Abstract
ROR1 is an orphan-receptor tyrosine-kinase-like surface antigen that is expressed by many tissues during embryogenesis, some B-cell malignancies, and various cancer cell lines but not by virtually all normal adult tissues. Here, we report that large proportions of many different human cancers also express ROR1, particularly those cancers that have high-grade histology. Primary cancers that expressed ROR1 more commonly expressed high levels of phosphorylated AKT (p-AKT) and phosphorylated cAMP response element binding-factor (p-CREB) than similar cancers that lacked expression of ROR1. Induced expression of ROR1 could enhance basal p-AKT and p-CREB levels and could promote the growth of a cancer cell line, MEC1. Conversely, silencing ROR1 resulted in lower levels of p-AKT and p-CREB, which was associated with impaired tumor cell growth. In summary, this study found that many different human cancers express ROR1 and that ROR1 may play a functional role in promoting tumor cell growth, suggesting that this orphan-receptor tyrosine-kinase-like protein may be a potential target for therapy directed against a variety of human cancers.
ROR1 is a type I orphan-receptor tyrosine-kinase-like surface protein that is expressed during embryogenesis and by certain leukemias and lymphomas but not on virtually all normal adult tissues.1–6 Some patients treated with vaccines of autologous leukemia cells genetically engineered to promote antileukemia immune responses generated autoantibodies specific for ROR1 that did not react with nontumor tissues.4 As such, ROR1 is considered an onco-embryonic antigen that currently is being targeted for immune therapy of patients with leukemias that express this surface antigen.7
More recent studies found that many human breast adenocarcinomas also expressed high levels of ROR1, which was not expressed by normal breast tissue.8 Expression of ROR1 appeared to contribute to activation of AKT and cAMP response element-binding factor (CREB) and was associated with enhanced tumor cell growth.8 The association between ROR1 with activated AKT also was noted in another study, which also found that ROR1 could associate with epidermal growth factor receptor, thereby enhancing signaling in response to relevant ligands.9
In this study, we used a high-affinity monoclonal antibody (mAb) highly specific for ROR1 (named 4A54) to examine for expression of this onco-embryonic antigen in other human cancers. In addition, we examined cancer cell lines and primary human lung, ovarian, and pancreatic cancer tissues for expression of phospho-AKT and phospho-CREB to define whether expression of ROR1 was associated with activated AKT/CREB in other human cancers.
Materials and Methods
Cell Culture
Cancer cell lines were purchased from American Type Culture Collection (Manassas, VA) and cultured at 37°C in a 5% CO2/95% humidified air incubator in Dulbecco's modified Eagle medium (Invitrogen, Carlsbad, CA) or RPMI 1640 (Gibco, Carlsbad, CA) with 10% fetal bovine serum (Invitrogen). Puromycin (1 μg/mL) or G418 (1.5 μg/mL) were used for selection of stable transfectants.
Immunohistochemistry
The project was reviewed and approved by one of the Institutional Review Boards of the University of California, San Diego, in accordance with the requirements of the Code of Federal Regulations on the Protection of Human subjects (45 CFR 46 and 21 CFR 50 and 56), including its relevant subparts (IRB no.080918). Tissue microarray slides were purchased from the National Development and Research Institutes, Inc. (Pan-cancer tissue microassay slides; NDRI, New York, NY) or the Cooperative Human Tissue Network (OvCa1; CHTN, Nashville, TN) Tissue Microarray, or were generated by Dr. Wendy Frankel (Ohio State University, Columbus, OH; see Supplemental Tables S1–S7 at http://ajp.amjpathol.org). Immunohistochemistry (IHC) was performed to detect ROR1, p-AKT, or p-CREB with the use of the streptavidin-biotin method as described in prior study.8 The level of ROR1, p-AKT, or p-CREB was scored on the following scale: 0 (negative) indicates that none of the cells within the sample bound to the antibody; + (moderate) indicates low-level binding of the mAb to tumor cells or moderate-level binding of the mAb to ≤50% of the tumor cells; ++ (strong) indicates moderate-level staining by >50% of the tumor cells or high-level staining of the tumor cells. A board-certified pathologist (J.W.-R.) scored each tissue microarray in a blinded fashion.
Flow Cytometry
Cells were stained with 4A5 conjugated with Alexa 647 (Alex 647-4A5) to detect surface ROR1. For cytoplasmic staining, the cells were fixed with paraformaldehyde and processed as described before staining the cells with anti-phosphoserine-133 CREB conjugated with Alexa-488 (Alex488-anti-p-CREB), or anti-phosphoserin-473 AKT conjugated with phycoerythrin (PE-anti-p-AKT). Stained cells were analyzed with a FACSCalibur (Becton Dickinson, San Jose, CA). Mean fluorescence intensity of each stained population was determined with FlowJo software version 6.4.7 (TreeStar, Inc., Ashland, OR). For analysis of cell DNA content, the cells were fixed in 70% ethanol before staining with propidium iodide (Sigma-Aldrich, St. Louis, MO). Sub-G1 apoptotic cells were calculated for analysis of cell survival. Viable single cells were analyzed for cell cycle distribution. Propidium iodide also was used to stain nonfixed, viable cells to examine for their capacity to exclude this dye, a measure of cell viability.
Silencing of Human ROR1
We used the Virapower lentivirus expression system (Invitrogen) to express short hairpin RNA (shRNA). ROR1 shRNA1, ROR1 shRNA2, or nonspecific control shRNA (Control) were purchased from Open Biosystems (Rockford, IL). The sequence of each shRNA is as follows: 5′-CTCATTTAGCAGACATCGCAA-3′ (shRNA1), 5′-CTTTACTAGGAGACGCCAATA-3′ (shRNA2), and 5′-AGCGGACTAAGTCCATTGC-3′ (Control).
Cell Proliferation Assay
Cell proliferation was analyzed with Cell-Counting Kit Solution (CCK-8) (Dojindo, Kumamoto, Japan). Cells were plated in 96-well plates at 1 to 5 × 103 cells/well and maintained at 37°C in a humidified incubator. CCK-8 (10 μL) was added to each well at different time points, and the cells were cultured for an additional 3 hours. The absorbance at 450 nm was measured to calculate the numbers of viable cells in each well.
Statistical Analysis
Statistical significance of the differences among various histologic subtypes and phenotypic subtype of various cancers was analyzed with the Kruskal–Wallis test. The correlation between expression of ROR1 and expression of p-AKT/p-CREB was analyzed with the Fisher's exact test and Pearson coefficiency. A one-way analysis of variance followed by Dunnett's multiple comparisons test was used to evaluate the statistical difference in rates of proliferation for cells. All analyses were performed with GraphPad Prism software version 5 (GraphPad Software, La Jolla, CA). A P value of <0.05 was considered significant.
Results
Expression of ROR1 in Human Cancer
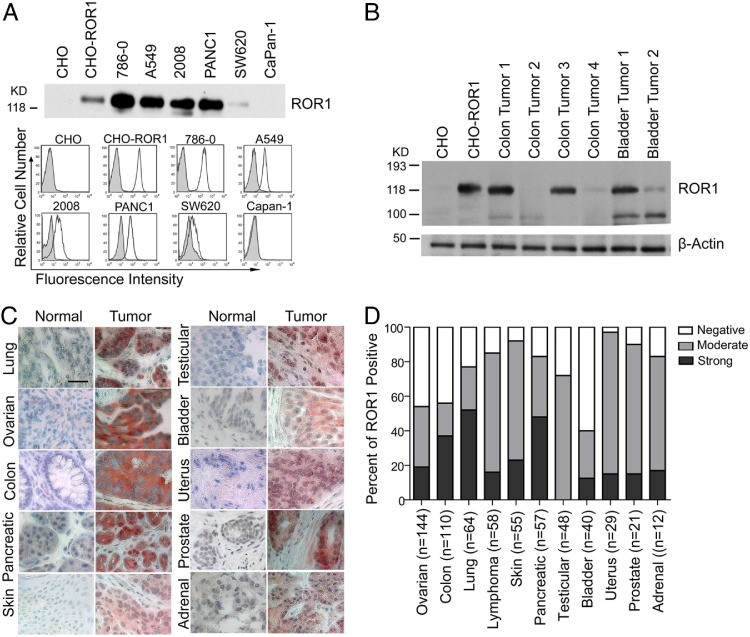
We examined for expression of ROR1 by various cancer cell lines by immunoblot analysis, immune-precipitation assays, and flow cytometry. Chinese hamster ovary (CHO) cells and CHO cells transduced to express human ROR1 (CHO-ROR1) were used as negative and positive control, respectively.4 We found that >50% of the cell lines examined expressed surface ROR1 at different levels by flow cytometry (Figure 1A; see also Supplemental Figure S1A and Supplemental Table S8 at http://ajp.amjpathol.org). Lysates from each cancer cell line that had detectable surface ROR1 also had proteins of ≈125 kDa and ≈100 kDa detectable by immunoblot analysis with the use of commercially available rabbit anti-ROR1 antibodies. Immune-precipitation analysis of these same lysates with the use of the 4A5 mAb identified only the 125-kDa band (Figure 1A; see also Supplemental Figure S1A at http://ajp.amjpathol.org). Lysates prepared from primary tumor tissues such as adenocarcinomas of the colon or bladder also had ROR1 detectable by immunoblot analysis (Figure 1B).
Figure 1.
ROR1 is expressed by a variety of different human cancer cells. A: Immunoblots of immune precipitates with 4A5, using lysates of various cell lines (as indicated at the top of each lane) were probed with rabbit anti-ROR1-peptide antibody (top panel). Chinese hamster ovary (CHO) cells made to express ROR1 (CHO-ROR1) were loaded as a positive control. Protein sizes are indicated on the left. Histograms in the bottom panel depict fluorescence of CHO, CHO-ROR1, or human cancer cell lines (786-0, A549, 2008, PANC1, SW620, or Capan-1; as indicated on the top of each panel) when stained with flurochrome-conjugated 4A5 (open histograms) or IgG2b isotope control monoclonal antibody (mAb; shaded histograms). B: Protein lysates from CHO cells, CHO cells made to express ROR1, or adenocarcinomas of the colon or bladder of different patients were probed with anti-ROR1 antibodies (top row) or β-actin (bottom row), which served to control for the amounts of protein in each lysate. Protein sizes are indicated on the left. C: Formalin-fixed, paraffin-embedded tissue microarray sections of normal or neoplastic tissues were stained with 4A5 or control IgG2b. Tissue-bound 4A5 is shown in red, and the nuclear staining with hematoxylin is in blue. Scale bar = 35 μm (top left). D: The proportion of each tumor type found negative (negative 4A5 staining on all tumor cells), weak to moderate (low-level binding of the mAb to the tumor cells or low-to-moderate-level binding of the mAb on ≤50% of tumor cells), or strong (moderate-level staining on >50% of tumor cells or high-level staining of the tumor cells) staining for ROR1 are indicated by the shading in each bar. The black shading represents the proportion of cases that have strong staining, the gray shading depicts the proportion of cases that have moderate staining, and the nonshaded open area indicates the proportion cases that lack staining for ROR1 of each of the various human cancers examined, as indicated at the bottom of each bar. The number of different cases examined for each tumor type is indicated in the parentheses.
We prepared cytospin slides of ROR1+ MDA-MB-231 cells to determine whether fixation in either paraformaldehyde or formalin affected the immunohistochemical staining pattern observed with 4A5. Although 4A5 detected the 125-kDa isoform found expressed on the plasma membrane of MDA-MB-231 cells (see Supplemental Figure S1B at http://ajp.amjpathol.org), we found that the apparent subcellular location of ROR1 in these cells was differentially affected by the fixation solution used to prepare the slide. We detected ROR1 prominently located at the cell membrane when the cells were fixed in paraformaldehyde. However, we detected mostly cytoplasmic ROR1 in formalin-fixed cells (see Supplemental Figure S1B at http://ajp.amjpathol.org). Nevertheless, the expression of the 125-kDa isoform of ROR1 was readily detected with the 4A5 mAb on formalin-fixed cells via IHC, allowing us to examine tissue microarrays of neoplastic and normal adult tissues for the isoform of ROR1 associated with plasma membrane expression.
High proportions of each of various human cancers expressed ROR1, which was not detectable on normal tissue counterparts (Figure 1, C and D; see also Supplemental Figure S1C and Supplemental Table S8 at http://ajp.amjpathol.org).2,4 Seventy-eight of 144 (54%) ovarian cancers, 63 of 110 (57%) colon cancers, 49 of 64 (77%) lung cancers, 52 of 58 (90%) lymphomas, 49 of 55 (89%) skin cancers, 38 of 48 (83%) pancreatic cancers, 35 of 48 (73%) testicular cancers, 17 of 40 (43%) bladder cancers, 28 of 29 (96%) uterus cancers, 19 of 21 (90%) prostate cancers, and 10 of 12 (83%) adrenal cancers had moderate-to-strong staining with 4A5 (Figure 1D). Similar to the formalin-fixed cytospin preparations of MDA-MB-231, the staining of the tumor cells had both membrane and cytoplasmic ROR1, which was not observed on contiguous nonneoplastic stromal tissue (Figure 1C).
The proportions of each tumor type that expressed ROR1 appeared variably distributed among subtypes of lung, ovarian, or testicular cancers, but they were evenly distributed among subtypes of pancreatic cancer. The expression of ROR1 was more prevalent among lung adenocarcinomas (34% moderate, 59% strong) than among lung squamous cell carcinomas (17% moderate, 38% strong) (Kruskal–Wallis test, P = 0.011). Serous papillary carcinoma had the smallest proportion of tumors that expressed ROR1 (20% moderate, 18% strong) among different subtypes of ovarian cancers (Kruskal–Wallis test, P = 0.001). We detected high-level expression of ROR1 in seminoma, mixed germ cell, and embryonal testicular carcinomas but not teratomas or other types of testicular cancers (Kruskal–Wallis test, P = 0.001) (Figure 2).
Figure 2.
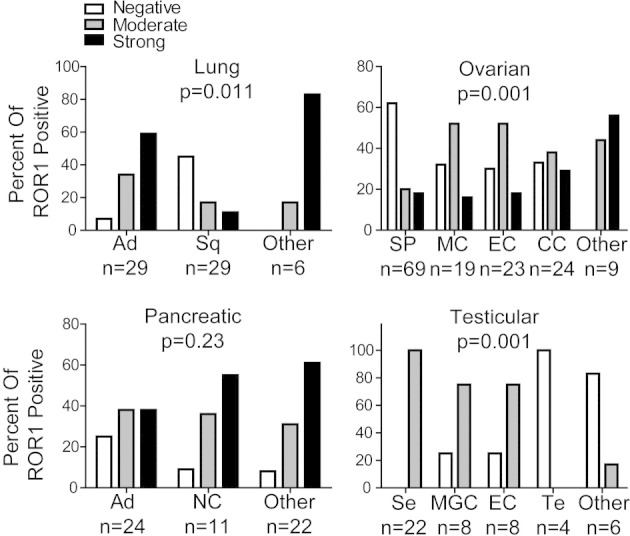
The distribution of ROR1 in different histologic subtypes of various cancers. The proportion of tumor cells found to have negative, moderate, or strong staining for ROR1 are indicated in each of the various histologic subtypes of various cancers examined as indicated at the bottom of each bar. The number of different cases examined for each tumor type is indicated at the bottom. Statistical significance of the differences was analyzed with the Kruskal–Wallis test. Lung cancer tissues: Ad, adenocarcinoma; Sq, squamous cell carcinoma. Ovarian cancer tissues: SP, serous papillary carcinoma; MC, mucinous adenocarcinoma; EC, endometrioid adenocarcinoma; CC, clear cell carcinoma. Pancreatic cancer tissues: Ad, adenocarcinoma; NC, neuroendocrine cancer. Testicular cancer tissues: Se, seminoma; MGC, mixed germ cell; EC, embryonal carcinoma; Te, teratoma.
Expression of ROR1 was associated with tumors that had high-grade, less-differentiated histology. For example, among ovarian serous papillary carcinomas (n = 69), a significantly higher proportion of carcinomas that were poorly differentiated expressed ROR1 (62%) than tumors that had grade 1 or 2 histology (21%; Fisher's exact test, P = 0.0007). Similarly, among pancreatic adenocarcinomas (n = 24), a significantly higher proportion of adenocarcinomas that were poorly differentiated expressed ROR1 (100%) than tumors that had grade 1 or 2 histology (54%; Fisher's exact test, P = 0.0093) (Table 1).
Table 1.
Correlation of ROR1 Expression with Poorly Differentiated Ovarian Serous Papillary Carcinomas or Pancreatic Adenocarcinomas
| Tissue type | ROR1 negative, n (%) | ROR1 positive, n (%) | P value⁎ |
|---|---|---|---|
| Ovarian | 0.0007 | ||
| Grade 1 to 2 | 34 (79) | 9 (21) | |
| Grade 3 to 4 | 9 (38) | 15 (62) | |
| Pancreatic | 0.0093 | ||
| Grade 1 to 2 | 6 (46) | 7 (54) | |
| Grade 3 to 4 | 0 (0) | 11 (100) |
Determined with Fisher's exact text.
Expression of ROR1 Is Associated with Higher Levels of Activated AKT or CREB and Enhanced Cell Growth
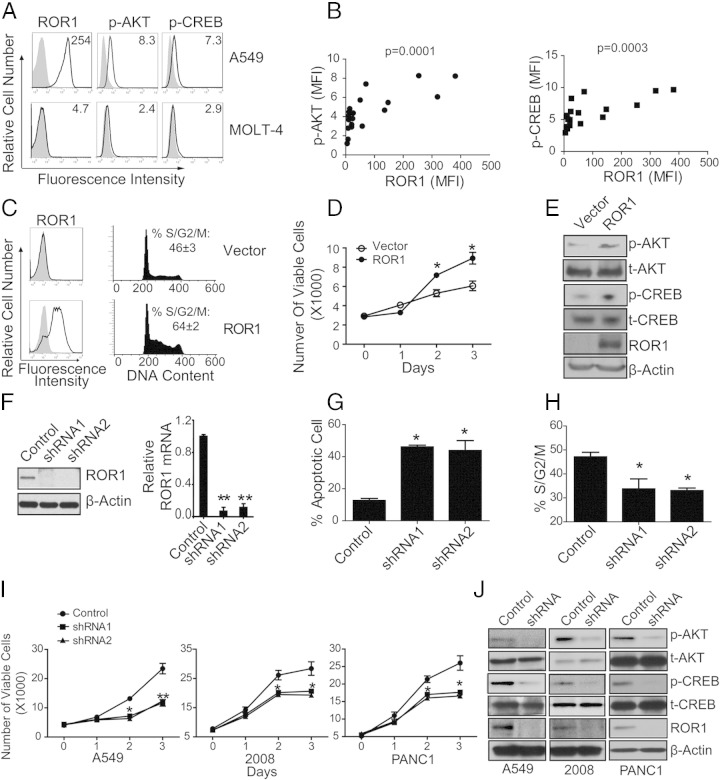
We performed cytoplasmic staining with the use of fluorochrome-conjugated mAb specific for phosphoserine-133 CREB (Alex 488-p-CREB) or PE-anti-p-AKT and examined the cells by flow cytometry. We found cancer cell lines expressed p-AKT or p-CREB at different levels (Figure 3A; see also Supplemental Table S9 at http://ajp.amjpathol.org), which significantly correlated with the relative expression level of ROR1 in the cell lines (Figure 3B) (ROR1 versus p-AKT: Pearson r = 0.7459, P = 0.0001; ROR1 versus p-CREB: Pearson r = 0.7106, P = 0.0003).
Figure 3.
Expression of ROR1 in cancer cell lines was associated with activation of AKT/CREB and silencing ROR1 expression impaired cell growth. A: Representative fluorescence histograms of cancer cell lines (A549 and MOLT-4) stained with 4A5, p-AKT, or p-CREB (open histograms) or isotype-control mAb (shaded histograms), respectively. Mean fluorescence intensity (MFI) was indicated on the top of each figure. B: Correlation of ROR1 expression level with either p-AKT or p-CREB in various cancer cell lines. ROR1 versus p-AKT: Pearson r = 0.7459, P = 0.0001; ROR1 versus p-CREB: Pearson r = 0.7106, P = 0.0003. C: MEC1 cells stably transfected with either empty vector (Vector) or vector encoding ROR1 (ROR1) were cultured in serum-free medium for 24 hours and then in full medium for 16 hours. Cell-cycle distributions were determined by staining with propidium iodide (PI). Fluorescence histograms depicts cells stained with either 4A5 (open histograms) or isotype-control mAb (shaded histograms), respectively. Representative histograms show DNA content in these cell lines. Live cells were gated with the forward and side scatter parameters, FL2W and FL2A parameters were used to gate on single cells and to exclude doublets, and the FL2A channel was used to measure DNA content. The fraction of cells in each phase of the cell cycle was determined according to the Watson model, using the Cell-cycle analysis tool of the FlowJo software. The mean percentage of cells in S/G2/M ± SEM (n = 3 experiments) is indicated on the top of each figure. D: Control MEC1 cells or MEC1 expressing ROR1 were cultured in serum-free medium overnight. Equal numbers of these cells were then seeded into separate, triplicate wells for culture in complete growth medium. The numbers of viable cells per well were assessed over time with the use of the WST-8 assay. The y axis indicates the average number of viable cells per well on day 0, 1, 2, or 3 after initiation of culture in complete growth medium. The error bars indicate the SEM for triplicate cultures. *P < 0.05. E: Protein lysates from control MEC1 cells or MEC1 cells made to express ROR1 were probed with the antibodies as indicated. F: A549 tumor cells were transduced with vectors encoding control short hairpin RNA (Ct-shRNA) or ROR1 shRNA and then selected for stable expression of the shRNA. Lysates of cells selected for stable expression of Ct-shRNA or ROR1 shRNA were examined for ROR1 or β-actin via immunoblot analyses. Relative expression of ROR1 mRNA quantitated by RT-PCR as indicated. The numbers on the y axis represent fold difference in ROR1 expression relative to GAPDH. Error bars indicate SEM (n = 3 experiments). G: The mean proportion of apoptotic sub-G1 population in A549 cells transiently transfected with control shRNA (control) or ROR1 shRNAs (shRNA). The error bars indicate the SE of triplicate samples. *P < 0.05 multiple comparison test. H: The percentage of cells in S/G2/M of A549 cells expressing control shRNA or ROR1 shRNA are indicated. The error bars indicate the SE of triplicate samples. *P < 0.05, multiple comparison test. I: Equal numbers of A549, 2008, or PANC1 cancer cells made to express control shRNA or ROR1 shRNA, as indicated in the legend, were cultured and monitored for growth over time. Data represent average number of viable cells assessed at times 0, 1, 2, or 3 days, using the WST-8 assay. The error bars indicate the SE of the mean for triplicate samples. *P < 0.05, **P < 0.01. J: Immunoblot analysis of CREB phosphorylation at ser-133 (p-CREB), total CREB (t-CREB), AKT phosphorylation at ser-473 (p-AKT), total AKT (t-AKT), ROR1, or β-actin for A549, 2008, or PANC1 tumor cells that had or had not been silenced for ROR1, as indicated on the top of each panel.
We transfected MEC1 cells10 with either an expression vector encoding human ROR1 or a control vector and then selected stable transfectants in selection media (Figure 3C). MEC1 cells typically have doubling time of ≥40 hours.10 However, MEC1 cells made to express ROR1 had significantly greater proportions of cells in S/G2/M phase than did control-transfected cells 16 hours after being transferred from serum-free medium to complete growth medium (Figure 3C), implying that expression of ROR1 increased the relative proportions of cells undergoing cell division. Consistent with this, we noted that ROR1+ MEC1 cells had significantly greater numbers of cells ≥48 hours after being transferred from serum-free medium than did comparably seeded cultures of MEC1 cells that did not express ROR1 (Figure 3D). Increased levels of p-AKT and p-CREB also were observed in MEC1 cells made to express ROR1 relative to MEC1 cells transfected with the control vector (Figure 3E).
We transduced the cells of various cancer cell lines with vectors encoding shRNAs specific for ROR1 or a random sequence (control shRNA). Cells transduced with ROR1 shRNA, but not control shRNA, lost expression of ROR1 mRNA and protein, as assessed via quantitative PCR and immunoblot analysis (Figure 3F). ROR1-expressing cancer cells [eg, A549 cells11 (lung adenocarcinoma cells)] had higher proportions of apoptotic cells and lower proportions of cells in S/G2/M phase when transfected with ROR1 shRNA than when transfected with control shRNA (Figure 3, G and H). Furthermore, ROR1-expressing cancer cells [eg, A549, 200812 (ovarian carcinoma) or PANC113(pancreatic carcinoma) cells] had poorer cell growth after transfection with ROR1 shRNA than with control shRNA (Figure 3I). Finally, cancer cells transfected with ROR1 shRNA had lower levels of p-CREB and p-AKT than cells transfected with control shRNA (Figure 3J).
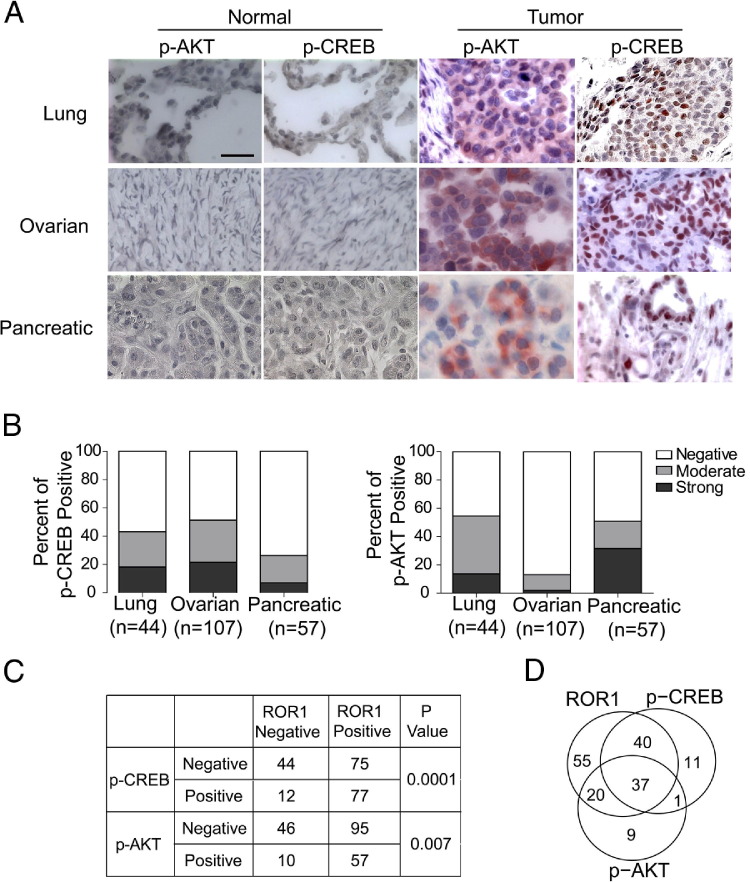
We examined primary tumor tissues for expression of ROR1, p-CREB, and p-AKT by immunohistochemistry. We detected high-level expression of p-CREB in the nucleus or p-AKT in the cytoplasm of many primary cancers derived from the lung, ovary, or pancreas, but not in normal human lung (n = 6), ovarian (n = 8), or pancreatic tissues (n = 4) (Figure 4A). We found moderate-to-strong staining of tumor cells for p-CREB or p-AKT in 43% or 54% of evaluated lung adenocarcinomas (n = 44), 51% or 13% of ovarian cancers (n = 107), and 26% or 51% of pancreatic cancers (n = 57), respectively (Figure 4B). We found that those tumors that had high levels of p-AKT or p-CREB were significantly more likely to express ROR1 (see Supplemental Table S10 at http://ajp.amjpathol.org).
Figure 4.
Activation of AKT and CREB in lung, ovarian, and pancreatic tumor tissues is associated with ROR1 expression. A: Representative immunohistochemistry staining for p-AKT and p-CREB in different normal or cancer tissue specimens. Tissue-bound p-AKT or p-CREB is shown in red, and the nuclear staining with hematoxylin is in blue. Scale bar = 35 μm (top left). B: The proportion of tumor cells found to have negative, weak-to-moderate, or strong staining for p-AKT or p-CREB are indicated for each tumor type. The number of different cases examined for each tumor type is indicated in parentheses. Negative indicates that there was no detectable monoclonal antibody (mAb) staining for all tumor cells; moderate indicates that there was low-level binding of the mAb to all tumor cells or low-to-moderate-level binding of the mAb on ≤50% of tumor cells; strong indicates that there was moderate-level staining on >50% of tumor cells or high-level staining on tumor cells. C: Significant association of ROR1 expression with p-CREB and/or p-AKT in various human cancers (eg, lung, ovarian, or pancreatic neoplasms), as assessed with Fisher's exact test. D: The Venn diagram shows the distribution and number of cases that express ROR (top left), p-AKT (bottom), and/or p-CREB (top right) in various human cancers (ie, lung, ovarian, and pancreatic neoplasms). The numbers indicate the numbers of cases that express ROR1, p-CREB, p-AKT, or a combination.
Detectable strong staining for p-AKT, p-CREB, or ROR1, respectively, was found in 67, 89, or 152 of 208 tumors. Moreover, 77 of the 152 (51%) tumor tissues that expressed ROR1 had high-level p-CREB. In contrast, a significantly lower proportion of 56 tumor tissues that lacked expression of ROR1 (n = 12; 21%) expressed p-CREB (P = 0.0001, Fisher's exact test) (Figure 4C). Similarly 57 of the 152 (38%) tumor tissues that expressed ROR1 also had high-level p-AKT. However, a significantly lower proportion of 56 tumor tissues that lacked expression of ROR1 (n = 10; 18%) expressed p-AKT (Fisher's exact test, P = 0.007) (Figure 4C). Finally, 37 of 152 (24%) tumor tissues that expressed ROR1 also had high levels of both p-AKT and p-CREB (Figure 4D).
Discussion
The results presented here indicate that human cancers derived from a diverse array of tissues expressed the 125-kDa isoform of ROR1 found on the plasma membrane. In contrast, the nonneoplastic adult tissues corresponding to these cancers lacked detectable ROR1 protein, as noted in prior studies.2,4 Two isoforms of ROR1 were detected in both cell lines and tumor tissues. However, 4A5, used for detection of ROR1 in formalin-fixed tissue sections, detected only the 125-kDa isoform of ROR1 that is expressed on the cell surface. We found that different fixation solutions affected the apparent subcellular location of ROR1, as has been noted for other proteins (eg, GATA factors).14 We readily detected ROR1 on the plasma membrane of paraformaldehyde-fixed cells, but mostly cytoplasmic ROR1 in formalin-fixed cells. For this reason, much of the surface/cytoplasmic staining of 4A5 on formalin-fixed tumor -tissue microarrays could be because of ROR1 expressed by primary tumor cells at the plasma membrane. Consistent with this notion, a variety of different tumor cell lines were observed to express surface ROR1 in this and prior studies.8
We found that cancer cells that were poorly differentiated with high-grade histology were more likely to express ROR1 than tumors with low-grade histology. This is similar to what we observed among human breast adenocarcinomas; expression of ROR1 was more common among less-differentiated adenocarcinomas that lacked expression of hormone receptors and human epidermal growth factor receptor 2/Neu and that were associated with more aggressive disease.8 The finding that ROR1 more commonly is expressed by tumors with high-grade histology is similar to what other investigators have observed for another onco-embryonic antigen, named 5T4, which also appears to be expressed predominantly in poorly differentiated tumors.15 Conceivably, expression of such embryonic antigens might be a feature associated with tumor cells that have de-differentiated and/or acquired biological features associated with embryogenesis.
We found expression of ROR1 was associated with activation of AKT and CREB. These proteins are overexpressed and/or constitutively phosphorylated in a number of human cancers and are postulated to play a direct role in tumor pathogenesis and progression.16–23 We found a significant association between the expression of ROR1 and activation of AKT in cancer cell lines or primary tumor tissues. Similarly, primary lung, ovarian, or pancreatic cancer tissues more frequently expressed p-CREB if they were ROR1 positive, as we noted previously for breast adenocarcinomas.8,24 However, it should be noted that some tumors expressed ROR1 but lacked expression of p-CREB or p-AKT. Conversely, some tumors expressed p-CREB or p-AKT but lacked expression of ROR1. For such tumors, the expression of ROR1 appears neither necessary nor sufficient to provide for activation of AKT or CREB. Nevertheless, there is a significant association supported by functional studies showing that forced expression or silencing of ROR1 can, respectively, enhance or suppress the levels of p-AKT/p-CREB in several different cancer cell lines. As such, our studies support the notion that ROR1 plays a role in the activation of AKT and/or CREB in a variety of different cancers.
A prior study reported that many tumor cell lines expressed ROR1 and that the activity of ROR1 depended on activation of the MET proto-oncogene.25 However, a recent study found that ROR1 could enhance the survival of tumor cells by either kinase-dependent or kinase-independent pathways.9 Moreover, we found that the neoplastic cells in chronic lymphocytic leukemia cells do not express MET,8 but they do express ROR1, which is implicated in supporting chronic lymphocytic leukemia cell survival.4,26 As such, the expression and/or activity of ROR1 might not be predicated on the expression of MET, at least not in all human cancers.
In summary, the results presented here indicate that many different human cancers express ROR1, which is not expressed on most normal adult tissues. We found ROR1 was more commonly expressed in tumors with high-grade histology than in tumors with more differentiated histology and identified a significant association between the expression of ROR1 and p-AKT/p-CREB in several different tumor types. Collectively, these studies support the notion that ROR1 plays a functional role in promoting tumor cell growth and suggest it may be a target for development of therapies against a variety of different human cancers.
Footnotes
Supported in part by NIH grant PO1-CA081534 (T.J.K.) and by the Moores UCSD Cancer Center Blood Cancer Research Fund.
S.Z. and L.C. contributed equally to this work.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at http://dx.doi.org/10.1016/j.ajpath.2012.08.024.
Supplementary data
A: Protein lysates from MDA-MB-231 cells or 786-0 cells were immune precipitated (IP) with monoclonal antibody 4A5. The bound IP products and whole cell lysate (WCL) were probed by anti-ROR1 antibody. B: Immunohistochemical staining with IgG control or 4A5 for MDA-MB-231 cells fixed with paraformaldehyde (left panel) or formalin (right panel). Cell-bound 4A5 in the left panel is shown in green, and the nuclear staining with DAPI is in blue. Image in the left panel was collected with confocal microscopy. Cell-bound 4A5 in the right panel is shown in red, which is localized in the cytoplasm. Hematoxylin is used as a counterstain in blue. Scale bar = 35 μm (bottom right). C: Immunohistochemical staining with 4A5 for different frozen tumor tissues. Cell-bound 4A5 is shown in red, which is localized to both cytoplasm and cell membranes. Hemotoxylin is used as a counterstain shown in blue. Scale bar = 35 μm (bottom right).
References
- 1.Barna G., Mihalik R., Timar B., Tombol J., Csende Z., Sebestyen A., Bodor C., Csernus B., Reiniger L., Petak I., Matolcsy A. ROR1 expression is not a unique marker of CLL. Hematol Oncol. 2011;29:17–21. doi: 10.1002/hon.948. [DOI] [PubMed] [Google Scholar]
- 2.Baskar S., Kwong K.Y., Hofer T., Levy J.M., Kennedy M.G., Lee E., Staudt L.M., Wilson W.H., Wiestner A., Rader C. Unique cell surface expression of receptor tyrosine kinase ROR1 in human B-cell chronic lymphocytic leukemia. Clin Cancer Res. 2008;14:396–404. doi: 10.1158/1078-0432.CCR-07-1823. [DOI] [PubMed] [Google Scholar]
- 3.Daneshmanesh A.H., Mikaelsson E., Jeddi-Tehrani M., Bayat A.A., Ghods R., Ostadkarampour M., Akhondi M., Lagercrantz S., Larsson C., Osterborg A., Shokri F., Mellstedt H., Rabbani H. Ror1, a cell surface receptor tyrosine kinase is expressed in chronic lymphocytic leukemia and may serve as a putative target for therapy. Int J Cancer. 2008;123:1190–1195. doi: 10.1002/ijc.23587. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda T., Chen L., Endo T., Tang L., Lu D., Castro J.E., Widhopf G.F., II, Rassenti L.Z., Cantwell M.J., Prussak C.E., Carson D.A., Kipps T.J. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc Natl Acad Sci U S A. 2008;105:3047–3052. doi: 10.1073/pnas.0712148105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabbani H., Ostadkarampour M., Danesh Manesh A.H., Basiri A., Jeddi-Tehrani M., Forouzesh F. Expression of ROR1 in patients with renal cancer–a potential diagnostic marker. Iran Biomed J. 2010;14:77–82. [PMC free article] [PubMed] [Google Scholar]
- 6.Shabani M., Asgarian-Omran H., Jeddi-Tehrani M., Vossough P., Faranoush M., Sharifian R.A., Toughe G.R., Kordmahin M., Khoshnoodi J., Roohi A., Tavoosi N., Mellstedt H., Rabbani H., Shokri F. Overexpression of orphan receptor tyrosine kinase Ror1 as a putative tumor-associated antigen in Iranian patients with acute lymphoblastic leukemia. Tumour Biol. 2007;28:318–326. doi: 10.1159/000121405. [DOI] [PubMed] [Google Scholar]
- 7.Hudecek M., Schmitt T.M., Baskar S., Lupo-Stanghellini M.T., Nishida T., Yamamoto T.N., Bleakley M., Turtle C.J., Chang W.C., Greisman H.A., Wood B., Maloney D.G., Jensen M.C., Rader C., Riddell S.R. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood. 2010;116:4532–4541. doi: 10.1182/blood-2010-05-283309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S., Chen L., Cui B., Chuang H.Y., Yu J., Wang-Rodriguez J., Tang L., Chen G., Basak G.W., Kipps T.J. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS One. 2012;7:e31127. doi: 10.1371/journal.pone.0031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamaguchi T., Yanagisawa K., Sugiyama R., Hosono Y., Shimada Y., Arima C., Kato S., Tomida S., Suzuki M., Osada H., Takahashi T. NKX2-1/TITF1/TTF-1-induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell. 2012;21:348–361. doi: 10.1016/j.ccr.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Stacchini A., Aragno M., Vallario A., Alfarano A., Circosta P., Gottardi D., Faldella A., Rege-Cambrin G., Thunberg U., Nilsson K., Caligaris-Cappio F. MEC1 and MEC2: two new cell lines derived from B-chronic lymphocytic leukaemia in prolymphocytoid transformation. Leuk Res. 1999;23:127–136. doi: 10.1016/s0145-2126(98)00154-4. [DOI] [PubMed] [Google Scholar]
- 11.Giard D.J., Aaronson S.A., Todaro G.J., Arnstein P., Kersey J.H., Dosik H., Parks W.P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 12.DiSaia P.J., Sinkovics J.G., Rutledge F.N., Smith J.P. Cell-mediated immunity to human malignant cells: A brief review and further studies with two gynecologic tumors. Am J Obstet Gynecol. 1972;114:979–989. doi: 10.1016/0002-9378(72)90109-3. [DOI] [PubMed] [Google Scholar]
- 13.Lieber M., Mazzetta J., Nelson-Rees W., Kaplan M., Todaro G. Establishment of a continuous tumor-cell line (panc-1) from a human carcinoma of the exocrine pancreas. Int J Cancer. 1975;15:741–747. doi: 10.1002/ijc.2910150505. [DOI] [PubMed] [Google Scholar]
- 14.Tate J.J., Cooper T.G. Formalin can alter the intracellular localization of some transcription factors in Saccharomyces cerevisiae. FEMS Yeast Res. 2008;8:1223–1235. doi: 10.1111/j.1567-1364.2008.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damelin M., Geles K.G., Follettie M.T., Yuan P., Baxter M., Golas J., DiJoseph J.F., Karnoub M., Huang S., Diesl V., Behrens C., Choe S.E., Rios C., Gruzas J., Sridharan L., Dougher M., Kunz A., Hamann P.R., Evans D., Armellino D., Khandke K., Marquette K., Tchistiakova L., Boghaert E.R., Abraham R.T., Wistuba I.I., Zhou B.B. Delineation of a cellular hierarchy in lung cancer reveals an oncofetal antigen expressed on tumor-initiating cells. Cancer Res. 2011;71:4236–4246. doi: 10.1158/0008-5472.CAN-10-3919. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal S., Kim S.W., Ryu S.H., Chung W.C., Koo J.S. Growth suppression of lung cancer cells by targeting cyclic AMP response element-binding protein. Cancer Res. 2008;68:981–988. doi: 10.1158/0008-5472.CAN-06-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng J.C., Kinjo K., Judelson D.R., Chang J., Wu W.S., Schmid I., Shankar D.B., Kasahara N., Stripecke R., Bhatia R., Landaw E.M., Sakamoto K.M. CREB is a critical regulator of normal hematopoiesis and leukemogenesis. Blood. 2008;111:1182–1192. doi: 10.1182/blood-2007-04-083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chhabra A., Fernando H., Watkins G., Mansel R.E., Jiang W.G. Expression of transcription factor CREB1 in human breast cancer and its correlation with prognosis. Oncol Rep. 2007;18:953–958. [PubMed] [Google Scholar]
- 19.Linnerth N.M., Greenaway J.B., Petrik J.J., Moorehead R.A. cAMP response element-binding protein is expressed at high levels in human ovarian adenocarcinoma and regulates ovarian tumor cell proliferation. Int J Gynecol Cancer. 2008;18:1248–1257. doi: 10.1111/j.1525-1438.2007.01177.x. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto K.M., Frank D.A. CREB in the pathophysiology of cancer: implications for targeting transcription factors for cancer therapy. Clin Cancer Res. 2009;15:2583–2587. doi: 10.1158/1078-0432.CCR-08-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao X., Li B.X., Mitton B., Ikeda A., Sakamoto K.M. Targeting CREB for cancer therapy: friend or foe. Curr Cancer Drug Targets. 2010;10:384–391. doi: 10.2174/156800910791208535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X., Odom D.T., Koo S.H., Conkright M.D., Canettieri G., Best J., Chen H., Jenner R., Herbolsheimer E., Jacobsen E., Kadam S., Ecker J.R., Emerson B., Hogenesch J.B., Unterman T., Young R.A., Montminy M. Genome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissues. Proc Natl Acad Sci U S A. 2005;102:4459–4464. doi: 10.1073/pnas.0501076102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellacosa A., Kumar C.C., Di Cristofano A., Testa J.R. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S., Chen L., Cui B., Chuang H.Y., Yu J., Wang-Rodriguez J., Tang L., Chen G., Basak G.W., Kipps T.J. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS One. 2012;7:e31127. doi: 10.1371/journal.pone.0031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gentile A., Lazzari L., Benvenuti S., Trusolino L., Comoglio P.M. Ror1 is a pseudokinase that is crucial for Met-driven tumorigenesis. Cancer Res. 2011;71:3132–3141. doi: 10.1158/0008-5472.CAN-10-2662. [DOI] [PubMed] [Google Scholar]
- 26.Choudhury A., Derkow K., Daneshmanesh A.H., Mikaelsson E., Kiaii S., Kokhaei P., Osterborg A., Mellstedt H. Silencing of ROR1 and FMOD with siRNA results in apoptosis of CLL cells. Br J Haematol. 2010;151:327–335. doi: 10.1111/j.1365-2141.2010.08362.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Protein lysates from MDA-MB-231 cells or 786-0 cells were immune precipitated (IP) with monoclonal antibody 4A5. The bound IP products and whole cell lysate (WCL) were probed by anti-ROR1 antibody. B: Immunohistochemical staining with IgG control or 4A5 for MDA-MB-231 cells fixed with paraformaldehyde (left panel) or formalin (right panel). Cell-bound 4A5 in the left panel is shown in green, and the nuclear staining with DAPI is in blue. Image in the left panel was collected with confocal microscopy. Cell-bound 4A5 in the right panel is shown in red, which is localized in the cytoplasm. Hematoxylin is used as a counterstain in blue. Scale bar = 35 μm (bottom right). C: Immunohistochemical staining with 4A5 for different frozen tumor tissues. Cell-bound 4A5 is shown in red, which is localized to both cytoplasm and cell membranes. Hemotoxylin is used as a counterstain shown in blue. Scale bar = 35 μm (bottom right).