Mukai C, Nomura I, Yamanishi K, Hanaoka M. Org. Lett. 2002;4:1755. doi: 10.1021/ol020051w. Brummond KM, Chen H, Fisher KD, Kerekes AD, Rickards B, Sill PC, Geib SJ. Org. Lett. 2002;4:1931. doi: 10.1021/ol025955w.
- 5.For vinylidenecyclopropane chemistry, see: Poutsma ML, Ibarbia PA. J. Am. Chem. Soc. 1971;93:440. Smadja W. Chem. Rev. 1983;83:263. Sugita H, Mizuno K, Saito T, Isagawa K, Otsuji Y. Tetrahedron Lett. 1992;33:2539. Mizuno K, Sugita H, Kamada T, Otsuji Y. Chem. Lett. 1994:449. and references therein; Sydnes LK. Chem. Rev. 2003;103:1133. doi: 10.1021/cr010025w. Mizuno K, Maeda H, Sugita H, Nishioka S, Hirai T, Sugimoto A. Org. Lett. 2001;3:581. doi: 10.1021/ol000379u. Shi M, Shao L-X, Lu J-M, Wei Y, Mizuno K, Maeda H. Chem. Rev. 2010;110:5883. doi: 10.1021/cr900381k. Xu G-C, Liu L-P, Lu J-M, Shi M. J. Am. Chem. Soc. 2005;127:14552. doi: 10.1021/ja054988e. Li W, Yuan W, Pindi S, Shi M, Li G-G. Org. Lett. 2010;12:64. doi: 10.1021/ol902832s. Lu B-L, Wei Y, Shi M. Chem. Eur. J. 2010;16:10975. doi: 10.1002/chem.201001433. Lu B-L, Shi M. Eur. J. Org. Chem. 2011:243. Lu B-L, Shi M. Angew. Chem. Int. Ed. 2011;50:12027. doi: 10.1002/anie.201105292.
- 6. Lu B-L, Wei Y, Shi M. Organometallics. 2012;31:4601. For selected reviews on the rhodium-catalyzed Pauson-Khand reaction, see: Jeong N, Sung BK, Kim JS, Park SB, Seo SD, Shin JY, In KY, Choi YK. Pure Appl. Chem. 2002;74:85. Baik M-H, Mazumder S, Ricci P, Sawyer JR, Song Y-G, Wang H, Evans PA. J. Am. Chem. Soc. 2011;133:7621. doi: 10.1021/ja107895g. Kim D-E, Park S-H, Choi Y-H, Lee SG, Moon DH, Seo J-J, Jeong NC. Chem. Asian J. 2011;6:2009. doi: 10.1002/asia.201100271. Turlington M, Pu L. Org. Lett. 2011;13:4332. doi: 10.1021/ol201670c. Kim DE, Ratovelomanana-Vidal V, Jeong N. Adv. Synth. Catal. 2010;352:2032. For selected recent reports on the Pauson-Khand-type reaction of heteroalkenes, see: Saito T, Furukawa N, Otani T. Org. Biomol. Chem. 2010;8:1126. doi: 10.1039/b924301a. Mukai C, Yoshida T, Sorimachi M, Odani A. Org. Lett. 2006;8:83. doi: 10.1021/ol052562z. For selected recent reports on the Pauson-Khand-type reaction of cyclopropanes, please see: Jiao L, Lin M, Zhuo L-G, Yu Z-X. Org. Lett. 2010;12:2528. doi: 10.1021/ol100625e. Fan X, Tang M-X, Zhuo LG, Tu YQ, Yu ZX. Tetrahedron Lett. 2009;50:155. Fan X, Zhuo L-G, Tu Y-Q, Yu Z-X. Tetrahedron. 2009;65:4709. Jiao L, Yuan C, Yu Z-X. J. Am. Chem. Soc. 2008;130:4421. doi: 10.1021/ja7100449. Kim SY, Lee SI, Choi SY, Chung YK. Angew. Chem. Int. Ed. 2008;47:4914. doi: 10.1002/anie.200800432. Wang Y, Wang J, Su J, Huang F, Jiao L, Liang Y, Yang D, Zhang S, Wender PA, Yu Z-X. J. Am. Chem. Soc. 2007;129:1006. doi: 10.1021/ja072505w. Wender PA, Gamber GG, Hubbard RD, Pham SM, Zhang L. J. Am. Chem. Soc. 2005;127:2836. doi: 10.1021/ja042728b. Wegner HA, de Meijere A, Wender PA. J. Am. Chem. Soc. 2005;127:6530. doi: 10.1021/ja043671w.
- 7.The crystal data of 2a have been deposited in CCDC with number 830675.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.