Abstract
We report significantly decreased white matter protein levels in the nucleus accumbens in an adult mouse model of chronic cocaine abuse. Previous studies from human cocaine abuse patients show disruption of white matter and myelin loss, thus supporting our observations. Understanding the neuropathological mechanisms for white matter disruption in cocaine abuse patients is complicated by polydrug use and other comorbid factors, hindering the development of effective therapeutic strategies to ameliorate damage or compliment rehabilitation programs. In this context, our data further demonstrate that cocaine-induced loss of white matter proteins is absent in mice treated with the β-lactam antibiotic, ceftriaxone, during cocaine withdrawal. Other studies report that ceftriaxone, a glutamate transporter subtype-1 activator, is neuroprotective in murine models of multiple sclerosis, thereby demonstrating potential therapeutic properties for diseases with white matter loss. Cocaine-induced white matter abnormalities likely contribute to the cognitive, motor, and psychological deficits commonly afflicting cocaine abusers, yet the underlying mechanisms responsible for these changes remain unknown. Our observations describe an adult animal model for the study of cocaine-induced myelin loss for the first time, and highlight a potential pharmacological intervention to ameliorate cocaine-induced white matter loss.
In 2009, it was estimated that nearly 5 million Americans ≥12 years of age had used cocaine during the past year.1 Imaging studies and postmortem microarray data from human cocaine abuser populations suggest that chronic abuse disrupts white matter (WM) integrity and myelin gene expression in discrete brain regions, including the nucleus accumbens (NA)2,3,4 As human studies of abuse are often confounded by polydrug use and co-morbid conditions, in vivo animal studies are needed to delineate pathways affected by cocaine that may contribute to myelin loss to highlight potential treatment targets. In a recent magnetic resonance imaging study, WM abnormalities were reported in active cocaine users.5 However, the WM abnormalities resolved to some degree in patients with prolonged abstinence, suggesting recovery potential, making results from our study relevant for adjuvant therapies during rehabilitation programs. The mechanisms involved in cocaine-induced myelin loss are unknown, but may involve glial excitotoxicity.6–13 Even less clear are mechanisms that may contribute to white matter recovery or protection.
Some studies have evaluated the effects of maternal cocaine abuse on myelination defects in offspring,14 however, investigations of cocaine-mediated myelin disruption in adult animal models have not been previously described. Recent studies in our laboratory uncovered a new model by which cocaine significantly decreases WM proteins in adult mice. Chronic cocaine administration followed by withdrawal and challenge resulted in a significant decrease in a number of myelin-associated proteins, including myelin basic protein (MBP), proteolipid protein (PLP), myelin oligodendrocyte glycoprotein (MOG), and myelin-associated glycoprotein (MAG) in the NA. However, when ceftriaxone, a glutamate subtype transporter-1 (GLT1) activator2,15,16 was administered during the withdrawal period, no evidence of WM loss was observed. Ceftriaxone has displayed anti-addictive and neuroprotective properties in murine models of cocaine abuse and multiple sclerosis, respectively,2,17,18 although the exact mechanisms through which it is acting are unknown. Additionally, recent studies by Sondheimer and Knackstedt19 reported that ceftriaxone prevented the induction of cocaine sensitization in a rat model of cocaine abuse.
Our results report two important observations in an adult mouse model: cocaine-induced myelin loss and attenuation of cocaine-induced WM protein loss in ceftriaxone-treated mice. The observation that ceftriaxone negates cocaine-induced WM loss provides clues as to the mechanism through which cocaine may exert its effects on WM protein levels and points to alterations in glutamatergic homeostasis. Expanded studies are underway to address this hypothesis.
Materials and Methods
Animals
This study used 10-week-old male C57BL/6 mice (Charles River Laboratories, Wilmington, MA). All procedures were approved by and conducted in accordance with Temple University Institutional Animal Care and Use Committee guidelines. Mice were singly housed in an animal facility with constant airflow, controlled temperature (21–23°C), and a 12-hour light/dark cycle, and the mice were supplied with food and water ad libitum. The mice were divided into five groups with 8 to 10 mice per group. Cocaine hydrochloride (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile water and administered at 15 mg/kg i.p. Ceftriaxone sodium (American Regent Inc., Shirley, NY) was dissolved in sterile water and administered (200 mg/kg i.p.). Injection volumes were 1 mL/100 g per body weight.
The paradigm for each group consisted of a total of 47 days of injections: 3 days pretreatment with the vehicle (sterile water) or ceftriaxone, 14 days of cocaine or vehicle with or without ceftriaxone, followed by 30 days of withdrawal with either vehicle or ceftriaxone on each day. The control group received a total of 47 days of vehicle. The ceftriaxone only (Ceft) group received ceftriaxone for 17 days, followed by 30 days of vehicle. The cocaine only (Coc) group received 3 days vehicle, followed by 14 days of cocaine, and 30 days of vehicle. The cocaine plus ceftriaxone (Coc with Ceft) group received 3 days of ceftriaxone, followed by 14 days of cocaine plus ceftriaxone, and then 30 days of vehicle. The cocaine then ceftriaxone (Coc then Ceft) group received 3 days of vehicle, 14 days of cocaine, and 30 days of ceftriaxone. All mice received a single challenge dose of cocaine (15 mg/kg i.p.) 24 hours before euthanasia.
Tissue Harvest
Animals were heavily anesthetized with 5% isoflurane and decapitated. Brains were removed and placed in ice-cold PBS. The NA was dissected from each brain, and the tissue was homogenized by mechanical dounce disruption on ice in lysis buffer (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 0.5% NP-40, 1:200 protease inhibitor cocktail; Calbiochem, San Diego, CA). Once completely homogenized, samples were centrifuged at 13,000 × g at 4°C for 5 minutes. The protein (contained in Supernatant) was collected, and protein concentrations were determined via the Bradford assay. Protein samples were stored at −80°C. For immunohistochemical analyses, whole brains were removed, fixed in formalin, embedded in paraffin, sectioned, and processed for immunolabeling, described as follows.
Western Analyses
Equal amounts of protein were loaded onto pre-cast midi-gels (4 to 12% Bis-Tris; Invitrogen, Carlsbad, CA), separated by electrophoresis and transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were blocked in 5% nonfat milk in 1× Tris-buffered saline, 0.1% Tween-20 for 30 minutes before incubation with primary antibodies. Primary antibodies included: myelin basic protein (1:10,000; Millipore, Danvers, MA), MOG (1:1000; Abcam, Cambridge, MA), MAG (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA), PLP, and PLP alternate isoform protein (DM20) (1:2000; Abcam), cleaved caspase-3, (1:500; Millipore), GLT1 (1:20,000; Millipore), or loading control glyceraldehydes-3-phosphate dehydrogenase (1:4000; Santa Cruz, CA). Membranes were incubated for either 2 hours at 23°C or overnight at 4°C, washed in 1× Tris-buffered saline, 0.1% Tween-20, incubated with appropriate secondary anti-mouse or anti-rabbit antibodies (1:10,000; Thermo Scientific, Lafayette, CO) for 1 hour at 23°C, and developed with enhanced chemiluminescence (ECL) or ECL PRIME (Amersham Pharmacia Biotech, Piscataway, NJ). Band intensities were calculated using ImageJ software (NIH, Bethesda, MD) and were normalized to loading control glyceraldehyde-3-phosphate dehydrogenase (GAPDH).20
Immunofluorescence Labeling
Coronal sections (4 μm) of formalin-fixed, paraffin-embedded tissues were placed on electromagnetically charged glass slides. The slides were deparaffinized in xylene and rehydrated through descending grades of ethanol up to water. After nonenzymatic antigen retrieval in 0.01 M sodium citrate buffer (pH 6.0) for 30 minutes at 97°C in a vacuum oven, tissue was permeabilized in 0.2% Triton X-100 in 1× PBS for 10 minutes, and slides were washed with 1× PBS and placed in blocking solution (5% normal goat or horse serum; Vector Laboratories, Burlingame, CA) for 2 hours. Primary antibodies consisted of MBP (1: 200; Millipore), neurofilament, (1:200; Covance, Princeton, NJ), CC1 (1:50; Abcam), and cleaved caspase-3 (1:50; Millipore). Sections were incubated with primary antibody overnight in a dark, humidified chamber at 23°C, rinsed three times with PBS, and incubated with fluorescein isothiocyanate (1:500; Vector Laboratories) or Texas Red (1:500; Vector Laboratories) conjugated secondary antibodies for 1 hour at 23°C in the dark. Sections were again washed three times with PBS, re-blocked (5% normal goat or horse serum), incubated overnight with the second primary antibody, washed, and again incubated with the appropriate secondary antibody. Slides were cover slipped with an aqueous-based mounting medium containing DAPI for nuclear labeling (Vectashield; Vector Laboratories), visualized with a Nikon UV inverted microscope, and processed with deconvolution software (Slidebook 4.0; Intelligent Imaging, Denver, CO), allowing acquisition of multiple 0.25 μm thick digital sections and 3-dimensional reconstruction of the image.
Statistical Analysis
All data were analyzed using one-way analysis of variance with post hoc testing where appropriate using GraphPad Prizm (GraphPad Software Inc., San Diego, CA) and results are expressed as mean ± SEM, n ≥ 8. Values of P ≤ 0.05 were considered statistically significant. The fewest mice required to detect differences with an α-level of 0.05 and 80% power were used.
Results
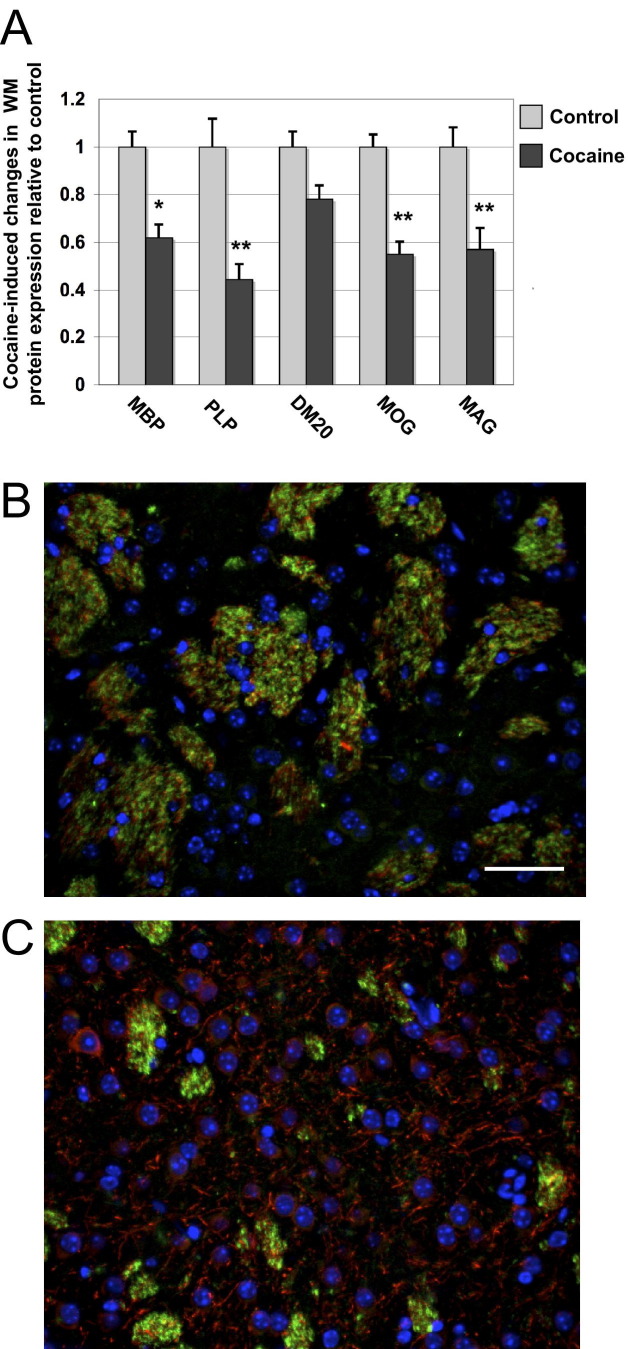
Chronic cocaine administration (15 mg/kg daily for 14 days) followed by 30-day withdrawal and cocaine challenge (15 mg/kg, once) resulted in significant decreases in myelin-associated proteins in the NA (Figure 1A). White matter deficits were not observed in the prefrontal cortex or other brain regions assessed (data not shown). During withdrawal, mice were given vehicle injections every day for 30 days, followed by a single dose cocaine challenge on the last day. After withdrawal, MBP levels in cocaine-treated animals were 38% lower than controls (P < 0.05), and PLP levels were 55% lower than controls (P < 0.001) (Figure 1A). Likewise, in cocaine-treated animals, DM20 was decreased by 33% compared to controls, but levels did not reach statistical significance (or were NS) (Figure 1A). Myelin basic protein and PLP/DM20 constitute between 60 and 80% of the total myelin sheath in human and rodent brains. Much of the remaining 20 to 40% of the myelin sheath consists of MOG and MAG. MOG and MAG were decreased significantly (P < 0.001) by 42 and 58%, respectively, compared to control levels (Figure 1A). Double-immunofluorescence labeling of NA tissue from a representative control mouse showed robust MBP immunoreactivity (green) and associated neurofilament (red) for axons (Figure 1B). On the other hand, in a cocaine-treated mouse, sparse MBP immunoreactivity (green) was observed in the NA (Figure 1C). Interestingly, neurofilament immunolabeling of the cocaine-treated mouse remained robust, suggesting the presence of unmyelinated axons (Figure 1C). These data indicate that 14 days of cocaine administration leads to significant loss of MBP, PLP, MOG, and MAG WM proteins after 30 days of cocaine withdrawal and a single challenge dose.
Figure 1.
Effects of cocaine on white matter protein levels in the nucleus accumbens. A: Levels of MBP (*P < 0.05) PLP, MOG, MAG (**P < 0.001), PLP alternate isoform (DM20) (NS), in mice administered cocaine (15 mg/kg) for 14 days, followed by 30 days vehicle during withdrawal and a single challenge dose of cocaine. n = 8 to 10 mice per group with one-way analysis of variance with Bonferroni's multiple comparison post hoc testing. B and C: Double immunofluorescence labeling of nucleus accumbens from control (B), and mice treated with cocaine for 14 days, 30 days withdrawal (C), followed by cocaine challenge. Tissues are labeled with MBP in green, neurofilament in red, and nuclei are labeled in blue with DAPI. Original magnification, ×40. Scale bar = 10 μm.
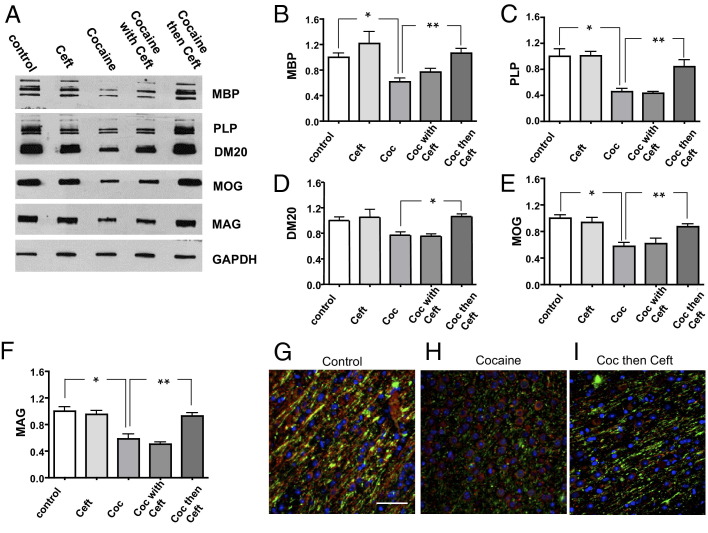
Mice administered cocaine for 14 days followed by daily injections of ceftriaxone (200 mg/kg) during the 30-day withdrawal showed no significant loss of myelin proteins (Figure 2) (compare the control group to the Coc then Ceft group). In mice in which ceftriaxone was given with cocaine for 14 days followed by a vehicle during withdrawal the loss of WM was similar to levels in the Coc group (Figure 2, A–F) (compare the Coc group to the Coc with Ceft group). Likewise, mice given ceftriaxone only for 14 days, followed by a vehicle during withdrawal had WM levels similar to the control group (Figure 2, A–F) (compare the control group to the Ceft group). These data show that administration of ceftriaxone during the withdrawal period after 14 days of cocaine administration prevents MBP, PLP, MOG, and MAG WM protein loss upon withdrawal and challenge.
Figure 2.
Effects of ceftriaxone administration on WM during withdrawal from cocaine. A: Representative Western blot of 10 μg protein from NAc from one mouse from each group. GAPDH was used as a loading control. B–F: WM protein levels in control; 17 days ceftriaxone followed by 30 days vehicle during withdrawal (Ceft); 14 days cocaine followed by 30 days vehicle during withdrawal (Coc); 3 days ceftriaxone pretreatment followed by 14 days of ceftriaxone and cocaine, and 30 days of vehicle during withdrawal (Coc with Ceft); 14 days of cocaine followed by 30 days ceftriaxone (200 mg/kg) during withdrawal (Coc then Ceft). B: Quantification of MBP. *P < 0.05, **P < 0.01. C: PLP. *P < 0.001, **P < 0.05. D: PLP alternate isoform (DM20). *P < 0.05. E: Myelin oligodendrocyte glycoprotein (MOG). *P < 0.001, **P < 0.01. F: MAG. *P < 0.001, **P < 0.01 levels. n = 8 to 10 mice per group with one-way analysis of variance with Bonferroni's multiple comparison post hoc testing. G–I: Double immunofluorescence labeling of nucleus accumbens from (G) control; (H) cocaine (15 mg/kg) for 14 days, followed by 30 days vehicle during withdrawal and a single dose challenge of cocaine; (I) cocaine (15 mg/kg) for 14 days, followed by 30 days ceftriaxone during withdrawal and a single dose challenge of cocaine. Tissues are labeled with MBP in green, neurofilament in red, and nuclei labeled in blue with DAPI. Original magnification, ×40. Scale bar = 10 μm. DM20, PLP alternate isoform; MAG, myelin-associated glycoprotein.
Double immunofluorescence labeling of NAc tissue from a representative control mouse showed robust MBP (green) and neurofilament (red) immunoreactivity (Figures 1B and 2G). On the other hand, in a cocaine-treated mouse, sparse MBP immunoreactivity (green) was observed in the NA (Figures 1C and 2H). In a mouse treated with cocaine for 14 days followed by ceftriaxone during withdrawal MBP labeling appeared similar to levels in the control (compare green) (Figure 2, G and I). However, co-localization (yellow) of neurofilament (red) with MBP (green) in mice given cocaine then ceftriaxone during withdrawal appears to be decreased (Figure 2I). This pattern may suggest that even though MBP levels are normal, association with axons may be altered.
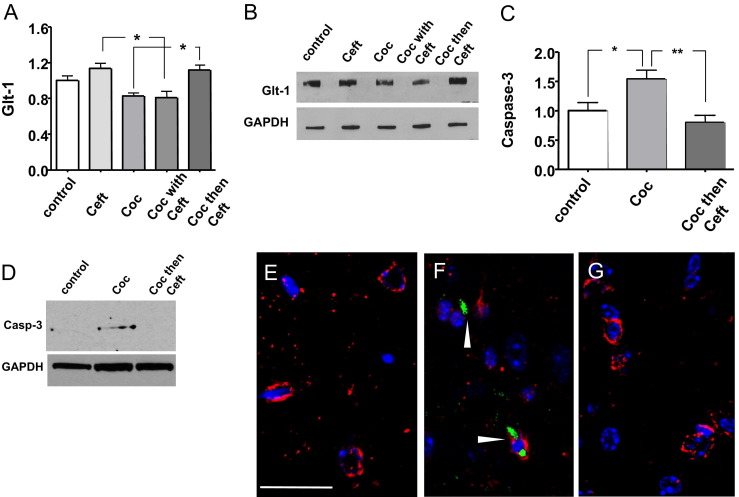
Cocaine-mediated changes in glutamate signaling may induce oligodendrocyte cell damage or death, and may be responsible in part for the loss of WM proteins observed in cocaine-treated mice. Likewise, ceftriaxone (a GLT1 activator) abolished cocaine-induced white matter protein loss, suggesting a contribution of glutamate excitotoxicity cell death. In this context, expression levels of GLT1 and cleaved caspase-3, which is indicative of apoptosis, were assessed. GLT1 levels in the cocaine-treated group were lower than levels in the control group, although changes did not reach statistical significance (Figure 3, A and B). Because GLT1 is responsible for the clearance of more than 90% of glutamate in the synaptic cleft, even slight changes in GLT1 expression could have a significant impact on synaptic glutamate levels.21 In the cocaine-treated group, GLT1 levels were significantly lower than Ceft only and the Coc then Ceft groups (P < 0.01) (Figure 3, A and B). In the cocaine-treated group, caspase-3 levels were significantly greater than the control (P < 0.05) and Coc then Ceft groups (P < 0.01) (Figure 3, C and D) in which caspase-3 was nearly undetectable. In this context, other studies have shown that glutamate excitotoxicity induces apoptosis in oligodendrocytes in a caspase-dependent manner.22,23 To assess if oligodendrocytes in cocaine-treated mice without Ceft showed increased caspase-3 cleavage, double immunofluorescence labeling for activated caspase-3 and an oligodendrocyte-specific marker, CC1, was conducted. CC1 immunoreactive oligodendrocytes (red) in cocaine-treated mice showed immunolabeling for caspase-3 (green) (Figure 3F, arrowheads), whereas, control mice (Figure 3E) and Coc then Ceft mice (Figure 3G) showed nearly undetectable caspase-3 immunolabeling.
Figure 3.
Evidence of decreased glutamate subtype transporter-1 (GLT1) and increased caspase-3 activity in oligodendrocytes of cocaine-treated mice. A: GLT1 levels in NAc of control; 17 days ceftriaxone only (Ceft) followed by 30 days vehicle during withdrawal; 14 days cocaine only (Coc) followed by 30 days vehicle during withdrawal; 3 days Ceft pretreatment followed by 14 days of ceftriaxone and cocaine (Coc with Ceft) and 30 days of vehicle during withdrawal; 14 days of cocaine followed by 30 days ceftriaxone (Coc then Ceft) during withdrawal. *P < 0.01. B: Representative Western blot of GLT1 levels in 10 μg protein from NAc from one mouse from each group. GADPH was used as a loading control. C: Representative using Western blot of cleaved caspase-3 levels in 40 μg protein from NAc from one mouse from each group. GAPDH was used as a loading control. *P < 0.05, **P < 0.01. D: Cleaved caspase-3 protein levels in control; 14 days cocaine followed by 30 days vehicle during withdrawal (Coc); 14 days of cocaine followed by 30 days ceftriaxone during withdrawal (Coc then Ceft). n = 8 to 10 mice per group with one-way analysis of variance with Bonferroni's multiple comparison post-hoc testing. E–G: NAc tissues from representative mice are labeled with an antibody against cleaved caspase-3 in green, CC1, an oligodendrocyte-specific maker in red, and nuclei in blue with DAPI. E: NAc tissues from control show robust CC1-immunoreactivity (red) with no evidence of caspase-3 activity. F: NAc tissues from cocaine-treated mice show decreased CC1-immunoreactivity (red) with evidence of caspase-3 activity (green, arrowheads) associated with CC1-1+ oligodendrocytes (red). G: NAc tissues from cocaine treated mice that received ceftriaxone during withdrawal show CC1-immunoreactivity (red) with undetectable caspase-3 activity (green). Original magnification, ×100. Scale bar = 10 μm.
Although detailed behavior assessments are beyond the scope of this report, we observed that ceftriaxone-treated mice and mice that received cocaine followed by ceftriaxone during withdrawal (Coc then Ceft) did not exhibit increased activity as observed in cocaine-treated mice (Coc) and in mice treated with cocaine and ceftriaxone simultaneously (Coc with Ceft) (data not shown). Thus, the prominent clinical signs observed in some of these groups included increased activity. Control, Ceft, and Coc then Ceft groups were not different from one another.
Discussion
Several pathways likely contribute to cocaine-mediated WM loss. Among potential contributors are changes in levels of dopamine, cellular retinoic acid, or glutamate. In this regard, acute central nervous system effects of cocaine administration include increased extracellular dopamine in the synaptic cleft due to the blockade of the dopamine transporter.24 Activation of dopaminergic D3 receptors decreases differentiation of oligodendrocyte precursor cells into mature oligodendrocytes and inhibits myelin formation.25 In addition, our laboratory has demonstrated that chronic cocaine abuse alters retinoic acid signaling and decreases expression of retinoid X receptor isoform-γ.26 These findings are significant in the context of the current study, because cellular levels of retinoic acid regulate both MBP and PLP.27,28 Furthermore, Huang et al29 recently demonstrated the importance of retinoid X receptor isoform-γ in oligodendrocyte maturation and remyelination after WM lesioning. Cocaine-induced disruption of the retinoic acid pathway in oligodendrocytes, therefore, may be responsible in part for the WM loss observed after paradigms of chronic abuse.
In our study, the cocaine-induced deficits in WM protein levels were normalized by the GLT1 activator ceftriaxone, suggesting a glutamate-related mechanism may be involved. Early studies reported that acute cocaine exposure increases extracellular glutamate in the NA.7,30 It is also reported that reintroduction to cocaine after forced abstinence elicits an increase in extracellular glutamate in the NA greater than increases observed after initial cocaine exposure.8,31 Moreover, recent studies by Sondheimer and Knackstedt19 report that in a rat model of cocaine abuse, ceftriaxone prevented the induction of cocaine sensitization. In light of these findings, all mice received a single challenge dose of cocaine (15 mg/kg i.p.) before euthanasia 24 hours later. Numerous studies from Kalivas et al10 have expanded on these findings to show that decreased extracellular glutamate levels in the NA after withdrawal from chronic cocaine potentiates reinstatement of cocaine-seeking behavior.2,8,32 Studies from Kalivas et al10 also show that the effects can be prevented by restoring extracellular glutamate levels. Thus, one potential explanation for the observations in our study is that the elevation in extracellular glutamate after cocaine challenge, coupled with the upregulation of N-methyl-d-aspartic acid and α-amino-3-hydroxy-5-methyl-4-isoxazole-propanoic acid receptors, which likely occurred during cocaine withdrawal, may have resulted in glutamate-mediated toxicity that was manifested as oligodendrocyte apoptotic death and a reduction in WM proteins. It is also important to note that the deficits in WM protein levels became more pronounced during the course of withdrawal. WM protein levels after 30 days of withdrawal were only 50% of the control levels in some cases (Figure 1), whereas, a shorter withdrawal interval of 2 days resulted in significant WM loss, but to a lesser degree than a 30-day withdrawal (data not shown). These observations are supported by previous studies showing that temporal decreases in basal glutamate levels are more pronounced at later stages of withdrawal.6,33 Thus, repeated cocaine exposure followed by treatment with ceftriaxone during the withdrawal interval may increase the expression of GLT1, an effect that is hypothesized to offset the upregulation of N-methyl-d-aspartic acid and α-amino-3-hydroxy-5-methyl-4-isoxazole-propanoic acid receptors.
Although neurons are reported to be most affected by increased extracellular glutamate on acute cocaine administration,30 the loss of WM proteins suggests damage to oligodendrocytes. In support of glutamate-mediated loss of WM, recent studies in multiple sclerosis, stroke, and cerebral palsy show that oligodendrocytes are damaged by glutamate excitotoxicity. However, other effects of ceftriaxone, such as activation of the GLT1 transporter, independent of changes in expression, reduction of T-cell activation by modulation of cellular antigen-presentation, or metal ion chelation cannot be excluded18,34 as contributors to our observations.
Important studies from Hanlon et al5 report that when compared to cocaine users that had been abstinent for at least 1 month (n = 24), current cocaine users (n = 24) had significantly lower WM tissue densities.5 Moreover, WM densities for abstinent cocaine users were similar to those of nondrug user controls, suggesting recovery of cocaine-induced WM loss upon prolonged abstinence. Given these findings, it is possible that with a longer withdrawal period in cocaine-treated mice, WM protein levels may have returned to normal in the absence of ceftriaxone. Expanded studies that include dose and time course responses are required to understand and characterize both the mechanisms through which cocaine decreases WM proteins and how ceftriaxone prevents this loss. However, the observation that ceftriaxone negates cocaine-induced WM loss provides clues as to the mechanism through which cocaine may exert its effects on white matter protein levels. One of the limitations of our study is the fact that a group of cocaine-treated withdrawal mice without challenge was not included. The NA plays an integral role in drug reward, as it receives dopaminergic projections from the ventral tegmental area and glutamatergic projections from the prefrontal cortex.35 Dysfunction of the NA is associated with impulsive decision-making, anxiety, and major depressive disorder.36,37 Thus, future studies addressing WM loss and connectivity networks are required to understand cocaine-mediated WM loss and ceftriaxone-induced interventions in myelin disruptions more clearly.
Footnotes
Supported in part by NIH grants R21DA029523 (D.L.) and RC1DA028153 and R21DA030676 (S.R.).
References
- 1.Administration SAMHS . Department of Health and Human Services; Rockville: 2011. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Edited by. [Google Scholar]
- 2.Knackstedt L.A., Melendez R.I., Kalivas P.W. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albertson D.N., Pruetz B., Schmidt C.J., Kuhn D.M., Kapatos G., Bannon M.J. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyoo I.K., Streeter C.C., Ahn K.H., Lee H.K., Pollack M.H., Silveri M.M., Nassar L., Levin J.M., Sarid-Segal O., Ciraulo D.A., Renshaw P.F., Kaufman M.J. White matter hyperintensities in subjects with cocaine and opiate dependence and healthy comparison subjects. Psychiatry Res. 2004;131:135–145. doi: 10.1016/j.pscychresns.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Hanlon C.A., Dufault D.L., Wesley M.J., Porrino L.J. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (Berl) 2011;218:681–692. doi: 10.1007/s00213-011-2360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson S.E., Kunko P.M., Smith J.A., Wallace M.J., Mo Q., Maher J.R. Extracellular aspartate concentration increases in nucleus accumbens after cocaine sensitization. Eur J Pharmacol. 1997;319:31–36. doi: 10.1016/s0014-2999(96)00923-5. [DOI] [PubMed] [Google Scholar]
- 7.Reid M.S., Hsu K., Jr., Berger S.P. Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: studies on the involvement of dopamine. Synapse. 1997;27:95–105. doi: 10.1002/(SICI)1098-2396(199710)27:2<95::AID-SYN1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 8.Pierce R.C., Bell K., Duffy P., Kalivas P.W. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFarland K., Lapish C.C., Kalivas P.W. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalivas P. Glutamate sytems in cocaine addiction. Curr Opin Pharmacol. 2004;4:23–29. doi: 10.1016/j.coph.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Hotsenpiller G., Giorgetti M., Wolf M.E. Alterations in behaviour and glutamate transmission following presentation of stimuli previously associated with cocaine exposure. Eur J Neurosci. 2001;14:1843–1855. doi: 10.1046/j.0953-816x.2001.01804.x. [DOI] [PubMed] [Google Scholar]
- 12.Du C., Yu M., Volkow N.D., Koretsky A.P., Fowler J.S., Benveniste H. Cocaine increases the intracellular calcium concentration in brain independently of its cerebrovascular effects. J Neurosci. 2006;26:11522–11531. doi: 10.1523/JNEUROSCI.3612-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornish J.L., Kalivas P.W. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiggins R.C., Ruiz B. Development under the influence of cocaine: II Comparison of the effects of maternal cocaine and associated undernutrition on brain myelin development in the offspring. Metab Brain Dis. 1990;5:101–109. doi: 10.1007/BF01001050. [DOI] [PubMed] [Google Scholar]
- 15.Rawls S.M., Zielinski M., Patel H., Sacavage S., Baron D.A., Patel D. Beta-lactam antibiotic reduces morphine analgesic tolerance in rats through GLT-1 transporter activation. Drug Alcohol Depend. 2010;107:261–263. doi: 10.1016/j.drugalcdep.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rothstein J.D., Patel S., Regan M.R., Haenggeli C., Huang Y.H., Bergles D.E., Jin L., Dykes Hoberg M., Vidensky S., Chung D.S., Toan S.V., Bruijn L.I., Su Z.Z., Gupta P., Fisher P.B. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 17.Ward S.J., Rasmussen B.A., Corley G., Henry C., Kim J.K., Walker E.A., Rawls S.M. Beta-lactam antibiotic decreases acquisition of and motivation to respond for cocaine, but not sweet food, in C57Bl/6 mice. Behav Pharmacol. 2011;22:370–373. doi: 10.1097/FBP.0b013e3283473c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melzer N., Meuth S.G., Torres-Salazar D., Bittner S., Zozulya A.L., Weidenfeller C., Kotsiari A., Stangel M., Fahlke C., Wiendl H. A beta-lactam antibiotic dampens excitotoxic inflammatory CNS damage in a mouse model of multiple sclerosis. PLoS One. 2008;3:e3149. doi: 10.1371/journal.pone.0003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sondheimer I., Knackstedt L.A. Ceftriaxone prevents the induction of cocaine sensitization and produces enduring attenuation of cue- and cocaine-primed reinstatement of cocaine-seeking. Behav Brain Res. 2011;225:252–258. doi: 10.1016/j.bbr.2011.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasband WS: ImageJ. Edited by Bethesda NIH, 1997–2009.
- 21.Danbolt N.C. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Gomez M.V., Alberdi E., Perez-Navarro E., Alberch J., Matute C. Bax and calpain mediate excitotoxic oligodendrocyte death induced by activation of both AMPA and kainate receptors. J Neurosci. 2011;31:2996–3006. doi: 10.1523/JNEUROSCI.5578-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald J.W., Althomsons S.P., Hyrc K.L., Choi D.W., Goldberg M.P. Oligodendrocytes from forebrain are highly vulnerable to AMPA/kainate receptor-mediated excitotoxicity. Nat Med. 1998;4:291–297. doi: 10.1038/nm0398-291. [DOI] [PubMed] [Google Scholar]
- 24.Volkow N.D., Fowler J.S., Wolf A.P., Schlyer D., Shiue C.Y., Alpert R., Dewey S.L., Logan J., Bendriem B., Christman D. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry. 1990;147:719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- 25.Bongarzone E.R., Howard S.G., Schonmann V., Campagnoni A.T. Identification of the dopamine D3 receptor in oligodendrocyte precursors: potential role in regulating differentiation and myelin formation. J Neurosci. 1998;18:5344–5353. doi: 10.1523/JNEUROSCI.18-14-05344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovalevich J., Corley G., Yen W., Kim J., Rawls S., Langford D. Cocaine decreases expression of neurogranin via alterations in thyroid receptor/retinoid X receptor signaling. J Neurochem. 2012;121:302–313. doi: 10.1111/j.1471-4159.2012.07678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopez-Barahona M., Minano M., Mira E., Iglesias T., Stunnenberg H.G., Rodriguez-Pena A., Bernal J., Munoz A. Retinoic acid posttranscriptionally up-regulates proteolipid protein gene expression in C6 glioma cells. J Biol Chem. 1993;268:25617–25623. [PubMed] [Google Scholar]
- 28.Pombo P.M., Barettino D., Ibarrola N., Vega S., Rodriguez-Pena A. Stimulation of the myelin basic protein gene expression by 9-cis-retinoic acid and thyroid hormone: activation in the context of its native promoter. Brain Res Mol Brain Res. 1999;64:92–100. doi: 10.1016/s0169-328x(98)00311-8. [DOI] [PubMed] [Google Scholar]
- 29.Huang J.K., Jarjour A.A., Nait Oumesmar B., Kerninon C., Williams A., Krezel W., Kagechika H., Bauer J., Zhao C., Evercooren A.B., Chambon P., Ffrench-Constant C., Franklin R.J. Retinoid X receptor gamma signaling accelerates CNS remyelination. Nat Neurosci. 2011;14:45–53. doi: 10.1038/nn.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith J.A., Mo Q., Guo H., Kunko P.M., Robinson S.E. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res. 1995;683:264–269. doi: 10.1016/0006-8993(95)00383-2. [DOI] [PubMed] [Google Scholar]
- 31.Reid M.S., Berger S.P. Evidence for sensitization of cocaine-induced nucleus accumbens glutamate release. Neuroreport. 1996;7:1325–1329. doi: 10.1097/00001756-199605170-00022. [DOI] [PubMed] [Google Scholar]
- 32.Baker D.A., McFarland K., Lake R.W., Shen H., Tang X.C., Toda S., Kalivas P.W. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 33.Baker D.A., Xi Z.X., Shen H., Swanson C.J., Kalivas P.W. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipski J., Wan C.K., Bai J.Z., Pi R., Li D., Donnelly D. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience. 2007;146:617–629. doi: 10.1016/j.neuroscience.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Kauer J.A., Malenka R.C. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 36.Creese I., Iversen S.D. The role of forebrain dopamine systems in amphetamine induced stereotyped behavior in the rat. Psychopharmacologia. 1974;39:345–357. doi: 10.1007/BF00422974. [DOI] [PubMed] [Google Scholar]
- 37.Daunais J.B., McGinty J.F. Cocaine binges differentially alter striatal preprodynorphin and zif/268 mRNAs. Brain Res Mol Brain Res. 1995;29:201–210. doi: 10.1016/0169-328x(94)00246-b. [DOI] [PubMed] [Google Scholar]