Abstract
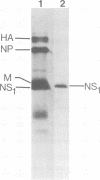
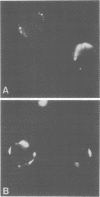
Rabbit antiserum to the influenza A virus nonstructural protein, NS1, was used for indirect immunofluorescence studies of infected cells. Nonstructural antigens were detected on surfaces of P815 cells as early as 4 h after infection with A/WSN/40 virus. Adsorption of the serum with virion structural proteins did not affect the observed fluorescence, and a progressive increase in surface fluorescence at later times postinfection indicated that the surface antigen was newly synthesized during the replication cycle.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biddison W. E., Doherty P. C., Webster R. G. Antibody to influenza virus matrix protein detects a common antigen on the surface of cells infected with type A influenza viruses. J Exp Med. 1977 Sep 1;146(3):690–697. doi: 10.1084/jem.146.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Braciale T. J. Immunologic recognition of influenza virus-infected cells. II. Expression of influenza A matrix protein on the infected cell surface and its role in recognition by cross-reactive cytotoxic T cells. J Exp Med. 1977 Sep 1;146(3):673–689. doi: 10.1084/jem.146.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Dimmock N. J. New virus-specific antigens in cells infected with influenza virus. Virology. 1969 Oct;39(2):224–234. doi: 10.1016/0042-6822(69)90042-7. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Effros R. B., Bennink J. Heterogeneity of the cytotoxic response of thymus-derived lymphocytes after immunization with influenza viruses. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1209–1213. doi: 10.1073/pnas.74.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros R. B., Doherty P. C., Gerhard W., Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J Exp Med. 1977 Mar 1;145(3):557–568. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett C. J., Askonas B. A., Webster R. G., van Wyke K. Quantitation of influenza virus antigens on infected target cells and their recognition by cross-reactive cytotoxic T cells. J Exp Med. 1980 May 1;151(5):1014–1025. doi: 10.1084/jem.151.5.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Koszinowski U. H., Allen H., Gething M. J., Waterfield M. D., Klenk H. D. Recognition of viral glycoproteins by influenza A-specific cross-reactive cytolytic T lymphocytes. J Exp Med. 1980 Apr 1;151(4):945–958. doi: 10.1084/jem.151.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morrongiello M. P., Dales S. Characterization of cytoplasmic inclusions formed during influenza/WSN virus infection of chick embryo fibroblast cells. Intervirology. 1977;8(5):281–293. doi: 10.1159/000148903. [DOI] [PubMed] [Google Scholar]
- Reiss C. S., Schulman J. L. Influenza type A virus M protein expression on infected cells is responsible for cross-reactive recognition by cytotoxic thymus-derived lymphocytes. Infect Immun. 1980 Aug;29(2):719–723. doi: 10.1128/iai.29.2.719-723.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULMAN J. L., KILBOURNE E. D. INDUCTION OF PARTIAL SPECIFIC HETEROTYPIC IMMUNITY IN MICE BY A SINGLE INFECTION WITH INFLUENZA A VIRUS. J Bacteriol. 1965 Jan;89:170–174. doi: 10.1128/jb.89.1.170-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M. W., Compans R. W. Isolation and characterization of cytoplasmic inclusions from influenza A virus-infected cells. J Virol. 1978 Feb;25(2):608–615. doi: 10.1128/jvi.25.2.608-615.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohrer R., Hunter E. Inhibition of Rous sarcoma virus replication by 2-deoxyglucose and tunicamycin: identification of an unglycosylated env gene product. J Virol. 1979 Nov;32(2):412–419. doi: 10.1128/jvi.32.2.412-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virelizier J. L., Allison A. C., Oxford J. S., Schild G. C. Early presence of ribonucleoprotein antigen on surface of influenza virus-infected cells. Nature. 1977 Mar 3;266(5597):52–54. doi: 10.1038/266052a0. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Courtneidge S. A., Skehel J. J., Crumpton M. J., Askonas B. A. Cytotoxic T cells kill influenza virus infected cells but do not distinguish between serologically distinct type A viruses. Nature. 1977 May 26;267(5609):354–356. doi: 10.1038/267354a0. [DOI] [PubMed] [Google Scholar]