Abstract
Botulinum Neurotoxins (BoNTs) are used therapeutically and in cosmetics, providing potential for bioterrorist activity, thus driving the search for small-molecule BoNT inhibitors. This report describes a 70,000-compound screen for inhibition of BoNT/A using a FRET assay to detect proteolysis of a peptide substrate. Hits were confirmed, followed by evaluation to determine compound specificity. Inhibitors fell into three main chemical classes, and on the basis of potency and specificity of inhibition, the activities of two chemotypes were examined further. Compounds exhibited specificity for BoNT/A LC inhibition with respect to other metalloproteases and displayed activity in a neuronal assay for botulinum intoxication.
Keywords: botulinum, neurotoxins, serotype A, bioterrorism, SNAP-25, high-throughput screening, benzimidazole acrylonitrile, hydroxyquinoline, small molecule inhibitors, drug discovery, metalloprotease
Introduction
BoNTs are the most potent of the biological toxins (Arnon et al., 2001; Dembek et al., 2007; Paddle, 2003), and due to their lethality, are listed as category A (highest priority) bioterrorism agents by the Centers for Disease Control and Prevention. BoNTs are easily produced and may be delivered by ingestion or by an aerosol route (Burnett et al., 2005; Paddle, 2003). Consequently, these toxins represent a serious threat to both military personnel and civilians (Clarke, 2005; Hicks et al., 2005; Josko, 2004).
Once ingested or inhaled into the lungs, BoNTs enter the blood stream, target the peripheral cholinergic nerve endings, and cause death by interrupting phrenic nerve function and resulting in the paralysis of the diaphragm (Rasetti-Escargueil et al., 2009). The zinc-dependent endopeptidase light chain (LC) portion of BoNTs impairs neuronal exocytosis through proteolysis of essential SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) components of neurotransmission (Simpson, 2004).
There are seven BoNT serotypes (A–G), which differ significantly in amino acid sequence, protein substrates, and substrate cleavage sites (Dembek et al., 2007; Simpson, 2004), as well as in the duration of resulting paralysis. BoNT/A paralysis lasts the longest, typically 4–6 months, and this is a primary reason that it has become popular for both medicinal and cosmetic applications (Charles, 2004). Examination of several BoNTs in a rat cerebellar neuron model system revealed a rank order of half-lives for inhibition of ≫31 days (BoNT/A), ≫25 days (BoNT/C), ~10 days (BoNT/B), ~2 days (BoNT/F), and ~0.8 days (BoNT/E) (Foran et al., 2003). The duration of paralysis from BoNT/A coupled with its potency and the fact that several high resolution crystal structures are available (Silvaggi et al., 2007; Silvaggi et al., 2008) have made it possibly the most tractable and relevant for immediate drug discovery efforts. Of the seven types of BoNT, only types A, B, E, and F cause illness in humans (CDC, 2009). The currently available BoNT toxoid vaccine, as well as experimental preventative antibodies cannot counter these toxins after they penetrate neurons (Willis et al., 2008). Currently, critical care mechanical ventilation is the only treatment option once neurons have been intoxicated and diaphragm muscles cease to function. However, the effects of internalized BoNTs can last for months (Eleopra et al., 1998; Keller et al., 1999; Meunier et al., 2003), and long-term mechanical ventilation would be impractical if even a limited number of individuals were simultaneously intoxicated. Therefore, there is an urgent need to identify and develop low molecular weight inhibitors that will serve as both prophylactics and post-exposure ‘rescue’ therapeutics.
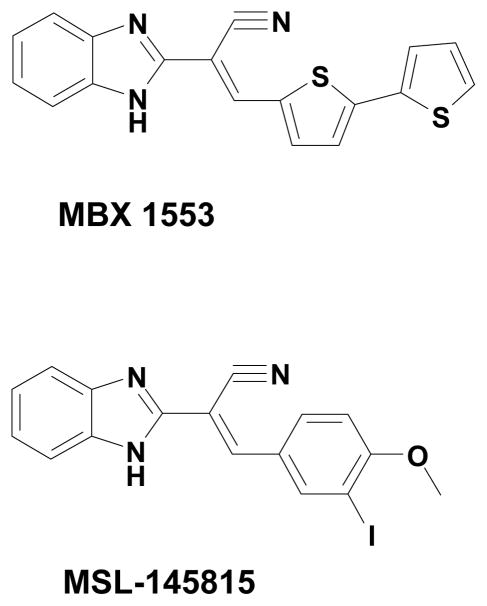
In order to survey libraries of diverse compounds for inhibitors of BoNT/A, a high-throughput screening assay based on fluorescence resonance energy transfer (FRET) was developed (Burnett et al., 2003). In this report, we describe application of this assay to screen a collection of over 70,000 compounds. Profiling of the hits yielded a benzimidazole acrylonitrile (MSL-145815) with significant selectivity for BoNT/A LC inhibition. Furthermore, an analog of the benzimidazole acrylonitrile synthesized in our laboratory (MBX-1553) demonstrated significant inhibition of SNAP-25 cleavage in a chick neuronal cellular assay.
MATERIALS AND METHODS
Compounds and Reagents
Chemical compound libraries were purchased from Chembridge Corporation (San Diego, CA), ChemDiv Incorporated (San Diego, CA), and from Microsource Corporation (Gaylordsville, CT). MBX 1553 was synthesized in-house (manuscript in preparation). Peptide substrates were purchased from Peptides International, (Louisville, KY) (BoNT/A-dacia/Dnp and BaLF-MCA/Dnp), List Biological Laboratories, (Campbell, CA) (Vamptide® BoNT/B) and Biomol, International, (Plymouth Meeting, PA) (OmniMMP™ fluorogenic substrate). Metalloproteases were purchased from List Biological Laboratories, Campbell, CA (BoNT/A LC, BoNT/B LC, BaLF) and Biomol, International, (Plymouth Meeting, PA) (MMP-1, MMP-2 and MMP-9). BoNT/A holotoxin was purchased from MetaBiologics, Inc., (Madison, WI). Chemical reagents were purchased from Sigma Aldrich (St. Louis, MO) and VWR (Bridgeport, NJ).
BoNT/A LC FRET Screening Assay-384 Well Format
The fluorescence resonance energy transfer (FRET) assay described previously (Schmidt and Stafford, 2003) was modified to a 384-well microplate format to increase throughput further and lower costs per assay. Briefly, 24 μM SNAP-25 substrate (aa 187–203) of sequence SNRTRIDEAN[DnpK]RA[daciaC]RML, was incubated at 37 °C for 60 min in the presence of buffer (50 mM HEPES–0.05% Tween [pH 7.4]) and 1 nM BoNT/A LC (Kadkhodayan et al., 2000) in a volume of 25 μL. Compounds at 83 μM [final] were placed into opaque black 384-well microplates (Costar, Corning, NY), utilizing a SciClone ALH 3000 liquid handling robot (Caliper Life Sciences, Hopkinton, MA). Reactions were stopped by addition of acetic acid to 0.5%, and the fluorescence of the cleaved substrate was measured at 485 nm following excitation at 398 nm in a Victor2V (Perkin Elmer, Boston, MA) plate reader. Any compound that inhibited BoNT/A LC activity greater than 90% was classified as a hit and was confirmed in a secondary assay utilizing a 96-well microplate format. Any compound that possessed intrinsic fluorescence was not analyzed further and was rejected to maintain screening efficiency.
BoNT/A LC FRET Assay-96 Well Format for Hit Confirmations, IC50 Determinations, and Inhibitor Kinetic analysis
Hit confirmations, IC50 determinations, and kinetic analysis for inhibitor mechanism of action were carried out utilizing a 96-well FRET assay microplate format. For hit confirmation, compound (25 μM [final]), 20 μM SNAP-25 substrate (see FRET assay for sequence), and 2 nM BoNT/A LC were incubated at 37 °C for 40 min, which was the initial linear rate phase of catalytic activity (Figure 1), in the presence of buffer (50 mM HEPES–0.05% Tween [pH 7.4]) in a volume of 100 μL. The reactions were stopped by the addition of acetic acid to 0.5% prior to measuring the fluorescence of the cleaved substrate at 485 nm following excitation at 398 nm in a Molecular Devices (Sunnyvale, CA) plate reader. Any compound that inhibited BoNT/A LC activity greater than 70 % at 25 μM was classified as a hit. For kinetic analysis of compounds, substrate concentration was varied in the presence of increasing inhibitor concentrations, and the results were analyzed utilizing Eadie-Hofstee transformations (Atkins and Nimmo, 1975). The FRET-based 96 well assay displayed linear kinetics at the SNAP-25 and enzyme concentrations used for hit confirmation (R2 greater than 0.98). The Km for the substrate ranged between 23–29 μM.
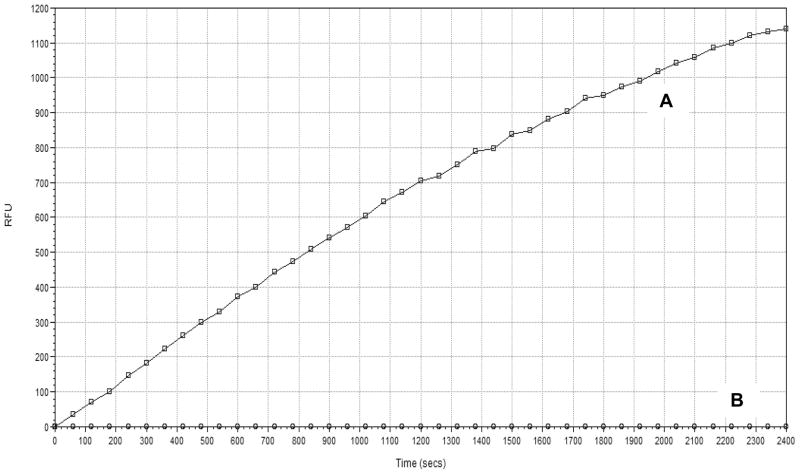
Figure 1.
Representative BoNT/A LC FRET assay kinetic trace of the SNAP-25 substrate cleavage over the 40 minute incubation period. (A) Native BoNT/A LC; (B) Heat Denatured BoNT/A LC.
HPLC Secondary Assay to Confirm FRET IC50 Values
The HPLC assay was carried out using a modified version of a published protocol (Schmidt and Bostian, 1995). Incubation conditions were the same as for the FRET assay except that the enzyme concentration in the assay was 6 nM, the substrate concentration was 60 μM, and 0.05% NP-40 was used in place of Tween-20. Assay mixes were incubated at 37 °C for 60 minutes and reactions stopped by addition of acetic acid to 0.5%. The extent of hydrolysis of the peptide substrate was determined by HPLC separation of the products from the substrate, followed by measurement of the peak areas (Figure 2). The samples were analyzed by reverse-phase HPLC (Gilson, Middleton, WI) (C18 column, 150 × 4.6 mm, 5 μM from Grace Alltima, Deerfield, IL) with a gradient of 35% B to 40% B over 21 min, 100% B for 8 min (solvent A: 0.1% trifluoroacetic acid (TFA), aqueous; solvent B: 0.1% TFA in 70% acetonitrile). The effluent was monitored at 365 nm and the resultant peaks quantified by integration utilizing Gilson Trilution® software.
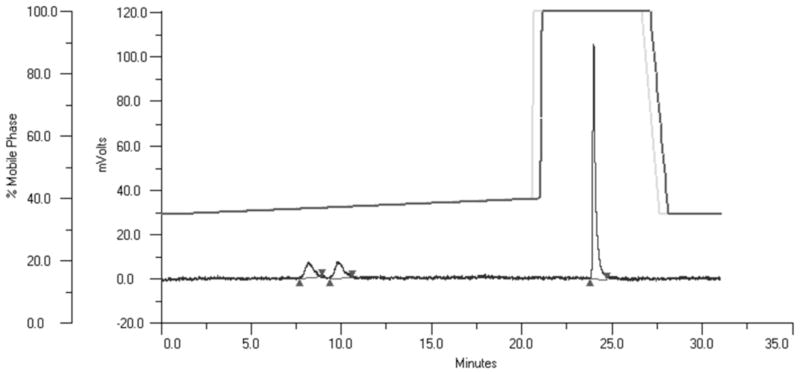
Figure 2.
Representative BoNT/A LC HPLC assay. The upper lines represent the programmed and actual gradients and the lower line the absorption at 365 nm of the HPLC effluent. The peak at ≈24 min represents the intact peptide while the peaks at ≈ 8 and 10 min represent the N -terminal and C -terminal pieces of the peptide, respectively.
BoNT/B LC Assay
The FRET assay was performed using 10 μM Vamptide® peptide substrate, 20 mM HEPES–0.05% Tween-20 [pH 7.4] and 13 nM BoNT/B LC with incubation at 37 °C for 60 min. Inactivation of the enzyme was achieved by addition of acetic acid to 0.5% and the fluorescent signal of the cleaved substrate was measured at 418 nm after excitation at 321 nm.
Bacillus anthracis Lethal Factor (BaLF) Assay
A BaLF FRET assay was performed using 20 μM peptide substrate (MCA-KKVYPYPME[dnp]K amide), 20 mM HEPES–0.05% Tween [pH 8.2] and 5.55 nM BaLF and incubating at 37 °C for 30 min as described previously (Panchal et al., 2004). Inactivation of the enzyme was achieved by addition of acetic acid to 0.5% and the fluorescent signal of the cleaved substrate was measured at 395 nm after excitation at 324 nm.
Human Matrix Metalloproteinase (MMP) 1, 2 and 9 Assays
MMP FRET assays were performed using 25 μM OmniMMP™ fluorogenic substrate, 50 mM MOPS–0.05% NP-40 [pH 6.0] and either MMP-1 (38 nM), MMP-2 (19 nM) or MMP-9 (13 nM). The enzyme reactions were incubated at 37 °C for 60 min. The fluorescent signal of the cleaved substrate was measured at 393 nm after excitation at 328 nm.
Chick Neuronal Cell Generation
Embryonic chicken spinal motor neurons were obtained by incubating fertilized chicken eggs (SPAFAS, Charles River Laboratories, North Franklin, CT) for 6 days and removing the ventral spinal cords from the embryos (Burnett et al., 2007; Kuhn, 2003). The dissociated cell population was enhanced for neuronal cells by briefly plating (to attach non-neuronal cells) and then treating with a mixture of the mitotic inhibitor, 5-fluorodeoxyuridine (295.4 μg/mL [Final]) (to prevent the growth of dividing non-neuronal cells) and uridine (732.6 μg/mL [Final]). Cells were then plated in 6-well tissue culture plates and incubated overnight at 37 °C prior to intoxication.
Chick Neuronal Cell SNAP-25 Cleavage Assay
As described previously (Burnett et al., 2007), cells were preincubated in Leibovitz L15 medium (Invitrogen; with N3 supplement and 10% fetal bovine serum) with inhibitor for 45 min, followed by a 3.5 h incubation with 5–10 nM BoNT/A holotoxin and inhibitor. Cells were rinsed with fresh growth medium, scraped, collected, washed with phosphate-buffered saline, lysed and assessed for protein content by Bradford assay prior to loading on a 12% Tris-glycine gel (Invitrogen). Gel contents were transferred to nitrocellulose and probed with SMI 81 mouse anti-SNAP-25 (Covance, Berkley, CA) as the primary antibody. A horseradish peroxidase-conjugated goat anti-mouse secondary antibody (Pierce) was used in combination with an ECL Western blotting detection system (Pierce) and densitometry was performed using an UN-SCAN-IT gel automated digitizing system (Silk Scientific, Inc., Orem, UT).
Zinc Chelation Assessments
Measurements of the effect of prior incubation of inhibitors with varying concentrations of Zn++ were carried out by a modification of a previously described procedure (Burnett et al., 2003). Compounds were diluted in DMSO without ZnCl2, DMSO solution containing 5 mM ZnCl2, or DMSO solution containing 10 mM ZnCl2, such that the final concentration of ZnCl2 was 0, 2.5, or 5 mM respectively. Likewise, N-Hydroxy-2,4-dichlorocinnanamide, a hydroxamate zinc non-chelator (Eubanks et al., 2007) was analyzed for zinc chelation and served as a negative control. The compounds were incubated in the ZnCl2 solutions for 15 minutes at room temperature (18–24°C) and then were diluted 100-fold to final concentrations of 0, 25 and 50 μM, respectively with assay mix and assessed for potency utilizing the BoNT/A LC 96 well FRET assay. The final concentrations of zinc chloride in the assay inhibit BoNT/A LC activity minimally (i.e., 4–5% inhibition, unpublished observations). Compounds were classified as zinc chelators if they displayed a zinc concentration-dependent decrease in potency.
Determination of Mammalian Cytotoxicity
Cytotoxicity of the compounds was measured by plating HeLa cells (ATCC# CCL-2) in 96-well plates (4 × 103 cells per well) in the presence or absence of compounds added from DMSO stock solutions (final DMSO concentration of 1%). The culture with compound, and an identical control culture containing only DMSO, were incubated at 37°C for 72 hr in Minimal Essential Medium (Gibco/Invitrogen, Carlsbad, CA) supplemented with 10% fetal calf serum, and cell viability was tested with the vital stain MTS (Marshall et al., 1995) according to the manufacturer’s instructions (Promega, Madison, WI). Cytotoxicity was quantified as the CC50, the concentration of compound that inhibited 50% of conversion of MTS to formazan (Marshall et al., 1995).
RESULTS
BoNT/A Inhibitor Screening
Over 70,000 compounds were screened for inhibition of BoNT/A LC using a FRET-based assay, yielding a primary hit rate of 0.47 %. Z′ scores, a measure of assay robustness that takes into account the numerical spread between negative and positive controls, as well as standard deviations of each, were very favorable, averaging about 0.75, and indicating that the assay was suitable for screening (Zhang et al., 1999). FRET-based assay results were confirmed in an HPLC-based assay using the same substrate and enzyme to ensure that compounds with intrinsic quenching capability did not interfere with the activity measurements. The final confirmed hit rate, representing compounds that exhibited inhibition in the repeated FRET assay as well as in the HPLC assay, was 0.16 %.
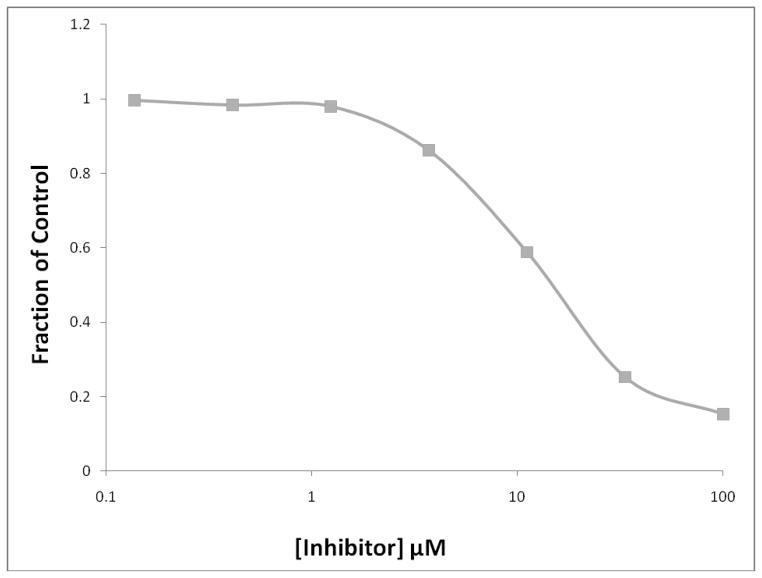
The most potent screening hits consisted of a variety of structural types, including, 8-hydroxyquinolines, benzimidazole acrylonitriles, arylamides, hydroxypyrazoles, vinylbenzothiazoles, and vinylbenzimidazoles (Figure 3). The concentration-dependence of inhibition (IC50 values) of BoNT/A LC and selectivity determinations of related enzymes BoNT/B LC and B. anthracis LF (BaLF) are shown in Table 1. Two benzimidazole acrylonitrile compounds were identified as hits, and the most potent and selective one, MSL-145815, was selected for further studies (See Figure 4 for the IC50 plot for MSL-145815).
Figure 3.
Structures of BoNT/A active screening hits.
Table 1.
BoNT/A Select Screening Hits IC50 Values
| IC50 (μM)* | ||||
|---|---|---|---|---|
| Compound MSL Number (structural type) | BoNT/A LC FRET | BoNT/A LC HPLC | BoNT/B LC FRET | BaLF FRET |
| 151862 (hydroxyquinoline) | 15 | 12 | >100 | >100 |
| 062516 (hydroxyquinoline) | 5.4 | 17 | 20 | 32 |
| 145005 (hydroxypyrazole) | 10 | 14 | 5.74 | >100 |
| 145815 (benzimidazole acrylonitrile) | 7.2 | 10 | >100 | >100 |
| 111029 (benzimidazole acrylonitrile) | 5.9 | 35 | >100 | 7.01 |
| 059336 (vinylbenzimidazole) | 3.1 | 59 | >100 | 59 |
| 111012 (arylamide) | 16 | 58 | >100 | >100 |
| 130541 (arylamide) | 10 | 27 | >100 | >100 |
| 059327 (vinylbenzothiazide) | 25 | 55 | 76 | 9.4 |
IC50 values represent averages of two separate assays.
Figure 4.
BoNT/A FRET-based IC50 plots for MSL-145815 (■).
Further Specificity Studies of MSL 145815
The benzimidazole acrylonitrile, MSL-145815, was characterized further for specificity of inhibition and for zinc chelation potential. MSL-145815 displayed considerable specificity, yielding IC50 values greater than 100 μM for BoNT/B LC, MMP-1, MMP-2, and MMP-9, while some inhibition of BaLF was noted with an IC50 of 74 μM (Table 2). Consistent with its specificity for BoNT/A, MSL-145815 was also not a zinc chelator, since its activity in the BoNT/A LC FRET assay was not dependent on prior incubation with Zn++ at concentrations up to 5 mM. However, the compound exhibited significant cytotoxicity (CC50 = 18.5 μM), yielding a selectivity index of ~2.6 (HeLa-CC50/BoNT/A-IC50).
Table 2.
Enzyme Specificity, Zinc Chelation and Cellular Cytotoxicity
| IC50 μM (FRET ASSAY)* | |||
|---|---|---|---|
| Enzyme | MSL-145815 | MBX 1553 | Hydroxamate |
| BoNT/A Lc | 7.2 | 26 | 7.33 |
| BoNT/B Lc | >100 | >100 | >100 |
| MMP-1 | >100 | >100 | >100 |
| MMP-2 | >100 | >100 | >100 |
| MMP-9 | >100 | >100 | >100 |
| Ba. Lethal Factor | 74 | >100 | >100 |
| Zinc chelation | No | No | No |
| HeLa cell cytotoxicity, CC50 (μM) | 19 | 3.6 | 4.4 |
IC50 values represent averages of two separate assays.
Given its potential to be a potent and specific inhibitor, MSL-145815 became the target of a preliminary SAR program. Roughly 50 compounds were synthesized (manuscript in preparation) and one, MBX 1553 (Figure 5), appeared to exhibit improved selectivity (Table 2). Like its parent compound, MBX 1553 inhibited BoNT/A, did not inhibit BoNT/B, MMP-1, MMP-2 nor MMP-9 or demonstrate zinc chelation (Table 2). Unlike its parent, MBX 1553 did not inhibit BaLF, and thus appeared to be a more specific BoNT/A inhibitor, albeit with a somewhat greater cytotoxicity to human cells (CC50 = 3.6 μM; Table 2).
Figure 5.
Structure of MBX 1553, resulting from an SAR study around screening hit, MSL-145815.
Chick Neuronal Cell SNAP-25 Cleavage Assay
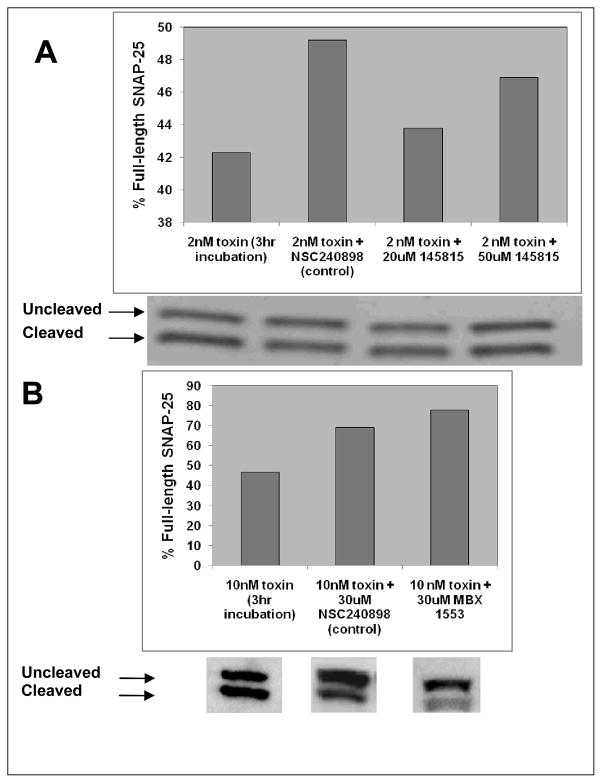
The benzimidazole BoNT/A LC inhibitor, MSL-145815 and the benzimidazole analog, MBX 1553, were evaluated for activity in cultured cells intoxicated with BoNT/A. In the chick neuronal cell assay, primary motor neurons were preincubated with test compounds and then exposed to BoNT/A. The ability of BoNT/A to cleave its substrate SNAP-25 was assessed using Western Blot analysis of cell extracts. MSL-145815 was tested at 20 and 50 μM (Figure 6A) and MBX 1553 was tested at a single concentration of 30 μM (Figure 6B). MSL-145815 exhibited little protection in the assay at the concentrations tested. However, the analog MBX 1553 inhibited BoNT/A intoxication of neuronal cells by approximately 58% at a concentration of 30 μM.
Figure 6.
Chick neuronal cell assay for BoNT/A inhibition. Dose response for screening compound, (A) MSL-145815 and single dose result for SAR compound, (B) MBX 1553.
Kinetic Analysis of BoNT/A LC Inhibition
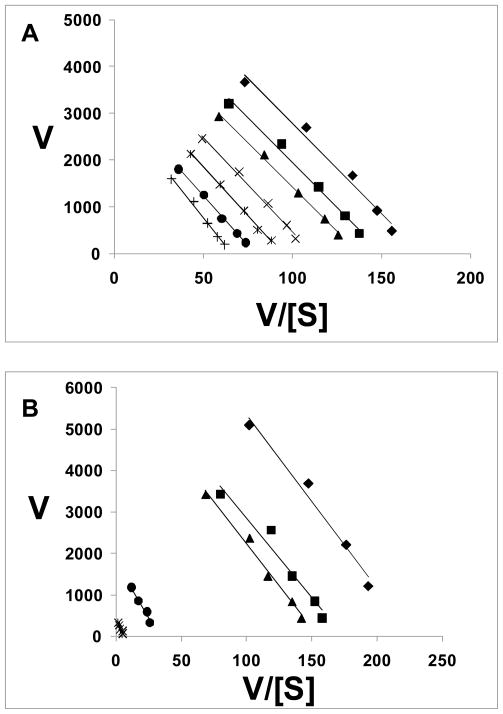
The kinetics of BoNT/A LC inhibition by the two benzimidazoles, MSL-145815 and MBX 1553, were examined in detail using Eadie-Hofstee transformations of the assay results (Atkins and Nimmo, 1975). MSL-145815 exhibited a noncompetitive mechanism of inhibition (Figure 7A), as evidenced by a decrease in Vmax (y intercept) and no change in Km (line slope) with increasing inhibitor concentrations, thus yielding parallel lines. MBX 1553 also appeared to act by a noncompetitive mechanism since the Eadie-Hofstee plots revealed a series of parallel lines, indicating no change in the Km values and decreasing Vmax values with increasing inhibitor concentrations (Figure 7B). The results observed for both compounds indicate that excess peptide substrate cannot compete out the effects of inhibitor. Analyses of the data using both Lineweaver-Burk and Hanes-Woolf plots yielded the same results (i.e. interpreted data indicated noncompetitive inhibition, data not shown).
Figure 7.
Inhibitor enzyme kinetics – linear transformation using Eadie-Hofstee plots. (A) Plot for benzimidazole acrylonitrile, MSL-145815: ◆, no inhibitor, ■, 6.25 μM; ▲, 12.5 μM; ×, 25 μM; *, 50 μM; ●, 75 μM; +, 100 μM. (B): Plot for benzimidazole acrylonitrile, MBX 1553: ◆, no inhibitor, ■, 6.25 μM; ▲, 12.5 μM; ●, 25 μM; *, 50 μM.
Discussion
In this report, we summarize the results of screening efforts to identify low molecular weight inhibitors of BoNT/A utilizing BoNT/A LC. The method employed was a 384 well automated high-throughput screen, followed by confirmation and characterization utilizing a 96 well format. We selected the most potent hits and subjected them to evaluation for specificity by testing them for their relative potency of inhibition of BoNT/B LC and BaLF. Several of these compounds exhibited specificity for BoNT/A LC and were examined for inhibition of a larger panel of metalloproteases.
In some cases the HPLC assay results were not in close agreement with the FRET assay results for the identical compound. There are several sources for this variation. First, there are higher concentrations of enzyme and substrate in the HPLC assay versus the FRET assay, which raises the stringency of the assay. Second, the detergent NP-40 is used in the HPLC assay versus Tween-20 in the FRET assay, which may affect the state of inhibitor aggregation, subsequently changing potency. Finally, although strong quenchers were dropped from the screen, some inherent mild quenching activity for true hits may also be a source of variation.
We focused on the benzimidazole acrylonitrile scaffold for further characterization, in particular compounds MSL-145815 and MBX 1553, due to their novelty and a large body of synthetic chemistry knowledge of this particular class of compounds. Neither MSL-145815 nor MBX 1553 were found to be zinc chelators. Kinetic studies indicate that mechanism of inhibition is of a noncompetitive nature. Shin et al. described a (Z)-3-(2,5-dimethoxyphenyl)-2-(4-methoxyphenyl) acrylonitrile that was a potent inhibitor of Sortase A (SrtA) and Sortase B (SrtB) which are cysteine proteases (Oh et al., 2006). The facts that SrtA and SrtB are not zinc containing metalloproteases and that benzimidazole acrylonitrile BoNT/A LC inhibition potencies are not neutralized by prior incubation in high concentrations of zinc are consistent with an inhibition mechanism for this class of compounds that does not involve coordination/interaction with the active site zinc.
We further characterized the specificity of MSL-145815 and MBX 1553 by testing the compounds for potency in a metalloprotease assay panel consisting of BoNT/B LC, MMP-1, MMP-2, MMP-9, and BaLF. Intriguingly, although it appears to act by a noncompetitive mechanism, MSL-145815 had notable specificity for BoNT/A LC, exhibiting a detectable but low level of inhibition of BaLF. MBX 1553 displayed specificity for BoNT/A, with no inhibition of the other metalloproteases examined. This remarkable specificity is consistent with the fact that, among this set of proteases, only BoNT/B LC has any detectable degree of similarity to BoNT/A LC, with an amino acid sequence similarity of 44% (BLAST Alignment). Moreover, these results are reassuring, given the noncompetitive nature of the mechanism of action for MSL-145815 and, thus, the likelihood that the compound is binding to BoNT/A LC somewhere other than the active site. The greater HeLa cell toxicity observed in Table 2 (CC50 = 3.6 μM vs. 19 μM for the parent MSL-145815) highlights a risk for further development of this chemotype, but the tolerance of the compound observed in the shorter duration chick neuronal cell assay indicates that its inhibitory activity in that assay is significant. An accurate understanding of the actual toxicity of the compound must await the completion of appropriate animal toxicity studies.
In brief, we have identified several types of small molecule BoNT/A LC inhibitors utilizing high throughput screening. Although MSL-145815, a benzimidazole acrylonitrile, exhibited only modest potency in the chick neuronal cell assay, it was a BoNT/A-specific inhibitor. Chemical modification of this inhibitor scaffold yielded MBX 1553, a benzimidazole acrylonitrile that not only exhibited improved enzyme specificity over that of its parent compound, but also demonstrated better potency in the chick neuronal cell assay. However, the results cannot clearly distinguish between the possible mechanisms of inhibition in the cellular assay, i.e., inhibition of internalized BoNT/A LC, inhibition of cell entry, or pre-cell entry inactivation of the toxin. This family of compounds will be pursued further to improve in vitro potency and specificity and to demonstrate activity in an in vivo model of BoNT/A intoxication.
In summary, we describe a novel benzimidazole scaffold that demonstrates notable specificity and a noncompetitive mechanism of action, both desirable characteristics when designing drugs to inactivate toxins. With only limited medicinal chemistry performed thus far on this scaffold, we have demonstrated this scaffold can be modified to further enhance its potential as a BoNT/A therapeutic.
Acknowledgments
This work was supported by NIAID in the form of Grant #5U01 AI070430 awarded to TLB. The authors wish to acknowledge Donald T. Moir for extensive editorial assistance.
Biographies
Steven Cardinale is currently a Research Scientist at Microbiotix Incorporated. At Microbiotix, he is participating in the small molecule inhibitor discovery efforts for BoNT Light Chain A and B and supporting in house Medicinal Chemistry efforts for inhibitor characterization. Steven’s areas of interest include metalloproteases and enzymes involved in RNA and DNA synthesis.
Michelle M. Butler received her Ph.D. in Pharmacology at the University of Massachusetts Medical School. She currently serves as a Senior Research Scientist at Microbiotix. Her group’s research efforts focus on the development of therapeutic products to counter the effects of bacterial toxins as well as to treat antibiotic-resistant bacterial infections.
Gordon Ruthel received his Ph.D. in Neuroscience at the University of Virginia. He currently works as a consultant contracted by Akimeka Technologies to the U.S. Army Medical Research Institute of Infectious Diseases.
Jonathan E. Nuss received his PhD in Biochemistry from Wright State University School of Medicine in Dayton, Ohio. He currently works at the US Army Medical Research Institute for Infectious Diseases where he works on the development of small molecules to counteract botulinum neurotoxins and the development of broad-spectrum anti-viral compounds.
Laura M. Wanner Laura M. Wanner received her BA in Biology at Hood College in Frederick, Maryland. She currently serves as a research associate at the U.S. Army Medical Research Institute for Infectious Diseases working on botulinum neurotoxin countermeasures.
Bing Li received his Ph.D. in Organic Chemistry at The University of Hong Kong. He currently serves as a Senior Research Scientist at Microbiotix. His research interest is focused on discovery and development of antiviral and antibacterial agents as well as development of small molecule inhibitors of botulinum neurotoxins.
Ramdas P. Pai currently works at Microbiotix Inc. as a synthetic medicinal chemist where he works on the design and synthesis of inhibitors of botulinum neurotoxin A protease inhibitors.
Norton P. Peet received his PhD degree in Organic Chemistry at the University of Nebraska at Lincoln. He currently is the Director of Chemistry at Microbiotix, Inc. The Chemistry Department at Microbiotix is designing new antiviral and antibacterial agents using structure-based drug design techniques with those projects where structural information is available.
Sina Bavari received his Ph.D. in Pharmaceutical Sciences and Immunotoxicology from the University of Nebraska Medical Center, College of Pharmacy. He is currently the Chief of Immunology, Target Identification, and Translational Research in the Bacteriology Division of the U.S. Army Medical Research Institute of Infectious Diseases where he oversees BSL 3 and 4 bacterial and viral projects. In addition, Dr. Bavari is a leading expert in bacterial toxins.
Terry L. Bowlin received his Ph.D. in Immunology from Cleveland State University. He currently is the CEO at Microbiotix, Inc., where he oversees projects covering the biology and chemistry of antibacterial drug development, antiviral drug development, modulators of bacterial and viral virulence and inhibitors of bacterial toxins. He has published 85 peer reviewed papers and book chapters and has 14 awarded patents to date.
Contributor Information
Steven C. Cardinale, Email: scardinale@microbiotix.com, Microbiotix, Inc., One Innovation Drive, Worcester, MA 01605, USA, Fax: 508-757-1999
Michelle M. Butler, Email: mbutler@microbiotix.com, Microbiotix, Inc., One Innovation Drive, Worcester, MA 01605, USA
Gordon Ruthel, Email: gordon.ruthel1@us.army.mil, US Army Medical Research Institute of Infectious Diseases, 1425 Porter St., Fort Detrick, MD 21702, USA.
Jonathan E. Nuss, Email: Jon.Nuss@AMEDD.ARMY.MIL, US Army Medical Research Institute of Infectious Diseases, 1425 Porter St., Fort Detrick, MD 21702, USA
Laura M. Wanner, Email: laura.wanner@us.army.mil, US Army Medical Research Institute of Infectious Diseases, 1425 Porter St., Fort Detrick, MD 21702, USA
Bing Li, Email: bli@microbiotix.com, Microbiotix, Inc., One Innovation Drive, Worcester, MA 01605, USA.
Ramdas Pai, Email: rpai@microbiotix.com, Microbiotix, Inc., One Innovation Drive, Worcester, MA 01605, USA.
Norton P. Peet, Email: npeet@microbiotix.com, Microbiotix, Inc., One Innovation Drive, Worcester, MA 01605, USA
Sina Bavari, Email: sina.bavari@us.army.mil, US Army Medical Research Institute of Infectious Diseases, 1425 Porter St., Fort Detrick, MD 21702, USA.
Terry L. Bowlin, Email: tbowlin@microbiotix.com, Microbiotix, Inc., One Innovation Drive, Worcester, MA 01605, USA
References
- Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Hauer J, Layton M, Lillibridge S, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Swerdlow DL, Tonat K. Botulinum toxin as a biological weapon: medical and public health management. Jama. 2001;285:1059–70. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- Atkins GL, I, Nimmo A. A comparison of seven methods for fitting the Michaelis-Menten equation. Biochem J. 1975;149:775–7. doi: 10.1042/bj1490775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett JC, Henchal EA, Schmaljohn AL, Bavari S. The evolving field of biodefence: therapeutic developments and diagnostics. Nat Rev Drug Discov. 2005;4:281–97. doi: 10.1038/nrd1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett JC, Ruthel G, Stegmann CM, Panchal RG, Nguyen TL, Hermone AR, Stafford RG, Lane DJ, Kenny TA, McGrath CF, Wipf P, Stahl AM, Schmidt JJ, Gussio R, Brunger AT, Bavari S. Inhibition of metalloprotease botulinum serotype A from a pseudo-peptide binding mode to a small molecule that is active in primary neurons. J Biol Chem. 2007;282:5004–14. doi: 10.1074/jbc.M608166200. [DOI] [PubMed] [Google Scholar]
- Burnett JC, Schmidt JJ, Stafford RG, Panchal RG, Nguyen TL, Hermone AR, Vennerstrom JL, McGrath CF, Lane DJ, Sausville EA, Zaharevitz DW, Gussio R, Bavari S. Novel small molecule inhibitors of botulinum neurotoxin A metalloprotease activity. Biochem Biophys Res Commun. 2003;310:84–93. doi: 10.1016/j.bbrc.2003.08.112. [DOI] [PubMed] [Google Scholar]
- CDC. Botulism. 2009 http://www.cdc.gov/nczved/dfbmd/disease_listing/botulism_gi.html.
- Charles PD. Botulinum neurotoxin serotype A: a clinical update on non-cosmetic uses. Am J Health Syst Pharm. 2004;61:S11–23. doi: 10.1093/ajhp/61.suppl_6.S11. [DOI] [PubMed] [Google Scholar]
- Clarke SC. Bacteria as potential tools in bioterrorism, with an emphasis on bacterial toxins. Br J Biomed Sci. 2005;62:40–6. doi: 10.1080/09674845.2005.11732685. [DOI] [PubMed] [Google Scholar]
- Dembek ZF, Smith LA, Rusnak JM. Botulism: cause, effects, diagnosis, clinical and laboratory identification, and treatment modalities. Disaster Med Public Health Prep. 2007;1:122–34. doi: 10.1097/DMP.0b013e318158c5fd. [DOI] [PubMed] [Google Scholar]
- Eleopra R, Tugnoli V, Rossetto O, De Grandis D, Montecucco C. Different time courses of recovery after poisoning with botulinum neurotoxin serotypes A and E in humans. Neurosci Lett. 1998;256:135–8. doi: 10.1016/s0304-3940(98)00775-7. [DOI] [PubMed] [Google Scholar]
- Eubanks LM, Hixon MS, Jin W, Hong S, Clancy CM, Tepp WH, Baldwin MR, Malizio CJ, Goodnough MC, Barbieri JT, Johnson EA, Boger DL, Dickerson TJ, Janda KD. An in vitro and in vivo disconnect uncovered through high-throughput identification of botulinum neurotoxin A antagonists. Proc Natl Acad Sci U S A. 2007;104:2602–7. doi: 10.1073/pnas.0611213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foran PG, Mohammed N, Lisk GO, Nagwaney S, Lawrence GW, Johnson E, Smith L, Aoki KR, Dolly JO. Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J Biol Chem. 2003;278:1363–71. doi: 10.1074/jbc.M209821200. [DOI] [PubMed] [Google Scholar]
- Hicks RP, Hartell MG, Nichols DA, Bhattacharjee AK, van Hamont JE, Skillman DR. The medicinal chemistry of botulinum, ricin and anthrax toxins. Curr Med Chem. 2005;12:667–90. doi: 10.2174/0929867053202223. [DOI] [PubMed] [Google Scholar]
- Josko D. Botulin toxin: a weapon in terrorism. Clin Lab Sci. 2004;17:30–4. [PubMed] [Google Scholar]
- Kadkhodayan S, Knapp MS, Schmidt JJ, Fabes SE, Rupp B, Balhorn R. Cloning, expression, and one-step purification of the minimal essential domain of the light chain of botulinum neurotoxin type A. Protein Expr Purif. 2000;19:125–30. doi: 10.1006/prep.2000.1227. [DOI] [PubMed] [Google Scholar]
- Keller JE, Neale EA, Oyler G, Adler M. Persistence of botulinum neurotoxin action in cultured spinal cord cells. FEBS Lett. 1999;456:137–42. doi: 10.1016/s0014-5793(99)00948-5. [DOI] [PubMed] [Google Scholar]
- Kuhn TB. Growing and working with spinal motor neurons. Methods Cell Biol. 2003;71:67–87. doi: 10.1016/s0091-679x(03)01005-7. [DOI] [PubMed] [Google Scholar]
- Marshall NJ, Goodwin CJ, Holt SJ. A critical assessment of the use of microculture tetrazolium assays to measure cell growth and function. Growth Regul. 1995;5:69–84. [PubMed] [Google Scholar]
- Meunier FA, Lisk G, Sesardic D, Dolly JO. Dynamics of motor nerve terminal remodeling unveiled using SNARE-cleaving botulinum toxins: the extent and duration are dictated by the sites of SNAP-25 truncation. Mol Cell Neurosci. 2003;22:454–66. doi: 10.1016/s1044-7431(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Oh KB, Oh MN, Kim JG, Shin DS, Shin J. Inhibition of sortase-mediated Staphylococcus aureus adhesion to fibronectin via fibronectin-binding protein by sortase inhibitors. Appl Microbiol Biotechnol. 2006;70:102–6. doi: 10.1007/s00253-005-0040-8. [DOI] [PubMed] [Google Scholar]
- Paddle BM. Therapy and prophylaxis of inhaled biological toxins. J Appl Toxicol. 2003;23:139–70. doi: 10.1002/jat.903. [DOI] [PubMed] [Google Scholar]
- Panchal RG, Hermone AR, Nguyen TL, Wong TY, Schwarzenbacher R, Schmidt J, Lane D, McGrath C, Turk BE, Burnett J, Aman MJ, Little S, Sausville EA, Zaharevitz DW, Cantley LC, Liddington RC, Gussio R, Bavari S. Identification of small molecule inhibitors of anthrax lethal factor. Nat Struct Mol Biol. 2004;11:67–72. doi: 10.1038/nsmb711. [DOI] [PubMed] [Google Scholar]
- Rasetti-Escargueil C, Jones RG, Liu Y, Sesardic D. Measurement of botulinum types A, B and E neurotoxicity using the phrenic nerve-hemidiaphragm: improved precision with in-bred mice. Toxicon. 2009;53:503–11. doi: 10.1016/j.toxicon.2009.01.019. [DOI] [PubMed] [Google Scholar]
- Schmidt JJ, Bostian KA. Proteolysis of synthetic peptides by type A botulinum neurotoxin. J Protein Chem. 1995;14:703–8. doi: 10.1007/BF01886909. [DOI] [PubMed] [Google Scholar]
- Schmidt JJ, Stafford RG. Fluorigenic substrates for the protease activities of botulinum neurotoxins, serotypes A, B, and F. Appl Environ Microbiol. 2003;69:297–303. doi: 10.1128/AEM.69.1.297-303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvaggi NR, Boldt GE, Hixon MS, Kennedy JP, Tzipori S, Janda KD, Allen KN. Structures of Clostridium botulinum Neurotoxin Serotype A Light Chain complexed with small-molecule inhibitors highlight active-site flexibility. Chem Biol. 2007;14:533–42. doi: 10.1016/j.chembiol.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Silvaggi NR, Wilson D, Tzipori S, Allen KN. Catalytic features of the botulinum neurotoxin A light chain revealed by high resolution structure of an inhibitory peptide complex. Biochemistry. 2008;47:5736–45. doi: 10.1021/bi8001067. [DOI] [PubMed] [Google Scholar]
- Simpson LL. Identification of the major steps in botulinum toxin action. Annu Rev Pharmacol Toxicol. 2004;44:167–93. doi: 10.1146/annurev.pharmtox.44.101802.121554. [DOI] [PubMed] [Google Scholar]
- Willis B, Eubanks LM, Dickerson TJ, Janda KD. The strange case of the botulinum neurotoxin: using chemistry and biology to modulate the most deadly poison. Angew Chem Int Ed Engl. 2008;47:8360–79. doi: 10.1002/anie.200705531. [DOI] [PubMed] [Google Scholar]
- Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]