Abstract
Patients with diabetes are at an increased risk for developing corneal complications including delayed wound healing and potential vision loss. To understand the cause of diabetic keratopathy, we investigated innervation and its correlation with delayed corneal epithelial wound healing in type 2 diabetic Goto-Kakizaki (GK) rats. GK rats are smaller than the age-matched control Wistar rats from which the GK rats were derived. The blood sugar levels of GK rats are significantly higher than those of Wistar rats. GK rats had increased rose bengal staining and cornea fragility. Fewer nerve fibers were detected compared with Wistar rats. Although nerve fiber densities detected by whole-mount immunohistochemistry were similar near the limbal region, in the central cornea the subbasal nerve plexuses were thinner, less abundant, and showed less branching in GK rats. Corneal epithelial wound closure was delayed and re-innervation was slow and incomplete in GK rats. These abnormalities were more apparent in older GK rats (12 months). Our data suggest that diabetic neuropathy occurs in the cornea of type 2 diabetic GK rats, and defects in the sensory nerve and/or tear film may contribute to diabetic keratopathy and delayed epithelial wound healing in diabetic corneas.
With the rapid increase in the prevalence of diabetes mellitus (DM), mostly in type 2 DM, ocular complications have become a leading cause of blindness in the world.1 In addition to abnormalities of the retina (diabetic retinopathy) and the lens (cataract), various types of ocular mucosal surface disorders are also relatively common in DM patients.2 They include impaired corneal sensation,3–6 reduced tear secretion,7,8 conjunctival squamous metaplasia and goblet cell loss,9 and corneal keratopathy.2,3 Diabetic keratopathy occurs in more than 70% of diabetic patients2–4 and increases the susceptibility of the cornea to trauma with epithelial erosions and ulcerations.5,6,10 Although the cornea is an avascular tissue, it is the most densely innervated part of the human body, containing Aδ and unmyelinated C fibers, and derives its innervation from the ophthalmic division of the trigeminal nerve.11 The sensory nerve fibers in diabetic patients with peripheral neuropathy probably undergo the earliest damage in diabetes.12,13 Therefore, the aforementioned abnormalities also can be attributed to the decrease or loss of the nerve ends and fibers, and diabetic keratopathy also can be thought of as a form of neuropathy.11–13 As a matter of fact, because corneal nerve structure and function can be assessed readily and accurately using in vivo corneal confocal microscopy and noncontact corneal esthesiometry, respectively, assessing corneal neuropathy has been proposed as a noninvasive and reliable way to diagnose peripheral diabetic neuropathy (keratopathy), a debilitating condition that affects approximately 50% of diabetic patients.14
The cornea is a transparent tissue consisting of three cellular layers: the epithelium, stroma, and a simple epithelial layer, termed endothelium. Many major hyperglycemia-caused pathologic changes in the cornea occur around the stratified epithelia, including alterations in the epithelial basement membrane such as thickening,6,15 a decreased number of hemidesmosomes,16 and the deposition of advanced glycation end products.6,17 Hyperglycemia also directly affects epithelial cells and significantly alters its structure and function, resulting in basal cell degeneration,10,18,19 decreased20,21 or increased22 cell proliferation, superficial punctate keratitis,23 breakdown of barrier function,24,25 and fragility,26 depending on the duration of DM and on the serum concentration of glycated hemoglobin HbA1c. Hence, diabetic keratopathy also was termed “diabetic corneal epitheliopathy.”27,28 Clinically, the cornea appears normal in patients with diabetic keratopathy in the absence of corneal injury. However, trauma and ocular surgeries, such as vitrectomy for vitreous hemorrhage, may require the removal of epithelial cells or damage the fragile structure, causing cell injury and/or removal of corneal epithelium. In diabetic patients, there is a considerable delay in corneal re-epithelialization after injury. The impairment of corneal epithelial wound healing could result in several types of epithelial disorders, such as persistent epithelial defects and recurrent erosion in patients after surgery.2,29–32 Furthermore, delayed healing of the epithelial defect may be associated with sight-threatening complications, such as stromal opacification, surface irregularity, and microbial keratitis.29 Hence, a better understanding of the mechanisms underlying delayed epithelial wound healing in diabetic corneas should lead to better management of the disease.
We previously used a streptozocin (STZ)-induced rat model of type 1 diabetes and showed that delayed wound healing in diabetic rats is associated with the impairment of epidermal growth factor receptor–mediated cell signaling in response to mechanical injury.21,25,28 These STZ rats had stronger rose bengal staining, decreased tear secretion, slightly attenuated sensitivity, and reduced nerve fibers. To better understand diabetic keratopathy in type 2 DM (T2D), which develops as a result of a failure to increase β-cell function and mass adequately to meet the demands of prevailing insulin resistance,33 we maintained a colony of Goto-Kakizaki (GK) rats, one of the best characterized animal models of spontaneous T2D that were derived from Wistar rats.34,35 We noticed that, unlike Sprague-Dawley (SD) rats, Wistar rats were less sensitive to aesthesiometer with thread and had reduced corneal sensitivity. In this study, we characterized the ocular alterations of GK rats. Our study revealed that, in addition to changes in nerve fibers, the subbasal nerve ends also were decreased. This decrease in corneal innervation may contribute to tear deficiency and delayed wound healing in GK rats.
Materials and Methods
Reagents
Neuronal class III β-tubulin (alias TUJ1) rabbit monoclonal antibody was from Covance (Princeton, NJ). The Alexa Fluor 488 donkey anti-rabbit IgG was from Invitrogen (Carlsbad, CA). All other reagents and chemicals were purchased form Sigma-Aldrich (St. Louis, MO).
Animals
GK rats were derived from GK breeding pairs obtained from Dr. Adviye Ergul (Georgia Health Sciences University) and were bred in house.36,37 In the first three to four generations in our animal facility, the GK rats had problems caring for their newborn pups and surrogate mothers of Wistar rats (Charles Rivers, Potage, MI) were used. This practice allowed us to raise both GK and Wistar rats from newborn pups to up to 1 year old simultaneously. After five generations, most GK rat mothers were able to feed and care for their youth, and age- and sex-matched nondiabetic control Wistar rats, some of which were retired breeding rats, were obtained from Charles Rivers. The animals were housed at the Wayne State University animal care facility, which is approved by the American Association for Accreditation of Laboratory Animal Care. All protocols were approved by the Institutional Animal Care and Use Committee. All animals were fed a pelleted standard rat chow ad libitum. They were kept in rooms with alternating 12-hour light cycles. Weight and blood glucose levels were measured once a week after 2 months of age. All investigations conformed to the regulations of the Association of Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research, the NIH, and the guidelines of the Animal Investigation Committee of Wayne State University.
Slit Lamp Examination
The corneal surface was examined with 1% fluorescein sodium and 1% rose bengal staining, and photographed with a digital camera (Pentax, Golden, CO) attached to the slit lamp under Cobalt blue or red-free filters, respectively. To quantitate the rose bengal staining pattern, each cornea was divided into four quadrants and each quadrant was scored based on the following scale: 0, no staining; 1, punctate staining; 2, continuous staining covering <50% area; 3, continuous staining covering ≥50% area but not confluent; and 4, confluent staining. The final score for each cornea is the totaled scores from all quadrants.
Evaluation of Cornea Sensitivity and Fragility
Corneal sensation was determined with an aesthesiometer (Cochet-Bonnet, Luneau, France) in unanesthetized rats. The test began with the nylon filament fully extended to its maximal length of 60 mm, and retracted by 5 mm each time a negative response was observed, until a positive response was noted. A positive blinking response was recorded by two observers and each test was repeated three times. Cornea fragility was measured by attaching a 4-mm round polyvinyl chloride (PVC) membrane onto the center of rat corneas for 30 seconds before gentle removal. The cornea was stained with 1% fluorescein immediately to highlight the denuded area. To quantitate the brightness of the fluorescein staining pattern, which indicates the fragility of the cornea, each cornea was divided into four quadrants and each quadrant was measured for the RGB value of one point by Photoshop version 6 (Adobe, San Jose, CA), and the RGB value of the center cornea also was valued. The green value stands for the brightness of the fluorescein staining and correlates with the amount of cells peeled off by the PVC membrane. The final green value for each cornea is the mean of values from all quadrants.
Confocal Microscopic Examination
Rat corneas were scanned with a digital confocal scanning microscope (ConfoScan-4; Nidek Technologies, Gamagori, Japan). Topical proparacaine 0.5% (Akron, Lake Forest, IL) was applied to the cornea of deeply anesthetized rats. A drop of GenTeal gel (Novartis Pharmaceuticals Corporation, East Hanover, NJ) was applied to the tip of a 40× objective lens, and the central cornea was scanned using the AutoFull mode at 25 frames per second with a scan step of 5 μm and a total of 350 images. Each cornea was scanned three times, and the corneal thickness was calculated as the peak-to-peak distance in the z-scan profile and was averaged among three scans. To analyze corneal innervation, three representative images of the subbasal nerve plexus from each rat were selected and the nerve fiber length was traced and measured (NeuronJ plug-in of ImageJ software version 1.44a, developed by Wayne Rasband, NIH, Bethesda, MD). The final length for each animal is the mean of three scans and is expressed in an arbitrary unit.
Evaluation of Tear Secretion and Corneal Sensitivity
Tear secretion was determined with phenol red–impregnated cotton threads (Zone-Quick, Tokyo, Japan). The threads were placed in the medial canthus for 1 minute and the length of the wetted part, turning red because of soaking tears, was measured. Corneal sensation was measured with an aesthesiometer (Cochet-Bonnet) in unanesthetized rats. The testing began with the nylon filament fully extended to its maximal length of 60 mm, and shortened by 5 mm each time a negative response was observed until a positive response was obtained. A positive blinking response was recorded by two observers and each test was repeated three times.
Determination of Corneal Epithelial Wound Healing
Topical proparacaine 0.5% was applied to 8-month-old male Wistar (Charles Rivers) and GK rats. A 4-mm circular wound first was demarcated with a trephine in the central cornea and the epithelium then was removed with a blunt scalpel blade under a dissecting microscope (Zeiss Monument Co). Bacitracin ophthalmic ointment (Fougera, Melville, NY) was applied to the cornea after surgery to prevent infection. To document the healing process, the remaining denuded area was visualized with fluorescein staining, photographed, and quantitated (Photoshop, version 6). The healing rate was calculated as follows: (original wound area − current wound area)/original wound area as a percentage.
Whole-Mount Staining of Corneal Nerve Fibers
Eyes were enucleated from euthanized rats and immersion-fixed for 1 hour at room temperature in 4% paraformaldehyde–0.2% picric acid in 0.1 mol/L PBS. The anterior segment then was removed with a razor blade, and the cornea and approximately 1 mm of attached scleral rim were isolated and returned to the fixative solution for an additional 30 minutes. Each cornea was mounted briefly on a dome-shaped post that approximated the radius of curvature of the rat cornea to facilitate subsequent cutting with a single-edge razor blade into eight standardized parts and not separated. The corneas were incubated at 37°C in 20 mmol/L EDTA (Sigma-Aldrich) for 30 minutes and then permeabilized by 3-day incubation in 0.025% hyaluronidase and 0.1% EDTA in 0.1 mol/L PBS, pH 5.3. Tissues were rinsed 4 times for 10 minutes each in PBS with 0.3% Triton X-100 (Sigma-Aldrich) and were blocked at room temperature for 2 hours in PBS–Triton X-100 containing 2% bovine serum albumin (Sigma-Aldrich). The corneas were incubated overnight at 4°C in a rabbit monoclonal antibody against neuronal class III β-tubulin. The neurotubulin antibody recognizes a cytoskeletal component expressed in all peripheral axons, regardless of phenotype, and was used here as a panneuronal marker for corneal nerves. The following morning, the corneas were rinsed 4 times for 10 minutes each in PBS with 0.3% Triton X-100 and 0.05% Tween20, followed by an overnight incubation at 4°C in the dark with a secondary antibody (Alexa Fluor 488 donkey anti-rabbit IgG). After 10-minute rinses, four times in PBS with 0.3% Triton X-100 and 0.05% Tween20 in the dark, the tissues were coverslipped with mounting medium (Vectashield; Vector Labs, Burlingame, CA) and examined under a confocal microscope (TCS SP2; Leica, Heidelberg, Germany).
Images then were cropped to an area of exactly 2.25 mm2, and the nerves were highlighted using the thresholding tool of Image J version 1.44a. The images were converted to black-and-white images and nerve density was calculated by analyzing particles, and then calculated as the percentage of each 2.25 mm2 area occupied by nerves.
Statistical Analysis
Each experiment used 8 to 10 rats in each group, Wistar or GK rats, unless otherwise noted in the figure legends. Results are the mean ± SEM. Statistical parameters were ascertained with the Student's t-test between two groups. P < 0.05 indicates a significant difference.
Results
GK Rats as a Type 2 DM Model to Study Diabetic Keratopathy
The GK rat is one of the best-characterized animal models of spontaneous T2D.35,38 As in humans, multiple factors are known to contribute to the defects in the GK rat because it was developed by repeated inbreeding of glucose-intolerant Wistar rats over generations.34 Although the degree of glucose intolerance has been maintained stably over 20 years, differences in β-cell number, insulin content, and islet metabolism and secretion have been noted between some of the different colonies, suggesting newly introduced genetic changes from different local breeding environments.35 Our colony has been maintained since 2009. In male GK rats, nonfasting plasma glucose levels typically are 180 to 260 mg/dL with an average of 200 mg/dL, which is substantially higher than the 90 mg/dL of Wistar rats (Figure 1A). The nonfasting plasma glucose levels in adult GK rats were reasonably consistent, and only those with glucose levels >180 mg/dL were used for the following experiments. The sugar levels for most female GK rats were lower than 180 mg/dL. Hence, only male GK rats and Wistar rats were used in the study. The average adult GK rat body weight (405 g) was 24.6% lower than that of age- and sex-matched control Wistar rats (537 g) at 6 months and 29% lower at 8 months (Figure 1B). The body length also decreased compared with normal Wistar rats (Figure 1C).
Figure 1.
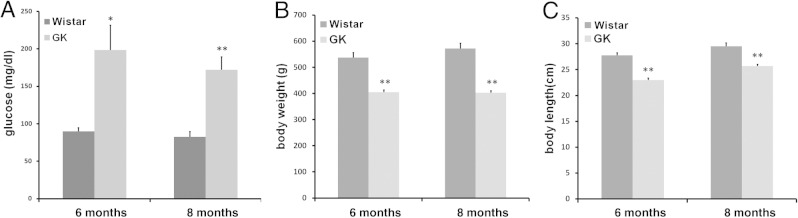
Hyperglycemia and growth retardation in GK rats. A: Blood glucose level was measured weekly. B: Body weight was measured monthly. C: The length of the body without tail was measured at 6 and 8 months of age. *P < 0.01, **P < 0.001.
Characterization of Ocular Surface Irregularity in GK Rats
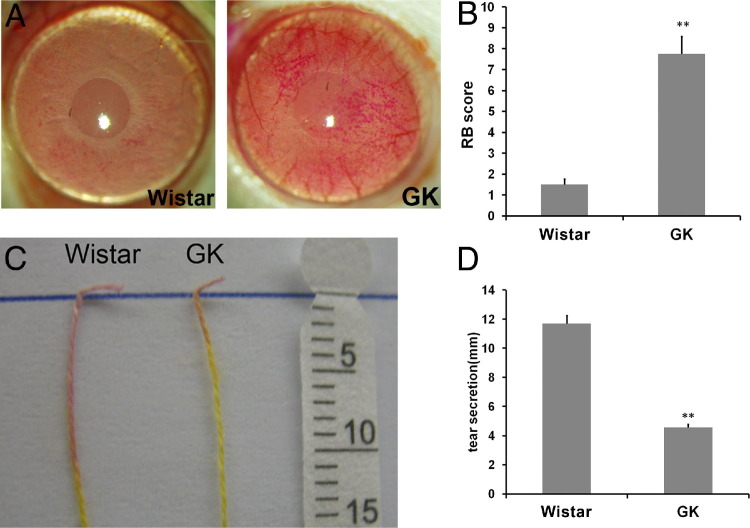
There were no visible defects at the ocular surface. Random ocular surface fluorescein staining revealed no significant punctate staining or loss of epithelial cells. As observed in STZ-SD rats, when the corneas of GK and Wistar rats were stained with rose bengal, a test used in the clinic to evaluate epithelial defects at the ocular surface, much stronger staining was observed in the GK rats than in the control rats (Figure 2A). The staining was quantitated as described in Materials and Methods. GK rats scored 7.75, whereas Wistar rats scored 1.5, indicating a deficiency of tear film protection (Figure 2B). Tear secretion also was measured with cotton threads (Figure 2C). Consistent with rose bengal staining, GK rats had a significantly reduced amount of tears compared with that of Wistar rats (Figure 2D).
Figure 2.
Impairment of tear film integrity in GK rats. A: Rat corneas were stained with rose bengal and representative images are shown. B: Rose bengal (RB) staining was scored (n = 16). **P < 0.001. C: Tear secretion was measured with cotton threads, and representative images are shown. D: Tear secretion was measured. **P < 0.001.
We also measured corneal fragility using PVC membrane. A 4-mm round dry PVC membrane was applied onto the center of rat corneas for 30 seconds and removed; fluorescein staining was performed immediately and photographed. Fluorescence remaining on the corneal surface indicated the rupture of epithelial cells. Stronger fluorescent staining was observed in GK rats, an indication of increased fragility in diabetic rat corneas (Figure 3A). Quantitation of the fluorescence retained revealed a 3.87-fold increase (N = 10) in diabetic rats, compared with age- and sex-matched Wistar rats (Figure 3B).
Figure 3.
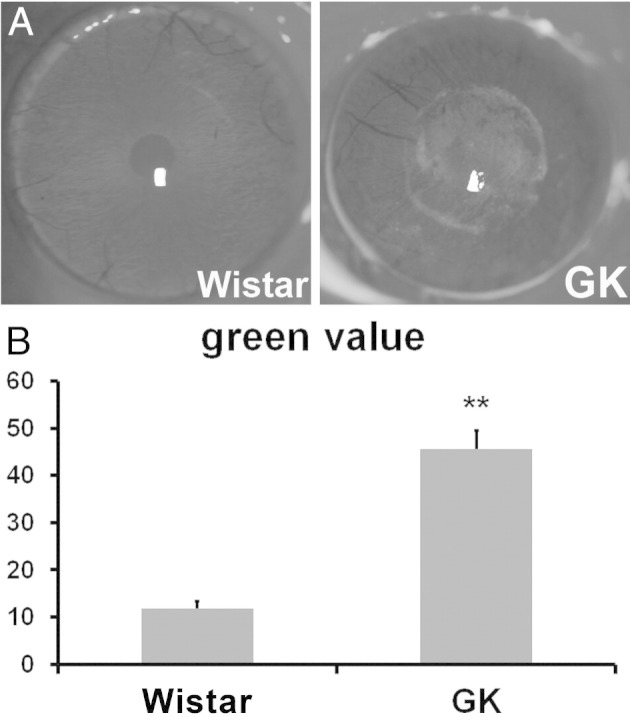
Increased fragility in GK rats cornea. A 4-mm-round PVC membrane was attached to the center of rat corneas for 30 seconds and taken off. Fluorescein staining was performed immediately to highlight the denuded area. A: Representative images are shown. B: The fragility of the cornea was qualified by measuring the green value (fluorescein staining level) (n = 10). **P < 0.001.
Impairment of Corneal Sensitivity and Innervations
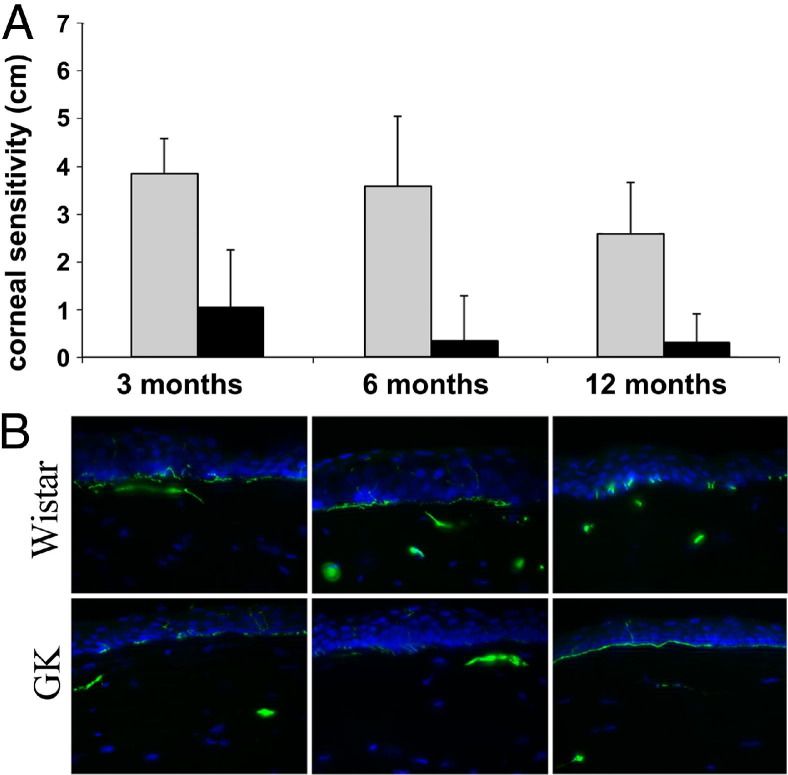
Corneal sensitivity was determined with an aesthesiometer and the values were expressed as the length of the aesthesiometer thread in centimeters to trigger blinking, with 6 being the longest or most sensitive (Figure 4A). On average, the normal control Wistar rats showed a positive blinking response at 3.94, 3.62, and 2.7 cm at 3, 6, and 12 months, respectively, whereas GK rats showed a significant decrease in sensitivity at 1.06, 0.25, and 0.25 cm, respectively. Although most GK rats had no detectable sensation to nylon thread after 6 months of age, there was a trend of decreased sensitivity in Wistar rats as well, although it was not statistically significant (Figure 4A). To assess long-term hyperglycemia on innervation, we compared the corneal sections of different ages of mice with a focus on the cross-section of nerve fibers in the stroma and epithelial layers (Figure 4B). In the cross-sections, there were nerve fibers detected in the stroma and subbasal nerve plexuses underneath the epithelial layers. The nerve staining also clearly showed the numerous sensory neurite projections between epithelial cells that reached to the apical cell layers. More sensory nerve fibers were found in normal Wistar rats than in GK rats, especially at 6 and 12 months of age.
Figure 4.
Age and duration of hyperglycemia-related decreases in corneal sensation and innervation. A: The age-matched GK and Wistar rats bred in the Kresge Eye Institute animal facility were subjected to cornea sensitivity measurements weekly with an aesthesiometer and the data were presented at ages 3, 6, and 12 months. At all three time points, corneal sensitivities of GK rats were significantly lower than that of Wistar rats (n = 12). P < 0.001. B: The corneas of GK and Wistar rats in each age group were subjected to Cryostat sectioning (Thermo Fisher Scientific, Barrington, IL) and immunostaining for β-tubulin III. The staining in the stroma represents nerve fibers and at the subbasal layer of epithelial are nerve ends, some of which are projected toward the apical cell layer of epithelia. The images are representative of 3 corneas in each group.
To visualize corneal innervation, the nerve fibers were assessed with in vivo confocal microscopy (ConfoScan). Compared with the Wistar rats, the nerve fibers of the GK rats are thinner and have fewer branches (Figure 5A). The length of the nerves in each frame was calculated; Figure 5B shows a significant reduction from 2169 arbitrary units in the Wistar to 1044 in the GK rat. The innervation also was examined with corneal whole-mount immunohistochemistry, which allows staining of all corneal subbasal nerves and their associated branches, from the points of nerve entry at the basal lamina to the most distal nerve terminals (Figure 5C). In general, near the limbal region, nerve fiber densities were similar between Wistar and GK rats (data not shown). In the central cornea, nerve fibers were significantly denser in Wistar than in GK rats. In addition, a swirling staining pattern of the nerve ends also was observed in the central cornea, and their density levels were less in GK than in Wistar rats. The difference of innervation was quantitated as described in Materials and Methods and showed an area fraction of 12.5% in GK rats versus 18.4% in the Wistar rats (Figure 5D).
Figure 5.
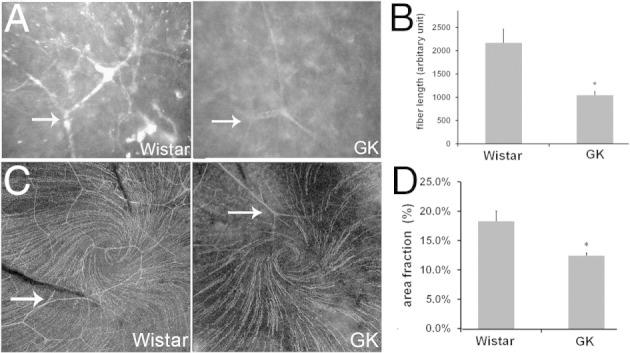
Defects of corneal innervations in 1-month-old GK rats. A: Subbasal plexus was visualized with confocal microscopy and representative images are shown. The arrow indicates the nerve branch. B: Nerve fiber length was quantitated (n = 7). *P < 0.05. C: Whole-mount immunostaining of β-tubulin in cornea epithelia showing the cornea nerve innervation. The arrow indicates the nerve branch. Representative images are shown. D: The whole-mount immunostaining was qualified by area fraction (n = 4). *P < 0.05.
Attenuation of Epithelial Wound Healing and Re-Innervation in the Corneas of GK Rats
We previously documented delayed epithelial wound healing in STZ rats, which mimics human T1D with more than 8 weeks of consistently high blood sugar levels (>450 mg/dL). In this study, we chose 6-month-old, T2D GK rats and created a 4-mm wound in the center of the cornea. The healing progress was monitored with fluorescein staining at 24 and 35 hours after wounding to highlight the denuded area and was photographed (Figure 6A). The size of the remaining wound in the GK rat cornea was larger than that of the Wistar rats at both time points. The average wound sizes (pixels) at 24 hours were 329,500 ± 69,681 in Wistar rats and 463,666 ± 69,692 in GK rats. At 35 hours, more than one third of the Wistar rat corneas healed completely yet none of the GK corneas healed completely. The average wound sizes were 278,417 ± 64,948 in GK rats, which was 3.96 times larger than in Wistar rats (70,250 ± 68,947).
Figure 6.
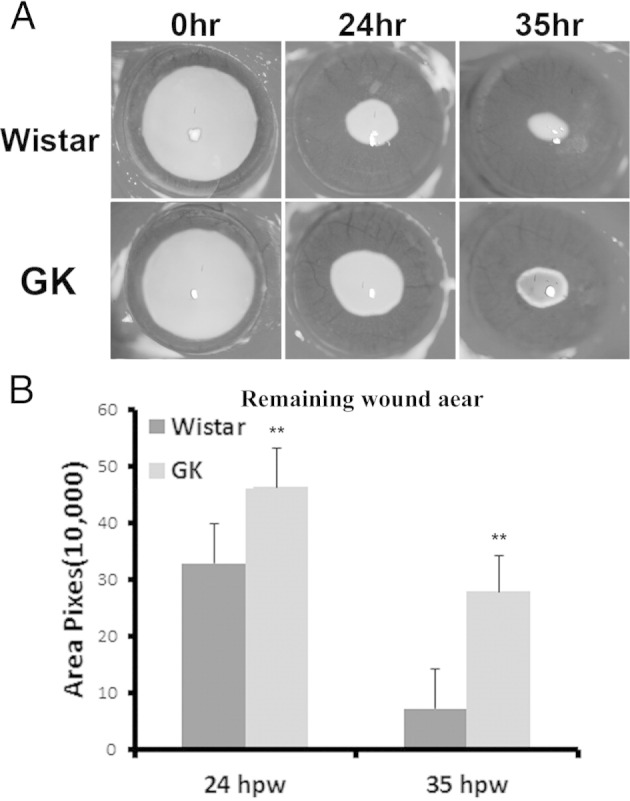
Attenuated wound healing in 6-month-old GK rat corneas. A 4-mm wound was made in the center of the cornea and the healing process was visualized with fluorescein staining to highlight the denuded area. A: Representative images are shown. B: The wound size was determined by and presented as the average of number of pixels of the fluorescent stained area. In GK rats, the average wound sizes (pixels) at 24 hours post wounding (hpw) were 329,500 ± 69,681 in Wistar rats and 463,666 ± 69,692 in GK rats; at 35 hpw the average wound sizes (pixels) were 70,250 ± 68,947 in Wistar rats and 278,417 ± 64,948 in GK rats (n = 12). **P > 0.01.
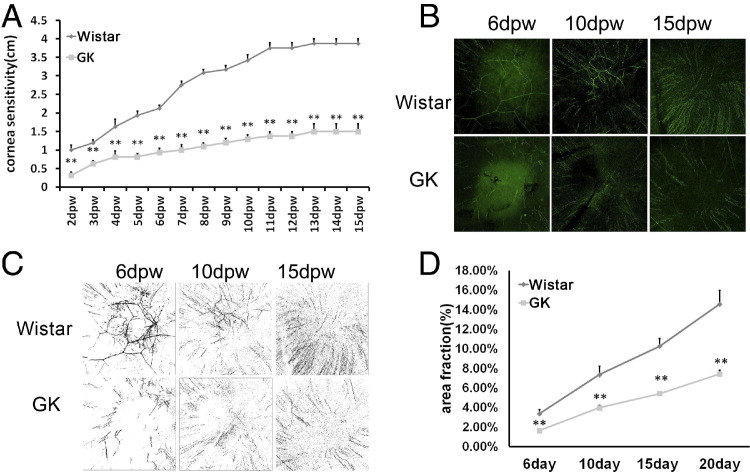
Having shown delayed epithelial wound healing, we next investigated the restoration of cornea sensitivity and reinnervation after wound closure (Figure 7). Starting from day 2 when the 4-mm wounds were healed in both types of rats, cornea sensitivity was measured daily (Figure 7A). The normal Wistar rats had some reflective response to aesthesiometer thread whereas most GK rats were nonresponsive at day 2. Afterward, corneal sensitivity consistently increased with an accelerated rate from day 6 to day 13, and then plateaued at day 15. On the other hand, the sensitivity remained remarkably low in GK rats. Whole-mount confocal immunohistochemistry revealed the formation of nerve fibers and a few nerve ends (Figure 7, B and C). The process continued with more nerve ends seen in the center of the cornea at day 10, and reached a relatively high density at day 15. However, the typical swirling pattern at the center of the cornea had not formed at day 15 after wounding, although corneal sensitivity was restored to a level similar to that before wounding, suggesting the swirling pattern may not be functionally required for corneal sensitivity. The density of subbasal nerve plexus in GK rats was low, compared with the Wistar rats at all time points (Figure 7D).
Figure 7.
Attenuated reinnervation in 6-month-old GK rat corneas. A 4-mm wound was made in the center of the cornea. Whole-mount immunostain of the cornea 6, 10, 15, and 20 days post wounding (dpw). A and B: Representative images and black-and-white contrasts are shown. C: The whole-mount immunostaining was quantitated by area fraction. D: Cornea sensitivity was measured with an aesthesiometer every day beginning 2 dpw (n = 6). **P < 0.001.
Older Mice with a Longer Duration of Hyperglycemia Show Greater Abnormalities in Delayed Wound Healing
We also performed wound healing using 12-month-old GK and Wistar rats by creating limbus-to-limbus epithelial debridement wounds (Figure 8). At 48 hours after wounding, the wounds were almost healed in Wistar rats whereas a large denuded region remained in GK rat corneas (Figure 8A). The average wound size (pixels) in GK rats 48 hours after wounding was 720,383 ± 207,661, which was 5.94 times larger than that in Wistar rats (121,372 ± 207,661).
Figure 8.
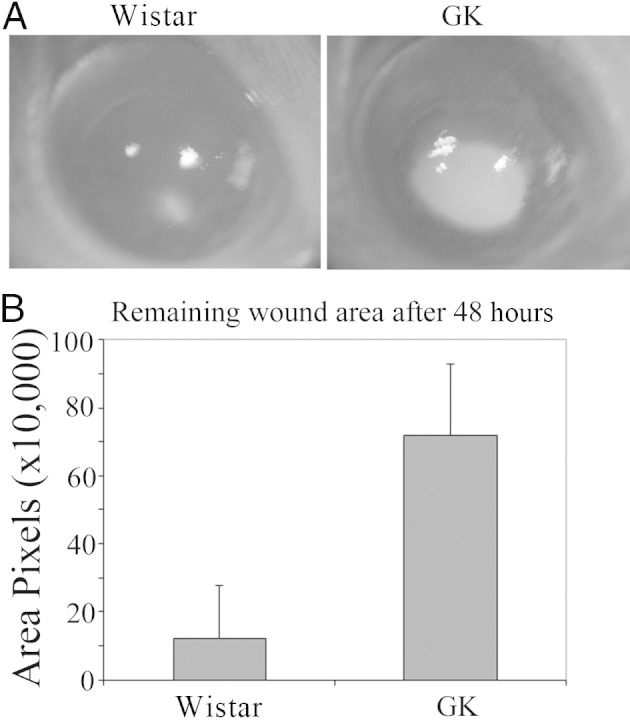
Impaired wound healing in 12-month-old GK rat corneas. A wound was made in the center of the cornea and the healing process was visualized with fluorescein staining to highlight the denuded area. A: Representative images are shown. B: The wound size was determined by and presented as the average of number of pixels of the fluorescent stained area. In GK rats, the average wound size (pixels) was 720,383 ± 207,661 and in Wistar rats was 121,372 ± 207,661 (n = 10). P < 0.01.
Discussion
The GK rat is one of the best-characterized animal models of spontaneous T2D and has proven to be a valuable tool for studying decreased functional β-cell mass, the hallmark of T2D.35,36,39 It also has been used to study diabetic complications such as retinopathy40,41 and corneal keratopathy.22 Similar to what was observed in diabetic patients, we found that these rats have increased rose bengal staining and decreased tear volume, which is indicative of tear deficiency. We used PVC paper to blot the center of the cornea, followed by fluorescence staining. Then we quantitated the increases in fluorescence staining, which is suggestive of increased fragility in the GK rat corneas, although a sticky surface as a result of less tears secreted also may be an underlying cause. For the first time, our study shows a decrease in nerve fiber density, size and branches formed, and reduced subbasal nerve plexuses, which is consistent with reduced corneal sensitivity in diabetic rats. Moreover, we showed delayed epithelial wound healing and re-innervation after wounding in GK rats compared with that of Wistar rats, thus linking diabetic neuropathy to delayed wound healing in the cornea. Our study further confirmed the potential use of confocal examination of corneal nerve as a rapid, effective, and, above all, noninvasive means to diagnose diabetic neuropathy.12,42–47 Moreover, therapeutic methods that target neuropathy also may be applicable to treat diabetic keratopathy or vice versa.48
Compared with our previous studies using STZ T1D, we observed many similarities in defects at the ocular surface, including increased rose bengal staining and decreased tear secretion. A major difference is the corneal sensitivity. Although no significant loss of sensitivity was detected in the STZ SD rat T1D model, GK rats have a dramatic decrease in sensitivity as tested with an aesthesiometer nylon thread. In the literature, there were contradicting reports regarding the corneal sensitivity in STZ SD rats. It was first reported that mean corneal sensitivity measured with nylon thread was the same between the control and DB groups.49 A later report stated that diabetic rats had markedly decreased corneal sensitivity compared with nondiabetic rats.49 We also noticed that all SD rats were responsive to 60-mm thread whereas most Wistar rats only respond to 50-mm thread or shorter. We assumed that SD rats were very sensitive to the nylon thread; indeed, when we pulled nylon thread to 90 mm, these rats were fully responsive. Hence, the observed lack of differences in corneal sensitivity in normal and STZ SD rats may be the result of inadequate sensitivity of nylon thread aesthesiometer as a technical modality. Nevertheless, we persistently observed very reduced corneal sensitivity in GK rats, compared with their age- and sex-matched Wistar rats. Hence, GK rats are also a valuable tool offering sufficient commonalities to study corneal innervation in the homeostatic state and re-innervation after epithelial wounding.
In vivo corneal confocal microscopy is an excellent method to detect corneal nerve fibers. Our study showed that the sensory nerve fibers in GK rats were fewer in number, smaller in diameter, and had fewer branches and/or interconnections, compared with Wistar rats. The alteration in nerve fibers is also illustrated by immunohistochemistry, which shows many more fibers in the center of the cornea, indicated by a swirling pattern of nerve ends on the smallest nerve fibers. Similar to that observed by ConfoScan, the fibers in Wistar rat corneas were well branched and interconnected, whereas in GK rats they were less so. The density of these nerve ends in GK rat corneas was clearly much lower than in Wistar rats. Interestingly, although the density of nerve fibers and ends were lower in GK rats, the subbasal surface still was covered with the fine fiber nerve ends. However, some animals had much reduced sensitivity and others were totally insensitive to the nylon thread, whereas the Wistar rats were sensitive to 30- to 50-mm thread stimulation. It appears that not only the quantity but also the quality of sensory nerves in the corneas are impaired. Hence, any therapeutic approach to improve peripheral neuropathy may need to preserve the peripheral sensory nerves structurally and functionally.
GK rats, a spontaneous nonobese model for T2D, grow slower with a smaller body, compared with the same age Wistar rats from which they are derived (Figure 1). Under such conditions, to choose a proper control (age- or weight-matched controls) for a corneal wound-healing study is problematic. In the type 1 STZ rat model, consistent very high levels of blood glucose (>450 mg/dL) cause diabetic corneal complications measurable within 8 weeks of hyperglycemia. In GK rats, blood glucose level increases as they grow and reaches the maximal level at 6 months of age. As in humans, the T2D in GK rats is an age-related disease, hence, only age-matched Wistar rats can be used as the control in wound-healing studies. This generates a problem: a defined size of wound (4 mm) (Figure 5) would represent a larger portion of the epithelium being removed in the diabetic than in the wild-type rat, leaving more epithelial cells in Wistar rats that can participate in wound closure. There is a possibility that the delayed wound healing observed in Figure 5 may be the result of the difference in the corneal size. To address the problem, we also performed limbus-to-limbus epithelial debridement wounding (Figure 8). Under such conditions, the wound size in GK rats is expected to be smaller than that in Wistar rats. However, our results showed more apparent delay in epithelial wound healing in these older mice. We conclude that the healing of corneal epithelial debridement wounds was impaired in T2D GK rats and that chronic hyperglycemia leads to diabetic complications, including poor wound healing, in animal models of human T2D.
Unlike STZ type 1 rats, the blood glucose levels were relatively low and most detectable abnormalities in these rats could be observed only around 6 months of age, suggesting the cumulative effects of hyperglycemia on corneal cells. In human T2D, the complications develop progressively.50 Hence, it would be expected that older mice with a longer duration of hyperglycemia show greater abnormalities. Indeed, we observed a reduced number of sensory nerve fibers crossing the corneal stroma of GK rat corneas, compared with that of Wistar rats. More importantly, the epithelial wound healing was delayed further in 12-month-old GK rats than in 6-month-old rats when compared with their age-matched Wistar rats (6 versus 4 times larger wound sizes in 12-month-old versus 6-month-old GK/Wistar rats, respectively). Similar to the rate at 35 hours, a third of the 4-mm wounds were healed in Wistar rats (counted as size 0), the actual ratio of wound sizes in these younger rats should be lower than 4. Thus, older mice with a longer duration of hyperglycemia have greater abnormalities (Figure 4) and the pathogenesis of corneal complications in GK rats resembles human T2D. Future studies that delineate the effects of age and the duration of hyperglycemia on corneal innervation, epithelial wound healing, and reinnervation after wounding is warranted.
In summary, using GK rats as a T2D model, we showed a correlation between defects in sensory neurons and abnormalities in corneal epithelial structure and function. Our results suggest that diabetic neuropathy is a contributing factor for delayed epithelial wound healing. Other factors such as decreased tear secretion, defects in tear film integrity, and the losses of epithelial barrier function25 also may be contributing factors for diabetic keratopathy. The combination of neurotrophic factors with other remedies may be required for maintaining epithelial barrier function and the ability to heal a wound properly in diabetic patients with a long duration of hyperglycemia.
Acknowledgments
We thank all of the members of the Yu Laboratory for assistance and comments on the work, Jessica Yu (Ohio State University Moritz School of Law) for proofreading the manuscript, and Dr. Keping Xu (New England College of Optometry) for his involvement in the early stage of the project, including maintaining the GK rat colony and noting some ocular abnormalities that were characterized in this manuscript.
Footnotes
Supported by NIH/National Eye Institute grants R01EY10869, EY17960, and P30EY004068 (F.-S.X.Y.), Midwest Eye Banks (N.G.), and Research to Prevent Blindness (Kresge Eye Institute).
References
- 1.Clark C.M., Lee D.A. Prevention and treatment of the complications of diabetes mellitus. N Engl J Med. 1995;332:1210–1217. doi: 10.1056/NEJM199505043321807. [DOI] [PubMed] [Google Scholar]
- 2.Kaji Y. Prevention of diabetic keratopathy. Br J Ophthalmol. 2005;89:254–255. doi: 10.1136/bjo.2004.055541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aiello L.P., Gardner T.W., King G.L., Blankenship G., Cavallerano J.D., Ferris F.L., 3rd, Klein R. Diabetic retinopathy. Diabetes Care. 1998;21:143–156. doi: 10.2337/diacare.21.1.143. [DOI] [PubMed] [Google Scholar]
- 4.Didenko T.N., Smoliakova G.P., Sorokin E.L., Egorov V.V. [Clinical and pathogenetic features of neurotrophic corneal disorders in diabetes]: Russian. Vestn Oftalmol. 1999;115:7–11. [PubMed] [Google Scholar]
- 5.Friend J., Thoft R.A. The diabetic cornea. Int Ophthalmol Clin. 1984;24:111–123. [PubMed] [Google Scholar]
- 6.Friend J., Ishii Y., Thoft R.A. Corneal epithelial changes in diabetic rats. Ophthalmic Res. 1982;14:269–278. doi: 10.1159/000265202. [DOI] [PubMed] [Google Scholar]
- 7.Cousen P., Cackett P., Bennett H., Swa K., Dhillon B. Tear production and corneal sensitivity in diabetes. J Diabetes Complications. 2007;21:371–373. doi: 10.1016/j.jdiacomp.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Saito J., Enoki M., Hara M., Morishige N., Chikama T., Nishida T. Correlation of corneal sensation, but not of basal or reflex tear secretion, with the stage of diabetic retinopathy. Cornea. 2003;22:15–18. doi: 10.1097/00003226-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Figueroa-Ortiz L.C., Jimenez Rodriguez E., Garcia-Ben A., Garcia-Campos J. [Study of tear function and the conjunctival surface in diabetic patients] Arch Soc Esp Oftalmol. 2011;86:107–112. doi: 10.1016/j.oftal.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Kabosova A., Kramerov A.A., Aoki A.M., Murphy G., Zieske J.D., Ljubimov A.V. Human diabetic corneas preserve wound healing, basement membrane, integrin and MMP-10 differences from normal corneas in organ culture. Exp Eye Res. 2003;77:211–217. doi: 10.1016/s0014-4835(03)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hossain P., Sachdev A., Malik R.A. Early detection of diabetic peripheral neuropathy with corneal confocal microscopy. Lancet. 2005;366:1340–1343. doi: 10.1016/S0140-6736(05)67546-0. [DOI] [PubMed] [Google Scholar]
- 12.Efron N. The Glenn A. Fry award lecture 2010: ophthalmic markers of diabetic neuropathy. Optom Vis Sci. 2011;88:661–683. doi: 10.1097/OPX.0b013e3182171020. [DOI] [PubMed] [Google Scholar]
- 13.Schultz R.O., Peters M.A., Sobocinski K., Nassif K., Schultz K.J. Diabetic keratopathy as a manifestation of peripheral neuropathy. Am J Ophthalmol. 1983;96:368–371. doi: 10.1016/s0002-9394(14)77829-8. [DOI] [PubMed] [Google Scholar]
- 14.Messmer E.M., Schmid-Tannwald C., Zapp D., Kampik A. In vivo confocal microscopy of corneal small fiber damage in diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 2010;248:1307–1312. doi: 10.1007/s00417-010-1396-8. [DOI] [PubMed] [Google Scholar]
- 15.Taylor H.R., Kimsey R.A. Corneal epithelial basement membrane changes in diabetes. Invest Ophthalmol Vis Sci. 1981;20:548–553. [PubMed] [Google Scholar]
- 16.Azar D.T., Spurr M.S.J., Tisdale A.S., Gipson I.K. Altered epithelial-basement membrane interactions in diabetic corneas. Arch Ophthalmol. 1992;110:537–540. doi: 10.1001/archopht.1992.01080160115045. [DOI] [PubMed] [Google Scholar]
- 17.McDermott A.M., Xiao T.L., Kern T.S., Murphy C.J. Non-enzymatic glycation in corneas from normal and diabetic donors and its effects on epithelial cell attachment in vitro. Optometry. 2003;74:443–452. [PubMed] [Google Scholar]
- 18.Friend J., Ishii Y., Thoft R.A. Corneal epithelial changes in diabetic rats. Ophthalmic Res. 1982;14:269–278. doi: 10.1159/000265202. [DOI] [PubMed] [Google Scholar]
- 19.Ljubimov A.V., Huang Z.S., Huang G.H., Burgeson R.E., Gullberg D., Miner J.H., Ninomiya Y., Sado Y., Kenney M.C. Human corneal epithelial basement membrane and integrin alterations in diabetes and diabetic retinopathy. J Histochem Cytochem. 1998;46:1033–1042. doi: 10.1177/002215549804600907. [DOI] [PubMed] [Google Scholar]
- 20.Zagon I.S., Sassani J.W., McLaughlin P.J. Insulin treatment ameliorates impaired corneal reepithelialization in diabetic rats. Diabetes. 2006;55:1141–1147. doi: 10.2337/diabetes.55.04.06.db05-1581. [DOI] [PubMed] [Google Scholar]
- 21.Xu K., Yu F.S. Impaired epithelial wound healing and EGFR signaling pathways in the corneas of diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:3301–3308. doi: 10.1167/iovs.10-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wakuta M., Morishige N., Chikama T., Seki K., Nagano T., Nishida T. Delayed wound closure and phenotypic changes in corneal epithelium of the spontaneously diabetic Goto-Kakizaki rat. Invest Ophthalmol Vis Sci. 2007;48:590–596. doi: 10.1167/iovs.05-1168. [DOI] [PubMed] [Google Scholar]
- 23.Inoue K., Okugawa K., Amano S., Oshika T., Takamura E., Egami F., Umizu G., Aikawa K., Kato S. Blinking and superficial punctate keratopathy in patients with diabetes mellitus. Eye. 2005;19:418–421. doi: 10.1038/sj.eye.6701497. [DOI] [PubMed] [Google Scholar]
- 24.Rehany U., Ishii Y., Lahav M., Rumelt S. Ultrastructural changes in corneas of diabetic patients: an electron-microscopy study. Cornea. 2000;19:534–538. doi: 10.1097/00003226-200007000-00026. [DOI] [PubMed] [Google Scholar]
- 25.Yin J., Huang J., Chen C., Gao N., Wang F., Yu F.S. Corneal complications in streptozocin-induced type I diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:6589–6596. doi: 10.1167/iovs.11-7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saini J., Khandalavla B. Corneal epithelial fragility in diabetes mellitus. Can J Ophthalmol. 1995;30:142–146. [PubMed] [Google Scholar]
- 27.Kenney M.C., Chwa M., Atilano S.R., Tran A., Carballo M., Saghizadeh M., Vasiliou V., Adachi W., Brown D.J. Increased levels of catalase and cathepsin V/L2 but decreased TIMP-1 in keratoconus corneas: evidence that oxidative stress plays a role in this disorder. Invest Ophthalmol Vis Sci. 2005;46:823–832. doi: 10.1167/iovs.04-0549. [DOI] [PubMed] [Google Scholar]
- 28.Xu K.P., Li Y., Ljubimov A.V., Yu F.S. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes. 2009;58:1077–1085. doi: 10.2337/db08-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pflugfelder S.C. Is autologous serum a tonic for the ailing corneal epithelium? Am J Ophthalmol. 2006;142:316–317. doi: 10.1016/j.ajo.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Brightbill F.S., Myers F.L., Bresnick G.H. Postvitrectomy keratopathy. Am J Ophthalmol. 1978;85:651–655. doi: 10.1016/s0002-9394(14)77099-0. [DOI] [PubMed] [Google Scholar]
- 31.Fukushi S., Merola L.O., Tanaka M., Datiles M., Kinoshita J.H. Reepithelialization of denuded corneas in diabetic rats. Exp Eye Res. 1980;31:611–621. doi: 10.1016/s0014-4835(80)80020-0. [DOI] [PubMed] [Google Scholar]
- 32.Schultz R.O., Van Horn D.L., Peters M.A., Klewin K.M., Schutten W.H. Diabetic keratopathy. Trans Am Ophthalmol Soc. 1981;79:180–199. [PMC free article] [PubMed] [Google Scholar]
- 33.Wright E.E., Jr, Stonehouse A.H., Cuddihy R.M. In support of an early polypharmacy approach to the treatment of type 2 diabetes. Diabetes Obes Metab. 2010;12:929–940. doi: 10.1111/j.1463-1326.2010.01255.x. [DOI] [PubMed] [Google Scholar]
- 34.Goto Y., Suzuki K., Ono T., Sasaki M., Toyota T. Development of diabetes in the non-obese NIDDM rat (GK rat) Adv Exp Med Biol. 1988;246:29–31. doi: 10.1007/978-1-4684-5616-5_4. [DOI] [PubMed] [Google Scholar]
- 35.Portha B., Lacraz G., Kergoat M., Homo-Delarche F., Giroix M.H., Bailbe D., Gangnerau M.N., Dolz M., Tourrel-Cuzin C., Movassat J. The GK rat beta-cell: a prototype for the diseased human beta-cell in type 2 diabetes? Mol Cell Endocrinol. 2009;297:73–85. doi: 10.1016/j.mce.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 36.Harris A.K., Hutchinson J.R., Sachidanandam K., Johnson M.H., Dorrance A.M., Stepp D.W., Fagan S.C., Ergul A. Type 2 diabetes causes remodeling of cerebrovasculature via differential regulation of matrix metalloproteinases and collagen synthesis: role of endothelin-1. Diabetes. 2005;54:2638–2644. doi: 10.2337/diabetes.54.9.2638. [DOI] [PubMed] [Google Scholar]
- 37.Song W., Ergul A. Type-2 diabetes-induced changes in vascular extracellular matrix gene expression: relation to vessel size. Cardiovasc Diabetol. 2006;5:3. doi: 10.1186/1475-2840-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Janssen U., Phillips A.O., Floege J. Rodent models of nephropathy associated with type II diabetes. J Nephrol. 1999;12:159–172. [PubMed] [Google Scholar]
- 39.Portha B., Lacraz G., Chavey A., Figeac F., Fradet M., Tourrel-Cuzin C., Homo-Delarche F., Giroix M.H., Bailbe D., Gangnerau M.N., Movassat J. Islet structure and function in the GK rat. Adv Exp Med Biol. 2010;654:479–500. doi: 10.1007/978-90-481-3271-3_21. [DOI] [PubMed] [Google Scholar]
- 40.Omri S., Behar-Cohen F., de Kozak Y., Sennlaub F., Verissimo L.M., Jonet L., Savoldelli M., Omri B., Crisanti P. Microglia/macrophages migrate through retinal epithelium barrier by a transcellular route in diabetic retinopathy: role of PKCzeta in the Goto Kakizaki rat model. Am J Pathol. 2011;179:942–953. doi: 10.1016/j.ajpath.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agardh C.D., Agardh E., Zhang H., Ostenson C.G. Altered endothelial/pericyte ratio in Goto-Kakizaki rat retina. J Diabetes Complications. 1997;11:158–162. doi: 10.1016/s1056-8727(96)00049-9. [DOI] [PubMed] [Google Scholar]
- 42.Tavakoli M., Quattrini C., Abbott C., Kallinikos P., Marshall A., Finnigan J., Morgan P., Efron N., Boulton A.J., Malik R.A. Corneal confocal microscopy: a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33:1792–1797. doi: 10.2337/dc10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quattrini C., Tavakoli M., Jeziorska M., Kallinikos P., Tesfaye S., Finnigan J., Marshall A., Boulton A.J., Efron N., Malik R.A. Surrogate markers of small fiber damage in human diabetic neuropathy. Diabetes. 2007;56:2148–2154. doi: 10.2337/db07-0285. [DOI] [PubMed] [Google Scholar]
- 44.Tavakoli M., Boulton A.J., Efron N., Malik R.A. Increased Langerhan cell density and corneal nerve damage in diabetic patients: role of immune mechanisms in human diabetic neuropathy. Cont Lens Anterior Eye. 2011;34:7–11. doi: 10.1016/j.clae.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tavakoli M., Quattrini C., Abbott C., Kallinikos P., Marshall A., Finnigan J., Morgan P., Efron N., Boulton A.J., Malik R.A. Corneal confocal microscopy a novel noninvasive test to diagnose and stratify the severity of human diabetic neuropathy. Diabetes Care. 2010;33:1792–1797. doi: 10.2337/dc10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson E.P., Coppey L.J., Holmes A., Yorek M.A. Changes in corneal innervation and sensitivity and acetylcholine-mediated vascular relaxation of the posterior ciliary artery in a type 2 diabetic rat. Invest Ophthalmol Vis Sci. 2012;53:1182–1187. doi: 10.1167/iovs.11-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cruzat A., Pavan-Langston D., Hamrah P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010;25:171–177. doi: 10.3109/08820538.2010.518133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdelkader H., Patel D.V., McGhee C., Alany R.G. New therapeutic approaches in the treatment of diabetic keratopathy: a review. Clin Experiment Ophthalmol. 2011;39:259–270. doi: 10.1111/j.1442-9071.2010.02435.x. [DOI] [PubMed] [Google Scholar]
- 49.Klocek M.S., Sassani J.W., McLaughlin P.J., Zagon I.S. Topically applied naltrexone restores corneal reepithelialization in diabetic rats. J Ocul Pharmacol Ther. 2007;23:89–102. doi: 10.1089/jop.2006.0111. [DOI] [PubMed] [Google Scholar]
- 50.Gartner V., Eigentler T.K. Pathogenesis of diabetic macro- and microangiopathy. Clin Nephrol. 2008;70:1–9. doi: 10.5414/cnp70001. [DOI] [PubMed] [Google Scholar]