ABSTRACT
Remodeling of the cervix is a critical early component of parturition and resembles an inflammatory process. Infiltration and activation of myeloid immune cells along with production of proinflammatory mediators and proteolytic enzymes are hypothesized to regulate cervical remodeling as pregnancy nears term. The present study standardized an approach to assess resident populations of immune cells and phenotypic markers of functional activities related to the mechanism of extracellular matrix degradation in the cervix in preparation for birth. Analysis of cells from the dispersed cervix of mice that were nonpregnant or pregnant (Days 15 and 18 postbreeding) by multicolor flow cytometry indicated increased total cell numbers with pregnancy as well as increased numbers of macrophages, the predominant myeloid cell, by Day 18, the day before birth. The number of activated macrophages involved in matrix metalloproteinase induction (CD147) and signaling for matrix adhesion (CD169) significantly increased by the day before birth. Expression of the adhesion markers CD54 and CD11b by macrophages decreased in the cervix by Day 18 versus that on Day 15 or in nonpregnant mice. The census of cells that expressed the migration marker CD62L was unaffected by pregnancy. The data suggest that remodeling of the cervix at term in mice is associated with recruitment and selective activation of macrophages that promote extracellular matrix degradation. Indices of immigration and activities by macrophages may thus serve as markers for local immune cell activity that is critical for ripening of the cervix in the final common mechanism for parturition at term.
Keywords: cervical remodeling, extracellular matrix, flow cytometry, immunology, inflammation, macrophage, parturition
Remodeling of the preterm cervix involves hyperplasia of cells, as well as increased presence and activities of macrophages that promote inflammation and degradation of the extracellular matrix.
INTRODUCTION
The cervix is the gatekeeper for mammalian parturition. As pregnancy nears term, the cervix virtually disappears into a lower uterine segment as part of the birth canal continuum between the uterus and vagina [1, 2]. The process of softening and remodeling begins well before labor, reflecting a transition in cervical structure from high tensile strength and rigidity to a compliant and disorganized assembly of collagen fiber during the prepartum period [3, 4]. The mechanism for remodeling and its accelerated phase near term, called ripening, resembles an inflammatory process [5–7]. Characteristic changes in the extracellular matrix of the cervix involve increased vascular permeability [8], immigration of immune cells [9], actions by proinflammatory mediators [5, 10], and degradation of collagen [11] and could represent loss of progestational hormone efficacy [12, 13]. Many of these processes could depend on the recruitment and activity of certain myeloid immune cells due to their capabilities to produce proinflammatory factors and proteolytic enzymes that degrade the extracellular matrix.
Several approaches have been taken to study the immigration and activation of leukocytes in the prepartum cervix. A greater presence of immune cells was observed in early studies of cervical morphology at term [14]. Standard bright-field microscopy has since provided evidence for a significant increase in the number of macrophages (Mφ) and neutrophils (Neu) in the cervix by the day before birth in various strains and several species of rodents [6, 15]. The census of Mφ is also reported to increase in biopsy specimens of cervix from peripartum women in labor at term compared to specimens from those not in labor [16–20]. Other reports using hemocytometry [15] or flow cytometry [21] have found no change in populations of Mφ or Neu in the cervix with pregnancy or before term; rather, monocytes were indicated to increase. These results are not consistent with previous biochemical and histological findings or expectations that immigrating myeloid cells differentiate into Mφ and enhance proinflammatory activities in the cervix near term.
To address methodological concerns about these reports, the approach of the present study was to standardize tissue dispersion methods and establish a rigorous, multiparameter gating sequence using flow cytometry to enumerate specific living leukocytes and functional activities of particular myeloid cell subsets in the cervix of mice before birth. The objective was to test the hypothesis that phenotypic markers of activities by specific populations of myeloid cells are enhanced in the cervix as pregnancy nears term. To accurately enumerate resident leukocytes in the cervix by flow cytometry, a standardized approach was needed to perfuse and disperse the cervix and eliminate systemic immune cells as well as to set gates based on critical controls (isotype, spleen, live cell). The findings indicate that the mechanism for remodeling the preterm cervix involves hyperplasia of the cervix as well as increases in Mφ that express proteins involved in adhesion and degradation of the extracellular matrix.
MATERIALS AND METHODS
General Procedures
Adult female nonpregnant (NP) and timed pregnant C3H/HeN mice were purchased from Harlan, Inc. Mice were housed in a light- and temperature-controlled room with access to food and water (photoperiod, 12L:12D; lights-on, 0700 h PST). NP mice were studied during estrus, a period of elevated levels of gonadal steroids in circulation, as determined by daily vaginal smears. Experimental procedures were approved by the Institutional Animal Care and Use Committee and conformed to the National Research Council standards for care and use of laboratory animals. Before 1000 h of the day of study, mice were deeply anesthetized with Nembutal (pentobarbital sodium injection, USP, Lundbeck Inc.) and euthanized by cervical dislocation. To confirm the hypothesis that cells in circulation do not affect retrieval of immune cells from the cervix [18], an initial study was conducted in which three mice on Postbreeding Day (D) 15 and D18 were euthanized by cervical dislocation and not perfused. For all other mice, the spleen was clamped with a hemostat and warm saline perfused through the heart to flush systemic blood from other tissues. The spleen and cervix were excised, trimmed of adherent tissues (fat as well as most of the adjacent uterus and vaginal folds), and then processed to disperse cells as described below.
Harvest and Staining of Cells from Spleen and Cervix for Flow Cytometry
Dispersed splenocytes were used to guide gate settings for flow cytometry of cells from dispersed cervix for each individual. Accordingly, the spleen was washed in PBS for 5 min at 4°C, pressed without mincing, then pressed through a 70-μm filter (Millipore) and rinsed with 500 μl of PBS. After centrifugation (1500 rpm for 5 min at 4°C), the pellet of splenocytes was resuspended in 1 ml of PBS for use as controls to identify labeled immune cells by flow cytometry. The cervix was washed in fresh PBS for 5 min at 4°C, minced, and incubated for 1.5 h at 37°C in 25 mM Hepes (10 ml) and Hanks balanced salt solution (Sigma) with collagenase B (1 mg/ml; Roche). The suspension was periodically agitated during the incubation, then passed through a 70-μm filter, washed in PBS for 5 min at 4°C, centrifuged (1500 rpm for 5 min at 4°C) to obtain a cell pellet, and resuspended in PBS for flow cytometry. Some spleens were cut in half, and one part was treated as described above or minced and mesh-filtered to verify that cell dispersal techniques did not affect counting of immune cells.
In preparation for flow cytometry, immune cell numbers were estimated in an aliquot from dispersed spleen and cervix with a hemocytometer, and the cell suspension from spleens was diluted to approximately 2 × 105 cells per 10 μl. An aliquot of cells was transferred into a 96-well polypropylene plate and incubated with combinations of fluorescent-conjugated antibodies for 30 min in the dark at 4°C. Antigen-specific fluorescent-labeled antibodies were used to identify immune cells that express markers of specific functional activity (Table 1). Plates were centrifuged (1500 rpm for 5 min at 4°C), and cells were washed with PBS for 5 min at 4°C, resuspended in 0.2 ml of PBS, then incubated with 2 μl of 7-aminoactinomycin D dye (7-AAD; eBioscience) to identify living cells. Plates were centrifuged (1500 rpm for 5 min at 4°C) after 5 min, washed in PBS for 5 min at 4°C, fixed in 1% paraformaldehyde, and passed through a 50-μm filter into a fluorescence-activated cell sorting tube. Samples were processed by flow cytometry within 24 h using a MACSQuant Analyzer (Miltenyi Biotec), with data analysis as described below. Spleen and cervical samples were processed to establish instrument and software settings for fluorescence compensation (FlowJo 7.6; Treestar). Spleen cells were incubated with the same fluorescent-labeled antibody combinations and concentration as those used for cervical tissue. Thus, compared to the approach used in a previous study of dispersed murine cervix [18], that used in the present study to analyze cells from the dispersed spleen from each mouse served as an added control to establish gates for immune cell subsets and to verify that the dispersal procedure did not adversely impact labeling of immune cells.
TABLE 1.
Antibody reagents for immune cells and markers of cellular activation.
APC, allophycocyanin; FITC, fluorescein isothiocyanate; PE, phycoerythrin.
Processing and Analyses of Flow Cytometry Data
A standard protocol was developed to analyze data acquired by flow cytometry to ensure specific, replicable, and accurate identification of immune cells and cell counts. The gating strategy for flow cytometry is summarized here, and more details are provided in Results. After isotype antibody-matched control adjustments, gates around labeled immune cells were sequentially set to include 1) total cells excluding debris (forward- and side-scatter), 2) CD45+ myeloid cells (forward- and side-scatter back-gated on CD45+ splenocytes), 3) living myeloid cell gate (7-AAD−), 4) leukocytes (CD45+ and side-scatter), 5) Mφ (F4/80+GR-1−) or Neu (Neu 7/4+GR-1+), and 6) Mφ activation markers (F4/80+ combined with CD54, CD147, CD169, CD11b, or CD62L). CD45 is specific for leukocytes. Because CD45+ cells are gated before evaluating other markers, analyses exclude nonimmune cells. The upregulation of CD11b in activated Mφ produces a distinct CD11bhi population that was quantitated as a percent of total F4/80+ Mφ. Median fluorescence intensity was used to evaluate increases in levels of the activation markers CD54, CD147, CD169 and CD62L.
This gating strategy was tested by incubating aliquots of dispersed spleen and cervix from NP mice in a combination of antibodies to identify living Mφ (CD45+7AAD−F4/80+), which were then processed immediately or fixed with 1% paraformaldehyde for 4 or 24 h before being passed through the flow cytometer (n = 3 per group per time). Using the gating strategy described above, the numbers of Mφ from spleen or cervical samples processed within 2 h of dispersal were equivalent to those in samples analyzed after 4 or 24 h in fixative. Thus, samples processed at multiple times relative to fixation accurately replicate the number of resident Mφ in spleen and cervix.
Statistical Analyses
Data were evaluated with the Levene test, and if normally distributed, ANOVA was performed. Individual comparisons were made with the Tukey test, unpaired independent t-test, or Mann-Whitney test (all two-tailed). A value of P < 0.05 was considered to be significant. If the Levene test was significant, data were log transformed to normalize variance and reanalyzed by ANOVA. Power analysis indicated P > 0.9 for this data set. Statistical analyses were performed using GraphPad Prism (v4; GraphPad Software).
RESULTS
Census of Immune Cells in the Cervix
We first studied how dispersal of cells from tissue and flow cytometry sample preparation affected the accuracy of the census of resident immune cells in the cervix. Cell counts as estimated by flow cytometry were compared to determinations made with a hemocytometer. The average number of immune cells from dispersed spleen and cervix from NP mice (n = 10) varied 4% and 9%, respectively, by flow cytometry, as compared to 12% and 44%, respectively, using the hemocytometer. Thus, flow cytometry provides a consistent and more accurate estimate of cell count in both spleen and cervix.
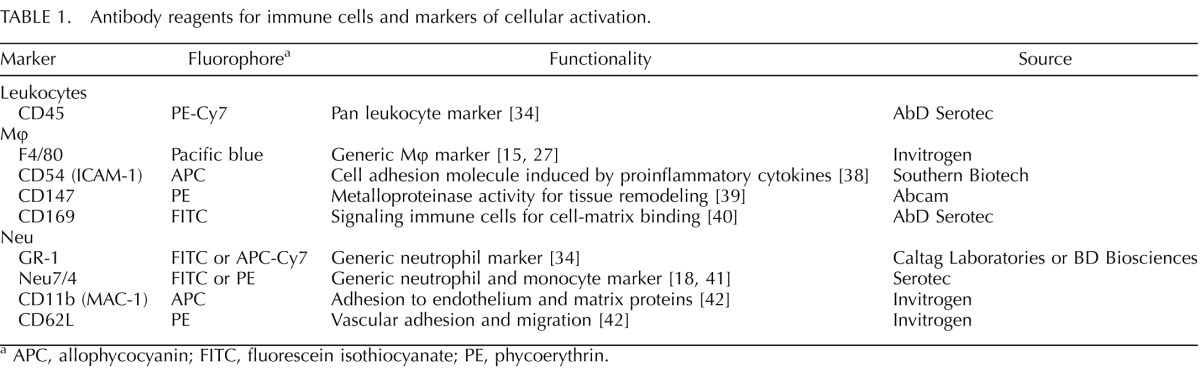
Given that the near-term cervix is enlarged and more vascularized compared with earlier in pregnancy or in NP mice [22, 23], we addressed the question of whether cells from the circulation affect enumeration of immune cells in the cervix [21]. The cervix from nonperfused and saline-perfused mice, both NP and prepartum on D18, were dispersed and processed by flow cytometry (Fig. 1). A population of living monocytes was found with the same immune-phenotype (CD45+7AAD−Neu7/4+GR-1+) and in the same proportions as reported by Timmons et al. [21]. However, this cell population was sparse and markedly diminished in cervices from perfused versus nonperfused NP and D18 pregnant mice (Fig. 1, A–C). These findings indicate that monocytes represent only a minor proportion of resident myeloid cells and that perfusion is necessary to accurately enumerate immune cells that actually reside outside of blood vessels in the cervix.
FIG. 1.
Preparation of tissue for flow cytometry of resident immune cells. Systemic perfusion reduces monocytes from preparations of dispersed cervix. A) Flow cytometry of living monocytes (7AAD−CD45+Neu7/4+ GR-1−) in dispersed cervix from NP mice that were perfused (green dots) or not perfused (red dots). B and C) The percentage of monocytes per total live cells in the dispersed cervix from individual mice that were NP or pregnant (D18). Data in A are enumerated in B for the same pair of NP individuals (perfused and not perfused). The NP and D18 mice were simultaneously processed on the same day and replicated in an additional pairing of mice. D) Study design to evaluate effects of tissue processing on Mφ viability. Samples I and II compared collagenase versus mesh dispersal of spleen. Sample III was seeded with splenocytes with a known number of Mφ, and sample IV assessed the effect of collagenase dispersal on census of living Mφ in the cervix from an NP mouse.
Finally, to determine whether collagenase dispersion of tissue affected the accuracy of immune cell counts, spleens from NP mice were halved and either minced and incubated with collagenase or minced, then pressed through a mesh-screen filter (n = 2 each) (see samples I and II, respectively, in Fig. 1D). Flow cytometry indicated that the number and proportion of living Mφ were not diminished by collagenase dispersal of either spleen or cervix. Rather, more Mφ were found in samples treated with collagenase than in those treated with minced/mesh-filtered dispersal alone. Moreover, the number and proportion of CD45+ leukocytes in spleen following collagenase dispersal—that is, living Mφ that express activation markers (CD54, CD147, CD163, CD169, or CD11b)—varied on average less than 12% in spleens subjected to mechanical versus collagenase dispersion. In addition, a 10-μl aliquot of mesh-filtered splenocytes was added to approximately half of a cervix obtained from a perfused mouse. The mixture as well as the remaining half of the cervix that had only PBS added were subjected to the same collagenase dispersal procedure and incubated with a mixture of antibodies as described above (n = 2 each) (see samples III and IV, respectively, in Fig. 1D). An average of greater than 85% of splenocytes that were added to the cervix before collagenase dispersal were accounted for when compared to the cervical samples lacking splenocytes. Collectively, these findings indicate that collagenase dispersal did not affect viability, retrieval, or activation status of Mφ from various tissues.
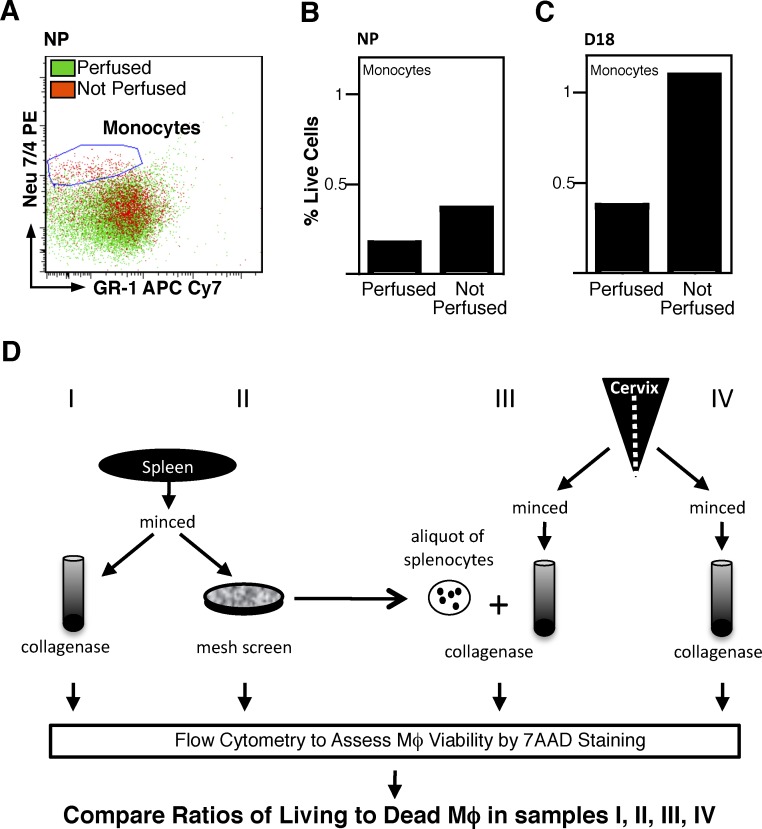
Gating Sequence to Assess Census of Immune Cells in Cervix with Pregnancy
To enumerate living resident myeloid cells, a series of flow cytometry gates were set based on morphological and immunofluorescence characteristics of cells in flow runs from dispersed spleen. Based on forward- and side-scatter morphology (Fig. 2, AI and BI), the gates were set to exclude debris. The clear conservative gate setting for CD45+ leukocytes in dispersed splenocytes was applied to analyze flow runs of dispersed cervical cells in the same mouse (Fig. 2, NP example in AII and BII). Subsequent gatings for living leukocytes that were Mφ (CD45+7AAD−F4/80+GR-1−) or Neu (CD45+7AAD−GR-1+) were determined from analysis of dispersed spleen and applied to dispersed cervical cells (Fig. 2, AIII–AV and BIII–BV). This gating strategy was applied to enumerate resident immune cells in the perfused cervix from pregnant mice on D15 or D18 (Fig. 2, CIV–CV and DIV–DV, respectively). The shift of living cells to the right into the F4/80+ gate indicates that the proportion of Mφ had increased in the D18 mouse (i.e., by the day before birth) as compared to that in the cervix on D15 of pregnancy.
FIG. 2.
Flow cytometry gating strategy to identify leukocyte populations within spleen and cervix. Dispersed cells were stained to detect living myeloid cells based on coexpression of CD45+, F4/80+, and GR-1 and gated as follows: light-scatter for intact cells (I), CD45+ leukocyte gate (II), 7AAD– gate to identify living cells (III), F4/80+ gate to identify myeloid cells (IV), and GR-1– to eliminate granulocytes and identify Mφ (V). The gates established in A were applied to dispersed cervical cells (B) to identify resident Mφ in cervix from each NP mouse. This same approach was used to establish gates to identify Mφ in the spleen from D15 and D18 mice (data not shown) and applied to identify resident Mφ in dispersed cells from the cervix of D15 mice (C) and D18 mice (D).
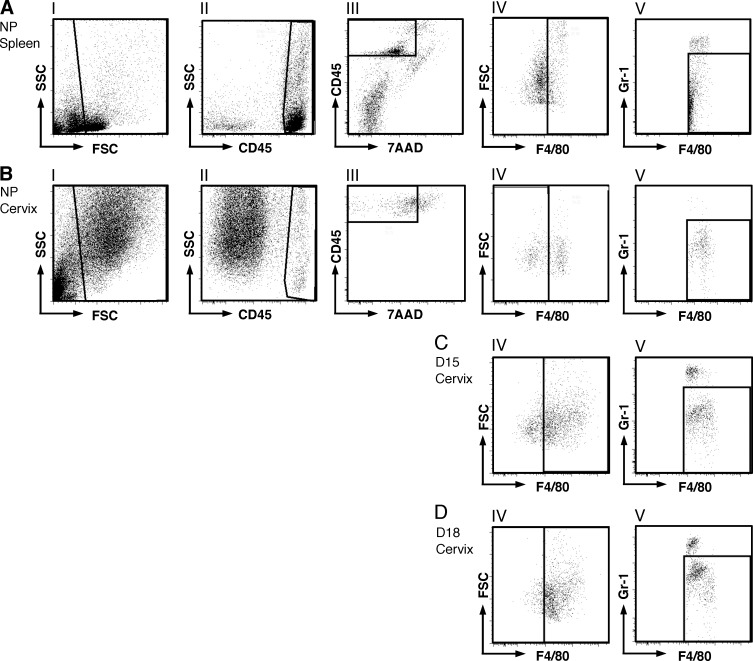
Using this systematic gating strategy, the numbers of living cells (total immune cells and subtypes) in the cervix were compared in pregnant and NP mice. Although the vast majority of cells within the cervix are nonimmune cells that include smooth muscle and fibroblast cells, immune cells represent approximately 10% of living cells (Fig. 2, BII–BIII). Compared to NP mice in estrus, the number of live cells per cervix was more than 2-fold greater by D15 (Fig. 3A). No further changes in living cell numbers were found by the day before birth (i.e., D18). Thus, the increase in total cell numbers indicates that in addition to the hypertrophy that has previously been reported [22, 23], hyperplasia of the cervix is present by D15 and maintained through D18 of pregnancy.
FIG. 3.
Mφ increase in the cervix before birth. The number of total living cells (7AAD–; A), leukocytes (CD45+; B), and Mφ (CD45+7AAD–F4/80+GR-1–; C) in the cervix as identified by flow cytometry and gated as described in Figure 2 is shown. Statistical significance (P < 0.05) for D18 (n = 5) versus groups of mice that were aNP (n = 6) or bD15 (n = 5) is also shown. D18 is pregnant mice on Day 18 postbreeding (n = 5). D) Representative plots of gated living Mφ and Neu in dispersed cervices from NP as well as D15 and D18 mice. E) Mφ stained dark brown by immunohistochemistry (arrows) and counterstained with hematoxylin in sections of cervix from NP and pregnant mice. Bar = 25 μm.
Analysis of living leukocytes (CD45+7-AAD−, exclusive of nonimmune cells, which lack CD45 expression) indicated that cell numbers were unchanged in D15 mice compared to that in NP controls. However, in cervices from D18 mice, leukocytes significantly increased more than 2-fold as compared to NP and D15 mice (Fig. 3B). Due to the 2-fold increase in total cervical cells, leukocytes as a percentage of total living cells in the cervix were reduced in the D15 versus NP and D18 groups (9 ± 2.7 vs. 15 ± 1.6 and 17 ± 1.8, respectively). Evaluation of leukocyte subsets indicated that the census of Mφ was significantly greater in the cervix from D18 versus NP and D15 groups (Fig. 3, C and D). Mφ as a percentage of leukocytes in the cervix were unchanged in D15 mice compare to the NP group but were significantly increased by D18 (69 ± 3.2 and 65 ± 7.8 vs. 89 ± 4.4, respectively). These results corroborate previous in situ findings [18, 19] (see examples in Fig. 3E) that indicate Mφ are increased in the prepartum cervix of mice by D18. The variability of living Neu in the cervix from D18 mice precluded significant differences.
Activation of Myeloid Cells in Cervix with Pregnancy
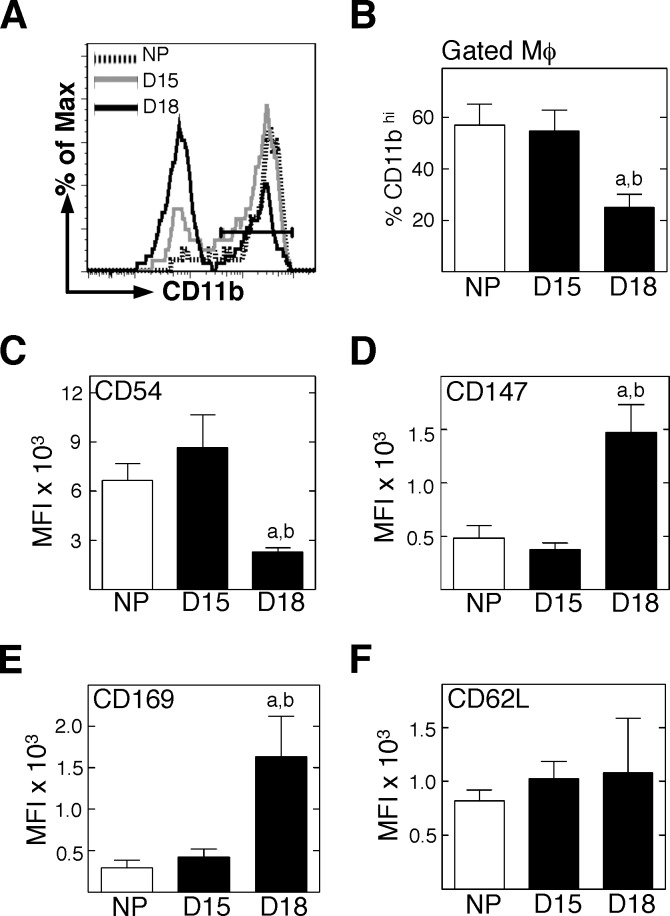
To evaluate the potential roles of immune cells as mediators of cervical remodeling, Mφ were costained for phenotypic markers of function and activation. In the cervix, Mφ that proportionally expressed high levels of CD11b (marker associated with adhesion to endothelium or extracellular matrix) (see CD11bhi in Fig. 4, A and B) or CD54 (migration-associated cell adhesion molecule induced by proinflammatory cytokine activation) (Fig. 4C) were significantly reduced in cervices from D18 mice as compared to that from D15 and NP groups. In contrast, Mφ that expressed markers for metalloproteinase activation (CD147) or remodeling associated with cell matrix binding (CD169) increased by D18 mice versus in D15 or in NP mice (Fig. 4, D and E). No change was evident among the three groups in Mφ that expressed the marker for vascular adhesion and migration (CD62L). These data suggest that Mφ in the cervix by the day before birth (i.e., D18) are less likely to be migrating or binding to vasculature and more likely to be involved with extracellular matrix remodeling processes than those in D15 and NP mice.
FIG. 4.
Mφ in the prepartum cervix increase expression of certain activation markers. Dispersed cells from perfused cervices were stained for flow cytometry, and a standardized gating protocol was used to identify F4/80+GR-1– Mφ. A) Representative histograms of CD11b expression by F4/80+GR-1− Mφ in the cervix of from mice that were NP, D15, or D18. B) Percentages of CD11b+hi Mφ/group (n = 3–5; Student t-test). C–F) Median fluorescence intensity of the various activation markers on F4/80+GR-1− Mφ/group (n = 4–6; Mann-Whitney test). For D18, statistical significance (P < 0.05) versus aNP or bD15 is shown.
DISCUSSION
The present findings support the hypothesis that recruitment and certain activities by Mφ increase in association with remodeling of the cervix near term. Consistent with previous immunohistochemical studies of rodent cervical sections [18, 19], the findings with flow cytometry of dispersed cervix indicate that Mφ were the predominant differentiated resident myeloid cell in the murine cervix. The number as well as percentage of Mφ among leukocytes significantly increased by the day before birth (i.e., D18). Compared to earlier in pregnancy or in NP mice, a larger proportion of Mφ appeared to be engaged in tissue remodeling or cell matrix-binding activities, as indicated by increased expression of molecules associated with metalloproteinase activity (CD147) or extracellular adhesion (CD169). Although Mφ-expressing epitopes reflect adherence or cross-vascular infiltration and migration (CD11b+ and CD54+ did not change), it is conceivable that CD54+ Mφ may recruit and locally activate other Mφ to express CD147 and CD169, which for whatever reasons do not express CD11b or CD54. A lack of a significant increase in Neu numbers or relevant markers of Neu activity in the prepartum cervix does not exclude the potential contribution of this immune cell to the remodeling process given its short life span and fragility [24]. Thus, the present findings raise the possibility that prepartum increases in Mφ with particular activities related to extracellular matrix degradation drive remodeling in the cervix by the morning of the day before birth.
These conclusions are dependent on an accurate census of resident leukocytes and subtypes in the cervix. Therefore, in the present study, careful attention was given to standardization of procedures for tissue processing and analysis of flow cytometry data. Three components of the methodology, including systemic perfusion of tissues, controls to maximize yield of live cells following dispersal (present study and [25]), and clearly defined gate settings with comparisons to living dispersed splenocytes in the same individual, provided assurances that flow cytometry would be useful to accomplish the objectives of the present study. Greater variability of hemocytometer cell counts from dispersed tissues, especially cervix, raise concerns about the accuracy of resident immune cells in the cervix [15] as compared to approaches that count specifically stained cells in cervical sections [18, 19] or use flow cytometry, as in the present report. In addition to increased Mφ, the total number of living cells was greater in cervices from pregnant versus NP mice. More cells in the cervix from pregnant mice on D15 and D18 compared to that in the cervix from NP mice is indicative of tissue hyperplasia with pregnancy. Increased numbers of cells in the cervix, possibly stroma, fibroblast, and smooth muscle cells, extends the results of previous studies that found cellular hypertrophy (i.e., increased size of cells as reflected by fewer nuclei per area) in the cervix from pregnant versus NP mice [18, 23, 26, 27]. Thus, along with hyperplasia of cells in the cervix, hypertrophy defines cervical growth during pregnancy.
The present study found an increase in Mφ by the day before birth but a low proportion of monocytes and no change in monocyte number in cervices from perfused NP and pregnant mice. A stable population of monocytes in circulation from women that deliver preterm or at term [28] further suggests that elimination of systemic cells are needed to accurately enumerate resident immune cells and may account for differences compared to findings in a previous study [21]. Even if a small number of monocytes reside in the cervix, their purpose is uncertain. Leukocyte invasion of the reproductive tract and cervix at term is suggested in a variety of species [6, 29] and is associated with labor in women [2]. In the present study, enhanced Mφ numbers with degradative activities in the peripartum cervix, before or coincident with cervical dilation, labor, or delivery, provides evidence for certain immune cell activity in cervical ripening. In addition to the expression of metalloproteinase that we observed, Mφ are a source for nitric oxide, prostaglandins, and proinflammatory cytokines, and along with Neu, they have collagenase activity [9, 30]. These factors could collectively promote local inflammatory processes in the prepartum uterine cervix at term, including vascular permeability, edema, and degradation of the extracellular matrix [31, 32], or contribute to inflammation-induced preterm cervical remodeling [2, 9, 33]. Other flow cytometry studies indicate that immigration and activation of immune cell subtypes are stimulus-specific (e.g., injury, infection, or endogenous signals) and vary over time with respect to local cellular functions in tissue [34, 35]. Moreover, heterogeneity in Mφ function that are tissue specific may not easily be categorized into the polarity of proinflammatory M1 or anti-inflammatory M2 subtypes [36, 37], which do not appear to change in the murine cervix before birth [21]. Thus, local signaling may be essential for the selective recruitment and differential activities by immune cells in the cervix at term or in association with preterm birth.
In summary, the present findings support and extend the hypothesis that increased numbers of Mφ and subtypes associated with certain functional activities occur late in the process of cervical remodeling in preparation for parturition. Replicable and accurate immune cell counts depended on the use of standardized procedures for tissue processing and analysis of flow cytometry data. The lack of changes in other myeloid cells and activities related to adhesion or migration in the preterm cervix is in contrast with Mφ functions associated with degradation of the extracellular matrix. These findings suggest that Mφ with specific biomarkers for immune-mediated tissue remodeling activities constitute most of the leukocytes in the prepartum cervix and may be a critical feature of the final mechanism pathway for cervical remodeling. The present results also raise the possibility that these processes occur within the cervix of mice while well-innervated [38], in advance of the decline in systemic progesterone, as previously discussed [22], and before the increase in contractions of the uterus with the onset of labor. Thus, novel approaches may be developed to diagnose or treat advanced or delayed cervical remodeling that are, respectively, associated with preterm birth or dystocia and delayed parturition.
ACKNOWLEDGMENT
We thank Thomas J. Lechuga for technical assistance and Jane Yun for her efforts to help develop flow cytometry methods of analysis of dispersed cervical cells.
Footnotes
Supported by National Institutes of Health grants R01 HD054931 and R25GM060507. Additional support provided by Loma Linda University School of Medicine Dean and the Department of Pediatrics.
REFERENCES
- Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med 2007; 25: 69 79 [DOI] [PubMed] [Google Scholar]
- Golightly E, Jabbour HN, Norman JE. Endocrine immune interactions in human parturition. Mol Cell Endocrinol 2011; 335: 52 59 [DOI] [PubMed] [Google Scholar]
- Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol 1995; 38: 267 279 [DOI] [PubMed] [Google Scholar]
- Read CP, Word RA, Ruscheinsky MA, Timmons BC, Mahendroo MS. Cervical remodeling during pregnancy and parturition: molecular characterization of the softening phase in mice. Reproduction 2007; 134: 327 340 [DOI] [PubMed] [Google Scholar]
- Hertelendy F, Zakar T. Prostaglandins and the myometrium and cervix. Prostaglandins Leukot Essent Fatty Acids 2004; 70: 207 222 [DOI] [PubMed] [Google Scholar]
- Mackler AM, Iezza G, Akin MR, McMillan P, Yellon SM. Macrophage trafficking in the uterus and cervix precedes parturition in the mouse. Biol Reprod 1999; 61: 879 883 [DOI] [PubMed] [Google Scholar]
- Kelly RW. Inflammatory mediators and cervical ripening. J Reprod Immunol 2002; 57: 217 224 [DOI] [PubMed] [Google Scholar]
- Pokabla MJ, Dickerson IM, Papka RE. Calcitonin gene-related peptide-receptor component protein expression in the uterine cervix, lumbosacral spinal cord, and dorsal root ganglia. Peptides 2002; 23: 507 514 [DOI] [PubMed] [Google Scholar]
- Yellon SM, Mackler AM, Kirby MA. The role of leukocyte traffic and activation in parturition. J Soc Gynecol Investig 2003; 10: 323 338 [DOI] [PubMed] [Google Scholar]
- Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition—a review. Placenta 2003; 24 (suppl A): S33 S46 [DOI] [PubMed] [Google Scholar]
- Akins ML, Luby-Phelps K, Bank RA, Mahendroo M. Cervical softening during pregnancy: regulated changes in collagen cross-linking and composition of matricellular proteins in the mouse. Biol Reprod 2011; 84: 1053 1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesiano S, Wang Y, Norwitz ER. Progesterone receptors in the human pregnancy uterus: do they hold the key to birth timing? Reprod Sci 2011; 18: 6 19 [DOI] [PubMed] [Google Scholar]
- Sfakianaki AK, Norwitz ER. Mechanisms of progesterone action in inhibiting prematurity. J Matern Fetal Neonatal Med 2006; 19: 763 772 [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Zugaib M, Montes GS, Toledo OM, Krisztan RM, Shigihara KM. Morphologic and histochemical evidence for the occurrence of collagenolysis and for the role of neutrophilic polymorphonuclear leukocytes during cervical dilation. Am J Obstet Gynecol 1980; 138: 273 281 [DOI] [PubMed] [Google Scholar]
- Timmons BC, Mahendroo MS. Timing of neutrophil activation and expression of proinflammatory markers do not support a role for neutrophils in cervical ripening in the mouse. Biol Reprod 2006; 74: 236 245 [DOI] [PubMed] [Google Scholar]
- Osman I, Young A, Ledingham MA, Thomson AJ, Jordan F, Greer IA, Norman JE. Leukocyte density and proinflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labor at term. Mol Hum Reprod 2003; 9: 41 45 [DOI] [PubMed] [Google Scholar]
- Sakamoto Y, Moran P, Bulmer JN, Searle RF, Robson SC. Macrophages and not granulocytes are involved in cervical ripening. J Reprod Immunol 2005; 66: 161 173 [DOI] [PubMed] [Google Scholar]
- Yellon SM, Ebner CA, Sugimoto Y. Parturition and recruitment of macrophages in cervix of mice lacking the prostaglandin F receptor. Biol Reprod 2008; 78: 438 444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon SM, Oshiro BT, Chhaya TY, Lechuga TJ, Dias RM, Burns AE, Force L, Apostolakis EM. Remodeling of the cervix and parturition in mice lacking the progesterone receptor B isoform. Biol Reprod 2011; 85: 498 502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyde LA, Lechuga TJ, Ebner CA, Burns AE, Kirby MA, Yellon SM. Transection of the pelvic or vagus nerve forestalls ripening of the cervix and delays birth in rats. Biol Reprod 2011; 84: 587 594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons BC, Fairhurst AM, Mahendroo MS. Temporal changes in myeloid cells in the cervix during pregnancy and parturition. J Immunol 2009; 182: 2700 2707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowa CN, Jesmin S, Sakuma I, Usip S, Togashi H, Yoshioka M, Hattori Y, Papka R. Characterization of vascular endothelial growth factor (VEGF) in the uterine cervix over pregnancy: effects of denervation and implications for cervical ripening. J Histochem Cytochem 2004; 52: 1665 1674 [DOI] [PubMed] [Google Scholar]
- Kirby LS, Kirby MA, Warren JW, Tran LT, Yellon SM. Increased innervation and ripening of the prepartum murine cervix. J Soc Gynecol Investig 2005; 12: 578 585 [DOI] [PubMed] [Google Scholar]
- Edwards SW, Moulding DA, Derouet M, Moots RJ. Regulation of neutrophil apoptosis. Chem Immunol Allergy 2003; 83: 204 224 [DOI] [PubMed] [Google Scholar]
- Kirillova I, Chaisson M, Fausto N. Tumor necrosis factor induces DNA replication in hepatic cells through nuclear factor kappaB activation. Cell Growth Differ 1999; 10: 819 828 [PubMed] [Google Scholar]
- Yellon SM, Ebner CA, Elovitz MA. Medroxyprogesterone acetate modulates remodeling, immune cell census, and nerve fibers in the cervix of a mouse model for inflammation-induced preterm birth. Reprod Sci 2009; 16: 257 264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellon SM, Burns AE, See JL, Lechuga TJ, Kirby MA. Progesterone withdrawal promotes remodeling processes in the nonpregnant mouse cervix. Biol Reprod 2009; 81: 1 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MA, Lee BS, Park YW, Seo K. Serum markers for prediction of spontaneous preterm delivery in preterm labor. Eur J Clin Invest 2011; 41: 773 780 [DOI] [PubMed] [Google Scholar]
- Mitchell BF, Taggart MJ. Are animal models relevant to key aspects of human parturition? Am J Physiol Regul Integr Comp Physiol 2009; 297: R525 R545 [DOI] [PubMed] [Google Scholar]
- Holt R, Timmons BC, Akgul Y, Akins ML, Mahendroo M. The molecular mechanisms of cervical ripening differ between term and preterm birth. Endocrinology 2011; 152: 1036 1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol 2008; 79: 50 57 [DOI] [PubMed] [Google Scholar]
- Garfield RE, Saade G, Buhimschi C, Buhimschi I, Shi L, Shi SQ, Chwalisz K. Control and assessment of the uterus and cervix during pregnancy and labor. Hum Reprod Update 1998; 4: 673 695 [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008; 371: 75 84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling DP, Yong VW. Dynamics of the inflammatory response after murine spinal cord injury revealed by flow cytometry. J Neurosci Res 2008; 86: 1944 1958 [DOI] [PubMed] [Google Scholar]
- Taylor ML, Noble PW, White B, Wise R, Liu MC, Bochner BS. Extensive surface phenotyping of alveolar macrophages in interstitial lung disease. Clin Immunol 2000; 94: 33 41 [DOI] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci 2008; 13: 453 461 [DOI] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Obstacles and opportunities for understanding macrophage polarization. J Leukoc Biol 2011; 89: 557 563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackler AM, Green LM, McMillan PJ, Yellon SM. Distribution and activation of uterine mononuclear phagocytes in peripartum endometrium and myometrium of the mouse. Biol Reprod 2000; 62: 1193 1200 [DOI] [PubMed] [Google Scholar]
- Zhu P, Ding J, Zhou J, Dong WJ, Fan CM, Chen ZN. Expression of CD147 on monocytes/macrophages in rheumatoid arthritis: its potential role in monocyte accumulation and matrix metalloproteinase production. Arthritis Res Ther 2005; 7: R1023 R1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartnell A, Steel J, Turley H, Jones M, Jackson DG, Crocker PR. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood 2001; 97: 288 296 [DOI] [PubMed] [Google Scholar]
- Rosas M, Thomas B, Stacey M, Gordon S, Taylor PR. The myeloid 7/4-antigen defines recently generated inflammatory macrophages and is synonymous with Ly-6B. J Leukoc Biol 2010; 88: 169 180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto TK, Jutila MA, Berg EL, Butcher EC. Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science 1989; 245: 1238 1241 [DOI] [PubMed] [Google Scholar]