ABSTRACT
Cavernous smooth muscle cells are essential components in penile erection. In this study, we investigated effects of estrogen exposure on biomarkers for smooth muscle cell differentiation in the penis. Neonatal rats received diethylstilbestrol (DES), with or without the estrogen receptor (ESR) antagonist ICI 182,780 (ICI) or the androgen receptor (AR) agonist dihydrotestosterone (DHT), from Postnatal Days 1 to 6. Tissues were collected at 7, 10, or 21 days of age. The smooth muscle cell biomarker MYH11 was studied in depth because microarray data showed it was significantly down-regulated, along with other biomarkers, in DES treatment. Quantitative real time-PCR and Western blot analyses showed 50%–80% reduction (P ≤ 0.05) in Myh11 expression in DES-treated rats compared to that in controls; and ICI and DHT coadministration mitigated the decrease. Temporally, from 7 to 21 days of age, Myh11 expression was onefold increased (P ≥ 0.05) in DES-treated rats versus threefold increased (P ≤ 0.001) in controls, implying the long-lasting inhibitory effect of DES on smooth muscle cell differentiation. Immunohistochemical localization of smooth muscle alpha actin, another biomarker for smooth muscle cell differentiation, showed fewer cavernous smooth muscle cells in DES-treated animals than in controls. Additionally, DES treatment significantly up-regulated Esr1 mRNA expression and suppressed the neonatal testosterone surge by 90%, which was mitigated by ICI coadministration but not by DHT coadministration. Collectively, results provided evidence that DES treatment in neonatal rats inhibited cavernous smooth muscle cell differentiation, as shown by down-regulation of MYH11 expression at the mRNA and protein levels and by reduced immunohistochemical staining of smooth muscle alpha actin. Both the ESR and the AR pathways probably mediate this effect.
Keywords: development, estrogen, microarray, MYH11, penis
Estrogen-induced loss of cavernous smooth muscle cells in the rat penis involves down-regulation of MYH11 expression, mitigated by estrogen receptor antagonist ICI 182,780 and dihydrotestosterone.
INTRODUCTION
The rat penis, similar to the human penis, contains cavernous spaces and smooth muscle cells (cavernous smooth muscle cells), two structures essential for erection in a vascular penis [1]. Unlike other body smooth muscle cells, cavernous smooth muscle cells remain contracted most of the time; they relax under sexual stimulation, which engorges cavernous spaces with blood and, consequently, produces erection [2]. Cavernous smooth muscle cells have been targets of pharmaceutical drugs that enhance erection by prolonging their relaxation [3]. It is estimated that 152 million males world wide experienced some degree of erectile dysfunction in 1995, and this number is expected to increase to 322 million in 2025 [4]. Vascular diseases impairing engorgement of cavernous spaces are considered a major cause for erectile dysfunction [5]. Cavernous smooth muscle cells were reduced in impotent men [6] and diabetic rats [7] compared to those in controls. The cells were reduced in castrated rabbits [8] and mice [9]; and a low testosterone level was correlated with their impaired relaxation in patients with erectile dysfunction [10]. These findings imply an important role for androgens in maintaining normal function of cavernous smooth muscle cells.
Testosterone or its metabolite dihydrotestosterone (DHT) is required for development and growth of the male reproductive tract, including the penis [11]. Perinatal exposure to antiandrogens, such as phthalates [12, 13] and vinclozolin [12], induce male reproductive tract abnormalities, including smaller phalluses. Infant boys whose mothers had higher level of phthalates had smaller penises [14]. Similarly, estrogen exposure has been linked with higher frequency of reproductive abnormalities [15]. A high prevalence of micropenis was reported in newborns from Brazil [16] and in infants and prepubescent boys from Denmark [17] whose mothers were exposed to pesticides during pregnancy. Laboratory animals treated prenatally with estrogens developed hypospadias [18, 19]. Bisphenol A treatment in rodents increased prostate size [20] and predisposed the gland to a precancerous growth [21]. Alligators from Lake Apopka (FL), contaminated with industrial estrogenic chemicals, had smaller phalluses [22]. Collectively, the above-mentioned studies suggest that exposure to antiandrogens or estrogens is detrimental for development of the male reproductive tract, including the penis. However, none of these studies reported effects on differentiation of cavernous spaces or cavernous smooth muscle cells.
Previously, we reported that adult rats treated neonatally with the antiandrogen GnRH-antagonist [23] or estrogens [24, 25] developed similar penile abnormalities, including loss of cavernous spaces and accumulation of fat cells. These abnormalities occurred in animals that were treated with DES before 12 days of age but not after. The magnitude of abnormalities was higher in animals treated from 1 to 6 days of age than in those treated from 7 to 12 days [26]. Collectively, these studies suggest that the rat penis is most sensitive to estrogen exposure from 1 to 6 days of age, the period just before the beginning of stromal cell differentiation into cavernous spaces and cavernous smooth muscle cells, which appeared at 6–7 days of age in rodents [27]. Although when cavernous smooth muscle cells differentiate in the human penis has not been investigated, differentiation most likely occurs at the end of the first trimester and the beginning of the second trimester of pregnancy when gross morphogenesis of the penis is completed [28]. This is also the period when 2 million women inadvertently continue to take the contraceptive ethinyl estradiol [29].
Hence, to understand the mechanism by which diethylstilbestrol (DES) exposure in neonatal rats alters differentiation of cavernous smooth muscles, the first aim of this study was to perform a microarray analysis to determine the differentially regulated genes in the penis. Based upon microarray data, we selected the smooth muscle biomarker MYH11 for further analysis. Thus, the second aim was to determine the effects of DES on MYH11 expression at the mRNA and protein levels, using quantitative real time-PCR (q-real time-PCR), Western blotting, and immunohistochemistry, and to determine whether the estrogen receptor (ESR) antagonist ICI 182,780 (ICI) and/or the androgen receptor (AR) agonist DHT can mitigate DES-induced alterations. Additionally, we studied DES effects on the neonatal testosterone surge and on Esr1, Esr2, Ar, and peroxisome proliferator-activated receptor gamma (Pparγ) expression because of their significance in penile development.
MATERIALS AND METHODS
Animals and Housing
Neonatal and adult Sprague-Dawley male and female rats (Harlan, Indianapolis, IN) were maintained at 22°C–23°C ambient temperature, 55%–60% relative humidity, in a 12L:12D cycle and had free access to food (Rodent Chow 5001; Purina Mills, St. Louis, MO) and water. Animals were handled in accordance with guidelines of the National Institutes of Health Guiding Principles for the Care and Use of Animal Research, and all animal procedures were approved by the Institutional Animal Care and Use Committee at Tuskegee University.
Treatment
Timed pregnancy female rats were housed individually. Within 24 h of delivery, five to eight male pups from different litters (one pup from each litter in order to avoid a litter effect) were randomly assigned to each group, and the number of pups per group was adjusted to eight with extra female pups where appropriate. Each pup in each group received daily s.c. injections of 50 μl of oil (control group) or oil containing DES (1 μg [0.1 mg/kg]; Sigma Aldrich, St. Louis, MO), with or without the ESR antagonist ICI (250 μg [25 mg/kg]; R&D Systems, Inc., Minneapolis, MN) or the AR agonist DHT (200 μg [20 mg/kg], Sigma Aldrich) for 6 consecutive days. The 6-day treatment was based on our previous data, wherein we showed that 100% of the adult rats or mice treated neonatally for 6 days with DES at a dose of 0.1 mg/kg developed permanently malformed penises and were infertile [26, 30]. Similarly, the above doses of ICI and DHT have been shown to mitigate estrogenic effects [24, 31]. Tissues were collected following CO2 asphyxiation of the pups at 7, 10, and 21 days of age. All samples were snap-frozen in liquid nitrogen upon collection and stored at −80°C. The reason for collecting tissues at these development periods was to determine DES-induced changes in gene expression soon after the end of the 6-day treatment (7 or 10 days) and at the onset of puberty (21 days).
Microarray Analysis
At least 50 mg of Day 7 snap-frozen penile (glans and body) and testicular tissue samples from control and DES-treated groups were shipped on dry ice to a commercial company for microarray analysis using the RatRef-12 Expression BeadChip for genome-wide expression analysis (Illumina; SABiosciences, Valencia, CA). At least five penises were pooled to obtain 50 mg of tissue for each replicate, and microarray analysis results were the combined data of three replicates each for control and treated samples. The microarray chips contained 21 910 probes selected primarily from the National Center for Biotechnology Information (NCBI) reference sequence database.
RNA Isolation and q-Real Time-PCR
At least 40 mg of penile (glans and body) tissue samples were homogenized, and total RNA was extracted using the RNeasy mini-kit (Qiagen, Valencia, CA), according to the manufacturer's instructions. cDNA was synthesized from 1 μg of the resulting total RNA by using the Quantitect reverse transcriptase kit (Qiagen), following the manufacturer's instructions. All primers were purchased from SABiosciences, and reactions were performed using 50 ng of cDNA, 10 μl of Brilliant III Ultra Fast SYBR Green q-real time-PCR Master Mix (Agilent Technologies, Santa Clara, CA) and primers at 500 nmol (Myh11, Esr1, Pparγ, Esr2, and Ar; catalog nos. PPR53316A, PPR44939A, PPR47599A, PPR48980A, and PPR44497A, respectively). Beta actin (Actb; catalog no. PPR06570B; Agilent Technologies) was used as the housekeeping control, and the final reaction mixtures were made to a total volume of 20 μl with DNase RNase-free water (Qiagen). All q-real time-PCR reactions were carried out in duplicate on a Stratagene model Mx3005P unit (Agilent Technologies). The cycling conditions were one cycle of initial denaturation at 95°C for 10 min, 40 cycles of denaturation at 95°C for 30 sec, annealing at 55°C, and extension at 72°C for 1 min each. The final segment involved the generation of a dissociation curve. This consisted of one cycle at 95°C for 1 min, followed by 55°C and 95°C for 30 sec each. Inclusion of a dissociation curve in each q-real time-PCR run ensured specificity of the amplicon.
Western Blotting
Three 10-day-old penis (glans and body) samples were pooled and protein extracted. Samples were homogenized in radioimmunoprecipitation assay lysis buffer solution (Santa Cruz Biotechnology, Santa Cruz, CA) on ice. Protein was separated out by centrifugation at 13 300 rpm for 30 min at 4°C and then stored at −80°C until ready for assay. Protein concentrations were determined using Coomassie Plus protein assay (Thermo Scientific, Rockford, IL), according to the manufacturer's instructions. Fifty micrograms per milliliter protein were used, and the total volume adjusted to 30 μl using a Laemmli sample buffer solution (Protea Biosciences, Inc., Morgantown, WV), which was mixed with beta-mercaptoethanol in a 17:1 ratio. The protein was denatured by heating for 5 min in a boiling water bath. Proteins were resolved on 10% Tris-HCl gels in a Mini Protean 3 Cell unit (Bio-Rad, Hercules, CA). After SDS-PAGE, gels were electroblotted at 120 V for 45 min and transferred onto polyvinylidene flouride membranes (Thermo Scientific) using a Mini Protean 3 Cell, by electrophoresis at 350 mA for 1 h. Nonspecific binding was blocked by incubation in 5% fat-free milk solution for 1 h at room temperature. Membranes were then incubated in the primary antibodies, rabbit polyclonal anti-MYH11, and mouse monoclonal anti-beta actin (loading control, catalog nos. sc-98705 and sc-47778, respectively, Santa Cruz Biotechnology) in 1% blocking buffer overnight at 4°C. After membranes were rinsed, they were incubated in horse radish peroxidase (HRP)-conjugated goat anti-rabbit and goat anti-mouse immunoglobulin G (IgG) secondary antibodies, respectively (catalog nos. sc-2004 and sc-2061, respectively, Santa Cruz Biotechnology) for 1 h at room temperature and developed using Luminata Crescendo Western HRP substrate (Millipore Corp., Billerica, MA).
Immunohistochemistry and Histochemistry
The body of the penis at 7, 10, and 21 days of age was fixed in 10 % neutral buffered formalin on a shaker for 24 h. For immunohistochemical localization of alpha actin protein (ACTA2), a smooth muscle cell biomarker (note, immunostaining with MYH11 was not successful in our hands), tissues were processed in an automatic tissue processor, embedded in paraffin, and sectioned at 5-μm thickness. Slides were de-paraffinized by three washes in xylene and rehydrated through a series of graded alcohol steps (100%, 95%, and 70%) and water, each for 5 min. Unless otherwise stated, all washes were performed three times in phosphate-buffered saline containing 0.05% Tween (pH 7.4) for 5 min each, and all incubations were carried out in a humid chamber at room temperature. Antigen retrieval was achieved by heating the slides in a microwave oven in a 0.01 M sodium citrate solution (pH 6.0) and subsequently cooling them for 30 min, followed by washing. Endogenous peroxidase activity was blocked by incubating the slides for 30 min in 1% hydrogen peroxide in methanol. Nonspecific binding was blocked by incubating the slides for 1 h with normal horse serum (Vector Laboratories, Inc., Burlingame, CA). Slides were then incubated with an anti-ACTA2 primary antibody (anti-alpha actin, catalog no. sc-130617, Santa Cruz Biotechnology) for 1.5 h at room temperature. After another wash, avidin-biotin-HRP complex (Vectastain Elite ABC kit, Vector Labs) was prepared and added according to the manufacturer's instructions. 3,3′-Diaminobenzidine was used as the chromogen substrate, and photomicrographs were taken using an Olympus BX73 microscope (Olympus, Center Valley, PA) under brightfield illumination.
For histochemical demonstration of fat, the formalin-fixed tissues from the body of the penis were rinsed in water for 5 min and then fixed in a solution consisting of 1% osmium tetroxide and 2.5% potassium dichromate for 8 h under a fume hood. This was followed by a 2-h wash in running water. Samples were then transferred to formalin, processed in an automatic tissue processor, and embedded in paraffin. Five-micrometer-thick sections were cut, and photographs were taken as described above. In addition to the 7-, 10-, and 21-day-old penis samples, paraffin sections from the body of the adult penis from a previous study [24] were stained for immunohistochemistry and histochemistry. Also, paraffin sections from each group were stained with hematoxylin and eosin.
Intratesticular and Plasma Testosterone
For intratesticular testosterone assay, a part of the right testis was collected at the time of necropsy. For plasma testosterone, a blood sample from the heart of each animal (21-day groups only) was collected just before complete CO2 asphyxiation, and plasma was collected by centrifugation. Both plasma and testicular samples were frozen at −20°C until assayed using a Coat-A-Count testosterone radioimmunoassay (Diagnostics Products Corp., Los Angeles, CA), as described previously from our laboratory [24]. The sensitivity of the assay was 0.2 ng/ml. All samples were quantified in a single assay, and the intra-assay coefficient of variation was 7%.
Body and Organ Weights
Each animal was weighed immediately after CO2 asphyxiation. Each testis was freed of the epididymis, and the adjoining fat and was weighed. The penis was exposed up to the ischial arch, freed from the adjoining tissues, and measured for length and weight as described previously [26]. The stretched length was measured from the tip of the glans penis to the midpoint of the ischial arch.
Statistics
Statistical analyses were performed using ProStat statistical software (Polysoftware International, Pearl River, NY). Analysis of variance was performed with all parameters. Treatment groups with significantly different means (P < 0.05) were identified using Duncan multiple range test or t-test when only comparisons to controls were made. When data were not distributed normally or heterogeneity of variance was identified analyses were performed with transformed or ranked data. Data are expressed as means ± SEM.
RESULTS
Microarray Analysis of Gene Expression Profiles in the Penis and Testis
The primary goal of the microarray analysis was to determine the immediate gene expression changes in the penis due to DES exposure. For this, we treated rat pups from Postnatal Days 1 to 6 with DES or vehicle and compared the genome-wide expression profiles of controls with those of DES-treated rats at 7 days of age. Of the 21 910 probes, approximately 4022 genes were differentially regulated between the two groups. To select genes with a high probability of differential expression, the threshold P value was set to 0.09. A total of 532 genes were up-regulated and 518 were down-regulated in the DES group, compared to controls. Supplemental Table S1 (available online at www.biolreprod.org) lists 297 up-regulated and 271 down-regulated genes that have a published symbol in the NCBI database. From that table, we selected biomarkers for smooth muscle cell, adipocytes, and angiogenesis and steroid receptors because of their significance in penile development [26, 32]. Among the selected genes that were differentially expressed in the DES-treated group, four smooth muscle cell markers, myosin heavy chain 11 (Myh11), Myh7, myosin light chain kinase (Mylk), and actin gamma 2 (Actg2) and the angiogenic factor angiopoietin 2 (Angpt2), were down-regulated (Fig. 1A). However, the gene expression profiles for the adipocyte marker (Pparγ) and steroid receptors (Esr1, Esr2, Ar) were not altered (not shown). Although microarray analysis of testicular genes was not the focus of the study, the gene expression profiles of five steroidogenic enzymes, hydroxy-delta-5-steroid dehydrogenase 3 beta delta-isomerase 1 (Hsd3β1), steroidogenic acute regulatory protein (Star), and cytochrome P450 family enzymes (Cyp11α1, Cyp17α1, Cyp2c22) that were down-regulated were included (Fig. 1B) because of their roles in testosterone production.
FIG. 1.
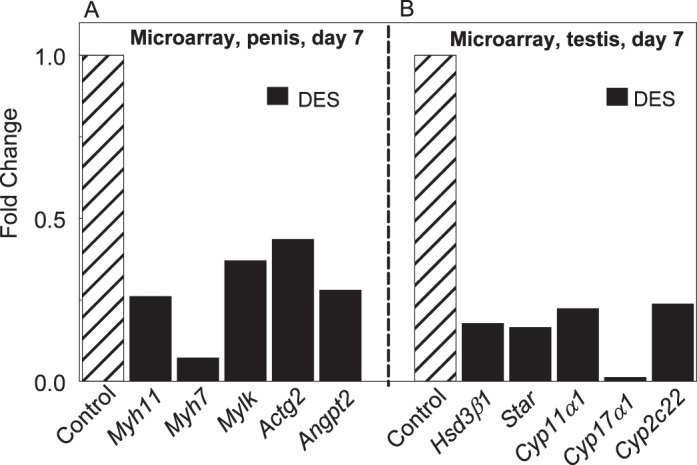
Microarray analysis from the penis (A) and testis (B) at 7 days of age in rats treated from 1 to 6 days of age with DES. Compared to controls, note in the penis 60%–90% reduction in gene expression profiles of markers for smooth muscle cell or angiogenesis and, in the testis, 80%–95% reduction in gene expression profiles of steroidogenic enzymes.
Based on microarray data and because of the significance of cavernous smooth muscle cells in penile erection, we studied effects of DES on smooth muscle cell biomarkers, including Myh11 expression at the mRNA and protein levels and smooth muscle alpha actin protein immunolocalization at the cellular level in different age groups. We also determined whether the ESR antagonist ICI and/or the AR agonist DHT could mitigate DES-induced alterations in Myh11 mRNA expression. Additionally, we studied effects of DES on mRNA expression of Pparγ, Esr1, Esr2, and Ar by using q-real time-PCR, and on fat accumulation by using histochemistry in the penises of different age groups.
Quantitative Real Time-PCR Analysis of Myh11 mRNA Expression in the Penis
DES treatment of neonatal pups significantly down-regulated Myh11 expression (50%–60% reduction) at both 7 and 21 days of age (Fig. 2, A and B). Coadministration of ICI or DHT with DES counteracted DES-induced down-regulation on both days, but the groups treated with ICI or DHT alone had Myh11 expression levels similar to those of the control group. A temporal analysis within the control group showed greater than threefold (300%) increase (P < 0.001) in Myh11 expression as animals grew from 7 to 21 days of age (Fig. 2C). Conversely, a similar comparison in the DES group showed a nonsignificant (P ≥ 0.05) onefold increase from 7 to 21 days of age, showing that neonatal DES treatment impeded differentiation of smooth muscle cells in the penis.
FIG. 2.
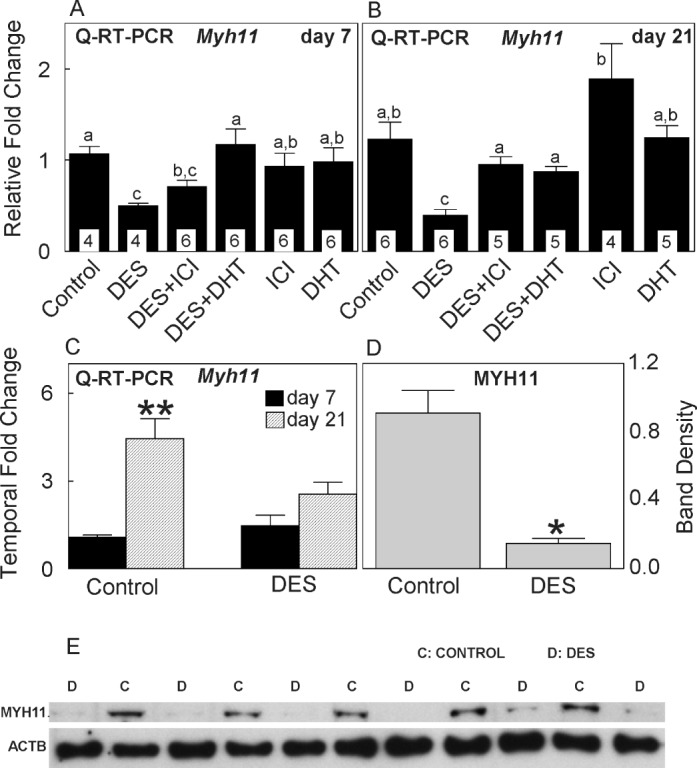
Quantitative real time-PCR of Myh11 expression at 7 (A) and 21 (B) days of age and a temporal change in Myh11 expression (C) from 7 to 21 days of age in rats treated from 1 to 6 days of age with DES, with or without coadministration of ICI or DHT. Note nearly 50%–70% reduction in Myh11 expression as a result of DES treatment and its reversal to the control level by ICI or DHT coadministration at 7 and/or 21 days. Note a temporal threefold increase in Myh11 expression in controls versus a nonsignificant onefold increase in the DES group. Temporal changes in other treatments are not shown. D) Note almost 80% reduction in MYH11 expression as a result of DES treatment. E) Western blot analysis of MYH11 protein expression at 10 days of age in control and DES-treated rats. Data are expressed as means ± SEM. Means with different lowercase letters are significantly different from each other (P < 0.05). Asterisks denote significant difference from controls at the level of *P < 0.05 or **P < 0.001. In A and B, numbers within bars indicate the number of animals in each experiment group.
Western Blot Analysis of MYH11 Protein Expression in the Penis
Western blot analysis was carried out in 10-day-old penises (note, the penis at 7 days of age was too small to collect enough protein). Compared to controls, where MYH11 band staining was clearly visible in all animals (n = 5, each sample was prepared from three pups, and the blotting was repeated twice), the band density was faintly visible in all DES-treated rats (n = 6) (Fig. 2E). Furthermore, digital analysis of band density revealed almost 80% reduction (P ≤ 0.05) in MYH11 protein expression as a result of DES treatment compared to that of controls (Fig. 2D).
Immunohistochemical Analysis of the Smooth Muscle ACTA2 Protein in the Body of the Penis
As reported earlier, MYH11 immunolocalization was not successful in our hands, and therefore, we immunolocalized ACTA2 protein, another smooth muscle cell biomarker, that has been successfully immunolocalized by other investigators [9, 33]. A brief description of the histology of the body of the penis is included for the sake of orientation. The body consists of two corpora cavernosa that lie dorsolateral to the urethra and a corpus spongiosum that lies ventrally and surrounds the urethra (Fig. 3, A and E). A partial intercrural septum containing blood vessels and nerves separates two corpora cavernosa. Each corpus cavernosum is surrounded by a thick connective tissue tunica albuginea capsule and contains endothelium-lined cavernous spaces (sinusoids), smooth muscle cells surrounding cavernous spaces, and connective tissue trabeculae separating adjacent cavernous spaces. The corpus spongiosum has similar morphology, except that cavernous spaces are less developed.
FIG. 3.
Immunohistochemical localization of smooth muscle alpha actin in the body of the penis at 7, 10, 21, days of age and at adulthood in control (A–D) and DES-treated (E–H) rats. Note cavernous spaces (arrowheads) as outlined by the surrounding alpha actin-positive cavernous smooth muscle cells in the corpora cavernosa (CC). Both structures are much less developed in DES-treated animals than controls. Note arterioles or metarterioles (arrows) in DES-treated animals. Other alpha actin-positive structures are blood vessels (BV) present in the intercrural septum located dorsally. Corpus spongiosum (CS) is located ventrally. The control section incubated with blocking serum in place of primary antibody did not stain smooth muscle cells surrounding the cavernous spaces or blood vessels in the intercrural septum and is not shown. All photomicrographs were obtained at the same magnification. Bar = 100 μm.
Immunohistochemical staining for ACTA2 demarcated the wide-channel cavernous spaces and ACTA2-positive smooth muscle cells that were present adjacent to the endothelium of cavernous spaces. These ACTA2-positive smooth muscle cells are termed “cavernous smooth muscle cells” to distinguish them from ACTA2-positive smooth muscle cells that surrounded other blood vessels present in the corpora cavernosa or the intercrural septum (Fig. 3, A–D). Cells of the tunica albuginea and those present between cavernous spaces, most likely mesenchymal cells or fibroblasts or fat cells, were negative for ACTA2. In controls, both cavernous spaces and cavernous smooth muscle cells were present at 7 days of age (Fig. 3A), and both were well developed by 10 days of age (Fig. 3B). The main change observed at 21 days and at adulthood was the widening of the lumen of cavernous spaces (Fig. 3, C and D). Compared to the cavernous spaces of controls, those of DES-treated animals of the same age group had fewer cavernous spaces as demarcated by ACTA2-positive cavernous smooth muscle cells (cf. Fig. 3, A–D, with 3, E–H, respectively). Many of these vessels in DES-treated animals are arterioles or metarterioles because they have a narrow lumen that is surrounded by a relatively thicker ACTA2-positive smooth muscle wall (Fig. 3G, the wall is thicker than the diameter of the lumen).
Histochemical Localization of Fat in the Penis
An examination of unstained, paraffinized, osmium tetroxide-stained sections from control and DES-treated rats of all age groups revealed that fat cells were limited to the corpora cavernosa of the body of the penis (Fig. 4, A–F). A comparison of sections from the control and DES-treated animals revealed that treated animals always had more fat cells and more cells containing larger fat droplets than controls of the same age (cf. Fig. 4, A–D, with 4, E–H). Furthermore, the number of fat cells and/or fat cells with larger fat droplets decreased with age in controls to the point that only a few fat cells were left at adulthood, whereas they increased with age in DES-treated rats to the point that they filled the corpora cavernosa at adulthood. Unlike the corpora cavernosa, the corpus spongiosum did not contain fat cells in the control or DES-treated animals in any age group.
FIG. 4.
Histochemical localization of fat cells in the body of the penis at 7, 10, and 21 days of age and at adulthood in control (A–D) and DES-treated (E–H) rats. Note accumulation of fat cells in DES-treated animals compared to that in controls. Also, note the absence of fat cells in the corpus spongiosum (CS) that is located ventrally and surrounds the urethra. En block staining with osmium tetroxide. CC, corpora cavernosa. Bar = 100 μm.
Quantitative Real Time-PCR Analysis of Pparγ, Esr1, Esr2, and Ar mRNA Expression in the Penis
Esr1 expression was significantly two- to fourfold increased by 7 and 21 days of age in the DES-treated group, respectively (Fig. 5, A and B). Temporally, from 7 to 21 days of age, while Esr1 expression significantly increased in both control and treated groups, there was less than onefold increase in the control group versus more than threefold increase in the DES group (Fig. 5C). Esr2 and Ar expression levels were similar between controls and DES groups in both age groups (Fig. 5, A and B). Temporally, Esr2 and Ar expression levels declined by 40%−70% in controls and in the DES group (Fig. 5, D and E). Pparγ expression was not significantly different between controls and the DES group at 7 days of age but was significantly higher at 21 days in the DES-treated group (Fig. 5, A and B). Temporally, while Pparγ was unaltered in the control group, it was more than twofold increased in the DES group (Fig. 5F).
FIG. 5.
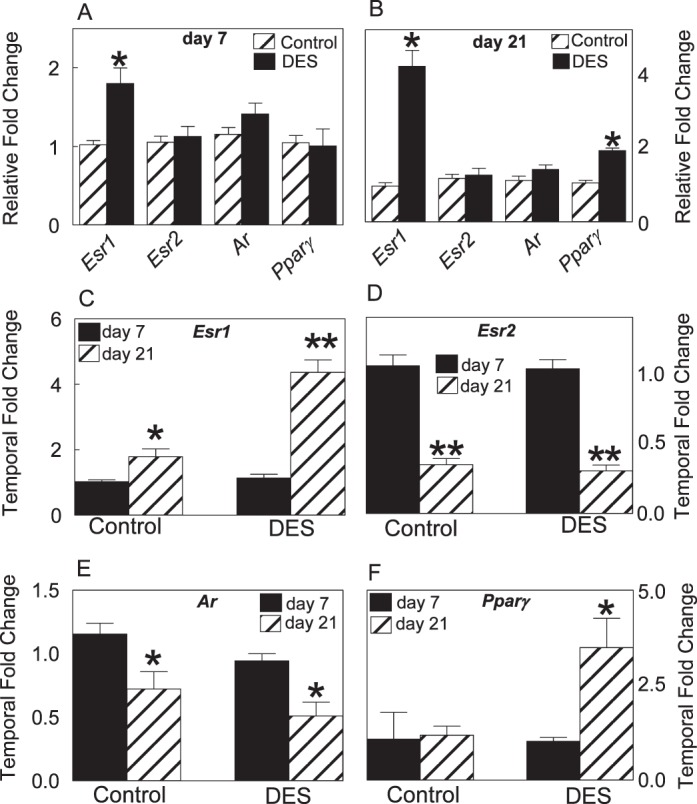
Quantitative real time-PCR shows a relative fold change in Esr1, Esr2, Ar, and Pparγ expression levels at 7 (A) and 21 days (B) of age as a result of DES treatment and shows a temporal fold change in Esr1 (C), Esr2 (D), Ar (E), and Pparγ (F) expression levels from 7 to 21 days of age in controls and DES-treated rats. Note significant up-regulation in Esr1 expression at 7 and 21 days and in Pparγ expression at 21 days as a result of DES treatment. Asterisks denote significant differences from controls at the level of *P < 0.05 or **P < 0.001.
Intratesticular and Plasma Testosterone
The mean intratesticular testosterone level at 7 days in controls was 419 ng/g, which was decreased by 90% as a result of DES treatment, compared to that in controls (Fig. 6). Coadministration of ICI with DES restored the intratesticular testosterone to the control level; however, coadministration of DHT with DES had no restorative effect. While treatment with ICI alone had no diminishing effect on intratesticular testosterone level, treatement with DHT alone reduced it to the level present in animals treated with DES alone or treated with DES plus DHT (Fig. 6). Compared to levels at 7 days, the mean intratesticular testosterone level at 21 days in controls was reduced to 56 ng/g, which was not different from any of the other treated groups (data not shown), except in the DES plus DHT group, where it was 415 ng/g. It is noteworthy that the testosterone level in animals of the latter group (n = 4) ranged from 322 to 490 ng/g, and data were similar when the assay was repeated. The plasma testosterone level was undetectable in all groups at 21 days of age.
FIG. 6.
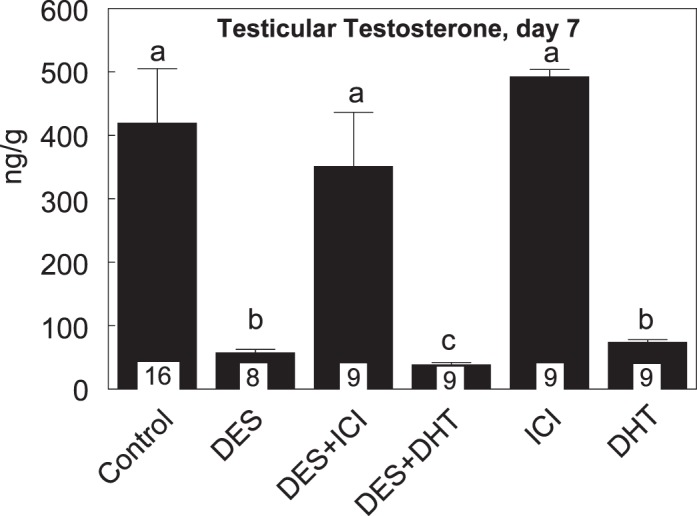
Testicular testosterone (ng/g) at 7 days of age in rats treated from 1 to 6 days of age with DES with or without coadministration of ICI or DHT. Note nearly 90% reduction in the neonatal testosterone surge as a result of DES treatment and its reversal to the control level by ICI coadministration, but not by DHT coadministration. Data are expressed as means ± SEM. Means with different lowercase letters are significantly different from each other (P < 0.05). Numbers within bars indicate the number of animals in each experiment group.
Body Weight and Penile Measurement
The emphasis of the study was to report estrogen-induced alterations at the subcellular and cellular levels. However, data for body weight and penile measurements are reported to validate the efficacy of experiments. The mean body weights were similar between controls and all treated groups at both 7 and 21 days of age (16.0 ± 0.5 g and 48.6 ± 1.2 g, respectively, in controls), except in the DES group, where it was significantly (P ≤ 0.05) lower by nearly 20% at 7 days and higher by nearly 15% at 21 days. The mean body weight at 10 days of age was not significantly different between the control and DES groups (18.6 ± 0.56 vs. 17.2 ± 0.62 mg, respectively). Both the weight and the length of the penis were significantly (P ≤ 0.05) decreased by 18%–40% at 7 and/or 21 days of age in the DES group, compared to that in controls; and both decreases were mitigated by ICI or DHT coadministration with DES (Fig. 7, A and B).
FIG. 7.
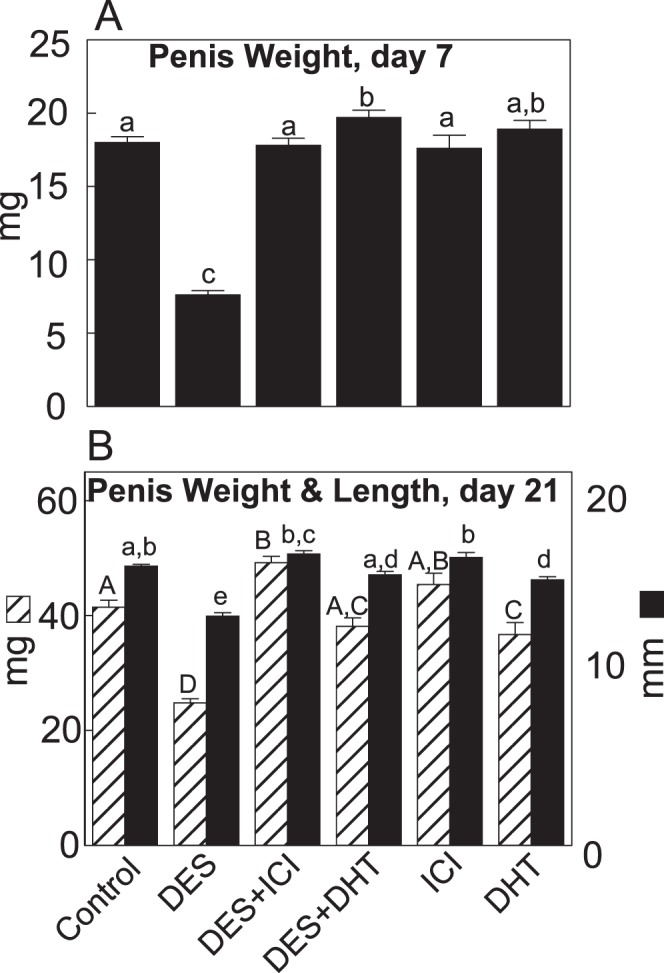
The penis weight at 7 days of age (A) and the penis weight and length at 21 days of age (B) in rats treated from 1 to 6 days of age with DES, with or without coadministration of ICI or DHT. Note that the coadministration of ICI or DHT mitigated DES-induced reductions in penile measurements in both age groups. Data are expressed as mean ± SEM. Means with different lowercase letters are significantly (P < 0.05) different from each another. Numbers within bars indicate the number of animals in each experiment group.
DISCUSSION
Results of the study provided evidence that DES-induced loss of cavernous smooth muscle cells in the body of the penis involved down-regulation of biomarkers for smooth muscle cell differentiation. This evidence is based on microarray analysis, where expression levels for Myh11, Myh7, Mylk, and Actg2 were significantly decreased, and on q-real time-PCR and Western blot analyses, where MYH11 expression was significantly lower in all DES-treated animals. Actually, MYH11 expression at the protein level was barely detectable in four of six DES-treated animals. Furthermore, results provided evidence that these effects were long-lasting because there was only onefold, nonsignificant (P ≥0.05), increase in temporal Myh11 mRNA expression from 7 to 21 days of age in DES-treated rats, in contrast to threefold (P ≤ 0.001) increase in controls.
Immunohistochemical localization of smooth muscle alpha actin, another biomarker for smooth muscle, corroborated molecular data where cavernous smooth muscle cells as well as cavernous spaces were much less developed in DES-treated rats, compared to controls of all age groups examined in this study. Interestingly, immunohistochemical observations also revealed that most vessels in the penis corpora cavernosa of DES-treated animals were arterioles or metarterioles, instead of cavernous spaces, implying that DES probably targeted these arterioles and inhibited their branching into cavernous spaces. These arterioles are unique because they are parts of a helicine (coiled) artery that is present only in the corpora cavernosa [34], and they also contain smooth muscle cells under the endothelium, similar to cavernous spaces in the corpora cavernosa. The unique blood supply to the corpora cavernosa might explain why DES exposure did not affect development of the corpus spongiosum because it is supplied by the bulbourethral artery, which is not a helical artery, and its cavernous spaces are not surrounded by smooth muscle cells [34]. To our knowledge, there is no report that describes effects of neonatal estrogen exposure on proliferation of cavernous smooth muscle cells in the rat; however, estrogen exposure in vitro was shown to inhibit proliferation of human fetal penile smooth muscle cells in a dose-dependent manner [35].
Another important effect of exposure of neonatal pups to DES was an accumulation of fat cells in the corpora cavernosa. This accumulation occurred in all age groups; however, compared to accumulation in pups at 7 and 10 days of age, the accumulation was more striking at 21 days of age and adulthood, where fat cells filled the corpora cavernosa. On the contrary, the number of fat cells as well as the area occupied by fat cells decreased with age to the point that only a few fat cells were left in the corpora cavernosa of control adult rats. It is noteworthy that the above identified age-related differences between control and treated animals, although descriptive, are dramatic and obvious as seen with the light microscope. Present observations that mRNA expression for Pparγ, a marker for fat cells, was nearly twofold increased at 21 days of age in DES-treated rats, compared to that in controls, provide support for the histochemical data. A possible reason for not observing similar differences in Pparγ mRNA expression at 7 days of age could be that the total number of fat cells was probably not different between control and treated animals at this time of penile development. The reason for an apparent increase in fat accumulation at 7 days may lie in the reduced thickness of the body of the penis in treated animals, thereby, giving an illusion of more fat cells being present.
Another noteworthy observation was the mitigation of DES-induced reduction in Myh11 mRNA expression at both 7 and 21 days in animals that simultaneously received DES and ESR antagonist ICI or DES and AR agonist DHT. These observations imply that the mechanism of DES-induced down-regulation of Myh11 expression probably involves both ESR- and AR-mediated pathways. As far as the ESR pathway is concerned, the present data support the involvement of ESR1-mediated pathway as DES treatment significantly increased Esr1 expression at both 7 and 21 days, without altering Esr2 expression in both age groups compared to controls. Observations that adult Esr1-knockout (Esr−1−) mice treated neonatally with DES were completely resistant to DES-induced abnormalities present in the wild-type littermates provide further support for the role of ESR1 in mediation of estrogen-inducible abnormalities in the developing penis [30]. Additional support for this role comes from observations that the prostate gland of adult Esr−1− mice treated neonatally with DES developed normally but that of the wild-type and Esr2-knockout (Esr−2−) mice developed epithelial hyperplasia and dysplasia following DES treatment [36]. Adult (Esr−1−) female mice treated with DES neonatally failed to develop pathological abnormalities that were present in the genital tract of similarly treated wild-type and (Esr−2−) female mice [37].
However, it remains unclear whether estrogens induce penile malformations via a direct effect on the penis or via an indirect effect through estrogen-induced reduced androgen action or both. The support for a direct effect comes from observations that estrogen receptors are present in the rat penis at birth [38] and from our observations that estrogen-induced penile maldevelopment was associated with ESR1 up-regulation [39] and was mitigated by ESR antagonist ICI coadministration with estrogen [24]. ESR1 up-regulation was associated with abnormal development of the female reproductive tract [40], mammary gland [41], prostate [42], and seminal vesicles [43] in rodents treated neonatally with estrogens. ESR1 up-regulation inhibited growth and angiogenic factors in the endometrial carcinoma cell line Ishikawa [44]. Furthermore, ESR1 is the main regulator of estrogenic effects on adipose tissue, and an alteration in estrogen signaling during development dramatically increased adipocyte number [45]. Estrogens inhibited proliferation of vascular smooth muscle cells [46], and the estrogen metabolite 2-methoxyestradiol inhibited angiogenesis [47].
Alternately, androgen receptors are also present in the neonatal rat penis [48, 49]. Present observations that DES treatment suppressed the neonatal testosterone surge by 90% at 7 days of age without altering Ar expression point to a lower androgen action at the hormone level as a probable cause for DES-inducible penile abnormalities. Additional observations that animals treated with DHT alone or DHT plus DES had normally developed penises and normal Myh11 expression levels despite nearly 90% decline in the neonatal testosterone surge imply that the exogenous DHT treatment circumvented a lower androgen action in both treatments. Thus, these observations highlight the significance of the neonatal testosterone surge in normal differentiation of cavernous smooth muscle cells and the lack thereof in their permanent loss. In this regard, it is noteworthy that testosterone stimulated proliferation of human fetal penile cavernous smooth muscle cells in vitro [35] and that low testosterone inhibited smooth muscle differentiation and promoted adipocyte differentiation in pluripotent mesenchymal cells in vitro [50, 51]. Additionally, castration induced loss of smooth muscle cells and deposition of fat cells in the corpora cavernosa of the rabbit penis [52]. The coadministration of testosterone with DES prevented most of the histopathological abnormalities affecting the male reproductive tract in rats [31].
Present results regarding DES-induced suppression of the neonatal testosterone surge are in agreement with a previous study where in utero exposure to DES at a dose similar to ours (0.1 mg/kg) on 13.5, 15.5, and 17.5 days of pregnancy reduced the intratesticular testosterone concentration on Day 19.5 of pregnancy to nearly 10% of the control value [53]. Similar to estrogens, antiandrogen-induced disruption in the prenatal testosterone surge during the “masculinization programming window” (15.5–18.5 gestation days of rats) reduced adult penile length [54]. Neonatal exposure to GnRH-antagonist reduced the neonatal testosterone surge by 70%–90% and induced penile malformations similar to those induced by estrogens [23]. Additionally, DES-induced down-regulation of steroidogenic genes (Hsd3β1, Star, Cyp11α1, Cyp17α1, Cyp2c22) that we observed in the microarray analysis results at 7 days of age is in agreement with previous studies where expression levels of the StAR protein [55] and that of cytochrome P450 17α-hydroxylase/C17-20 lyase [56] were reduced in the testis as a result of DES exposure. Leydig cells in Esr−1− mice had higher testosterone production and higher steroidogenic enzyme activity than Leydig cells in wild-type mice [57]. DES exposure to fetal or neonatal Leydig cells decreased testosterone production in wild-type mice, but not in Esr−1− mice, implying an ESR1-mediated role of estrogen in testosterone production [58].
In conclusion, results of the present study provided evidence that DES treatment to neonatal rat pups down-regulated expression of MYH11 and ACTA2, biomarkers for smooth muscle cell differentiation, leading to the loss of cavernous smooth muscle cells in the corpora cavernosa of the body of the penis. Coadministration of the ESR antagonist ICI or the AR agonist DHT mitigated DES-induced effects, implying the role of ESR and AR pathways in mediating DES effects. These effects were probably induced by a lower androgen action resulting from DES-induced suppression of the neonatal testosterone surge. These findings are significant because cavernous smooth muscle cells are an essential component in penile erection and also because both humans and wild-life organisms are continuously exposed to a mixture of environmental estrogens and antiandrogens throughout prenatal and postnatal life.
ACKNOWLEDGMENT
The authors thank Carol Williams for comments and editorial corrections, LaDissa Moore for technical help, and Dr. Abdalla Eljack, department head, and Dr. Tsegaye Habtemariam, Dean, for their encouragement and support.
Footnotes
Supported by National Institutes of Health grants 5SC1ES019355 (to H.O.G.) and RCMI-5-G12RR03059.
REFERENCES
- Yiee JH, Baskin LS. Penile embryology and anatomy. ScientificWorldJournal 2010; 10:1174 1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res 2008; 20:17 29 [DOI] [PubMed] [Google Scholar]
- Burnett AL. Molecular pharmacotherapeutic targeting of PDE5 for preservation of penile health. J Androl 2008; 29:3 14 [DOI] [PubMed] [Google Scholar]
- Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 1999; 84:50 56 [DOI] [PubMed] [Google Scholar]
- Nehra A, Barrett DM, Moreland RB. Pharmacotherapeutic advances in the treatment of erectile dysfunction. Mayo Clin Proc 1999; 74:709 721 [DOI] [PubMed] [Google Scholar]
- Wespes E, Goes PM, Schiffmann S, Depierreux M, Vanderhaeghen JJ, Schulman CC. Computerized analysis of smooth muscle fibers in potent and impotent patients. J Urol 1991; 146:1015 1017 [DOI] [PubMed] [Google Scholar]
- Wei AY, He SH, Zhao JF, Liu Y, Liu Y, Hu YW, Zhang T, Wu ZY. Characterization of corpus cavernosum smooth muscle cell phenotype in diabetic rats with erectile dysfunction. Int J Impot Res. 2012; 24(5):196 201 [DOI] [PubMed] [Google Scholar]
- Traish AM, Park K, Dhir V, Kim NN, Moreland RB, Goldstein I. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology 1999; 140:1861 1868 [DOI] [PubMed] [Google Scholar]
- Palese MA, Crone JK, Burnett AL. A castrated mouse model of erectile dysfunction. J Androl 2003; 24:699 703 [DOI] [PubMed] [Google Scholar]
- Aversa A, Isidori AM, De Martino MU, Caprio M, Fabbrini E, Rocchietti-March M, Frajese G, Fabbri A. Androgens and penile erection: evidence for a direct relationship between free testosterone and cavernous vasodilation in men with erectile dysfunction. Clin Endocrinol (Oxf) 2000; 53:517 522 [DOI] [PubMed] [Google Scholar]
- George FW, Wilson JD. Sex determination and differentiation : Knobil E, Neil J.(eds.), The Physiology of Reproduction. New York: Raven Press; 1994; 3 28 [Google Scholar]
- Gray LE, Ostby J, Furr J, Wolf CJ, Lambright C, Parks L, Veeramachaneni DN, Wilson V, Price M, Hotchkiss A, Orlando E, Guillette L. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum Reprod Update 2001; 7:248 264 [DOI] [PubMed] [Google Scholar]
- van den Driesche S, Scott HM, MacLeod DJ, Fisken M, Walker M, Sharpe RM. Relative importance of prenatal and postnatal androgen action in determining growth of the penis and anogenital distance in the rat before, during and after puberty. Int J Androl 2011; 34:e578 586 [DOI] [PubMed] [Google Scholar]
- Swan SH, Main KM, Liu F, Stewart SL, Kruse RL, Calafat AM, Mao CS, Redmon JB, Ternand CL, Sullivan S, Teague JL; Study for Future Families Research Team. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect 2005; 113:1056 1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ Jr, Jegou B, Jensen TK, Jouannet P, Keiding N, Leffers H, McLachlan JA, et al. Male reproductive health and environmental xenoestrogens. Environ Health Perspect 1996; 104(suppl 4):741 803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspari L, Sampaio DR, Paris F, Audran F, Orsini M, Neto JB, Sultan C. High prevalence of micropenis in 2710 male newborns from an intensive-use pesticide area of Northeastern Brazil. Int J Androl 2012; 35:253 264 [DOI] [PubMed] [Google Scholar]
- Wohlfahrt-Veje C, Andersen HR, Jensen TK, Grandjean P, Skakkebaek NE, Main KM. Smaller genitals at school age in boys whose mothers were exposed to non-persistent pesticides in early pregnancy. Int J Androl 2012; 35:265 272 [DOI] [PubMed] [Google Scholar]
- McLachlan JA, Newbold RR, Bullock B. Reproductive tract lesions in male mice exposed prenatally to diethylstilbestrol. Science 1975; 190:991 992 [DOI] [PubMed] [Google Scholar]
- Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol 2004; 199:142 150 [DOI] [PubMed] [Google Scholar]
- Gupta C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc Soc Exp Biol Med 2000; 224:61 68 [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Tang WY, Ho SM. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod Toxicol 2007; 23:374 382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette LJ Jr, Pickford DB, Crain DA, Rooney AA, Percival HF. Reduction in penis size and plasma testosterone concentrations in juvenile alligators living in a contaminated environment. Gen Comp Endocrinol 1996; 101:32 42 [DOI] [PubMed] [Google Scholar]
- Simon L, Avery L, Braden TD, Williams CS, Okumu LA, Williams JW, Goyal HO. Exposure of neonatal rats to anti-androgens induces penile mal-developments and infertility comparable to those induced by oestrogens. Int J Androl 2012; 35:364 376 [DOI] [PubMed] [Google Scholar]
- Goyal HO, Braden TD, Williams CS, Williams JW. Estrogen-induced developmental disorders of the rat penis involve both estrogen receptor (ESR)- and androgen receptor (AR)-mediated pathways. Biol Reprod 2009; 81:507 516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews E, Braden TD, Williams CS, Williams JW, Bolden-Tiller O, Goyal HO. Mal-development of the penis and loss of fertility in male rats treated neonatally with female contraceptive 17alpha-ethinyl estradiol: a dose-response study and a comparative study with a known estrogenic teratogen diethylstilbestrol. Toxicol Sci 2009; 112:331 343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal HO, Braden TD, Williams CS, Dalvi P, Mansour M, Williams JW. Estrogen-induced abnormal accumulation of fat cells in the rat penis and associated loss of fertility depends upon estrogen exposure during critical period of penile development. Toxicol Sci 2005; 87:242 254 [DOI] [PubMed] [Google Scholar]
- Murakami R. A histological study of the development of the penis of wild-type and androgen-insensitive mice. J Anat 1987; 153:223 231 [PMC free article] [PubMed] [Google Scholar]
- Klonisch T, Fowler PA, Hombach-Klonisch S. Molecular and genetic regulation of testis descent and external genitalia development. Dev Biol 2004; 270:1 18 [DOI] [PubMed] [Google Scholar]
- Smithells RW. Oral contraceptives and birth defects. Dev Med Child Neurol 1981; 23:369 372 [PubMed] [Google Scholar]
- Goyal HO, Braden TD, Cooke PS, Szewczykowski MA, Williams CS, Dalvi P, Williams JW. Estrogen receptor alpha mediates estrogen-inducible abnormalities in the developing penis. Reproduction 2007; 133:1057 1067 [DOI] [PubMed] [Google Scholar]
- Rivas A, McKinnell C, Fisher JS, Atanassova N, Williams K, Sharpe RM. Neonatal coadministration of testosterone with diethylstilbestrol prevents diethylstilbestrol induction of most reproductive tract abnormalities in male rats. J Androl 2003; 24:557 567 [DOI] [PubMed] [Google Scholar]
- Mansour MM, Goyal HO, Braden TD, Dennis JC, Schwartz DD, Judd RL, Bartol FF, Coleman ES, Morrison EE. Activation of penile proadipogenic peroxisome proliferator-activated receptor gamma with an estrogen: interaction with estrogen receptor alpha during postnatal development. PPAR Res 2008; 2008:651419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Kobayashi M, Saitoh S, Someya T, Hosaka T, Sezaki H, Suzuki Y, Suzuki F, Akuta N, Arase Y, Kumada H. Origin of neovascular structure in an early stage of hepatocellular carcinoma: study of alpha-smooth muscle actin immunohistochemistry in serial thin sections of surgically resected cancer. J Gastroenterol Hepatol 2006; 21:183 190 [DOI] [PubMed] [Google Scholar]
- Aboseif SA, Tanagho EA.(eds.) Anatomy of the Penis. San Fransisco, CA: Allen Press, Inc.; 1999 [Google Scholar]
- Crescioli C, Maggi M, Vannelli GB, Ferruzzi P, Granchi S, Mancina R, Muratori M, Forti G, Serio M, Luconi M. Expression of functional estrogen receptors in human fetal male external genitalia. J Clin Endocrinol Metab 2003; 88:1815 1824 [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res 2001; 61:6089 6097 [PubMed] [Google Scholar]
- Couse JF, Korach KS. Estrogen receptor-alpha mediates the detrimental effects of neonatal diethylstilbestrol (DES) exposure in the murine reproductive tract. Toxicology 2004; 205:55 63 [DOI] [PubMed] [Google Scholar]
- Jesmin S, Mowa CN, Matsuda N, Salah-Eldin AE, Togashi H, Sakuma I, Hattori Y, Kitabatake A. Evidence for a potential role of estrogen in the penis: detection of estrogen receptor-alpha and -beta messenger ribonucleic acid and protein. Endocrinology 2002; 143:4764 4774 [DOI] [PubMed] [Google Scholar]
- Goyal HO, Braden TD, Williams CS, Dalvi P, Mansour MM, Mansour M, Williams JW, Bartol FF, Wiley AA, Birch L, Prins GS. Abnormal morphology of the penis in male rats exposed neonatally to diethylstilbestrol is associated with altered profile of estrogen receptor-α protein, but not of androgen receptor protein: a developmental and immunocytochemical study. Biol Reprod 2004; 70:1504 1517 [DOI] [PubMed] [Google Scholar]
- Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod 2005; 72:1344 1351 [DOI] [PubMed] [Google Scholar]
- Diaz-Cruz ES, Sugimoto Y, Gallicano GI, Brueggemeier RW, Furth PA. Comparison of increased aromatase versus ERalpha in the generation of mammary hyperplasia and cancer. Cancer Res 2011; 71:5477 5487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology 1997; 138:1801 1809 [DOI] [PubMed] [Google Scholar]
- Williams K, Fisher JS, Turner KJ, McKinnell C, Saunders PT, Sharpe RM. Relationship between expression of sex steroid receptors and structure of the seminal vesicles after neonatal treatment of rats with potent or weak estrogens. Environ Health Perspect 2001; 109:1227 1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SH, O'Donnell AL, Mohamed S, Mousa S, Dandona P. Overexpression of estrogen receptor-alpha in the endometrial carcinoma cell line Ishikawa: inhibition of growth and angiogenic factors. Gynecol Oncol 2004; 95:637 645 [DOI] [PubMed] [Google Scholar]
- Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med (Maywood) 2004; 229:1127 1135 [DOI] [PubMed] [Google Scholar]
- Ortmann J, Veit M, Zingg S, Di Santo S, Traupe T, Yang Z, Volzmann J, Dubey RK, Christen S, Baumgartner I. Estrogen receptor-alpha but not -beta or GPER inhibits high glucose-induced human VSMC proliferation: potential role of ROS and ERK. J Clin Endocrinol Metab 2011; 96:220 228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotsis T, Zhang Y, Pepper MS, Adlercreutz H, Montesano R, Nawroth PP, Schweigerer L. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature 1994; 368:237 239 [DOI] [PubMed] [Google Scholar]
- Rajfer J, Namkung PC, Petra PH. Identification, partial characterization and age-related changes of a cytoplasmic androgen receptor in the rat penis. J Steroid Biochem 1980; 13:1489 1492 [DOI] [PubMed] [Google Scholar]
- Takane KK, George FW, Wilson JD. Androgen receptor of rat penis is down-regulated by androgen. Am J Physiol 1990; 258:E46–E50 [DOI] [PubMed] [Google Scholar]
- Bhasin S, Taylor WE, Singh R, Artaza J, Sinha-Hikim I, Jasuja R, Choi H, Gonzalez-Cadavid NF. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J Gerontol A Biol Sci Med Sci 2003; 58:M1103 1110 [DOI] [PubMed] [Google Scholar]
- Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology 2003; 144:5081 5088 [DOI] [PubMed] [Google Scholar]
- Traish AM, Toselli P, Jeong SJ, Kim NN. Adipocyte accumulation in penile corpus cavernosum of the orchiectomized rabbit: a potential mechanism for veno-occlusive dysfunction in androgen deficiency. J Androl 2005; 26:242 248 [DOI] [PubMed] [Google Scholar]
- Haavisto TE, Adamsson NA, Myllymäki SA, Toppari J, Paranko J. Effects of 4-tert-octylphenol, 4-tert-butylphenol, and diethylstilbestrol on prenatal testosterone surge in the rat. Reproductive Toxicology 2003; 17:593 605 [DOI] [PubMed] [Google Scholar]
- Welsh M, MacLeod DJ, Walker M, Smith LB, Sharpe RM. Critical androgen-sensitive periods of rat penis and clitoris development. Int J Androl 2010; 33:e144 152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkila TF, Toppari J, Paranko J. Effects of neonatal exposure to 4-tert-octylphenol, diethylstilbestrol, and flutamide on steroidogenesis in infantile rat testis. Toxicol Sci 2006; 91:456 466 [DOI] [PubMed] [Google Scholar]
- Majdic G, Sharpe RM, O'Shaughnessy PJ, Saunders PT. Expression of cytochrome P450 17alpha-hydroxylase/C17-20 lyase in the fetal rat testis is reduced by maternal exposure to exogenous estrogens. Endocrinology 1996; 137:1063 1070 [DOI] [PubMed] [Google Scholar]
- Akingbemi BT, Ge R, Rosenfeld CS, Newton LG, Hardy DO, Catterall JF, Lubahn DB, Korach KS, Hardy MP. Estrogen receptor-alpha gene deficiency enhances androgen biosynthesis in the mouse Leydig cell. Endocrinology 2003; 144:84 93 [DOI] [PubMed] [Google Scholar]
- Delbes G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R. Endogenous estrogens inhibit mouse fetal Leydig cell development via estrogen receptor alpha. Endocrinology 2005; 146:2454 2461 [DOI] [PubMed] [Google Scholar]




