ABSTRACT
Several studies have demonstrated that maternal undernutrition or overnutrition during pregnancy can have negative consequences for the health of children born to these pregnancies, but the physiological mechanisms by which this occurs are not completely understood. During periods of food restriction, concentrations of leptin decline, whereas leptin is elevated in obesity, suggesting that it may play a role in the response to altered nutrition during pregnancy. This study compares placental development and global placental gene expression profiles at Day 11.5 in pregnant control mice, mice that were undernourished, and mice that were undernourished but given leptin. Placentas from mothers exposed to food restriction preserved the placental labyrinth zone at the expense of the junctional zone, an effect abrogated in the restricted plus leptin group, which had a significant decrease in the labyrinth zone area compared with controls. Similarly, there were more significant differences in gene expression between placentas from control and restricted plus leptin mothers (1128 differentially expressed genes) than between placentas of control and restricted mothers (281 differentially expressed genes). We conclude that the presence of high concentrations of circulating leptin during food restriction disrupts the normal adaptive response of the placenta to reduced energy availability.
Keywords: developmental origin of adult health and disease (DOHAD), leptin, trophoblast, undernutrition
Elevated maternal leptin disrupts the response of the placenta to maternal food restriction during early pregnancy.
INTRODUCTION
The peptide hormone leptin was initially discovered as the missing factor in the Lepob/ob mouse, which has a spontaneous mutation in the leptin gene and is therefore obese, hyperphagic, cold intolerant, and infertile [1]. Administration of leptin to these mice restored normal weights, raising the prospect that leptin could be a cure for obesity. However, it was soon found that serum leptin concentrations are generally proportional to body mass index, and most people who are obese are leptin resistant, with very high concentrations of circulating leptin [2, 3]. These findings gave rise to a different understanding of the function of leptin. It was proposed that leptin signals that fat storage is adequate and that loss of leptin, as occurs during fasting or sustained weight loss, functions to conserve energy [4, 5]. The characteristics of the leptin-deficient Lepob/ob mouse–hyperphagia, reduced activity and metabolic rate, and a shut-down reproductive system–are adaptive in an individual who is undernourished, allowing him or her to conserve resources [4].
We hypothesized that high concentrations of leptin would therefore disrupt the adaptive response to food restriction during pregnancy, specifically placental adaptations. We have previously found that in mice that are restricted to 50% of their normal food consumption from Days 0.5 to 11.5 of pregnancy, serum leptin concentrations decline significantly [6]. Similarly, in sheep, food restriction prevents the increase in serum leptin that occurs during normal pregnancy [7]. The placenta is directly exposed to maternal serum leptin, and leptin has been shown to influence multiple placental functions in vitro, including trophoblast differentiation [8], hormone production [9], trophoblast invasion [10], and nutrient transport [11]. However, its role in the placenta in vivo, particularly in relation to changing nutrient availability, has not been characterized.
By midpregnancy, the placenta is responsible for the exchange of all nutrients and growth factors between maternal and fetal circulations, and is a major determinant of fetal growth. It has been proposed that the effects of early pregnancy nutrient restriction on fetal growth may be mediated by effects on placental growth and development [12, 13]. Thus, understanding the role of leptin in the response to undernutrition during pregnancy may be important in uncovering mechanisms of the developmental origins of adult health and disease. It has been shown that both maternal undernutrition and overnutrition during pregnancy can lead to obesity and other negative health consequences for offspring [14]. One of the earliest and clearest lines of evidence for developmental origins of adult health and disease is the Dutch Hunger Winter Study, in which men whose mothers were food restricted during early pregnancy, but not restricted during later pregnancy, had increased rates of metabolic and cardiovascular disease as adults [15]. This has been modeled in classic studies in the rat, in which food restriction during the first half of pregnancy results in increased offspring weights in adulthood [16, 17]. We have therefore chosen to focus on the effects of food restriction during this time period. We previously compared offspring from three groups of mouse mothers treated from Days 0.5 to 11.5 of pregnancy: controls, mothers that were food restricted, and mothers that were food restricted and given excess leptin. There was a reduction in body fat percentage in the male offspring of food-restricted mothers but not in the male offspring of restricted mothers treated with leptin. Female offspring of restricted, leptin-treated mothers were heavier, had higher body fat percentage, and were more glucose intolerant when fed a high-fat diet than offspring of control or restricted mothers [18]. Thus, the artificial presence of high leptin concentrations during food restriction was more deleterious to offspring health than food restriction alone, and it resembled the effects of maternal obesity. In the present study, we used the same three maternal treatment groups but analyzed placental morphology and gene expression at Day 11.5, the last day of the food restriction period, to begin to elucidate mechanisms by which offspring health is affected by altered maternal leptin concentrations.
MATERIALS AND METHODS
Animals
All animal procedures were approved by the University of Missouri Animal Care and Use Committee and were conducted in accordance with the National Institutes of Health's (NIH's) Guide for Care and Use of Laboratory Animals. Female Swiss Webster (ND4) mice, age 6–8 wk, were obtained from Harlan Laboratories or born at the University of Missouri to Swiss Webster mice obtained from Harlan, and they were individually housed at the University of Missouri. Mice were acclimated for 1 wk to AIN-93G diet for pregnancy and lactation (Research Diets) in a powdered, blue-dyed form presented in specialized dishes for weighing [19]. Average daily individual food consumption levels were determined during 10–14 days. The females were then introduced to Swiss Webster males, and the day in which a copulatory plug was detected was considered pregnancy Day 0.5. Females were returned to individual cages, and at Day 1.5 they were randomly assigned to one of three treatment groups: 1) ad libitum-fed controls (n = 8), 2) food restricted to 50% of prior consumption levels (n = 13), or 3) 50% restricted and given 1 μg of leptin per gram of body weight per day (n = 12). Leptin (restricted + leptin group) or saline (control and restricted groups) was given once daily at 1400 h via intraperitoneal injection. The leptin dose used was previously shown to reverse fasting-induced infertility in mice [4]. All groups were fed at 1600 h. Food intake was not measured during the treatment period. However, the restricted and restricted + leptin mice were observed to consume all of the food provided. Mice were euthanized at Day 11.5 for collections. Blood was collected by cardiac puncture. The placentas from one horn (selected randomly) were used for morphology, and the other placentas were used for RNA collection. Tissue was not collected from sites of fetal resorption.
To test the effects of saline injection on serum leptin concentrations, blood was collected from 10 female Swiss Webster mice maintained on ad libitum feed via the saphenous vein. They were then restricted to 50% of their daily food consumption levels for 10 days, with either no further treatment (n = 5) or daily saline injection (n = 5). Blood was collected again on the 10th day for leptin measurement.
Placental Morphology
Placentas were weighed and counted, and then half of them were fixed overnight in 4% paraformaldehyde. Each of these placentas was sliced in half midsagitally and embedded in paraffin, with the cut-face down (RADIL). Three pairs of serial 5-μm sections were cut at 50-μm intervals from each placenta and were stained with hematoxylin and eosin. The largest face on each slide was chosen for morphological analysis. Overlapping images were photographed with a 4× objective lens on an Olympus IX81 microscope and assembled into a single high-resolution image covering the entire placental cross section. Total, junctional zone, and labyrinth zone cross-sectional areas were measured by manual outline with the freehand selection tool and area calculation feature of ImageJ software (NIH) by two independent operators blinded to treatment group. Cross-sectional areas were averaged for three placentas, selected randomly, from each mother, and mothers for which these were not available were excluded from analysis. Three frames per placenta, and two to three placentas per mother were photographed with a 40× objective for analysis of blood spaces. Maternal and fetal blood spaces were identified by the presence of nucleated (fetal) or enucleated (maternal) red blood cells, and were outlined with the freehand selection tool, followed by area determination with ImageJ.
Microarray Analysis
The remaining placentas from each mother were bisected, and one half was frozen in TriReagent (Sigma). Following homogenization and phase separation according to TriReagent instructions, the aqueous phase was further purified using the RNEasy Mini Kit (Qiagen) according to manufacturer's instructions for “RNA cleanup.” Samples from four mothers from each treatment group were analyzed by microarray. Each sample was obtained by pooling RNA from two placentas per mother, selected randomly. Preparation of cRNA, microarray hybridization, and scanning and statistical analysis were carried out by GenUs Biosystems. Mouse 4×44 whole-genome arrays (design ID 026655; Agilent) containing more than 39 000 probes were scanned on an Agilent G2565 Microarray Scanner. Data were analyzed with Agilent Feature Extraction and GeneSpring GX v7.3.1 software. Genes were considered differentially expressed among pairs of treatment groups (control, restricted, and restricted + leptin) with expression difference >150% or <66.7%, P < 0.05. The functional annotation clustering feature of the DAVID Bioinformatics Resource (NIH) was used to identify significantly overrepresented biological pathways, structural domains, cell localization, or other meaningful commonalities among the differentially expressed genes.
Real-Time RT-PCR Analysis
RNA was reverse transcribed using Superscript III First-Strand Synthesis System (Invitrogen) with random hexamer primers according to the manufacturer's protocol. Real-time PCR was then performed using RT2 SYBR Green ROX qPCR Mastermix (Qiagen) on an Applied Biosystems 7500 real-time PCR system. Primers (Integrated DNA Technologies) were designed using Primer Express software (Applied Biosystems/Life Technologies), except for Pdhb [20] and Tpbpa [21] primers, and were validated by performing serial dilutions of template. Primer sequences are shown in Table 1.
TABLE 1.
Primer sequences used for real-time RT-PCR.
Leptin Measurement
Terminal blood samples were collected from each pregnant mother by cardiac puncture. Serum was obtained by centrifugation of blood samples at 1000 × g for 12 min. Leptin concentration was measured by Mouse Leptin ELISA according to the manufacturer's instructions (Linco/Millipore).
Oxidative Phosphorylation Complexes
Relative amounts of the mitochondria oxidative phosphorylation complexes I, III, and V were measured using the Milliplex Rat/Mouse Oxidative Phosphorylation Magnetic Bead Panel (Millipore), a multiplex antibody-based assay. Briefly, frozen samples of two placentas each from seven mothers per treatment group were homogenized in Cell/Mitochondria lysis buffer, and lysate protein content was assessed by BCA assay (Pierce) according to the microplate assay protocol. After plate washing, 10 μg of each lysate was loaded into each well, followed by incubations with microbeads, detection antibodies, and streptavidin-phycoerythrin according to the manufacturer's protocol. Washes were performed using the MAGx3 program on a Bio-Rad magnetic plate washer. After the final wash, beads were resuspended in 125 μl of sheath fluid, and the plate was read on a Bio-Plex 200 fluorescent bead reader (Bio-Rad).
Statistical Analysis
Dams were considered the experimental unit, such that fetal weights, placental weights, and placental areas were averaged for all pups within each mother to obtain n = 1. Treatment effects were assessed by 1-way ANOVA using GraphPad Prism software, with Tukey posthoc test for pairwise comparisons. Bartlett test was used to assess homogeneity of variance, one of the assumptions for ANOVA. For analysis of placental weights by ANOVA, data were inverse transformed to overcome significant differences in variability among treatment groups. In the test of the effects of saline injection, a paired t-test was used to compare leptin concentrations before and after food restriction.
RESULTS
Maternal Characteristics
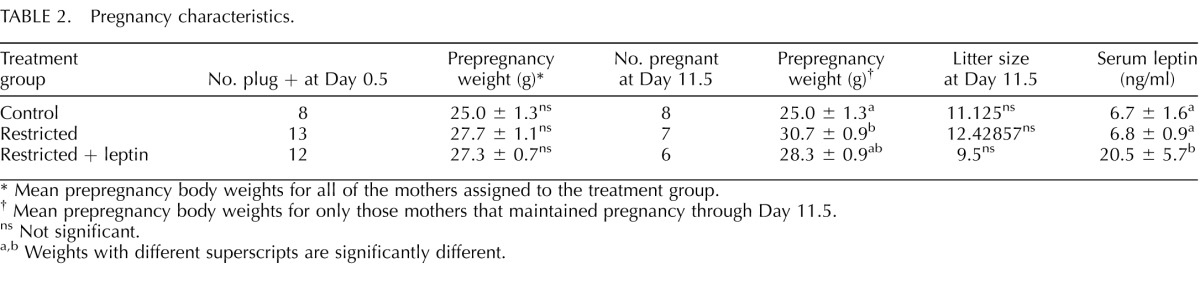
Whereas 94% of ad libitum-fed (control) dams remained pregnant after initial detection of a copulatory plug, approximately half of food-restricted and restricted + leptin dams failed to maintain pregnancies through Day 11.5 (Table 2). Those that maintained pregnancies after food restriction were, on average, heavier prior to pregnancy than those that aborted (Table 2). Two hours after the final leptin injection, serum concentrations in the restricted + leptin group were significantly higher than in the other groups, which were not different from each other (Table 2). Because we had previously found a highly significant decrease in serum leptin in food-restricted mice over the same time period [6], we tested whether saline injections, which were used as a control for leptin injections in the current study, raise leptin concentrations. Leptin concentrations declined significantly from 7.1 ± 1.9 ng/ml to 2.4 ± 0.9 ng/ml after female mice were placed on food restriction for 10 days. However, when food restriction was accompanied by daily saline injections, the decline in serum leptin, from 9.9 ± 2.0 ng/ml to 5.6 ± 1.38 ng/ml, was not statistically significant.
TABLE 2.
Pregnancy characteristics.
* Mean prepregnancy body weights for all of the mothers assigned to the treatment group.
† Mean prepregnancy body weights for only those mothers that maintained pregnancy through Day 11.5.
ns Not significant.
a,b Weights with different superscripts are significantly different.
Placental and Fetal Weights
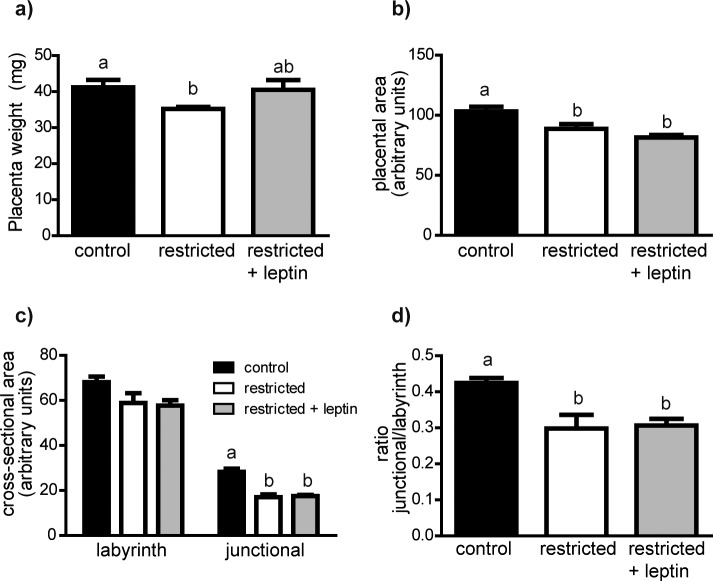
There were no differences in litter sizes at Day 11.5 among treatment groups (Table 2). Placental wet weights were significantly reduced in the restricted group, but the restricted + leptin group was not statistically different from controls (Fig. 1a). There was also significantly different variability across groups (Bartlett test P = 0.0064), suggesting that food restriction limited the normal range of placental weights.
FIG. 1.
a) Average placental wet weights were determined for each litter (control, n = 8; restricted, n = 13; restricted + leptin, n = 12). Midsagittal sections were used to manually select and measure in ImageJ: cross-sectional area (b), cross-sectional area of each major placental zone (c), and the ratio of junctional zone:labyrinth zone area (d). Control, n = 7; restricted, n = 5; restricted + leptin, n = 6. Bars with different letters are significantly different (Tukey multiple comparison test). All measures shown (placental wet weight, placental area, labyrinth area, junctional zone area, and ratio of junctional:labyrinth zone) differed significantly among treatment groups (ANOVA, P < 0.05).
Placental Morphology
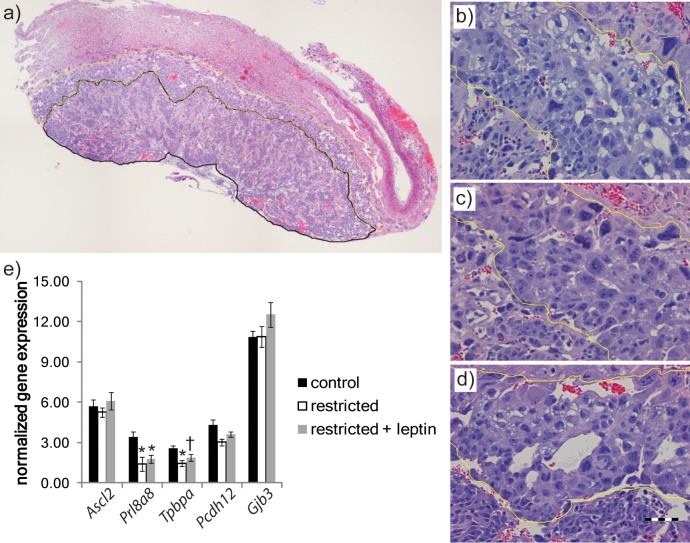
Placental morphology was first assessed by examining cross sections of three placentas from each mother (Figs. 1 and 2a). At Day 11.5, total placental areas were significantly reduced in the restricted group relative to controls. In contrast to the effect of restriction on placental weights, which was reversed by leptin, the decrease in area was not modified by leptin injection (Fig. 1b). The decrease in total area was the result of a highly significant decrease in the area of the junctional zone (P < 0.0001) in both restricted groups (Fig. 1c). The proportionally smaller reduction in labyrinth area (P < 0.05) was consistent only in the restricted + leptin group (Fig. 1c). The relative proportion of the placenta occupied by the junctional zone was significantly decreased in both the restricted and restricted + leptin groups (Fig. 1d). In addition to this quantitative analysis, we observed an apparent reduction in the number of glycogen vacuoles within the junctional zone of placentas in the restricted and restricted + leptin groups (Fig. 2). The morphological observations were reinforced by gene expression data. In the microarray analysis, expression of Tpbpa, a junctional zone marker, was reduced in the restricted and restricted + leptin groups relative to controls (Fig. 2e), a result confirmed by real-time RT-PCR (Table 3). Other junctional zone-expressed genes, Ascl2, Prl8a8, and Pcdh12, exhibited similar patterns of expression among treatment groups, although not all differed significantly (Fig. 2e). There was a tendency for these genes to be less severely reduced in the restricted + leptin group than in the restricted only group, and this corresponded to an observation of a less severe reduction in the number of glycogen vacuoles in the restricted + leptin group.
FIG. 2.
a) Representative placental cross section showing labyrinth (black) and junctional (yellow) zones selected. Representative images of junctional zone (outlined in yellow) from control (b), restricted (c), and restricted + leptin (d) placentas showing glycogen vacuoles. Bar in d = 100 μm. e) Selected microarray data showing relative expression of genes characteristic of major junctional zone cell types, spongiotrophoblast (spongioTB), and glycogen cells. Error bars indicate mean ± SEM. *P < 0.05, fold change >1.5. †P < 0.05, fold change <1.5.
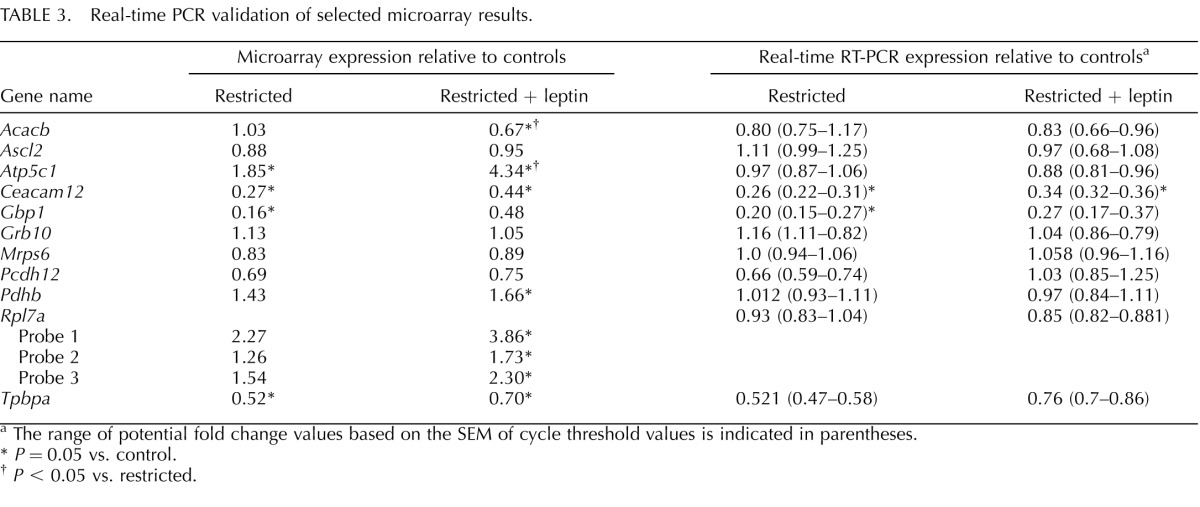
TABLE 3.
Real-time PCR validation of selected microarray results.
The range of potential fold change values based on the SEM of cycle threshold values is indicated in parentheses.
P = 0.05 vs. control.
P < 0.05 vs. restricted.
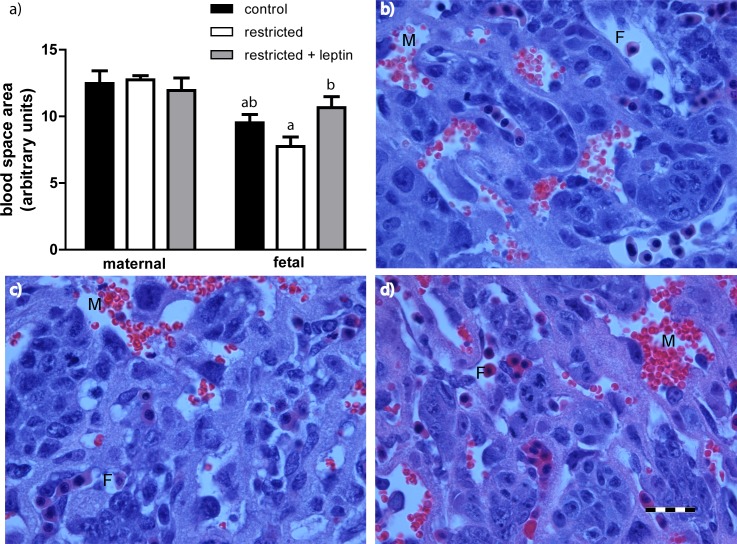
Maternal and fetal blood space areas were assessed in randomly selected areas of the labyrinth zone (Fig. 3). There was significantly lower cross-sectional area of the fetal blood spaces in the food-restricted group compared with the restricted + leptin group, and a numeric, but not statistically significant, reduction in restricted versus controls (Fig. 3a).
FIG. 3.
a) Average cross-sectional area of fetal and maternal blood spaces within three randomly selected images of the labyrinth zone from three placentas per mother (control, n = 8; restricted, n = 7; restricted + leptin, n = 6). Bars with different letters are significantly different (Tukey multiple comparison test). Representative images of blood spaces from control (b), restricted (c), and restricted + leptin (d) placentas. F, fetal blood space (distinguished by nucleated red blood cells); M, maternal blood space. Bar = 50 μm.
Microarray Analysis
We analyzed changes in gene expression among placentas of the control, restricted, and restricted + leptin mothers by whole-genome microarray (GEO accession no. GSE38095). All possible pairwise comparisons were made to identify significant differences among groups (Fig. 4 and Supplemental Table S1, available online at www.biolreprod.org). Selected individual gene expression changes were validated by real-time RT-PCR, along with related genes that were not shown to be differentially expressed by microarray (Table 3). There were six different patterns of gene expression differences among the groups. The first pattern was of transcripts significantly upregulated or downregulated by food restriction but not affected by leptin treatment, which consisted of 87 genes (Fig. 4, yellow). Conversely, 241 genes were significantly changed by leptin treatment but not affected by food restriction (Fig. 4, pink). Other changes induced by food restriction were either completely (25; Fig. 4, cyan) or partially (169; Fig. 4, green) reversed when the food-restricted mothers also were injected with leptin. An additional 188 genes were expressed differently between the restricted and restricted + leptin groups (Fig. 4, blue) but weren't different between controls and either restricted or restricted + leptin groups. Finally, the pattern that accounted for the most gene expression differences (800; Fig. 4, red) was that in which leptin injection exacerbated the effects of food restriction; these genes were expressed differently in the restricted + leptin and control groups, with expression in the restricted group not significantly different from either restricted + leptin or control. Thus, although leptin tended to reverse the effects of food restriction on the expression of 382 genes (Fig. 4, cyan, green, and blue), it intensified the effects of food restriction on more than twice that number of genes (Fig. 4, red and pink).
FIG. 4.
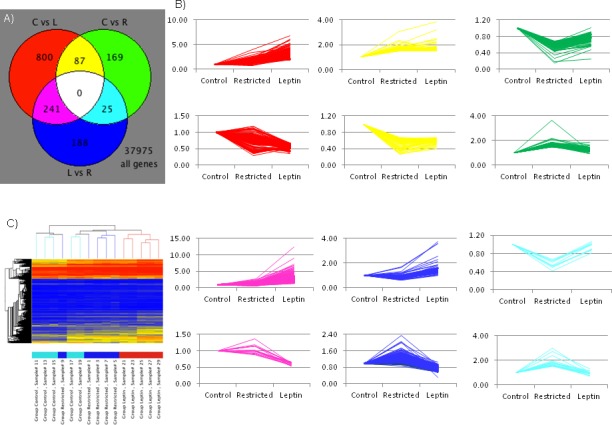
Microarray analysis of global gene expression profiles in RNA pooled from two placentas each from five mothers per group. A) Venn diagram indicates numbers of differentially expressed genes (P < 0.05, fold change >1.5) in each pairwise comparison. C, control; R, restricted; L, restricted + leptin. B) Normalized gene expression (1 = 75th percentile) is indicated in the line graphs for each of the differentially expressed genes, with line colors corresponding to the Venn diagram (e.g., expression patterns of the 800 genes differentially expressed between control and restricted + leptin placentas are shown with red lines). C) Clustering of samples based on genes differentially expressed in at least one of the three pairwise comparisons. In the sample key shown on the bottom: light blue, controls; blue, restricted; red, restricted + leptin. In the tree: red, high expression; yellow, medium expression; blue, low expression.
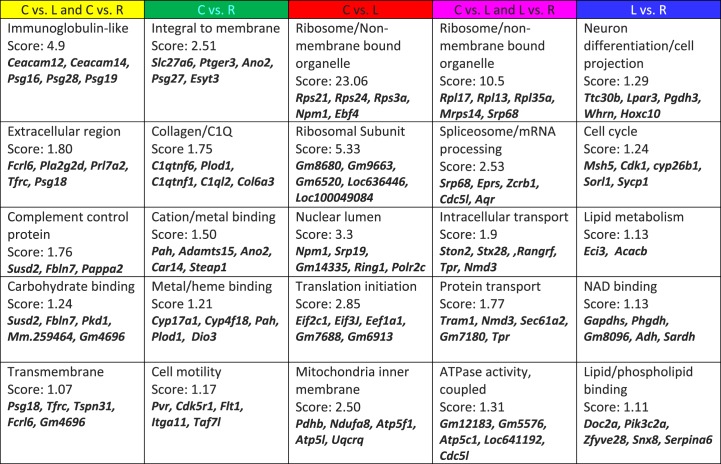
To further analyze the observed gene expression changes, DAVID functional annotation clustering was used to identify pathways, physiological functions, cellular localization, or other meaningful commonalities among the regulated genes (Fig. 5). Among the genes (yellow) that were upregulated or downregulated by food restriction compared with controls, but not different between restricted and restricted + leptin mice, five clusters were identified as being significantly overrepresented (Fig. 5). The most significant cluster, genes related to immunoglobulins during pregnancy, was dominated by members of the carcinoembryonic antigen family—genes encoding four CEA-related adhesion molecules (CEACAMS) and six pregnancy-specific glycoprotein (PSG) family members were downregulated by food restriction.
FIG. 5.
DAVID analysis of functional categories of differentially expressed genes in the placenta. Each column indicates categories significantly overrepresented among the genes differentially expressed between pairs of maternal treatment groups (e.g., between placentas of control and food-restricted mothers [green]). C, control; R, food restricted; L, food restricted and leptin injected.
The top five functional clusters identified among the genes that were altered by restriction + leptin, but not by food restriction alone (Fig. 4, pink), are shown in Figure 5. Ribosomal genes were highly overrepresented among the leptin-upregulated genes, and a subset of these was related to RNA splicing and processing, the second most overrepresented cluster. Leptin also upregulated genes associated with intracellular transport, particularly RAS-RAN-associated nuclear export. The fifth cluster includes genes related to both ATP use and ATP generation, including components of the mitochondrial ATPase complex. Not shown in Figure 5 is a cluster related to glucose metabolism, with an enrichment score of 1.05. Leptin upregulated genes for glucose-6-phosphate dehydrogenase (G6pd2), the rate-limiting enzyme in the pentose phosphate pathway, and for an inhibitory subunit of protein phosphatase 1 (Pp1r2), the rate-limiting step in glycogen synthesis.
Among the genes that were differentially expressed between restricted and restricted + leptin placentas, but not different between controls and either group (Fig. 4, red), were key genes in glucose metabolism. Pdhb, the rate-limiting step for entry into the tricarboxylic acid cycle, trended higher in restricted versus controls (1.43-fold) and was significantly different between restricted + leptin (1.66-fold) and controls, although this change was not detected by real-time RT-PCR (Table 3). Pgm1, which can participate in either glycogen breakdown or synthesis, showed a similar expression pattern.
There were other similarities in the functional clusters identified among genes differentially expressed between the restricted + leptin group and the restricted group (Fig. 4, red) or the restricted and control groups (Fig. 4, pink). In both expression patterns, the two most significant functional clusters were related to ribosomes, and the fourth cluster for the red genes, translation initiation, also included several ribosomal protein genes. The fifth clusters in the two categories were also related, with mitochondrial ATPase components represented in each.
There were only 25 gene expression changes induced by food restriction that were completely reversed by leptin treatment (Fig. 4, cyan, different in restricted vs. control and vs. restricted + leptin). Thus, for functional clustering, these were combined with gene changes that were only partially reversed by leptin treatment (Fig. 4, green, different in restricted vs. control, intermediate in restricted + leptin). The most significant cluster, encoding membrane-inserted proteins, accounted for approximately half of the genes in the combined list (Fig. 5). The next category identified by DAVID analysis was genes encoding proteins that contain the complement component C1q domain. The third category, cation- and metal-binding proteins, includes metalloproteinases and cation transporters, and it overlaps somewhat with the fourth category, which consists mainly of heme-binding proteins (Fig. 5). These include Cyp17a1, the enzyme responsible for progesterone synthesis, which was decreased under food restriction but partially restored by leptin injection. Dio3, which inactivates thyroid hormone T4, was increased under food restriction, and this was reversed by leptin injection.
The final pattern of gene expression changes is of those significantly different between restricted + leptin and restricted, but not different between controls and either restricted + leptin or restricted (Fig. 4, blue). The top annotation clusters for genes with this pattern were mostly different from the other leptin-regulated genes, and enrichment scores were not as high as for the other gene expression patterns (Fig. 5). The third cluster contained only two genes, Eci3 and Acacb, which are nonetheless significant to lipid metabolism. Acacb, which encodes the rate limiting enzyme in fatty acid oxidation, was decreased in restricted + leptin placentas versus restricted and controls.
Oxidative Phosphorylation Complexes
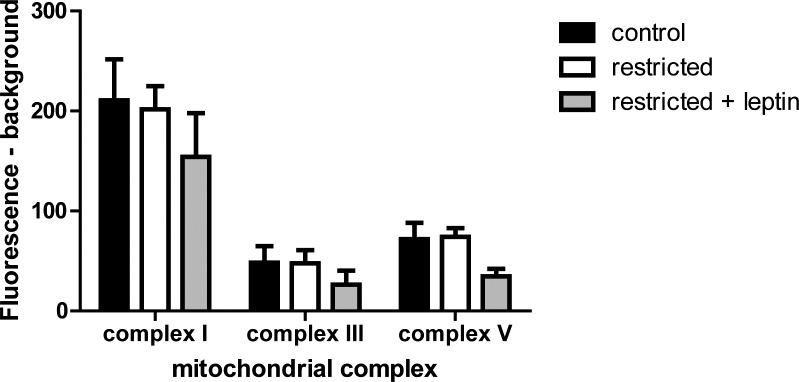
The microarray analysis indicates modestly higher expression of several mitochondrial oxidative phosphorylation components in the placentas of restricted + leptin mice, including multiple subunits of ATPase synthase (complex V) and NADH-ubiquinone oxidoreductase (complex I; Fig. 5 and Supplemental Table S1). Therefore, the relative amount of each protein complex (I, III, and V) was assessed by multiplex immunobead assay. There were no significant differences among treatment groups (Fig. 6). Additionally, the change in expression in Atp5c1 detected by microarray was not detected by real-time RT-PCR (Table 3).
FIG. 6.
Immunobead measurement of mitochondrial oxidative phosphorylation complexes I, III, and V in placental protein lysates (control, n = 8; restricted, n = 7; restricted + leptin, n = 6).
DISCUSSION
We designed a series of studies to determine the role of leptin depletion in the response to food restriction during pregnancy. We previously found that obesity and insulin resistance were increased in female offspring of restricted + leptin-treated mothers relative to offspring of control mothers or mothers that were only food restricted [18]. Results of the present study are consistent with these findings from adult offspring. Although leptin treatment prevented a significant loss of placental weights and fetal placental capillary area during food restriction, it otherwise led to placentas that were more abnormal than those from mothers subjected to food restriction alone.
There were 281 genes differentially expressed between placentas of control and restricted mothers, but there were 1128 significant expression differences between placentas of control and restricted + leptin-injected mothers. Among these changes, the most numerous (800) were for genes that were differentially expressed between controls and restricted + leptin placentas but expressed at an intermediate level in restricted placentas. In other words, addition of leptin exacerbated effects of food restriction on expression of many genes.
Among genes whose expression was disrupted by leptin treatment, the most represented functional category was ribosomal genes, and other translation-related genes were also overrepresented. This finding is in agreement with a previous in vitro study that showed that leptin stimulates protein synthesis in a concentration-dependent manner in JEG-3 cells and human placental explants [22]. Although we did not see the same increase in Eif4e reported by Perez-Perez et al. [22], expressions of Eif3j and Eif2c1 were significantly increased with leptin treatment. For many of the ribosomal and other translation-related genes, expression was significantly different between placentas of control and restricted + leptin mothers, and was intermediate in the food-restricted-only group—not significantly different from either controls or restricted + leptin. Thus, there was some tendency for food restriction alone to increase expression of these genes and leptin to exacerbate this response. Upregulation of a large number of ribosomal proteins was previously demonstrated in response to 24-h starvation on pregnancy Days 12–13 [23]. In that study, the increased ribosomal gene expression was accompanied by increased expression of genes encoding proteosome and ubiquitinylating factors, leading the authors to speculate that placental cells may use ribosomes as an energy source during food restriction in the process of autophagy, as yeast do [23]. We did not see the same evidence of proteasome upregulation, and although there was a modest increase in expression of genes for ubiquitin-conjugating enzymes Ube2e1 and Ube2w, ubiquitination was not a significantly regulated pathway overall. In previous studies, leptin has exhibited mixed effects on autophagy, with both leptin treatment and leptin receptor knockout leading to increased autophagy [24]. Although it is not clear whether the increase in large numbers of ribosomal proteins that we observed is indicative of increased protein synthesis, autophagy, or both, it is clear that food restriction is driving a small change in ribosomal gene expression that is exacerbated by leptin treatment.
Another area in which leptin treatment most altered gene expression was in mitochondrial genes, particularly those associated with oxidative phosphorylation. There were increases in expression of multiple components of complex I, NADH dehydrogenase; one of complex III, cytochrome c reductase; and multiple components of complex V, ATP synthase (Fig. 5 and Supplemental Table S1). However, at the protein level, there were no significant differences in the amount of complexes I, III, and V, with if anything a tendency toward decreases in all three (Fig. 6). Nor was a change in Atp5c1 detected by PCR (Table 3). Further work is needed to resolve the apparent discrepancy between the microarray and other results. There may be a shift in the relative expression of some subunits rather than in the total amount of the complexes, and we did not determine whether there were changes in activity of any of the complexes. A similar increase in transcription of mitochondrial genes related to oxidative phosphorylation was reported in the hypothalamus in response to leptin treatment during fasting [25, 26]. It has also been shown in rat muscle, where leptin and caloric restriction, but not leptin alone, increased mitochondrial oxidative phosphorylation genes [27]. This suggests that the combination of food restriction and high leptin results in a unique response not seen in normal physiological conditions of low energy stores/low leptin or high energy stores/high leptin, but its functional significance is not clear.
When analyzing placental morphology, we found evidence of adaptation to food restriction. Although the total areas of the placenta and junctional zone were smaller in the food-restricted groups than in controls, the labyrinth makes up a larger proportion of the placenta in the restricted groups. The labyrinth is the site of nutrient exchange between maternal blood spaces and fetal capillaries, and thus sparing this region may minimize the effects of nutrient restriction on fetal growth. Similar results were reported by Coan et al. [28], who showed an increase on Day 16 in the relative size of the labyrinth zone in the lightest placentas in a litter relative to that in the heaviest placentas. This research group also showed that the area of the junctional zone, but not the labyrinth zone, was significantly reduced at Day 16 when dams were restricted to 80% of stage-matched ad libitum food consumption [29]. The relative sparing of labyrinth zone area under nutrient restriction was not significantly altered by leptin.
The change in fetal blood space area was leptin dependent. There was a significant reduction in the cross-sectional area of fetal blood spaces in placentas from food-restricted mothers compared with those from restricted + leptin mothers. Leptin is generally thought to be proangiogenic [30], although it also has been shown to inhibit VEGF secretion by cultured human cytotrophoblast cells [31]. Our microarray analysis did not show changes in Vegf expression, and although expression of the receptor, Flt1, was significantly decreased in restricted placentas, it was similar in controls and restricted + leptin placentas (Supplemental Table S1). Another class of genes associated with vascular remodeling whose expression was altered by food restriction was the pregnancy-specific glycoproteins [32, 33], although these changes were not further altered by leptin treatment. One of the most overrepresented categories among gene expression changes caused by food restriction but reversed by leptin treatment was the complement component 1q (C1q) family. This includes C1qtnf6 expression, which was decreased in the food-restricted placentas, but not in the restricted, leptin-treated placentas, relative to controls. C1QTNF6 has been implicated in tumor angiogenesis [34]. Furthermore, mice that lack C1q have a pre-eclampsia/IUGR-like phenotype, with inadequate trophoblast invasion and incomplete vascular remodeling [35, 36]. Further study of the role of this pathway in placental angiogenesis is needed, but it may be associated with the morphological differences in fetal blood space that we observed.
We previously demonstrated that pregnant mice restricted to 50% of normal food consumption from Days 1.5 to 11.5 of pregnancy show a sharp drop in serum leptin concentrations (7.6 ± 1.6 vs. 0.3 ± 0.2 ng/ml) [6], and thus we were surprised to find no difference in serum leptin between the control and restricted animals at Day 11.5 in this study. We reasoned that the only difference between the current and prior study was the addition of saline injections to both control and restricted groups that were given to control for leptin injections. This indeed prevented a significant decrease in leptin when we directly compared saline-injected and noninjected mice following food restriction, although there was a numeric decrease, unlike in the full study. Glucocorticoids, which are likely released in response to injection stress, have been shown to induce leptin expression [37], although this may be blunted by fasting [38]. Because leptin was only measured at 2 h after injection, we do not know whether the saline injection affected leptin levels in a sustained way or only transiently. Leptin concentrations in food-restricted mice were, however, much lower than in the restricted + leptin mice, still allowing us to identify effects of leptin during food restriction by comparing these two groups.
Overall, our findings suggest that elevated leptin disrupts the adaptive response to food restriction in the placenta. Placentas exposed to high leptin during food restriction had many more gene expression differences from controls than those that were only food restricted and showed less morphological evidence of adaptation to food restriction. Although the combination of elevated leptin and nutrient restriction does not occur naturally, this model allows us to separate out the effects of altered leptin concentrations from other factors that coexist with changes in leptin concentrations in normal physiology. By artificially increasing leptin during food restriction, we show the importance of low leptin in the normal physiological response to restriction. Conversely, by examining high leptin concentrations in the absence of obesity, we also uncovered effects of leptin that cannot normally be distinguished from other aspects of obesity. We have previously shown in this model that the female offspring of the mothers exposed to high leptin during food restriction resembled the offspring of obese mothers, which suggests that high concentrations of leptin are responsible for some of the effects of maternal obesity on offspring [18]. In the present study, we identify changes in placental gene expression and morphology that occur in response to high leptin in the absence of obesity. Because the placenta is responsible for the exchange of all nutrients and growth factors between maternal and fetal circulations, and it is directly exposed to maternal leptin, it is likely to mediate the observed effects on offspring health [12, 13]. Further work will be needed to establish precisely how changes in fetal blood space area, junctional space area, translational machinery, etc. alter fetal development.
ACKNOWLEDGMENT
The authors wish to thank Lisa Mao for technical assistance. Thanks to Luis Martinez-Lemus for assistance with oxidative phosphorylation assays.
Footnotes
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development grant HD055231.
REFERENCES
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372: 425 432 [DOI] [PubMed] [Google Scholar]
- Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab 1996; 81: 4406 4413 [DOI] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med 1995; 1: 1155 1161 [DOI] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature 1996; 382: 250 252 [DOI] [PubMed] [Google Scholar]
- Ahrén B, Månsson S, Gingerich RL, Havel PJ. Regulation of plasma leptin in mice: influence of age, high-fat diet, and fasting. Am J Physiol 1997; 273: R113 R120 [DOI] [PubMed] [Google Scholar]
- Schlitt JM, Schulz LC. The source of leptin, but not leptin depletion in response to food restriction, changes during early pregnancy in mice. Endocrine 2012; 41: 227 235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH. Broughton Pipkin F, Stephenson T, Symonds ME. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology 2003; 144: 3575 3585 [DOI] [PubMed] [Google Scholar]
- Schulz LC, Widmaier EP, Qiu J, Roberts RM. Effect of leptin on mouse trophoblast giant cells. Biol Reprod 2009; 80: 415 424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameo P, Bischof P, Calvo JC. Effect of leptin on progesterone, human chorionic gonadotropin, and interleukin-6 secretion by human term trophoblast cells in culture. Biol Reprod 2003; 68: 472 477 [DOI] [PubMed] [Google Scholar]
- Schulz LC, Widmaier EP. The effect of leptin on mouse trophoblast cell invasion. Biol Reprod 2004; 71: 1963 1967 [DOI] [PubMed] [Google Scholar]
- Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab 2003; 88: 1205 1211 [DOI] [PubMed] [Google Scholar]
- Sibley CP, Brownbill P, Dilworth M, Glazier JD. Review: Adaptation in placental nutrient supply to meet fetal growth demand: implications for programming. Placenta 2010; 31 (suppl): S70 S74 [DOI] [PubMed] [Google Scholar]
- Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ 1990; 301: 259 262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers MH. Developmental programming of the metabolic syndrome—critical windows for intervention. World J Diabetes 2011; 2: 137 148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz LC. The Dutch Hunger Winter and the developmental origins of health and disease. Proc Natl Acad Sci U S A 2010; 107: 16757 16758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Friedman MI. Obesity and adipocyte abnormalities in offspring of rats undernourished during pregnancy. Science 1982; 215: 1518 1519 [DOI] [PubMed] [Google Scholar]
- Jones AP, Simson EL, Friedman MI. Gestational undernutrition and the development of obesity in rats. J Nutr 1984; 114: 1484 1492 [DOI] [PubMed] [Google Scholar]
- Pennington KA, Harper JL, Sigafoos AN, Beffa LM, Carleton SM, Phillips CL, Schulz LC. Effect of food restriction and leptin supplementation on fetal programming in mice. Endocrinology 2012; 153 (9): 4556 4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend KL, Lorenzi MM, Widmaier EP. High-fat diet-induced changes in body mass and hypothalamic gene expression in wild-type and leptin-deficient mice. Endocrine 2008; 33: 176 188 [DOI] [PubMed] [Google Scholar]
- Czibere L, Baur LA, Wittmann A, Gemmeke K, Steiner A, Weber P, Putz B, Ahmad N, Bunck M, Graf C, Widner R, Kuhne C. et al. Profiling trait anxiety: transcriptome analysis reveals cathepsin B (Ctsb) as a novel candidate gene for emotionality in mice. PLoS One 2011; 6: e23604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sferruzzi-Perri AN, Macpherson AM, Roberts CT, Robertson SA. Csf2 null mutation alters placental gene expression and trophoblast glycogen cell and giant cell abundance in mice. Biol Reprod 2009; 81: 207 221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Perez A, Gambino Y, Maymo J, Goberna R, Fabiani F, Varone C, Sanchez-Margalet V. MAPK. and PI3K activities are required for leptin stimulation of protein synthesis in human trophoblastic cells. Biochem Biophys Res Commun 2010; 396: 956 960 [DOI] [PubMed] [Google Scholar]
- Broad KD, Keverne EB. Placental protection of the fetal brain during short-term food deprivation. Proc Natl Acad Sci U S A 2011; 108: 15237 15241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik SA, Marino G, BenYounes A, Shen S, Harper F, Maiuri MC, Kroemer G. Neuroendocrine regulation of autophagy by leptin. Cell Cycle 2011; 10: 2917 2923 [DOI] [PubMed] [Google Scholar]
- Jovanovic Z, Tung YC, Lam BY, O'Rahilly S, Yeo GS. Identification of the global transcriptomic response of the hypothalamic arcuate nucleus to fasting and leptin. J Neuroendocrinol 2010; 22: 915 925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung YC, Ma M, Piper S, Coll A, O'Rahilly S, Yeo GS. Novel leptin-regulated genes revealed by transcriptional profiling of the hypothalamic paraventricular nucleus. J Neurosci 2008; 28: 12419 12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barazzoni R, Zanetti M, Bosutti A, Biolo G, Vitali-Serdoz L, Stebel M, Guarnieri G. Moderate caloric restriction, but not physiological hyperleptinemia per se, enhances mitochondrial oxidative capacity in rat liver and skeletal muscle–tissue-specific impact on tissue triglyceride content and AKT activation. Endocrinology 2005; 146: 2098 2106 [DOI] [PubMed] [Google Scholar]
- Coan PM, Angiolini E, Sandovici I, Burton GJ, Constancia M, Fowden AL. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J Physiol 2008; 586: 4567 4576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sferruzzi-Perri AN, Vaughan OR, Coan PM, Suciu MC, Darbyshire R, Constancia M, Burton GJ, Fowden AL. Placental-specific Igf2 deficiency alters developmental adaptations to undernutrition in mice. Endocrinology 2011; 152: 3202 3212 [DOI] [PubMed] [Google Scholar]
- Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science 1998; 281: 1683 1686 [DOI] [PubMed] [Google Scholar]
- Islami D, Bischof P, Chardonnens D. Modulation of placental vascular endothelial growth factor by leptin and hCG. Mol Hum Reprod 2003; 9: 395 398 [DOI] [PubMed] [Google Scholar]
- Wu JA, Johnson BL, Chen Y, Ha CT, Dveksler GS. Murine pregnancy-specific glycoprotein 23 induces the proangiogenic factors transforming-growth factor beta 1 and vascular endothelial growth factor a in cell types involved in vascular remodeling in pregnancy. Biol Reprod 2008; 79: 1054 1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne F, Ball M, McLellan AS, Dockery P, Zimmermann W, Moore T. Mouse pregnancy-specific glycoproteins: tissue-specific expression and evidence of association with maternal vasculature. Reproduction 2006; 131: 721 732 [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Adachi Y, Nagayama T. Expression of a secretory protein C1qTNF6, a C1qTNF family member, in hepatocellular carcinoma. Anal Cell Pathol (Amst) 2011; 34: 113 121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostinis C, Bulla R, Tripodo C, Gismondi A, Stabile H, Bossi F, Guarnotta C, Garlanda C, De Seta F, Spessotto P, Santoni A, Ghebrehiwet B. et al. An alternative role of C1q in cell migration and tissue remodeling: contribution to trophoblast invasion and placental development. J Immunol 2010; 185: 4420 4429 [DOI] [PubMed] [Google Scholar]
- Singh J, Ahmed A, Girardi G. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension 2011; 58: 716 724 [DOI] [PubMed] [Google Scholar]
- De Vos P, Saladin R, Auwerx J, Staels B. Induction of ob gene expression by corticosteroids is accompanied by body weight loss and reduced food intake. J Biol Chem 1995; 270: 15958 15961 [DOI] [PubMed] [Google Scholar]
- Laferrere B, Fried SK, Hough K, Campbell SA, Thornton J, Pi-Sunyer FX. Synergistic effects of feeding and dexamethasone on serum leptin levels. J Clin Endocrinol Metab 1998; 83: 3742 3745 [DOI] [PMC free article] [PubMed] [Google Scholar]